1. INTRODUCTION
Infectious diseases are one of the major causes of death in tropical countries. For a long time, chemotherapy based on the use of antibiotics remained the fastest and most effective way to treat infections. Unfortunately, the upsurge of microbial resistance to antibiotics and the spread rate of resistant bacterial species became major public health concerns. In recent years, research has turned to therapeutic alternatives to prevent the upsurge of bacterial resistance to existing molecules. Among the strategies developed, the inhibition of virulence factors or the inhibition of quorum sensing could be found [1,2]. All of which is characterized by the ability of the bacterium to control gene expression leading to the secretion of proteins involved in virulence, bioluminescence, or biofilms formation [3–5]. Recently, the regulation of bacterial infection pathogenicity was carried out through the development of antipathogenic agents. All of which modulate bacterial diseases by inhibiting the bacterial communication process called bacterium quorum sensing (QS). The quorum communication system regulates the release of Pseudomonas virulence factors, such as protease, elastase, pyocyanin, alginate, biofilm formation, bacterial motility, and toxin production [6]. Similarly, human exposure to environmental pollution including electromagnetic radiation, cosmic radiation, UV light, ozone, cigarette smoke, and low wavelength electromagnetic radiations has been identified in several studies as a leading cause of main diseases [7–10]. Among them are cardiovascular and neurodegenerative diseases in humans due to an imbalance of redox states, involving either excessive generation of reactive oxygen species (ROS) or dysfunction of the antioxidant system [7–10]. Several mechanisms are used by the body to counteract the harmful effects caused by oxidative stress. Endogenous antioxidants systems with catalase, glutathione/thiol regulation, food-derived antioxidants, or other exogenous antioxidants enhance the body’s defense by trapping ROS and thus reducing the oxidation of cell molecules, thereby reducing oxidative stress [11,12]. Natural antioxidants, especially those derived from plants, could be considered as molecules that can effectively fight the damage caused by oxidative stress in human cells with little or no side effects compared to synthetic antioxidants [13]. Previous reports showed that herbal antioxidants were successfully used as rejuvenators or as supplements in boosting the endogenous antioxidant defenses of the body; and was found to be a promising method of countering the side effects of oxidative stress [14,15]. Most medical applications of antimicrobial and antioxidant drugs have been completed. Therefore, plants could contribute to the development of new therapeutic agents facilitating the control of bacteria pathogenicity and allowing the reduction of reactive oxygen species in the body.
In the search for plants with effective antimicrobial activity, we investigated the traditional use of species from genus Acacia in Benin. Different databases were consulted to examine the literature concerning these species. Consequently, the present study aimed to investigate the antibacterial, anti-quorum sensing, antioxidative effects, and oral acute toxicity of extracts and fractions from the leafy stems of Acacia macrostachya (Fabaceae) which is used in alternative medicine to treat infections and gastrointestinal disorders [16].
2. MATERIALS AND METHODS
2.1. Plant Material
The leafy stems of A. macrostachya were collected in April 2015 from Tanguiéta, Department of Atacora, in Bénin. Botanical determination was performed by taxonomists from the National Herbarium of the University of Abomey-Calavi. The collected plant was then registered under a number AA6616/HNB.
2.2. Preparation of Plant Extracts
Plant was washed quickly with distilled water and dried under laboratory temperature (22°C ± 3°C) and then crushed using a grinder (Longyue LY-989). The aqueous and ethanolic extracts were prepared according to the method previously described with minor modifications [17]. After extraction, the filtrates were evaporated in vacuo to obtain dry aqueous and ethanolic extracts.
2.3. Fractionation of Ethanolic Extract
The most active ethanolic extract after preliminary antibacterial assay was subjected to fractionation using the liquid-liquid partition method. An amount of dry ethanolic extract (75 g) from A. macrostachya was dissolved in 700 ml of ethanol/water (20:80, v/v). The mixture was subjected to a successive partition using hexane, dichloromethane, and ethyl acetate. The biological activity of crude extracts and obtained fractions were evaluated.
2.4. Total Phenolic Content
Total phenolic content was determined using the Folin–Ciocalteu reagent method as described previously with slight modification [18]. The reaction mixture, composed of 1 ml of extract, 1 ml of Folin–Ciocalteu reagent, and 800 μl of a sodium carbonate solution (75 g/l), was incubated at room temperature for 2 hours. Absorbance was read at 765 nm. Gallic acid was used as a reference and the results were expressed as mg GAE/100 mg of plant materials.
2.5. Total Flavonoids Content
The total flavonoids content was determined using the aluminum trichloride method and Quercetin as a reference compound [19]. One milliliter of extract at 100 μg/ml was added to 1 ml of aluminum trichloride (10%). After 10 minutes of incubation, absorbance was measured at 415 nm. The total flavonoids content was expressed as mg Equivalent Quercetin/100 mg of plant materials.
2.6. Total Condensed Tannin Contents
Vanillin and hydrochloric acid method was used to determine the total condensed tannins content [20]. Catechin was used as a reference compound. The optical densitometry absorbance was read at 500 nm. The condensed tannin was stated as mg Equivalent Catechin/100 mg of plant dry weight materials.
2.7. Antioxidant Activity
2.7.1. DPPH scavenging activity
The antioxidant activity of extracts was investigated using a method described previously [21]. Eight concentrations of extracts ranged from 30 to 0.23 μg/ml were tested. Each assay was done by mixing 0.75 ml of extract and 1.5 ml of solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) (0.1 mM) prepared in methanol. In the blank, the sample was replaced by methanol. Ascorbic acid was used as a positive control. Each test was performed three times. Absorbances were then taken at 517 nm using a spectrophotometer (VWR UV-1600 PC). The DPPH scavenging activity of extracts and fractions expressed as inhibition percentage (IP) was determined as described below:
As: represents the extract absorbance; Ab: represents the blank absorbance. The IC50 was calculated using the equation of curve IP = f([C]).
2.7.2. Ferric-Reducing antioxidant power assay
Ferric-Reducing antioxidant power was accessed using the method described by Adjileye et al. [22]. Ascorbic acid was used for calibration curve (y = 0.0069 × +0.015; R2 = 0.995). Each assay was performed in triplicate. The iron (III) reducing activity was expressed in μMol equivalent ascorbic acid/100 μg of extract.
2.7.3. Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging ability was determined according to the method described by Mohan et al. [23]. 100 μg/ml of extract were dissolved in phosphate buffer (0.1 nM, pH 7.4) and mixed with 600 μl of hydrogen peroxide solution. The absorbance values of the reaction mixture were read at 230 nm after 10 minutes. Gallic acid was used as a reference compound. The hydrogen peroxide scavenging ability was calculated as below:
Ac: control absorbance; As: extract absorbance.
2.8. Identification of Phenolic Compounds and Flavonoids
The most active fraction on antioxidant, antibacterial activity, and in the inhibition of virulence factors was analyzed to identify and quantify the presence of phenolic compounds and flavonoids. Phenolic compounds and flavonoids were identified as described previously [24].
2.9. Determination of Antibacterial Activity
2.9.1. Tested microorganisms
Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis CIP8039, Staphylococcus aureus methicillin resistant, Escherichia coli CIP 53126, and P. aeruginosa CIP 82118 were used to evaluate antimicrobial potential of A. macrostachya extracts and fractions. All microorganisms’ strains were obtained from Laboratoire de Biophotonique et Pharmacologie, the University of Strasbourg in France.
2.9.2. Minimum inhibitory concentration (MIC)
The microdilution method using p-iodonitrotetrazolium (INT) reagent was used [25]. The effects of extracts and fractions were evaluated at different concentrations (5–0.078 mg/ml). To determine the MIC, a mix of 100 μl of each overnight bacterial culture [106 colony-forming unit (CFU)/ml] and 100 μl of extracts or fractions was incubated at 37°C for 18 hours. The growth of bacteria was revealed by adding 40 μl of methanolic solution of INT (2%). The MICs values were recorded after further incubation for 1 hour.
2.10. Bioautography Assay
The bioautography test of hexane, dichloromethane, and ethyl acetate fractions was performed on six selected bacteria as described previously [26] with minor modifications. 10 ml of each fraction at 20 mg/ml were loaded onto Thin Layer Chromatography plates [Pre-coated Thin Layer Chromatography (TLC)-sheets ALUGRAM® silica gel 60] and eluted using two different mobile systems, dichloromethane/methanol, 99.8:0.2 and Toluene/ethyl acetate/formic acid/water, 5:100:10:10. The plates were dried overnight, visualized under UV light (365 nm, 254 nm), sprayed with bacterial culture (106 CFU/ml), and incubated at 37°C. After 24 hours, each plate was sprayed with a methanolic solution of p-iodonitrotetrazolium (2%). Bioautography allowed to select the most active fraction for the evaluation of anti-quorum sensing activity.
2.11. Anti-quorum Sensing Activity
2.11.1. Bacterial strains and culture conditions
Two Gram-negative bacteria, Chromobacterium violaceum (CV026) and P. aeruginosa (PAO1), were used to evaluate the antiquorum sensing activity of the ethanol extract and the ethyl acetate fraction from A. macrostachya. These bacteria were obtained from the laboratory of biotechnology (Laboratoire de Biotechnologie Vegetale, Université Libre de Bruxelles, Gosselies, Belgium). These bacteria were grown in Luria-Bertani broth (LB) broth under stirring and temperature conditions which were 175 rpm at 37°C for C. violaceum CV026 and 175 rpm at 30°C for P. aeruginosa PAO1.
2.11.2. Determination of MIC and Minimum Bactericidal Concentration (MBC)
Before evaluated anti-quorum-sensing effects, the MIC of extract and fraction against PAO1 and CV026 was determined as described above [25]. The minimum bactericidal concentration was also determined [27]. This stage of our study allowed the selection of extract or fraction concentrations which has no effect on bacterial growth, and which will be used for the evaluation of the anti-quorum sensing activity.
2.11.3. Effect of extract on the growth and violacein production in C. violaceum CV026
The effect of the ethanolic extract and the ethyl acetate fraction of A. macrostachya on violacein production was evaluated using a method described previously [24]. C. violaceum is a Gram-negative bacterium deficient in the homoserine-lactone synthase gene cviI. The production of violacein is therefore induced by adding N-hexanoyl-l-homoserine lactone (HHL; Sigma-Aldrich Chemie GmbH, Darmstadt, Germany) to the culture medium. Extract and fraction at 10 mg/ml were prepared in dimethyl sulfoxide (DMSO). For the test, 100 μl of C. violaceum CV026 culture (107 CFU/ml) were added to 1,860 μl Luria Bertani broth supplemented with 20 μl HHL (10 mM in DMSO) and 20 μl extract or fraction. The final extract and fraction concentrations were 100 μg/ml and DMSO was used as control.
2.11.4. Effect of extract on growth and pyocyanin production in P. aeruginosa PAO1
Effect of extract and fraction on inhibition of pyocyanin was estimated according to a method described previously [24]. P. aeruginosa PAO1 overnight culture was washed twice with fresh LB medium by centrifugation (7,000 rpm for 15 mn). To evaluate the effet of extract and fraction on pyocyanin production, 100 μl of PAO1 [Optical density (OD)600 nm between 0.02 and 0.03] was added to 1,880 μl LB medium supplemented with 20 μl sample at 10 mg/ml in DMSO. Each assay was done three times and all tubes were incubated at 37°C stirring for 18 hours. At 3 hour periodic intervals, the tubes contents were sampled to assess bacterial growth and pyocyanin production. The pyocyanin was extracted from the supernatant obtained by centrifugation (8,000 rpm, 10 minutes) of the P. aeruginosa culture. 2 ml of chloroform was added to 4 ml of supernatant and the solution was mixed vigorously. The chloroform layer containing pyocyanin was extracted again with 1 ml of HCl (0.2 M). The pyocyanin was quantified spectrophotometrically at 380 nm using a plate reader (Epoch Biotek).
2.12. Statistical Analysis
Data were recorded, analyzed with Microsoft Excel, and reported as mean ± standard deviation of triplicate tests.
3. RESULTS
3.1. Total Polyphenol, Flavonoid, and Condensed Tannin Contents
The preliminary chemical analysis showed a diversity of secondary metabolites. The results were summarized in Table 1. The evaluation of polyphenol content showed significant differences when comparing ethanolic and aqueous extracts. Ethanolic extract had the highest phenolic content (31.85 ± 0.31 mg GAE/100 mg) compared to aqueous extract (21.26 ± 0.67 mg GAE/100 mg).
The total flavonoids and condensed tannins content of ethanolic and aqueous extracts ranging from 26.35 ± 0.71 to 25.42 ± 0.36 mg quercetin equivalent (QE)/100 mg and 18.24 ± 0.13 to 15.9 ± 0.17 mg Catechin equivalent (CE)/100 mg, respectively. Ethanolic extract showed the highest total flavonoids and condensed tannins content.
3.2. Antioxidant Activity
The DPPH scavenging activity was dose-dependent and varying from 4.08% to 97.53% (Fig. 1). Among the two extracts, the ethanolic extract presented more promising results with an IC50 value of 4.15 ± 0.05 μg/ml followed by aqueous extract with IC50 of 4.57 ± 0.7 μg/ml close to that of ascorbic acid (3.83 ± 0.04 μg/ml). Ethyl acetate fraction was the most active with an IC50 value of 4.38 μg/ml followed by aqueous fraction 6.98 μg/ml. Hexane and dichloromethane fractions exhibited the lowest activity with IC50 values of 18.35 and 12.24 μg/ml, respectively.
 | Table 1: Qualitative phytochemical constituents of leafy stems of A. macrostachya. [Click here to view] |
The Ferric-reducing antioxidant activity of extracts and fractions of A. macrostachya was also evaluated for their ability to reduce 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ)-Fe3+ (2,4,6-tri(2-pyridyl)-1,3,5-triazine) complex to TPTZ-Fe2+ [28]. Ethanolic extract exhibited the most important activity (3747.32 μMol Equivalent ascorbic acid (EAA)/100 μg extract) while aqueous extract activity was 3066.99 μMol EAA/100 μg of extract. Among fractions, the highest activity was noted for ethyl acetate fraction (3451.05 μMol EAA/100 μg extract) followed by dichloromethane fraction (2556.74 μMol EAA/100 μg extract). Hexane fraction showed weak Ferric reducing antioxidant power (FRAP) activity (444.41 μMol EAA/100 μg extract). Ethanolic extract and ethyl acetate fraction showed the highest activity with 97.65% and 94.02% scavenging capacity close to gallic acid activity (93.97%). Other extracts and fractions showed low activity ranging from 9.3% to 30.1% (Fig. 2).
3.3. Identification of Phenolic Compounds and Flavonoids
The analysis of ethyl acetate fraction revealed the presence of phenolic compounds (gallic, chlorogenic, tannic, and ferulic acids) and flavonoid (isorhamnetin). Other non-identified phenolic acid and flavonoids (peaks 12, 14, 16, 18) were also revealed on the High Pressure Liquide Chromatography (HPLC) chromatogram (Table 2, Fig. 3).
3.4. Antibacterial Activity
3.4.1. Minimum Inhibitory Concentrations (MIC)
The preliminary antibacterial activity showed that extracts at 10 mg/ml were active against one or more bacteria. Therefore, the minimum inhibitory concentration of all extracts and fractions were evaluated. Ethanolic extract showed the best antibacterial activity by inhibiting the growth of Gram-positive and Gram-negative whereas aqueous extract inhibited only Gram-negative. The MIC values of the extract ranged from 0.312 to 5 mg/ml and results are summarized in Table 3. Ethanolic and aqueous extracts were active against P. aeruginosa with an MIC of 0.312 mg/ml. Among fractions, ethyl acetate was the most active against all tested bacteria followed by dichloromethane fractions (MIC varying from 1.25 to 5 mg/ml) whereas hexane and aqueous fractions were not active (MIC ? 5 mg/ml).
3.4.2. Identification of antimicrobial compounds using bioautography
The bioautography assay revealed inhibition zones for ethyl acetate fraction against both Gram-positive and Gram-negative. Presence of white bands means that ethyl-acetate fraction contains compounds responsible for the antimicrobial activity of the plant. No inhibition was observed with n-hexane and dichloromethane fractions.
 | Figure 1: DPPH scavenging activity and inhibitory concentration 50 (IC50) of extracts and fractions from Acacia macrostachya. Hex = hexane, DcM = dichloromethane, AcOEt = ethyl acetate, Aq = aqueous. [Click here to view] |
 | Figure 2: Ferric-reducing antioxidant power (A) and superoxide scavenging activity (B) of A. macrostachya fractions. EtOH = ethanol, H20 = aqueous, Hex = hexane, DcM = dichloromethane, AcOEt = ethyle acetate, Aq = aqueous. [Click here to view] |
3.5. Effect of Extract and Fraction on Violacein Production
The ethyl-acetate fraction and the ethanolic extract at 100 μg/ml did not inhibit the growth of C. violaceum CV026. At the same concentration, the extract and fraction inhibited the production of violacein. The ethyl acetate fraction was the most active by reducing the production of violacein by 56.45% while the ethanol extract by 17.74% (Fig. 4).
 | Table 2: Phenolic compounds, flavonoids identified in A. macrostachya by U-HPLC 3000. [Click here to view] |
3.6. Effect of Extract and Fraction on Pyocianin Production
Our results showed that the ethanolic extract and the ethyl acetate fraction at 100 μg/ml had no effect on the growth of P. aeruginosa PAO1. At the same concentration, the ethanolic extract and ethyl acetate fraction inhibited the production of pyocyanin. The ethyl acetate fraction was the most active by reducing the production of pyocyanin by 48.88% and ethanolic extract 21.11% (Fig. 5).
4. DISCUSSION
For centuries, herbal medicines have been used and are considered safe and effective because people believe, rightly or wrongly, that herbal medicines would have no toxic effects. However, over the past decade, the effectiveness of many plants has been studied to determine their biological potential and toxicity. In this logic, this study was conducted to investigate the antimicrobial, anti-quorum sensing, and antioxidant activity of A. macrostachya, used in Benin to treat infectious diseases.
 | Figure 3: U-HPLC profile and UV spectrum of ethyl acetate fraction from Acacia macrostachya. Peaks 12 and 18 = non-identified phenolic acid, Peaks 14 and 16 = non-identified flavonoids. [Click here to view] |
The phenolics compounds concentrations of the ethanolic and aqueous extracts are variable. This variability indicates that the extraction of phytoconstituents from plants depends strongly on the type of solvents used. Many authors have reported ethnopharmacological studies on A. Macrostachya. According to the literature, no study has been carried out on the total phenolic content of leafy stems extracts of A. Macrostachya. However, interesting results were reported in phenolic compounds for related species; Acacia albida, Acacia nilotica, and Acacia polyacantha [29,30]. Based on the fascinating results of phytochemical screening and quantification of phenolic, flavonoid, and tannin contents, the ethanolic extract was subjected to a liquid fractionation using three solvents with increasing polarity (hexane, dichloromethane, and ethyl acetate). The biological properties of extracts and obtained fractions were then evaluated.
Biological activities of plant extracts depend highly on the nature and number of active compounds. The complexity of the extracts due to mixtures of secondary metabolites with variable physicochemical properties could lead to scattered results. Accordingly, the approach based on the use of various tests to investigate the antioxidant potential would be the most appropriate procedure. In this study, the antioxidant activity of extracts and fractions was accessed using DPPH scavenging activity, ferric reducing antioxidant power assay, and hydrogen peroxide scavenging activity.
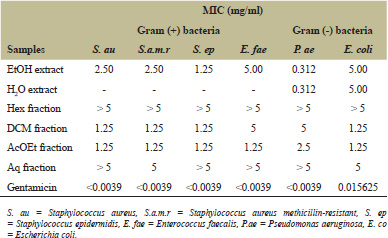 | Table 3: MIC (mg/ml) of extracts and fractions. [Click here to view] |
.png) | Figure 4: Chromobacterium violaceum CVO26 growth kinetics (A) and violacein production (B). AM1 = ethanolic extract, AM2 = ethyl acetate fraction, DMSO 1% = dimethyl sulfoxide 1% used as negative control. [Click here to view] |
The DPPH scavenging activity is a first-line method with relatively easy implementation. Ethanolic, aqueous extracts, and ethyl-acetate fraction showed more promising results in the evaluation of the antioxidant activity. The interesting inhibition of DPPH radical could be related to the presence of the phenolic compounds and flavonoids in the ethanolic extract and ethyl acetate fraction. These molecules, with many antioxidant properties, can neutralize ROS by transferring protons or by acting as electron [31]. It is also known that polyphenols play an important role in maintaining the body through their ability to scavenge ROS, to act as reducing agents or hydrogen donors [32]. The ability of extracts and fractions to reduce TPTZ-Fe3+ complex to TPTZ-Fe2+ suggests that they contain molecules that have the ability to modify free radicals into more stable compounds. It was reported that the scavenging potential and metal chelating ability dependent upon phenolic compounds redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers [33,34]. Hydrogen peroxide (H2O2) scavenging is also an oxidizing agent that inactivates directly some enzymes, usually by oxidation of essential thiol groups as it can cross cell membranes rapidly. Inside the cell, it is converted into hydroxyl radicals that could be responsible for many oxidative effects [35]. The obtained results in H2O2 scavenging assay could be due to the presence of secondary metabolites having antioxidant properties in extracts and fractions. A. macrostachya extracts revealed the presence of flavonoids and phenolics which act as antioxidants or free radical scavengers [36,37].
 | Figure 5: Pseudomonas aeruginosa PAO1 growth kinetics (A) and pyocyanin production (B) AM1 = ethanolic extract, AM2 = ethyl acetate fraction, DMSO 1% = dimethyl sulfoxide 1% used as negative control.w [Click here to view] |
The ethanol extract of the leafy stems of A. macrostachya exhibited a broad spectrum of antibacterial activity by inhibiting Gram-positive and Gram-negative tested bacteria whereas aqueous extract inhibited Gram-positive bacteria. The most important inhibition was found against P. aeruginosa with MIC of 0.312 mg/ml. Phytochemical screening of extracts from A. macrostachya revealed the presence of various actives phytoconstituents such as flavonoids, alkaloids, saponins, coumarins, terpenoids, and tannins, which are known to produce an inhibitory effect on bacterial strains [36,38]. The antimicrobial effect of these extracts could be due to the presence of these secondary metabolites. Several mechanisms such as inhibition of deoxyribonucleic acid and ribonucleic acid synthesis, cytoplasmic membrane function, and energy metabolism used by flavonoids to inhibit bacterial growth have been described [37]. It has been reported that the antibacterial effect of terpenoids was related to the damage caused by these molecules to the bacterial membrane [39]. Furthermore, coumarins are secondary metabolites that have demonstrated various biological activities, including antimicrobial properties [40,41]. Also, tannins were known for their antimicrobial property by inhibiting the activity of certain bacterial proteins that promote the growth of bacteria [42]. Compared to the ethanol extract, the ethyl-acetate fraction from the liquid partition of the ethanolic extract showed a better activity on all tested bacteria. This activity could be justified by the presence of many phenolic acids and flavonoids such as gallic, chlorogenic, tannic, ferulic acids, and isorhamnetin. As far as we know, it is the first time that the antibacterial activity of A. macrostachya was described. However, previous studies on related species such as A. polyacantha, Acacia etbaica showed comparable results with MICs from 0.312 to 5 mg [43,44].
The bioautography which aimed to identify directly the antimicrobial active compounds was performed on the dichloromethane and ethyl-acetate fractions, which were active on the bacterial strains tested. These yellow or white areas on a purple red background indicate the presence of antimicrobial compounds [38]. The ethyl-acetate fraction showed a large white area corresponding to the inhibition area of bacteria whereas the spots of the dichloromethane fraction showed no inhibition. The lack of activity in the dichloromethane fraction could be due to the little number of active secondary metabolites [45]. Indeed, this difference between fraction activities could be explained by the liquid fractionation of the ethanolic extract which would have clustered the active compounds in the ethyl-acetate fraction while the other fractions contain little.
The anti-quorum-sensing activity was assessed by evaluating the effect of the most active ethanolic extract and ethyl acetate fraction on QS-regulated violacein in CV026 and pyocyanin production in PAO1. In this study, at a subinhibitory concentration of 100 μg/ml, the ethyl acetate fraction was the most active by reducing the production of violacein and pyocyanin, respectively, by 56.45% and 48.88%, while the ethanol extract reduced by 17.74% and 21.11% compared to control (DMSO 1%). At the same concentration (100 μg/ml), ethanolic extract and ethyl acetate fraction have no effect on the growth of C. violaceum CV026 nor on P. aeruginosa PAO1. Therefore, the inhibition of the production of the violacein could be due to the quenching of the QS system of the bacteria but not to the antibacterial effects of the extracts. Likewise, the reduction in pyocyanin production could be due to some interferences with the QS mechanism controlling the production of pyocyanin by PAO1. In this study, a culture of C. violaceum with tested extract or fraction did not lead to the production of violacein. This result indicates that the tested samples do not contain any mimic HHL metabolites. The anti-quorum sensing activity of some phenolic compounds identified in ethyl acetate fraction was previously evaluated against C. violaceum. These results showed that the activity of these compounds could be due to their capability to regulate the activity and synthesis of homoserine lactone [46]. Numerous studies showed the antioxidant and anti-quorum sensing potential of the compounds identified in extract and ethyl acetate fraction during this study [47]. Reduction of pyocyanin and violacein production observed here could be due to previous identified polyphenols and flavonoids which were known for their anti-quorum sensing activity [48]. It was reported that flavanones, naringenin, quercetin, and taxifolin significantly reduced the generation of pyocyanin and violacein without affecting bacterial growth. Moreover, some flavonoids reduced the expression of several QS-controlled genes (lasR, lasI, lasA, lasB, rhlA, rhlI, rhlR, phzA1) in PAO1 [24,49]. In addition, pyocyanin is involved in host cell deleterious effects by inhibiting their growth, disturbing their respiration, mediating ROS production, and then increasing the oxidative stress on the host cells [50,51]. Pyocyanin induces cell death through different mechanisms that could be related to its redox properties and its capability to reduce molecular oxygen [52,53]. Polyphenols and flavonoids known as antioxidant compounds could participate in the decreasing of the oxidative stress caused by pyocyanin. The anti-quorum sensing activity of ethyl acetate fraction would be an asset for cell protection while inhibiting or reducing the production of the virulence factor. It is well documented that flavonoids have antimicrobial and antioxidant properties [54].
5. CONCLUSION
The present study highlighted the antioxidant and antimicrobial activities of aqueous and ethanolic extracts of A. macrostachya leafy stems. The results also demonstrated the anti-quorum sensing activity of the ethyl acetate fraction from the ethanolic extract. Polyphenols and flavonoids in these extract and fraction might be responsible for the anti-quorum sensing activity. These results could be investigated in the management of infectious diseases and in the control of oxidative stress. These results confirm the traditional use of A. macrostachya leafy stems in the management of infected diseases and digestive system disorders. By reducing the production of pyocyanin and violacein and the associated oxidative stress in infected tissues, A. macrostachya extract is therefore beneficial to the treatment process of infectious diseases caused by resistant bacteria.
ACKNOWLEDGMENTS
The authors would like to thank and acknowledge Mr. Honoré Zoclanclounon, Technician in Laboratory of Humain Biology, Faculty of Medicine, the University of Abomey-Calavi for his help and Dr. Capo-Chichi D. Callinice who corrected the manuscript for this latest version.
REFERENCES
1. Brooks BD, Brooks AE. Therapeutic strategies to combat antibiotic resistance. Adv Drug Deliv Rev 2014;78:14–27. CrossRef
2. Tang K, Zhang XH. Quorum quenching agents: resources for antivirulence therapy. Mar Drugs. 2014;12(6):3245–82. CrossRef
3. Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the luxR-luxI family of cell density-responsive transcriptional regulators. J Bacteriol 1994;176:269–75. CrossRef
4. Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic resistance related to biofilm formation in Klebsiella pneumonia. Pathogens 2014;3: 743–58. CrossRef
5. Arendrup MC, Patterson TF. Multidrug-resistant candida: epidemiology, molecular mechanisms and treatment. J Infect Dis 2017;15:216(Suppl 3):445–51. CrossRef
6. Zhang LH, Dong YH. Quorum sensing and signal interference: diverse implications. Mol Microbiol 2004;53(6):1563–71. CrossRef
7. Oyedemi SO, Bradley G, Afolayan AJ. In-vitro and -vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr J Pharm Pharmacol 2010;4:70–8.
8. Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM. Biological and physiological role of reactive oxygen species-the good, the bad and the ugly. Acta Physiol (Oxf) 2015;214(3):329–48. CrossRef
9. Kim GH, Kim JE, Rhie SJ, Yoon S. The Role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 2015;24(4):325–40. CrossRef
10. Liu Z, Ren Z, Zhang J, Chuang C-C, Kandaswamy E, Zhou T, et al. Role of ros and nutritional antioxidants in human diseases. Front Physiol 2018;9:477. CrossRef
11. Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001;40(8):959–75. CrossRef
12. Kerksick CM, Zuh M. Mechanisms of oxidative damage and their impact on contracting muscle. In: Lamprecht M (ed.). Antioxidants in Sport Nutrition, CRC Press/Taylor & Francis, Boca Raton, FL, 2015. CrossRef
13. Akira Y, Ichinose A, Kawai C, Kuribayashi F. The phagocyte NADPH oxidase and bacterial infections. Kawasaki Med J 2012;3:11–8.
14. Panchawat S, Rathore KS, Sisodia SS. A review on herbal antioxidants. IntJ PharmTech Res 2010;2(1):232–9
15. Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of Antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 2015;11(8):982–91. CrossRef
16. Arbonnier M. Arbres, arbustes et lianes des zones sèches de l’Afrique de l’Ouest. CIRAD/ MNHN/ UICN, 541 p, 2000.
17. Lagnika L, Tchatchedre M, Amoussa A, Latoundji K, Sanni A. Phytochemical assessment, in vitro antimicrobial and antioxidant activities of Acacia hockii De Wild. Adv Biol BioMed 2016;3(1):1–8.
18. Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 2007;102:771–6. CrossRef
19. Nadhiya K, Vijayalakshmi K. Evaluation of total phenol, flavonoid contents and in vitro antioxidant activity of benincasa hispida fruit extracts. Int J Pharm, Chem Biolo Sci 2014;4:332–8.
20. Sun B, Richardo da-Silvia JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 1998;4267–74. CrossRef
21. Velazquez E, Tournier HA, Buschiazzo MP, Saavedra G, Schinella GR. Antioxydant activity of Paraguayan plant extracts. Fitoterapia 2003;74:91–7. CrossRef
22. Adjileye RAA, Amoussa AMO, Lagnika L. Trema orientalis L. and Dialium guineense Wild. used to manage hypertension in Bénin: phytochemical study and antioxidant activity. J Med Plants Stud 2019;7(3):43–8.
23. Mohan SC, Balamurugan V, Thiripura SS, Rekha R. Metal ion chelating activity and hydrogen peroxide scavenging activity of medicinal plant Kalanchoe pinnata. J Chem Pharm Res 2012;4(1):197–202.
24. Mounirou Tchatchedre., et al. “Anti-Quorum Sensing, Antibacterial, Antioxidant Activity and Acute Oral Toxicity of Acacia hockii De Wild. (Fabaceae)”. EC Microbiol 2019 ; 15(8): 744–758.
25. Keymanesh K, Hamedi J, Moradi S, Mohammadipanah F, Sardari S. Antibacterial, antifongique and toxicity of rare Iranian plants. Int J Pharm 2009;5:81–5. CrossRef
26. Srinivas P, Rajashekar V, Upender RE, Venkateshwarulu L, Anil KCH. Phytochemical screening and in vitro antimicrobial investigation of the methanolic extract of Xanthium strumarium leaf. Int J Drug Dev Res 2011;3:286–93.
27. Escalona-Arranz JC, Péres-Roses R, Urdaneta-Laffita I, Camacho-Pozo MI, Rodríguez-Amado J, Licea-Jiménez I. Antimicrobial activity of extracts from Tamarindus indica L. leaves. Pharmacogn Mag 2010;6:242–7. CrossRef
28. Kandhasamy S, Sun CK. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci 2013;20(4):319–25. CrossRef
29. Mohammede N, Ahmed A, Mirghani MES, Kabbashi NA, Alam MZ, Musa KH, et al. Effect of solvent types on phenolics content and antioxidant activities of Acacia polyacantha gum. Int Food Res J 2017;(Suppl 24):369–77.
30. Shirwaikan A, Rajendran K, Dinesh K. In vitro antioxidant studies of Annona squamosa. Indian J Exp Biol 2004;42(8):803–7.
31. Ghasemzadeh A, Omidvar V, Jaafar HZE. Polyphenolic content and their antioxidant activity in leaf extract of sweet potato (Ipomoea batatas). J Med Plants Res 2012;6(15):2971–6. CrossRef
32. Kandhasamy S, Sun CK. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci 2013;20(4):319–25. CrossRef
33. Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compound. Trends Plant Sci 1997;2(4):152–9. CrossRef
34. Pazos M, Gallardo JMM, Torres JL, Medina I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem 2005;92:547–7. CrossRef
35. Parshuram S, Leena P, Vilasrao K. In vitro evaluation of antioxidant activity of dillenia indica Linn. Leaf extract. Int J Pharm Sci Res 2011;2(7):1814–8.
36. Chatoui K, Talbaoui A, Aneb M, Bakri B, Harhar H, Tabyaoui M. Phytochemical screening, antioxidant and antibacterial activity of Lepidium sativum seeds from Morocco. J Mater Environ Sci 2016;7:2938–46. CrossRef
37. Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005;26:343–56.
38. Masola SN, Mosha RD, Wambura PN. Assessment of antimicrobial activity of crude extracts of stem and root barks from Adansonia digitata (Bombacaceae) (African baobab). Afr J Biotechnol 2009;8:5076–83.
39. Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Raiz N, et al. Antimicrobial natural products: an update on future antibiotic drug candidates. Nat Prod Rep 2010;27:238–54. CrossRef
40. Widelski J, Popova M, Graikou K, Glowniak K, Chinou I. Coumarins from Angelica lucida L. antibacterial activities. Mol 2009;14:2729–34. CrossRef
41. Nitiema LW, Savadogo A, Simpore J, Dianou D, Traore AS. In vitro antimicrobial activity of some phenolic compounds (Coumarin and Quercetin) against gastroenteritis bacterial strains. Int J Microbiol Res 2012;3:183–7.
42. Obasi NL, Egbuonu ACC, Ukoha PO, Ejikeme PM. Comparative phytochemical and antimicrobial screening of some solvent extracts of Samanea saman (fabaceae or mimosaceae) pods. Afr J Pure Appl Chem 2010;4:206–12.
43. Koudoro YA, Agbangnan DCP, Yèhouénou B, Tchobo FP, Alitonou GA, Avlessi F, et al. Chemical characterization and biological activities of extracts from two plants (Cissus quadrangularis and Acacia polyacantha) used in veterinary medicine in Benin. J Pharmacogn Phytochem 2015;3(6):91–6.
44. Getachew B, Getachew S, Mengiste B, Mekuria A. In-vitro antibacterial activity of Acacia etbaica against Staphylococcus aureus and Escherichia coli. Int J Pharmacogn 2015; 7(4):219–22.
45. Masoko P, Picard J, Eloff JN. Antifungal activities of six South African Terminalia species (Combretaceae). J Ethnopharmacol 2005; 99:301–8. CrossRef
46. Luís Â, Silva F, Sousa S, Duarte AP, Domingues F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014;30(1):69–79. CrossRef
47. Phenolic compoundsbiological activity. In: Soto-Hernandez M, Palma-Tenango M, Garcia- Mateos MdelR (eds.). Phenolic compounds with anti-virulence properties, IntechOpen, London Bridge, United Kingdom, pp 139–67, 2017.
48. Cho HS, Lee JH, Cho MH, Lee J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015;31(1):1–11. CrossRef
49. Asfour HZ. Anti-quorum sensing natural compounds. J Microsc Ultrastruct 2018;6:1–10 CrossRef
50. Gloyne LS, Grant GD, Perkins AV, Powell KL, McDermott CM, Johnson PV, et al. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol In Vitro 2011;25:1353–8. CrossRef
51. Mossine VV, Waters JK, Deborah LC, Mawhinney TP. Transient proteotoxicity of bacterial virulence factor pyocyanin in renal tubular epithelial cells induces er-related vacuolation and can be efficiently modulated by iron chelators. Toxicol Sci 2016;154(2):403–15. CrossRef
52. Muller M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic Biol Med 2006;41:1670–7. CrossRef
53. Muller M, Li Z, Maitz PK. Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: role for p38 mitogen-activated protein kinase. Burns 2009;35:500–8. CrossRef
54. Jagani S, Chelikani R, Kim DS. Effects of phenol and natural phenolic compounds on biofilm formation by Pseudomonas aeruginosa. Biofouling 2009;25(4):321–4. CrossRef