1. Introduction
Higher organisms are dependent on oxygen for their survival, as it accepts released electrons during biological oxidations in its presence. Oxidative stress arises in consequence of imbalance linking the reactive nitrogen species and/or release of reactive oxygen species (ROSs), and mechanisms with an antioxidant potential [1]. When present at high levels, ROSs should be viewed as a result of oxidative damage [2]. These poorly regulated ROSs are likely to exert deleterious effects on proteins, carbohydrates, nucleic acids, and membrane lipids, resulting in profound alterations in cellular reinforcement, and subsequently, cell death [3]. Numerous age-related illnesses, including cancer, osteoarthritis and atherosclerosis, and neurological disorders including Huntington’s, amyotrophic lateral sclerosis, Alzheimer’s, and Parkinson’s diseases, can be brought on by oxidative stress [4]. The long-term consumption of synthetic antioxidants including butylated hydroxytoluene (BHT), propyl gallate, tert-butylhydroquinone, and butylated hydroxyanisole has potential toxicity to human body [5]. Therefore, it is necessary to replace these molecules with natural antioxidants with high efficiency and low side effect. The phenolic compounds are potential agents that can help prevent and heal several illnesses related to oxidative stress, such as cancer, cardiovascular diseases, aging, neurological disorders, and mellitus diabetes [6]. They are thought to have a high potential for scavenging free radicals and act as antioxidants. Their mechanism consists on blocking the enzymes that produce highly oxidized ROS and lower their production [7].
Marine invertebrates owe their richness in phenolic compounds to their phytoplankton consumption [8]. Among them, sea cucumber presents a potential marine source for high added-value compounds with medicinal properties, especially phenolic compounds that have shown an antioxidant potency that is similar to plants and seaweeds [9,10]. Sea cucumbers are soft flesh marine invertebrates from Holothuroidea class (Animalia, Echinodermata, and Echinozoa). They are characterized by an elongated tube-like body with wrinkled skin and a single branching gonad [11]. Sea cucumbers have a large diversity with up to 1700 species over the globe [12]. While some species are free swimmers, most are sedentary and slow-moving [13]. They have a wide range of habitats. Depending on the species and their age, they can be found in proximity to seaweed as well as in deep waters [13]. Their food is mainly sediments that are rich in nutrients, absorbing microphytobenthic biomass, bacteria, and detrital matter [13]. Sea cucumber received great attention as a sea food, given the promising therapeutic advantages, thanks to its medicinal properties [6,12,14]. Efforts at international level are attempting to preserve marine biodiversity and farming of sea cucumber while sustainably managing it to generate opportunities [15]. In Morocco, the government regulates and controls illegal fishing of this species [16]. The most common species are Holothuria tubulosa, Pearsonothuria graeffei, Isostichopus badionotus, Holothuria nobilis, Holothuria polii, Holothuria forskali, Cucumaria japonica, Cucumaria frondosa, Actinopyga mauritiana, Acaudina molpadioides, and Apostichopus japonicus [14].
Various works on sea cucumbers showed a high antioxidant potential thanks to their phenolic content [8,10]. Ezz et al. [17] used high-performance liquid chromatography (HPLC) method to detect eight phenolic compounds from sea cucumber (Holothuria atra) aqueous extracts (AEs), and demonstrated cardiopreventive activity against isoproterenol-induced cardiac injury in rats. On another work, Alper and Günes [18] evaluated the phenolic contents of methanolic and AEs of H. tubulosa and their cytotoxic effects against different cancer cells. Besides, despite the health benefits of bioactive molecules, the product powdering method can affect application range. At an industrial scale, freeze drying (FD) and spray drying (SD) are generally applied to obtain the desired dry products [19]. FD is a process that requires sublimation of ice in the frozen product under vacuum and low temperature [20]. This method has been reported to maintain nutritional properties and product quality. On the other hand, SD is a unique and effective drying technique for different products, due to the low cost, controlled operational conditions, and short processing duration. In addition, it preserves high quality of product properties such as nutrients, flavor, and color. As well as maintaining a good stability of the final product [21].
The phenolic composition and antioxidant activity of sea cucumber extracts are directly associated with the drying method. Thus, it would be beneficial to investigate the relationship between phenolic composition and the antioxidation of extracts of sea cucumber (H. tubulosa) using different powdering method. After a thorough search of the relevant literature, no work has been found on the phenol composition of spray-dried and freeze-dried AEs of H. tubulosa from Moroccan littorals. The use of numerous techniques for the evaluation of antioxidant capacity is necessary for a comprehensive exploration of the antioxidant potential of the produced extracts, as conclusions could be inaccurate or even contradictory when utilizing only one technique [22]. The aims of this study are: The synthesis of bioactive AEs of H. tubulosa sea cucumber from Moroccan littorals, the investigation of chemical and morphological characteristics of the AEs, the evaluation of the antioxidant capacity of the produced extracts as function of extraction time and the drying method (spray-drying or freeze-drying), and the characterization of the phenolic composition of the AEs.
2. Materials and methods
2.1. Sample Collection and Preparation
The collection of H. tubulosa specimens was under the supervision and authorization of the Moroccan National Institute of Fisheries Research INRH (Agadir, Morocco). The collection site is the shore of Sidi Boulfdail in the region of Agadir, during the month of September 2020. The samples were collected in fresh condition. The species were brought to the Biotechnology Laboratory (INRH) and kept in an ice box. Inner organs of the sea cucumbers were immediately removed. Tap water was utilized for the body wall rinsing. The samples were sliced and dried. The dried samples were mechanically powdered and sieved using a ≤500-μm sieve. Before tests, the powder was kept in a sealed, dark bottle at 4°C.
2.2. Production of Sea Cucumber AEs
AEs were obtained from sea cucumber H. tubulosa. Slight modifications were applied to the extraction technique reported by Fredalina et al. [23]. An amount of 200 g of the dry sample was suspended in 400 mL of phosphate-buffered saline (PBS, pH = 7.4) in a 1000 mL flask. The suspension was mixed for 3 and 4 h using an overhead stirrer at room temperature. The samples were separated using a centrifuge for 20 min at 3000 rpm at 4°C. The supernatant was spray dried (L-8i spray dryer, Ohkawara Kakohki, Japan) or freeze dried (ALPHA 1-2 LD plus freeze dryer, Martin Christ Gefriertrocknungsanlagen GmbH, Germany) and then stored at 4°C for further analysis. This process yielded the Freeze-dried AEs (FAE3 and FAE4) and Spray-dried AEs (SAE3 and SAE4), respectively, for 3 and 4 h extraction time.
2.3. Chemical and Morphological Characterization
The influence of SD and FD processes was studied by an energy dispersive X-ray spectroscopy coupled scanning electron microscopy (SEM/EDS, Tescan Vega 3, TESCAN ORSAY HOLDING a.s., Czech republic). The specimen is sticked into a double-sided adhesive tape mounted to stubs. Carbon coating was performed on the sample surface under vacuum using a sputter coater. Morphological characterization of samples was done under an accelerating voltage of 10 kV.
The functional groups of all AEs were distinguished by Fourier transform infrared spectroscopy (Fourier-transform infrared [FT-IR], Spectrum 3 Tri-Range, Perkin Elmer, USA). Each AE sample (1 mg) is grounded and crushed to quite a powder and mixed with 99 mg potassium bromide (KBr) to make compressed pellet. The transmittance of each sample is then recorded between 4000 and 400 cm-1.
2.4. Total Phenolic and Total Flavonoid (TF) Contents
The Folin Ciocalteu phenol reagent was used for approximate assessment of total phenolic (TP) contents of AEs following a slightly modified protocol of the one reported by Slinkard and Singleton [24]. 0.5 mL of extract sample and 2 mL of Na2CO3 (75 g.L−1) were transferred into the test tubes containing 2.5 mL Folin–Ciocalteu reagent (10% (v/v)). After intense mixing, the sample tubes were kept until the characteristic blue color appears after 30 min at ambient temperature. A UV spectrophotometer (160-UV, Shimadzu Co., Japan) was served to measure the absorbance of the mixtures at 725 nm versus a blank comprised of the same solution but with distilled water instead of the Folin-Ciocalteu reagent. Gallic acid calibration curve was plotted. The linear regression equation of this curve was developed to assess the phenolic content in the sample. Gallic acid equivalents (GAEs) per mg of extract are used to express the results.
The TF content of sea cucumber AEs was estimated by the AlCl3 colorimetric technique of Köksal and Gülçin, which is a slightly modified version of Ahn et al. [25] method. It consists on mixing 0.5 mL of sea cucumber AE solution with aluminum chloride-ethanol (2%, 0.5 mL). The mixture was kept for incubation for 1 h at ambiant temperature, and then the UV absorbance was determined at 420 nm. The same method described above for TP content was used to create the curve of TF content using a quercetin standard solution (0–100 mg/L). All measurements were done in triplicate.
2.5. In Vitro Antioxidant Activity
Three distinct in vitro antioxidant methods were used in this work, including the 2,2-Diphenyl-1-picrylhydrazyl (DPPH)• radical inhibition, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)•+ radical inhibition, and ferrous ion chelating test. As a positive control, BHT was used.
2.6. DPPH Free Radical Scavenging Activity
Concentrations ranging from 0.5 to 5 mg/mL each sample (1 mL) were placed in a cuvette and mixed with 1 mL of DPPH• reagent (0.002% (w/v)/methanol water solution) as per the instructions of Burits and Bucar [26]. DPPH was procured from Sigma Aldrich (Ref. D9132, Sigma Chemical Co., St. Louis, MO, USA). After a reaction of 15 min at ambient temperature, UV absorbance measurements were made at 517 nm. The tests were done 3 times and BHT is employed as the positive control. The values of radical scavenging activity percentage were evaluated following this formula:
 |
The blank solution absorbance is referred to as Abs. blank, and the absorbance of the tested samples is Abs. sample.
2.6.1. ABTS radical scavenging assay
Sea cucumber AEs were tested for their ability to scavenge ABTS•+ radicals applying a modified version of Li et al. [27] technique. ABTS is from Sigma Aldrich (Ref. A1888, Sigma Chemical Co., St. Louis, MO, USA). ABTS•+ has a characteristic absorbance at 734 nm with a blue-green color. The preparation of ABTS•+ cation radical is a result of a reaction of 7 mM aqueous ABTS solution and 2.45 mM of K2S2O8 for 12–16 h, at room-temperature and dark incubation. Ethanol was used to dilute the ABTS•+ solution to get a value of 0.750 ± 0.025 for the absorbance at 734 nm. Mixtures using different concentrations of sea cucumber AE ranging from 0.5 to 5 mg/mL (1 mL) with ABTS•+ solution (1 mL) were prepared. After being homogenized, the samples were kept for 15 min of dark incubation. For each concentration, the absorbance at 734 nm was calculated in comparison to a blank. BHT was used as positive control. The samples’ decreasing absorbance is an indicator of ABTS•+ cation radical scavenging activity. The whole experiment was conducted in triplicate.
2.6.2. Ferrous ion chelating assay
The reductive potential of the sea cucumber AEs was assessed using the protocol of Le et al. [28]. Ferrozine (3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p’-disulfonic acid monosodium salt hydrate) was purchased from Sigma Aldrich (Ref. 160601, Sigma Chemical Co., St. Louis, MO, USA). The reaction mixture contains 500 μl of the AEs of sea cucumbers with concentrations between 0.5 and 5.0 mg/mL mixed with 50 μl of Iron(II) chloride (0.6 mM in distilled water) and methanol (900 μl). 100 μl of Ferrozine (5 mM) is added to the mixture after stirring for 5 min, the mixture is then kept for 20 min to react at room temperature. A volume of methanol is added in the place of the tested extract in the control. The absorbance is then determined at 562 nm. BHT is used as a reference.
The results of this test are expressed as a chelating effect according to the following equation:
 |
Where the blank control absorbance is referred to as Abs. blank, and the absorbance of the tested samples is Abs. sample.
2.7. HPLC-UV Analysis
Methanol was used to dissolve 17 phenolic compounds to create stock standard solutions in a 10 mL volumetric flask. Appropriate volumes of each stock solution were mixed together to prepare two mixtures, Pyrogallic mixture: Pyrogallic acid, Vanillic acid, Caffeic acid, Furelic acid, Hesperidin, and Salicylic acid with retention times of 6.23, 13.82, 14.21, 19.13, 20.85, and 21.48 min, respectively, and the Gallic mixture: Gallic acid, Catechin, Chlorogenic acid, Epicathechin, Vanilin acid, p-Coumaric acid, Sinapic acid, Naringin, Rutin, Quercetin, and Kaempferol with retention times of 6.47, 10.96, 12.02, 13.63, 15.40, 18.55, 19.15, 20.49, 21.65, 26.60, and 27.55 min, respectively. All polyphenol standards were procured from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO, USA). Standard solutions of work were prepared through serial dilutions. All solutions were kept at a refrigerator for storage.
The AEs were analyzed on a Shimadzu HPLC system (LC-20 AD, Shimadzu Co., Japan) equipped with DAD detectors (SDP-M20A module, Shimadzu Co., Japan). Each sample, at a concentration of 10 mg/mL (10 μL/injection), was separated on a SunFire C18 column (3.5 μm, 150 × 3.0 mm i.d.; Waters, USA) equipped with a guard column (10 mm × 3.0 mm i.d.; Waters, USA). Solvent system is composed of (A) H2O with 0.1% CH2O2 and (B) methanol with 0.1% CH2O2. The gradient was used as follows: Linear gradient from 5% to 25% B, from 0 to 3 min; at 25% B, from 3 to 6 min; from 25 to 37% B, from 6 to 9 min; at 37% B, from 9 to 13 min; from 37 to 54% B, from 13 to 18 min; at 54% B, from 18 to 22 min; from 54 to 95% B, from 22 to 26 min; at 95% B, from 26 to 29 min; back to initial conditions at 5% B, from 29 to 29.15 min; and at 5 % B, from 29.15 to 36 min. The flowrate of mobile phase was 1 mL/min.
2.8. Statistical Analysis
Homogeneity of variance and normality of the data were checked before performing the analysis. The Kruskal–Wallis test was applied to detect significant differences in antioxidant activity and TF and polyphenols with post hoc Tukey HSD test using IBM SPSS Statistics 25 for Windows. A statistically significant difference was determined to exist when the probability value was P < 0.05. Pearson correlation between antioxidant activity and flavonoid or phenol content was investigated. All experiments were presented as mean values ± standard deviation after being conducted in triplicate. All graphics were constructed using origin 8.0 software.
3. Results and discussion
3.1. Product Yield
The practical recovery yield was calculated using the ratio (%) of the amount of recovered AE per the entire amount of sea cucumber dry powder sample in the feed mixture. The yield of each AE was estimated by the following formula:
 |
According to the findings, FD method yielded slightly more AE than SD method. Specifically, 16.8 ± 0.8%, 16.5 ± 0.9%, 14.7 ± 0.5%, and 14.5 ± 0.7% respectively, for FAE4, FAE3, SAE4, and SAE3 samples. Given an identical solution was used for all samples; the results suggest that freeze-drying produced better yield. This pattern was also observed in FD and SD of liquid smoke [29], strawberry flavour [30], black glutinous rice [31], and orange powder [32]. Slightly lower yield from SD might be due to the gummy nature of the spray-dried sample which makes it relatively hard to be recovered from the spray drier wall.
3.2. FT-IR Analysis
FT-IR is an efficient way to analyze the biochemical composition without complex sample preparation and in a short period of time. The FT-IR spectroscopy was used to understand the biochemical composition based on peak transmittance and wavenumber of the spectra of sea cucumber AEs [Figure 1]. The most important frequencies are between 1000 and 4000 cm−1; these regions are known as the functional areas or characteristic areas [33]. All AE samples exhibited similar characteristics indicating polyphenols with no significant difference. FAE3, FAE4, SAE3, and SAE4 showed characteristic bands of the hydroxyl group –OH stretching in 3404 and 3424 cm-1 which is a characteristic of polyphenolic compounds [34]. The presence of a strong peak at 2933 cm and 2960 cm-1 is assigned to –OH probably from the carboxylic acid [35]. A C=O stretching vibration appears as a strong peak at 1635–1647 cm-1, this might be due to a carbonyl compound presence from high content of flavonoids in AEs. These results are similar to reported FT-IR spectra of polyphenolic compounds in the literature [34].
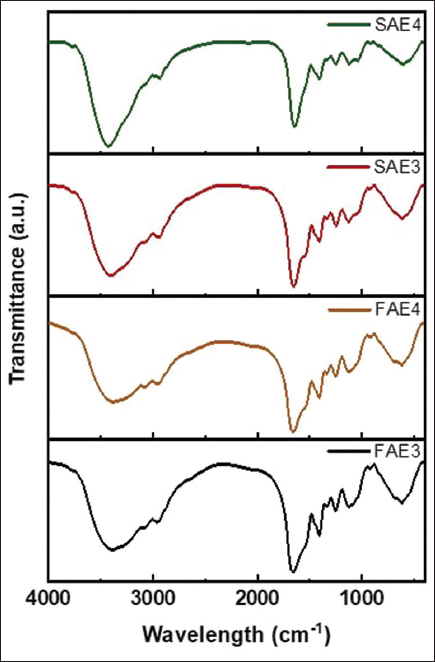 | Figure 1: Fourier-transform infrared spectra of aqueous extract samples. [Click here to view] |
The band around 1547 cm-1 can be assigned to the aromatic C=C bending. The band around 1410-1403 cm-1 might be attributed to C-H bending vibration. While the infrared bands around 1340 cm-1 indicate the O-H bending that might be due to the presence of phenolic extracts, the band at 1120 cm-1 is assigned to C-O stretching [36]. The FT-IR spectra indicate that sea cucumber extracts are rich in phenolic compounds. Further analyses are needed to confirm this conclusion.
3.3. Microstructural Characterization
The microstructure of the AEs of H. tubulosa was observed under a SEM. The drying techniques of AEs gave particles with different morphologies. SEM images indicate that they have a hollow morphology and dimpled spherical shape [Figure 2]. FAEs have an irregular flake-like morphology of porous structure. Freeze-dried materials have typically the same morphology [37]. SAEs are irregularly spherical; the same thing was remarked in reported spray-dried materials [38]. This difference may be because of slower ice sublimation and to the drying mechanism [39]. Ice crystal growth during the freezing process of the freeze-dried AEs probably shaped the observed porosity on the crack [40]. The development of spherical structures may be connected to the biopolymer’s high surface activity, which results in more homogeneous structures [41].
 | Figure 2: Scanning electron microscopy images of aqueous extract samples. [Click here to view] |
3.4. Total Phenolic and Flavonoids Contents
The quantitative study of sea cucumber AEs studied by spectroscopic assays aims to quantify total polyphenols content (TP) along with TFs content (TF). The calibration curve for gallic acid was used to determine the TP in each extract (y = 0.0042x; R² = 0.9897) and presented in milligrams of GAEs per gram of dry matter (DW). While the colorimetric assay with aluminum chloride using quercetin as a standard was used to determine TF content (y = 0.2778x - 0.5018; R² = 0.9285) and expressed in milligrams of quercetin equivalents (QE) per gram of dry matter (DW). The obtained TF and TP results are evaluated for different AE samples [Table 1].
Table 1: Total phenols and total flavonoids of different AFs.
| Sample | FAE3 | FAE4 | SAE3 | SAE4 |
|---|---|---|---|---|
| TP (mg GAE/g of dw)1 | 21.57±0.73ab | 30.15±1.24c | 19.43±0.61a | 24.50±0.78b |
| TF (mg QE/g of dw)2 | 2.10±0.21a | 4.41±0.23b | 1.40±0.18c | 2.53±0.41d |
1 Data expressed as gallic acid equivalent (GAE), mean±SD (n=3).
2 Data expressed as Quercetin Equivalent (QE), mean±SD (n=3). Values with different subscripts a-d within the same row differ significantly at P<0.05. , TP: Total polyphenols, TF: Total flavonoids, AFs: Aqueous extracts
The highest TP content is measured in FAE4 (30.15 ± 1.24 mg GAE/g DW) followed consecutively by SAE4, FAE3, and SAE3 (24.50 ± 0.78, 21.57 ± 0.73, and 19.34 ± 0.61 mg GAE/g DW respectively). The same sequence was observed for TF content, with the highest measured value for FAE4 followed, respectively, by SAE4, FAE3, and SAE3 (4.41 ± 0.23, 2.53 ± 0.41, 2.10 ± 0.21, and 1.40 ± 0.18 mg QE/g DW). There is an obvious influence of extraction time and the type of the drying treatment on the TF and TP content. FD is less aggressive to bioactive molecules compared to SD that uses heat, which explains the difference in polyphenol contents [42]. In our case, longer extraction time produced higher TF and TP contents. Therefore, long extraction periods provide enough time for the migration of the targeted molecules [43].
TF and TP contents of different sea cucumber species were reported in the literature. They were measured in extracts from respiratory apparatus, gonads, muscles, and digestive tract of C. frondosa [10]. Reported values of TP ranged between 22.5 and 236.0 mg of GAE/100 g of dry weight, while TF ranged between 2.9 and 59.8 mg of rutin equivalents/100 g of dry weight. AEs of Stichopus chloronotus, Holothuria leucospilota, and Holothuria scabra were studied, their respective TP values were found as 4.85, 9.7, and 8.27 mg of GAE/g extract [44]. High-Pressure Processing (HPP) pre-treatment was reported for the preparation of freeze-dried extracts of insoluble-bound, esterified, and free phenolics from inner organs of the C. frondosa [9]. The TP content for all phenolic compounds was 302.82 mg GAE/100 g for HPP treated extracts and 232.67 mg GAE/100 g for untreated ones. While the TF content was 124.42 mg catechin equivalent/100 g for HPP treated extracts and 101.04 mg catechin equivalent/100 g for untreated ones. In our study, AEs of H. tubulosa have significantly higher levels of phenols and flavonoids compared to many other sea cucumber species. These differences are probably due to the different extraction types and different used parts of the species. As the extraction techniques vary in terms of the operating temperature, extraction duration, and efficiency this variation impacts bioactive molecules [37]. Furthermore, the used body part of the species was reported to influence the polyphenolic content [14].
3.5. In Vitro Antioxidant Activity
Since a single technique cannot precisely assess the antioxidant capacity, the antioxidant activity of the studied AEs has been evaluated with three different methods, namely: Trapping of the ABTS•+ cation radical, DPPH free radical scavenging test, and the ferrous iron chelation test, which will allow us to better assess the antioxidant effect. In the following, we present the results of the antioxidant potential of our AEs of H. tubulosa.
3.5.1. DPPH• scavenging activity
With a maximum absorbance at 517 nm, the DPPH• is a stable free radical, it gets transformed into 1,1-diphenyl-2-picrylhydrazine when it is trapped by the extract. The ability to scavenge free radicals by the extracts determines the antioxidant activity. The level of discoloration reveals the antioxidant’s scavenging capacity in the sample [45]. At all the tested concentrations, the AEs significantly inhibited the DPPH radical in a dose-dependent manner (p <0.05 versus negative control) [Figure 3]. The maximum antioxidant activity of AEs is determined as 75.41 ± 0.26% for FAE4, followed by 74.67 ± 0.30%, 67.38 ± 0.34% and 64 ± 0.28% respectively for FAE3, SAE4 and SAE3, with a significant difference (P < 0.05) between the four samples. Compared to the reference antioxidant BHT, all AEs showed lower ability to reduce the DPPH free radical.
 | Figure 3: 2,2-Diphenyl-1-picrylhydrazyl scavenging activity of sea cucumber aqueous extracts for different concentrations (n = 3). Error bars represent the standard deviation. [Click here to view] |
To better compare the antioxidant potential of the different AEs, the IC50 values were determined [Table 2]. IC50 is the necessary AE concentration under the specified experimental conditions for 50% reduction of the initial DPPH concentration. The IC50 values are 1.14 ± 0.08 mg/mL, 1.85 ± 0.08 mg/mL, 2.6 ± 0.4 mg/mL, and 2.91 ± 0.6 mg/mL, respectively, for FAE4, FAE3, SAE4, and SAE3. The TP content (r = 0.668; P < 0.332) and the TF content (r = 0.760; P < 0.240) are significantly positively correlated with the IC50 value for DPPH• radical scavenging [Table 3].
Table 2: The IC50 values of sea cucumber AFs from different assays.
| Sample | DPPH assay | ABTS assay | Ferrous ion chelating assay |
|---|---|---|---|
| FAE4 | 1.453±0.012a | 1.982±0.021a | 1.198±0.048a |
| FAE3 | 1.804±0.024b | 2.435±0.011b | 1.336±0.162a |
| SAE4 | 2.621±0.047c | 2.154±0.001c | 2.100±0.197b |
| SAE3 | 2.914±0.033d | 2.378±0.013d | 2.220±0.020b |
Different subscripts a-d indicates significant difference at P<0.05. DPPH: 2,2-Diphenyl-1-picrylhydrazyl, ABTS: 2,2’- azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), AFs: Aqueous extracts
Table 3: Pearson’s correlations between values obtained from each assay.
| DPPH | ABTS | Ferrous ion chelating assay | TP | TF | |
|---|---|---|---|---|---|
| DPPH | 1 | 0.484 | 0.956* | 0.668 | 0.760 |
| ABTS | 1 | 0.390 | 0.950* | 0.919 | |
| Ferrous ion chelating assay | 1 | 0.636 | 0.719 | ||
| TP | 1 | 0.991** | |||
| TF | 1 |
3.5.2. ABTS•+ scavenging activity
For chain breaking and hydrogen-donating antioxidants, an effective method to assess the antioxidant activity is the ABTS•+ test [45]. ABTS+ is a blue-green stable cationic radical that is generated by the oxidation of ABTS by active oxygen. For AEs, ABTS+ can react with the antioxidant compounds and lighten the color of the substance [46].
The findings of the ABTS free radical scavenging assay are nearly identical to those of the DPPH experiment [Figure 4]. At higher concentration, the amount of free radical scavenging increases. At 5 mg/mL, the respective scavenging rates of FAE4, FAE3, SAE4, and SAE3 were 87.51 ± 0.25, 78.31 ± 0.16, 77.29 ± 0.15, and 73.54 ± 0.19%, with a significant difference (P < 0.05). However, these scavenging abilities are still lower than that of the positive control BHT.
 | Figure 4: 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) scavenging activity of sea cucumber aqueous extracts for different concentrations (n = 3). Error bars represent the standard deviation. [Click here to view] |
The IC50 values are 1.98 ± 0.02 mg/mL, 2.43 ± 0.01 mg/mL, 2.15 ± 0.001 mg/mL, and 2.37 ± 0.01 mg/mL, respectively, for FAE4, FAE3, SAE4, and SAE3 [Table 2]. ABTS•+ radical scavenging correlates significantly with TP content (r = 0.950; P < 0.05), as well as with TF content (r = 0.919; P < 0.81) [Table 3].
This reveals the ability of the examined AEs to scavenge free radicals and confirms the findings of the DPPH• scavenging assay. Consequently, the confirmed antioxidant activity suggests that AEs of H. tubulosa can be a source of several naturally occurring substances with antioxidant potential that function as hydrogen donors to stop the oxidation process through stabilizing free radicals [47].
3.5.3. Ferrous ion chelating assay
The evaluation of the chelating effect of the AEs showed a good antioxidant activity and an increasing trend with increasing concentration [Figure 5]. The results clearly indicate that the active compounds which could act as antioxidant agents were released during the preparation of the AEs. The AEs of sea cucumber, with concentrations ranging from 1 to 5 mg/mL, gave a respective chelating effect of 45.68 ± 0.36% to 78.05 ± 0.18% for FAE4, 37.42 ±0.33% to 75.08 ± 0.17% for FAE3, 32.73 ± 0.19% to 69.55 ± 0.21% for SAE4, and 35.20 ± 0.45% to 69.20 ± 0.63% for SAE3. BHT has significantly higher chelating effect when compared to all AEs.
 | Figure 5: Ferrous ion chelating activity of sea cucumber aqueous extracts for different concentrations (n = 3). Error bars represent the standard deviation. [Click here to view] |
The IC50 values for the ferrous ion chelating assay were 1.19 ± 0.04 mg/mL, 1.33 ± 0.16 mg/mL, 2.22 ± 0.02 mg/mL, and 2.10 ± 0.19 mg/mL for FAE4, FAE3, SAE4, and SAE3 [Table 2]. A close correlation was remarked between the IC50 values of the ferrous ion chelating assay with TP (r = 0.636; P = 0.364) and TF (r = 0.719; P = 0.281) [Table 3].
The observed difference in the scavenging activities of different samples against the free radicals can be assigned to the time, type of drying, and thus to the phenolic composition/content that exists in the extract. The positive impact of freeze-drying and longer extraction time on the preservation of polyphenols was also visible on the scavenging activities.
Esmat et al. [48] reported that mixed organic/AEs of sea cucumber Holothuria atra exhibit an antioxidant activity (using DPPH) of 16.8% and 17.01% at concentrations of 150 mg/L and 600 mg/L, respectively. While Dakrory et al. [49] revealed that Holothuria atra extracts produced concentration dependent scavenging rate of DPPH radical from 81 to 94% at concentrations between 10 and 80 mg/mL. Holothuria atra AE exhibit a scavenging activity for NO• radical (40.8% at 600μg/mL), DPPH• radical (1.78–2.97% at 150-600 μg/mL), and iron chelating activity (32.5% at 600 μg/mL) [17]. In addition, Mamelona et al. [10] found that the oxygen radical absorbance capacity values of extracts from the respiratory tract, muscles, gonads, and digestive tract of sea cucumber, C. frondosa, varied greatly from 140 to 800 µmol of Trolox equivalents/g of DW. Antioxidant activity of different phenolic extracts of C. frondosa was studied, the obtained values ranged from 76.54 to 589.18 mg Trolox equivalents (TE)/100g, 39.76–346.48 mg TE/100 g, 131.89–598.93 mg TE/100 g and 4.09 to 25.67 mg TE/100 g, respectively, using ABTS, DPPH, hydroxyl radical scavenging activity, and metal chelation activity [9].
The obtained results in this study display higher antioxidant activity values in comparison with reported works on other sea cucumber species. This could be owed to the variation in extraction technique and drying method, as well as the studied species. Thus, AEs of H. tubulosa exhibit an important antioxidant efficacy.
3.6. Characterization of Phenolic Compounds
Polyphenolic compounds are of considerable therapeutic and scientific interest, thanks to their antioxidant properties and their use in preventing numerous pathologies. The phenolic compounds and quantities were characterized by HPLC-UV by comparing 17 used standards with the obtained retention times.
According to this analysis, nine phenolic compounds were determined in the AEs [Figure 6]. The determined phenolic acids are: Gallic acid, Vanillic acid, Caffeic acid, and Pyrogallic acid. For flavonoids, the identified compounds are Rutin, Quercetin, Vanillin, p-Coumaric acid, and Kaempferol. Characterization of the extracts by HPLC showed the existence of 12 phenolic compounds. Only three of them were not identified under the specified conditions. The major component is Pyrogallic acid (166.00 mg/L), while the minor component is Quercetin (0.08 mg/L). Other components were also detected, and the respective concentrations of each compound were quantified [Table 4]. When comparing samples, the obtained concentrations for some identified components are slightly different. This can be due to the varying level of sensitivity of some phenolic compounds toward applied temperature, vacuum, and grinding [50]. Hence, FD and SD can produce uneven degrees of loss or preservation of phenolic compounds.
 | Figure 6: High-performance liquid chromatograph-ultra violet chromatograms of aqueous extract samples, peak numbers correspond to the phenolic compounds: Unidentified (peak 1, 2 and 5), pyrogallic acid, gallic acid, vanillic acid, caffeic acid, vanillin, p-Coumaric acid, Rutin, Quercetin, and Kaempferol (peak 3, 4, 6, 7, 8, 9, 10, 11, and 12). [Click here to view] |
Table 4: Concentration of phenolic compounds from sea cucumber AEs
| Phenolic compound | Concentration (mg/L) | |||
|---|---|---|---|---|
| SAE3 | SAE4 | FAE3 | FAE4 | |
| Pyrogallic acid | 102.94 | 121.36 | 141.41 | 166.00 |
| Gallic acid | 3.58 | 1.47 | 2.51 | 4.10 |
| Vanillic acid | 0.63 | - | - | 2.60 |
| Caffeic acid | 0.44 | 0.42 | 0.67 | 2.10 |
| Vanillin | 0.59 | 0.68 | 0.95 | 0.40 |
| p-Coumaric acid | - | - | 0.10 | 4.30 |
| Rutin | 3.89 | 1.72 | 3.80 | 3.10 |
| Quercetin | 0.08 | 0.12 | 0.09 | 0.10 |
| Kaempferol | 8.52 | 8.29 | 8.81 | 9.00 |
AEs:
The most prevalent class of polyphenolic compounds in the human diet is flavonoids. They exist in many plants and in some marine species. Flavonoids are recognized for their diverse therapeutic properties which make them a unique class of therapeutic molecules [51]. Among them, Rutin has been known for the numerous pharmacological effects [51]. Whereas Pyrogallol is a phenolic compound with a remarkable ability to scavenge free radicals [52]. Vanillic and vanillin acid are known flavoring additives in the cosmetic, food and pharmaceutical industries [53]. Epicatechin is thought to have antioxidant activity which induces neuroprotective effects [54]. Due to its powerful antioxidant activity, the bioactive molecule known as quercetin is frequently employed in traditional Chinese medicine and botanical medicine. [55]. Kaempferol is a flavonoid antioxidant; numerous studies have described its dietary beneficial effects on minimizing the risk of chronic illnesses, particularly cancer [56].
Althunibat et al. [44] studied different species of sea cucumber and revealed the presence of phenolic compounds in them, which explains their potential of scavenging free radicals. These phenols could therefore contribute to the antioxidant properties of the AEs. Esmat et al. [48] performed HPLC analysis of mixed organic/AE of the sea cucumber and showed the existence of many phenolic compounds. They confirmed the presence of Pyrogallol (2.95%) and Rutin (1.83%) in sea cucumber Holothuria atra mixed organic/AE. Dakrory et al. [49] reported that Holothuria atra extract contains Rutin (0.82%) and Pyrogallol (2.25%). In addition, Alper and Günes [18] mentioned the presence of Gallic acid (205.871 - 139.19 µg/g), Vanillic acid (3.42 - 7.483 µg/g), and Epicatechin (790.091 - 0.726 µg/g) in methanolic and AEs of H. tubulosa. Holothuria arenicola extract were reported to contain Rutin (1.06%) and Pyrogallol (1.88%) (1.88%) [57]. Hossain et al. [9] identified the presence of Vanillic acid (1.37 ± 0.12 - 0.7 ± 0.06 mg/100g), Quercetin (3.05 ± 0.32 – 0.7 ± 0.06 mg/100g), and Gallic acid (3.22 ± 0.32 - 0.85 ± 0.1 mg/100g) in phenolic extracts from the internal organs of (C. frondosa) using HPP, respectively. Furthermore, Ezz et al. [17] highlighted the presence of Pyrogallol (36.9%) and Rutin (31.48%) in sea cucumber AEs.
The body wall of sea cucumbers has shown to have active phenolic compounds, which is explained by the food source of these species, that are phenol-rich materials, such as particles resulting from the degradation of marine macro-algae and phytoplankton [44]. These phenolic compounds can contribute in the management and treatment of many diseases, like hypertension, inflammatory diseases, and cancers in addition to neurological disorders [6]. On the other hand, flavonoids can help control Type 2 diabetes [58] and inhibit tyrosinase [59]. The powerful antioxidant properties of polyphenols play an important role in these applications.
4. Conclusion
The study that we carried out on the AEs of H. tubulosa highlighted the impact of extraction time and the type of drying on the content of bioactive compounds and the antioxidant activity. The analysis of antioxidant activity of different AEs showed a promising potential for scavenging free radicals and a high total ratio of polyphenols and flavonoids. Freeze-drying method and longer extraction time exhibited a higher efficiency in the retention of phenolic compounds. Twelve bioactive compounds were identified by HPLC-UV analysis. In addition, a noteworthy correlation was observed between phenolic and flavonoid compounds and antioxidant capacities. This indicates that phenolic compounds might be a primary factor of the significant antioxidant activities of AEs of sea cucumbers. This study creates a paradigm for future research on the use of drying technology to prepare AEs as well as preserving phenolic compounds.
5. AUTHORS CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
The authors acknowledge the support of the Moroccan-Tunisian cooperation in the framework of the project “Biotechnological valorization of sea cucumbers from the Tunisian and Moroccan coasts”.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data supporting this study are available upon request.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling:Revisited. Oxid Med Cell Longev 2016;2016:1656450. [CrossRef]
2. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47-95. [CrossRef]
3. Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, Ramirez-Tortosa CL, Granados-Principal S, Lorente JA, et al. Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells. Biological bases to develop oxidative-based therapies. Crit Rev Oncol Hematol 2011;80:347-68. [CrossRef]
4. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443:787-95. [CrossRef]
5. Huang D, Li C, Chen Q, Xie X, Fu X, Chen C, et al. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem 2022;377:131922. [CrossRef]
6. Rahman MM, Rahaman MS, Islam MR, Rahman F, Mithi FM, Alqahtani T, et al. Role of phenolic compounds in human disease:Current knowledge and future prospects. Molecules 2022;27:233. [CrossRef]
7. Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients 2014;6:6020-47. [CrossRef]
8. Zhong Y, Khan MA, Shahidi F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa). J Agric Food Chem 2007;55:1188-92. [CrossRef]
9. Hossain A, Yeo JD, Dave D, Shahidi F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP). Antioxidants (Basel) 2022;11:337. [CrossRef]
10. Mamelona J, Pelletier É, Girard-Lalancette K, Legault J, Karboune S, Kermasha S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem 2007;104:1040-7. [CrossRef]
11. Gajdosechova Z, Palmer CH, Dave D, Jiao G, Zhao Y, Tan Z, et al. Arsenic speciation in sea cucumbers:Identification and quantitation of water-extractable species. Environ Pollut 2020;266:115190. [CrossRef]
12. Pangestuti R, Arifin Z. Medicinal and health benefit effects of functional sea cucumbers. J Tradit Complement Med 2018;8:341-51. [CrossRef]
13. Slater M, Chen J. Sea cucumber biology and ecology. In:Echinoderm Aquaculture. Hoboken:John Wiley and Sons Inc.;2015. 47-55. [CrossRef]
14. Hossain A, Dave D, Shahidi F. Northern sea cucumber (Cucumaria frondosa):A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar Drugs 2020;18:274. [CrossRef]
15. USAID. The United States Provides an Additional $13.9 Million to Support Madagascar. Press Release;2022. Available from:https://www.usaid.gov/news-information/press-releases/apr-29-2022-united-states-provides-additional-139-million-support-madagascar [Last accessed on 2023 Feb 02].
16. Ministry of Agriculture, Maritime Fisheries, Rural Development, Waters and Forests. Arrêtén 853-20 du 17 Rejeb 1441 (2 Mars 2020) Relatif àL'interdiction Temporaire de Pêche et de Ramassage du “Concombre de mer “(Holuthuria sp) Dans les Eaux Maritimes Marocaines. Ministerial Decree;2020. Available from:http://www.mpm.gov.ma/wps/wcm/connect/ff7b4839-98bc-4596-b093-7705d8f17ac2/65_n%c2%b0853-20.pdf?mod=ajperes [Last accessed on 2023 Feb 02].
17. Ezz MK, Atef AA, Esmat AY. In vivo and in vitro studies on the antioxidant activity of sea cucumber aqueous extract against isoproterenol-induced myocardial injury in a rat model. Egypt J Biochem Mol Biol 2012;30:37-56.
18. Alper M, Günes M. Evaluation of cytotoxic, apoptotic effects and phenolic compounds of sea cucumber Holothuria tubulosa (Gmelin, 1791) extracts. Turk J Vet Anim Sci 2020;44:641-55. [CrossRef]
19. Ng ZX, Yong PH, Lim SY. Customized drying treatments increased the extraction of phytochemicals and antioxidant activity from economically viable medicinal plants. Ind Crops Prod 2020;155:112815. [CrossRef]
20. Oetjen GW, Haseley P. Freeze-Drying. 2nd ed. Weinheim:Wiley-VCH;2004. [CrossRef]
21. Ma JJ, Mao XY, Wang Q, Yang S, Zhang D, Chen SW, et al. Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. LWT Food Sci Technol 2014;56:296-302. [CrossRef]
22. Liu G, Zhu W, Zhang J, Song D, Zhuang L, Ma Q, et al. Antioxidant capacity of phenolic compounds separated from tea seed oil in vitro and in vivo. Food Chem 2022;371:131122. [CrossRef]
23. Fredalina BD, Ridzwan BH, Abidin AA, Kaswandi MA, Zaiton H, Zali I, et al. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen Pharmacol 1999;33:337-40. [CrossRef]
24. Slinkard K, Singleton VL. Total phenol analyses:Automation and comparison with manual methods. Am J Enol Vitic 1977;28:49-55. [CrossRef]
25. Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH, Kim TG, et al. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol Pharm Bull 2004;27:544-7. [CrossRef]
26. Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res 2000;14:323-8. [CrossRef]
27. Li Y, Li X, Wong YS, Chen T, Zhang H, Liu C, et al. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 2011;32:9068-76. [CrossRef]
28. Le K, Chiu F, Ng K. Identification and quantification of antioxidants in Fructus lycii. Food Chem 2007;105:353-63. [CrossRef]
29. Xin X, Essien S, Dell K, Woo MW, Baroutian S. Effects of spray-drying and freeze-drying on bioactive and volatile compounds of smoke powder food flavouring. Food Bioproc Technol 2022;15:785-94. [CrossRef]
30. Pellicer JA, Fortea MI, Trabal J, Rodríguez-López MI, Gabaldón JA, Núñez-Delicado E. Stability of microencapsulated strawberry flavour by spray drying, freeze drying and fluid bed. Powder Technol 2019;347:179-85. [CrossRef]
31. Laokuldilok T, Kanha N. Effects of processing conditions on powder properties of black glutinous rice (Oryza sativa L.) bran anthocyanins produced by spray drying and freeze drying. LWT Food Sci Technol 2015;64:405-11. [CrossRef]
32. Barbosa J, Borges S, Amorim M, Pereira MJ, Oliveira A, Pintado ME, et al. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J Funct Foods 2015;17:340-51. [CrossRef]
33. Othman N. IR spectroscopy in qualitative and quantitative analysis. In:Infrared Spectroscopy-perspectives and Applications. London, UK:IntechOpen;2023. [CrossRef]
34. Fernandez K, Agosin E. Quantitative analysis of red wine tannins using fourier-transform mid-infrared spectrometry. J Agric Food Chem 2007;55:7294-300. [CrossRef]
35. Kannan RR, Arumugam R, Anantharaman P. Fourier transform infrared spectroscopy analysis of seagrass polyphenols. Curr Bioact Compd 2011;7:118-25. [CrossRef]
36. Dos Santos Grasel F, Ferrão MF, Wolf CR. Development of methodology for identification the nature of the polyphenolic extracts by FT-IR associated with multivariate analysis. Spectrochim Acta A Mol Biomol Spectrosc 2016;153:94-101. [CrossRef]
37. Chranioti C, Chanioti S, Tzia C. Comparison of spray, freeze and oven drying as a means of reducing bitter aftertaste of steviol glycosides (derived from Stevia rebaudiana Bertoni plant)--evaluation of the final products. Food Chem 2016;190:1151-8. [CrossRef]
38. Liu T, Wang Y, Yu X, Li H, Ji L, Sun Y, et al. Effects of freeze-drying and spray-drying on the physical and chemical properties of Perinereis aibuhitensis hydrolysates:Sensory characteristics and antioxidant activities. Food Chem 2022;382:132317. [CrossRef]
39. Xu X, Zhang L, Feng Y, Yagoub AE, Sun Y, Ma H, et al. Vacuum pulsation drying of okra (Abelmoschus esculentus L. Moench):Better retention of the quality characteristics by flat sweep frequency and pulsed ultrasound pretreatment. Food Chem 2020;326:127026. [CrossRef]
40. Vardanega R, Muzio AF, Silva EK, Prata AS, Meireles MA. Obtaining functional powder tea from Brazilian ginseng roots:Effects of freeze and spray drying processes on chemical and nutritional quality, morphological and redispersion properties. Food Res Int 2019;116:932-41. [CrossRef]
41. Silva RS, Santos CD, Mar JM, Kluczkovski AM, Figueiredo JD, Borges SV, et al. Physicochemical properties of tucumã(Astrocaryum aculeatum) powders with different carbohydrate biopolymers. LWT Food Sci Technol 2018;94:79-86. [CrossRef]
42. Pérez-Gregorio MR, Regueiro J, González-Barreiro C, Rial-Otero R, Simal-Gándara J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011;22:1108-13. [CrossRef]
43. Sulaiman IS, Basri M, Masoumi HR, Chee WJ, Ashari SE, Ismail M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem Cent J 2017;11:54. [CrossRef]
44. Althunibat OY, Hashim RB, Taher M, Daud JM, Ikeda MA, Zali BI. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur J Sci Res 2009;37:376-87.
45. Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol 2013;50:687-95. [CrossRef]
46. Sheng Y, Ma Z, Wang X, Han Y. Ethanol organosolv lignin from different agricultural residues:Toward basic structural units and antioxidant activity. Food Chem 2022;376:131895. [CrossRef]
47. Guenaou I, Irahal IN, Errami A, Lahlou FA, Hmimid F, Bourhim N. Bioactive compounds from Ephedra fragilis:Extraction optimization, chemical characterization, antioxidant and antiglycation activities. Molecules 2021;26:5998. [CrossRef]
48. Esmat AY, Said MM, Soliman AA, El-Masry KS, Badiea EA. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition 2013;29:258-67. [CrossRef]
49. Dakrory AI, Fahmy SR, Soliman AM, Mohamed AS, Amer SA. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced Hepatorenal diseases in rats. BioMed Res Int 2015;2015:563652. [CrossRef]
50. Buratto AP, Carpes ST, Pereira EA, Diedrich C, Oldoni TL, da Silva LD. Effect of drying method in the maintenance of bioactive compounds and antioxidant activity of feijoa pulp (Acca sellowiana). Orbital Electron J Chem 2019;11:386-93. [CrossRef]
51. Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J 2017;25:149-64. [CrossRef]
52. Rafiee SA, Farhoosh R, Sharif A. Antioxidant activity of gallic acid as affected by an extra carboxyl group than pyrogallol in various oxidative environments. Eur J Lipid Sci Technol 2018;120:1800319. [CrossRef]
53. Salau VF, Erukainure OL, Islam MS. Phenolics:Therapeutic applications against oxidative injury in obesity and Type 2 diabetes pathology. In:Pathology:Oxidative Stress and Dietary Antioxidants. London:INC;2020. 297-307. [CrossRef]
54. Shokrzadeh M, Javanmard H, Zadeh GG, Emran HA, Modanlou M, Yaghubi-Beklar S, et al. Evaluation of the anti-apoptotic and anti-cytotoxic effect of epicatechin gallate and edaravone on SH-SY5Y neuroblastoma cells. Basic Clin Neurosci 2019;10:619-30. [CrossRef]
55. Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019;24:1123. [CrossRef]
56. Dos Santos JS, Cirino JP, de Oliveira Carvalho P, Ortega MM. The pharmacological action of kaempferol in central nervous system diseases:A review. Front Pharmacol 2021;11:565700. [CrossRef]
57. Fahmy SR. Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats. BMC Complement Altern Med 2015;15:14. [CrossRef]
58. Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, Omelka R. Pharmaceutical drugs and natural therapeutic products for the treatment of Type 2 diabetes mellitus. Pharmaceuticals (Basel) 2021;14:806. [CrossRef]
59. Lee SY, Baek N, Nam TG. Natural, semisynthetic and synthetic tyrosinase inhibitors. J Enzyme Inhib Med Chem 2016;31:1-13. [CrossRef]