1. INTRODUCTION
One of the recognized plants from the Zingiberaceae family, Curcuma xanthorrhiza roxb., is famous as a medicinal plant included in the rhizome group [1]. Although the rhizome of C. xanthorrhiza is primarily used in folk medicine, it is often used as a spice. Notably, In Indonesia, the rhizome of C. xanthorrhiza has been vastly utilized in a household to small-medium scale industries. Furthermore, the pharmacological values of C. xanthorrhiza comprise antibacterial, anti-inflammatory, antidepressant, hepatoprotective, and antioxidant properties [2,3]. Rahmat et al. [4] reported that more than 40 secondary metabolites were identified in C. xanthorrhiza, including phenolic, terpenoid, and curcuminoid compounds. C. xanthorrhiza contains a specific secondary metabolite known as xanthorrhizol. Several studies have investigated the presence of xanthorrhizol in C. xanthorrhiza and its biological activities, i.e., antifungal, antibacterial, and antimetastatic [5]. Besides xanthorrhizol, curcuminoids, essential oils, and starch fractions are essential constituents of C. xanthorrhiza [6,7].
The utilization of plant secondary metabolites has been a concern for many years because of its health benefits for the human body. The group of secondary metabolites most often studied for their function for health is polyphenolic compounds [8]. As a type of plant-based compound, polyphenols are one of the major groups of secondary metabolites found in plants, alongside alkaloids and terpenoids [9]. Most fundamentally, the biosynthesis of plant endogenous polyphenolic compounds occurs as a protective response to environmental stresses (abiotic stresses), as well as pathogen and insect attacks, which are biotic stresses [10,11]. Polyphenolic compounds are broadly distributed in all parts of the plant. Various studies have noted the pharmacological effects of polyphenols as anti-cancer, anti-inflammatory, anti-androgen, and anti-diabetic [12,13]. The antioxidant capacity is one of the essential pharmacological properties of polyphenolic compounds [14-16]. Polyphenolic compounds have the antioxidant capacity to counter various types of free radical species. Free radicals are reactive and unstable molecules because they have unpaired electrons [17]. Moreover, Free radicals tend to find their electron pair by taking electrons from other stable molecules. Free radicals in the body ultimately damage the integrity of biomolecules such as DNA, lipids, and proteins [18,19]. A large number of free radicals can cause oxidative stress that leads to the emergence of degenerative diseases. Thus, secondary metabolites with antioxidant potential significantly eliminate free radicals and their multiplication chain.
The potency of plant-based antioxidants can be increased by improving the production of endogenous plant polyphenolic compounds. Genetic and environmental factors contribute greatly to the production of plant metabolites [20,21]. Particularly for C. xanthorrhiza, Nurcholis et al. [22] stated the factors that affect the quantity and quality of C. xanthorrhiza bioactive compounds, such as nutrient sufficiency, pest and disease control, plant genetic potential, and post-harvest handling. Fertilization, one of the plant cultivation techniques, affects plants’ growth and physiological characteristics and the production of plant essential metabolites [23,24]. NPK fertilizer is an inorganic fertilizer with three macronutrients (nitrogen, phosphorus, and potassium) that play a significant role in plants’ vegetative and generative growth phases. In general, the three macronutrients have an essential role in the formation of biomolecules, as well as plant metabolism. Nitrogen is a major component of DNA, RNA, amino acids, and chlorophyll [25]. Phosphorus is responsible for the binding between DNA (phospho-diester bonds), and the formation of chemical energy (ADP, ATP) [26]. Potassium has a function in transfer assimilates and enzyme cofactor [27]. Ahmed et al. [28] reported an increase in the content of flavonoids and carbohydrates due to the modification of the dose of fertilization of Daucus carota L. Sun et al. [29] found an increase in polyacetylene and volatile sesquiterpene oils of the Atractylodes chinensis as a result of the application of phosphorus in high doses. Gradually increasing the dose of NPK fertilization can improve the content of carotenoid metabolites in Solanum muricatum [30]. The former findings provide an insight that optimization of the production of plant endogenous secondary metabolites can be achieved through modifying the fertilization techniques. Thus, the present study aims to grasp the role of the application of NPK fertilizer on the polyphenol contents and antioxidant properties in C. xanthorriza.
2. MATERIALS AND METHODS
2.1. Materials
The list of solutions and materials used in this study included methanol (Merck), aluminum chloride (Merck), potassium acetate (Merck), quercetin (Sigma-Aldrich), Folin-Ciocalteu reagent (Merck), Na2CO3 (Merck), gallic acid (Merck), DPPH powder (Sigma-Aldrich), tripyridyl-s-triazine (TPTZ) (Sigma-Aldrich), FeCl3 (Merck), HCl solution (Merck), acetic acid (Merck), neocuproine (Merck), ammonium acetate (Merck), ABTS powder (Sigma-Aldrich), K2S2O8 (Merck), and Trolox (Sigma-Aldrich).
2.2. Experimental Sites
Cultivation until harvesting of C. xanthorrhiza rhizomes was carried out in the Biopharmaca Conservation and Cultivation Station, West Java of Indonesia (6°3’49” and 106°42’57” E). Analysis of polyphenols and antioxidant activity of C. xanthorrhiza was located at Tropical Biopharmaca Research Center, Bogor Agricultural University (IPB) (6°32’25.47” N and 106°42’53.22” E) and Biochemical Research Laboratory, Department of Biochemistry, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University (IPB).
2.3. NPK Treatment and Experimental Design
In this study, the rhizome of C. xanthorrhiza was used as planting material. The planting material used in this study was the Cursina-2 variety obtained from the Indonesian Spice and Medicinal Crops Research Institute in Bogor, Indonesia. The rhizomes are sown in polybags (15 × 10 cm) for 2 months. After 2 months of growth, the C. xanthorrhiza seedlings were transferred to bigger polybags (40 × 30 cm). The mature rhizome was then harvested 5 months after planting. The field experiment was designed with a randomized complete block design. Eight treatments consisted of control (C/untreated), N, P, K, NP, NK, PK, and NPK, each treatment was replicated three times, and one experimental unit consisted of five C. xanthorrhiza plants. Thus, there are 120 plants planted in this research. NPK treatment was given twice, 1 month after transplanting and 3 months after transplanting. The dose of each nutrient was designated as follows: n = 250 kg/ha (3.75 g/pot), P = 200 kg/ha (3 g/pot) and K = 200 kg/ha (3 g/pot). The combination of nutrients and fertilizer doses was based on prior research conducted by Nihayati et al. [31] and Peng and Ng [32]. Throughout the research, plant maintenance activities such as watering, weeding, and pest and disease control were conducted to ensure the plants remained in good condition. These activities were performed from the seedling stage until harvest.
2.4. Extraction Method
Fresh rhizomes of C. xanthorrhiza were washed and dried in an oven at 45°C for 8 h. The dried rhizomes of C. xanthorrhiza were weighed and then mashed into powder. Extraction of test samples of C. xanthorrhiza using a maceration technique which refers to Khumaida et al. [33] with a slight change. To prepare the sample, 25 grams of C. xanthorrhiza powder was mixed with 250 mL of methanol (95%) and shaken for 24 h using a water bath shaker (WiseBath, Korea) at a speed of 120 rpm and a temperature of 37°C. The mixture was then filtered using filter paper (Whatman-type 4) to obtain the first filtrate. The maceration technique was repeated on the first residue until the second filtrate was obtained. The first and the second filtrates were then combined and concentrated using a vacuum evaporator (OGAWA, Japan) at a temperature of 45°C and a pressure of 80 cmHg. Evaporation of the filtrate of C. xanthorrhiza produces a viscous extract that is used for polyphenols and antioxidant activity analyses.
2.5. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
The analysis of DPPH scavenging activity refers to Nurcholis et al. [34] with minor modifications. 100 μL methanol extract of C. xanthorrhiza was mixed into a microplate with 100 μL of DPPH solution (125 M). Soon after, the test solution, a mixture of extracts and DPPH solutions, was incubated in the dark for 30 min. The absorbance of the test solution was measured using a nano-spectrophotometer (SPECTROstarNano BMG LABTECH) at a wavelength of 517 nm. Trolox is used to make standard curves for determining antioxidant activity, converted as μmol TE (Trolox equivalent)/g dry weight. The standard trolox solution consisted of seven concentration levels: 0, 20, 40, 60, 80, 100, and 120 μM. Antioxidant activity was calculated using the equation y = 0.4252x - 1.3922, where y represents the absorbance value and x represents the trolox concentration.
2.6. 2,2′-Azino-Bis-3-Ethylbenzthiazoline-6-Sulphonic Acid (ABTS) Assay
The ABTS scavenging activity evaluation refers to Kumar et al. [19] with minor changes. ABTS reagent consisted of two solutions, i.e., ABTS stock solutions (7 mM) and K2S2O8 stock solutions (2.4 mM). ABTS stock solution and K2S2O8 stock solution were combined in a ratio of 2:1 and incubated for 12 h. 20 μL methanol extract of C. xanthorrhiza and 180 μL of ABTS reagent were mixed in a microplate and incubated in the dark for 6 min. The absorbance of the mixed solution was measured using a nano-spectrophotometer (SPECTROstarNano BMG LABTECH) at a wavelength of 734 nm. Trolox is used to make standard curves for determining antioxidant activity, expressed as mol TE (Trolox equivalent)/g dry weight. The standard trolox solution was prepared with ten different concentration levels, ranging from 0 to 450 μM in intervals of 50 μM (i.e., 0, 50, 100, 150, 200, 250, 300, 350, 400, and 450 μM). Quantifying the antioxidant activity involved using the equation y = 0,1323x + 0,5284, where y represents the absorbance value and x represents the trolox concentration.
2.7. Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
CUPRAC antioxidant analysis based on Nurcholis et al. [35] with slight modifications. CUPRAC reagent was formed from a mixture of Neocuproin (7.5 × 10–3 M), CuCl2.2H2O (10–2 M), and ammonium acetate buffer solution at pH 7 (1 M). 50 μL methanol extract of C. xanthorrhiza and 50 μL of CUPRAC reagent were homogenized and incubated in the dark for 30 min. The absorbance of the mixed solution was measured using a nano spectrophotometer (SPECTROstarNano BMG LABTECH) at a wavelength of 450 nm. Trolox is used to make standard curves for determining antioxidant activity, represented as μmol TE (Trolox equivalent)/g dry weight. To determine the CUPRAC antioxidant activity of the samples, a standard trolox solution was made at seven concentration levels: 0, 100, 200, 300, 400, 500, 600, and 700 μM. The absorbance values were then converted using the equation y = 0.0017x + 0.0039, where y represents the absorbance value and x represents the concentration of trolox.
2.8. Ferric Reducing Antioxidant Power Assay (FRAP) Assay
The FRAP analysis refers to Suleria et al. [36] with modifications. The FRAP reagent consisted of three partial solutions, including acetate buffer with pH 3.6 (300 mM), 2,4,6 TPTZ (10 mM) dissolved in HCl (40 mM), and FeCl3.6H2O (20 mM). Acetate buffer, TPTZ, and FeCl3 were mixed in a ratio of 10:1:1, and before analysis, the FRAP reagent was incubated in a dark room at 37°C for 30 min. The FRAP assay was performed by incubating the combined 10 μL of C. xanthorrhiza methanol extract with 300 μL of FRAP reagent in the dark at 37°C for 6 min. The absorbance of the mixed solution was measured using a nano spectrophotometer (SPECTROstarNano BMG LABTECH) at a wavelength of 593 nm. Trolox is used to make standard curves for determining antioxidant activity, defined as μmol TE (Trolox equivalent)/g dry weight. Trolox standards were formulated at seven concentrations: 0, 100, 200, 300, 400, 500, and 600 μM. The antioxidant activity was quantified using the following equation: y = 0.0013x + 0.0107, where y represents the absorbance value and x represents the trolox concentration.
2.9. Total Phenolic Content (TPC) Analysis
TPC measurement is based on the Folin-Ciocalteu method, which refers to Zheng et al. [37] with minor modifications. The TPC assay solution was prepared by combining 10 μL of methanol extract from C. xanthorrhiza with 10 μL of 10% Folin-Ciocalteu reagent and incubating for 5 min. Next, 20 μL of 7.5% Na2CO3 was added to the mixture, which was then incubated in a dark room for 30 min. The absorbance of the mixed solution was measured using a nano spectrophotometer (SPECTROstarNano BMG LABTECH) at a wavelength of 750 nm. Gallic acid is used to make standard curves for determining the TPC of C. xanthorrhiza, defined as mg GAE (gallic acid equivalent)/g dry weight. The gallic acid standard was designed in seven concentration levels: 0, 50, 100, 150, 200, 250, and 300 ppm. The equation used to calculate TPC was y = 0.0058x + 0.1982, where y represents the absorbance value and x represents the gallic acid concentration.
2.10. Total Flavonoid Content (TFC) Analysis
TFC was estimated using the AlCl3-colorimetric method, referred to Sahid et al. [13] 10 μL methanol extract of C. xanthorrhiza was mixed with 60 μL methanol, 10 μL AlCl3 (10%), 10 μL CH3COOK (1M), and 110 μL distilled water. The absorbance of the mixed solution was measured using an ELISA reader (Epoch Biotek, USA) at a wavelength of 415 nm. Quercetin is used to make standard curves for estimating the TFC of C. xanthorrhiza, defined as mg quercetin equivalent (QE)/g dry weight. The quercetin standard was composed of six different concentration levels, varying from 0 to 500 ppm in increments of 100 ppm (i.e., 0, 100, 200, 300, 400, and 500 ppm). To quantify TFC, the following equation was used: y = 0,0038x + 0,0623, where y represents the absorbance value and x represents the quercetin concentration.
2.11. Data Analysis
The effect of NPK fertilization on the polyphenol content and antioxidant capacity of C. xanthorrhiza was statistically tested using analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test at a significance level of 5%. ANOVA and posthoc test were analysed using SPSS (version 26). Bar graph visualization was processed using Microsoft Excel (2013). Correlation analysis was performed with R studio software (version 4.1.3) using the analysis package “corrplot.”
3. RESULTS
3.1. Total Phenolics Content
The NPK fertilizers treatment using a single nutrient, a combination of two nutrients, or a complete combination of NPK significantly affected the TPC of C. xanthorrhiza [Figure 1]. TPC levels of C. xanthorrhiza after treatment with different combinations of NPK fertilizers ranged from 8.61 mg GAE/g dry weight (NP treatment) to 34.54 mg GAE/g dry weight (NPK treatment). Interestingly, It is noteworthy that the single nutrient (N, P, K) contributed better to the increase in TPC than the combination of two nutrients (NP, NK, PK). Moreover, the TPC in the control sample was increased compared to the two-nutrient combination treatment. TPC in phosphorus and NPK treatment was higher and remarkably different compared to the control treatment.
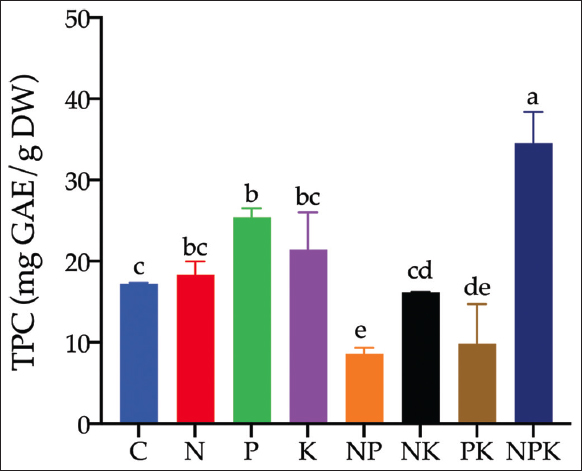 | Figure 1: Variation of total phenolic content ofCurcuma xanthorrhiza through combination of NPK nutrient treatments. C refers to control (no fertilizer treatment), N refers to nitrogen, P refers to phosphorus, and K refers to potassium. Different letters in each bar showed significant differences based on Duncan’s Multiple Range Test (P < 0.05). [Click here to view] |
3.2. TFC
The effect of NPK fertilizer treatment on the TFC of C. xanthorrhiza is illustrated in [Figure 2]. The highest TFC was recorded in the NPK treatments at 50.00 mg QE/g dry weight, while the lowest TFC was recorded in the control treatment at 11.83 mg QE/g dry weight.
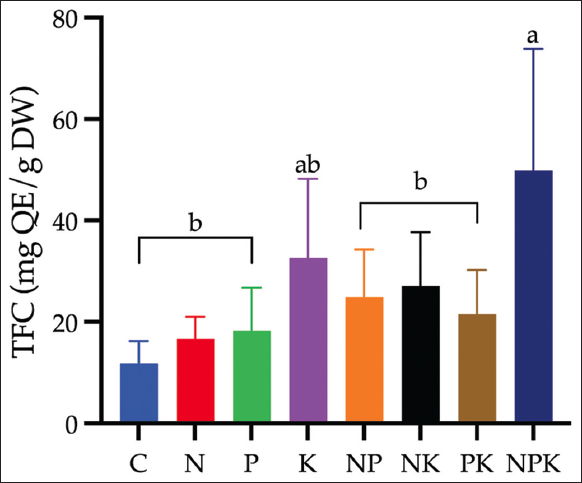 | Figure 2: Variation of total flavonoid content ofCurcuma xanthorrhiza through combination of NPK nutrient treatments. C refers to control (no fertilizer treatment), N refers to nitrogen, P refers to phosphorus, and K refers to potassium. Different letters in each bar showed significant differences based on Duncan’s Multiple Range Test (P < 0.05). [Click here to view] |
3.3. Antioxidant Activities
The influence of NPK fertilization on the antioxidant activity of C. xanthorrhiza according to the DPPH assay is presented in Table 1. The mean antioxidant activity values of C. xanthorrhiza measured by the DPPH method ranged from 76.33 μmol TE/g dry weight (nitrogen treatment) to 169.67 μmol TE/g dry weight (nitrogen and potassium treatment). The combination of nitrogen and potassium treatment resulted in the highest antioxidant capacity, as determined by the DPPH method, and this capacity was significantly different from the nitrogen treatment alone. These findings suggest that NPK fertilization can affect the antioxidant activity of C. xanthorrhiza, particularly its scavenging activity, as measured by the DPPH method. In the DPPH method, samples that exhibit antioxidant activity produce a yellow-orange color change. The NPK treatment exhibited the highest mean scavenging activity of 261.67 μmol TE/g dry weight, as determined by the ABTS assay. The antioxidant activity potential, which was assessed based on the ABTS method, changed the colorless solution to a green solution. As opposed to the NPK treatment, the combination treatment of nitrogen and potassium was recorded with the lowest scavenging activity of 120.33 μmol TE/g dry weight. There were two other treatments with lower antioxidant activity than the control (220.00 μmol TE/g dry weight), including phosphorus treatment (145.33 μmol TE/g dry weight) and potassium treatment (148.67 μmol TE/g dry weight).
Table 1: Antioxidant assays of Curcuma xanthorrhiza extracts.
| Treatments | DPPH (µmol TE/g dry weight) | ABTS (µmol TE/g dry weight) | FRAP (µmol TE/g dry weight) | CUPRAC (µmol TE/g dry weight) |
|---|---|---|---|---|
| C | 146.33±49.34a | 220.00±39.37a,b | 15.78±2.26b | 155.32±14.18d |
| N | 76.33±16.59b | 227.00±48.37a | 20.97±2.26b | 216.03±28.88b,c |
| P | 157.67±42.82a | 145.33±67.17b,c | 22.89±3.16b | 147.76±32.01d |
| K | 139.33±51.54a | 148.67±6.60b,c | 24.40±2.02b | 222.16±16.51b,c |
| NP | 113.67±38.24a,b | 230.00±23.28a | 27.69±6.08b | 175.23±38.17cd |
| NK | 169.67±4.87a | 120.33±34.88c | 43.24±2.06a | 227.87±15.36b,c |
| PK | 147.33±19.67a | 216.67±5.14a,b | 25.61±5.17b | 259.60±55.30a,b |
| NPK | 139.00±17.68a | 261.67±16.21a | 41.76±3.46a | 307.11±43.05a |
C: Control (no fertilizer treatment), N: Nitrogen, P: Phosphorus, K: Potassium, DPPH: 2,2 diphenyl-1-picrylhydrazyl scavenging activity, ABTS: 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid scavenging activity, FRAP: Ferric reducing antioxidant power, CUPRAC: Cupric reducing antioxidant capacity. Different letters within column showed significant differences based on Duncan’s Multiple Range Test (P<0.05). +refers to the standard error for each mean value.
The NPK fertilizer treatment significantly affected the antioxidant capacity of C. xanthorrhiza, as determined by the FRAP method. The highest antioxidant capacity was observed in the NPK and NK treatments, with values of 41.67 μmol TE/g dry weight and 43.24 μmol TE/g dry weight, respectively, according to the FRAP assay. The reaction of FRAP solution with samples that possess antioxidant activity shows a change in color from dense brown to intense blue. These two treatments were significantly different from the other treatments. Furthermore, The lowest antioxidant capacity of FRAP was found in the control treatment (15.78 μmol TE/g dry weight), followed by N, P, K, and combinations of NP and PK treatments. The highest antioxidant activity was observed in the NPK treatment (307.11 μmol TE/g dry weight), followed by the PK combination treatment (259.60 μmol TE/g dry weight), based on the CUPRAC assay. The control treatment and phosphorus treatment had the lowest antioxidant activity, with values of 155.32 μmol TE/g dry weight and 147.76 μmol TE/g dry weight, respectively. The color change reaction that occurs with the CUPRAC assay is blue-green to yellow.
3.4. Correlation Analysis
Figure 3 demonstrates the relationship between polyphenols and antioxidant assays of C. xanthorrhiza. TFC and TPC showed a positive and strong correlation value of 0.79. Furthermore, TPC and TFC have weak correlation values with ABTS of 0.06 and 0.08, respectively. While the correlation value between TPC and DPPH is 0.29, lower than the correlation value between TFC and DPPH (0.58). The correlation value between TPC and FRAP is 0.31, while The higher and stronger correlation values showed in TFC and FRAP (0.76). TFC and CUPRAC have the highest correlation value of 0.92, while the correlation value between TPC and CUPRAC is 0.57.
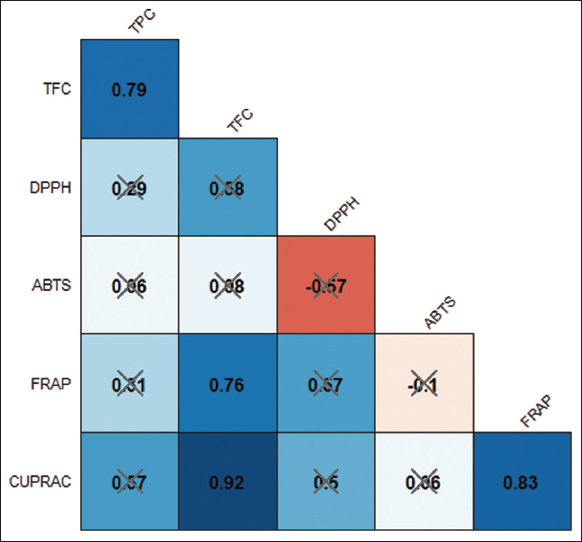 | Figure 3: The correlation plot of polyphenols and antioxidant capacity of Curcuma xanthorrhiza. The value inside each box represents the correlation coefficient between the variables, which was statistically tested at a 5% level of significance. The presence of a cross indicates that the correlation is not statistically significant. [Click here to view] |
4. DISCUSSION
Recently, various studies related to the analysis of the antioxidant activity in diverse medicinal plants have been of significant interest and have evolved into an essential research topic in increasing medicinal plants’ potency. C. xanthorrhiza has various pharmacological effects, one of which is antioxidant (22)]. In this study, It was found that the combined NPK fertilization affected the biochemical content of C. xanthorrhiza, in this particular case, its polyphenol content and antioxidant activity. The combination of three NPK increased the polyphenol content and affected the antioxidant potential of C. xanthorrhiza (except for DPPH and FRAP analysis). However, Our findings tend to be different to other experimental results [30] reported that the phenolic content in the control (untreated) treatment of Pepino melon was remarkably higher than in the NPK treatment. The study conducted by [38] stated that combining fertilization with organic and bioorganic fertilizers was more effective in increasing the polyphenol content of broccoli (Brassica olaracea, var. Italica). The decrease in the content of flavonoids and saponins in Panax notoginseng also occurred due to NPK treatment [39]. It is highly likely that nitrogen application increases the synthesis of substrates which act as precursors. It is well-established that nitrogen leads to an increase in the synthesis of phenylalanine, which is the main precursor in phenolic biosynthesis [40]. Therefore, it can be confidently assumed that nitrogen application plays a significant role in enhancing phenolic biosynthesis. Phosphorus plays a crucial role in plant biochemical processes, acting as an agent for the formation and release of energy in the form of ATP and ADP [41]. The synthesis of chemical energy initiated by phosphorus fertilization is utilized by plants for various cellular processes, including the biosynthesis of phenolic compounds. Potassium plays a crucial role in numerous physiological activities in plants, including the activation and stabilization of enzymes and proteins [42]. These effects may indirectly influence the biosynthesis of phenolic compounds, as many proteins and enzymes are involved in their production.
Several results uncovered in this study appeared to be ‘counter-intuitive’. For instance, TPC in the combination treatment of NP (8.61 mg GAE/g dry weight), NK (16.19 mg GAE/g dry weight), and PK (9.84 mg GAE/g dry weight) were significantly lower than the control treatment (17.0 mg GAE/g dry weight) [Figure 1]. Furthermore, the antioxidant capacity of the control treatment (based on the ABTS assay) was higher than that of the P, K, and NK treatment combinations [Table 1]. Several studies have shown an opposite effect between nutrient supply and quantity of plant metabolites [43] reported a decrease in the levels of phenolic compounds when the dose of nitrogen treatment was gradually raised. On the other hand, the results of [44] stated that low-dose nitrogen treatment increased phenolic accumulation and activated enzymes in phenolic biosynthesis in lettuce. The latter result implies the role of phenolic compounds in various environmental stresses such as such as drought, salinity, cold, heat, heavy metal toxicity, and UV radiation. [20,21,45].
There are several hypotheses as the explanation of the phenomena in this study. First, although the research results of Radušien? et al. [38] stated a decrease in the levels of phenolic compounds due to nitrogen treatment, on the other side, there was a significant increase in naphtodianthrones and emodin, both of which are derivatives of anthraquinone. It is in line with Carol et al. [30] which stated a decrease in the amount of TPC Pepino melon due to NPK treatment, but a drastic increase in lutein, lycopene, and β-carotene. Furthermore, the research of Narvekar and Tharayil [46] stated that the nitrogen supply increased the quantity of proanthocyanidins in strawberries, one of the phenolic oligomers. However, in the other phenolic oligomers, it did not occur. The exogenous nitrogen supply increases the saponin content in Anchomanes difformis [47]. Thus, although there was a decrease in TPC, there was an increase in other compounds that were still included in the phenolic group, or other compounds from other groups. Plant biosynthetic systems involve complex interactions between enzymes and substrates. Further research needs to examine a wider biochemical spectrum of C. xanthorrhiza to obtain a bigger picture regarding the role of NPK on the biochemical content of C. xanthorrhiza. Second, There are metabolites known as nitrogen-containing secondary metabolites [48]. From their chemical structure, these compounds are rich in nitrogen. Alkaloids, glucosinolates, cyanogenic glycosides, alkamides, and lecithin are nitrogenous secondary metabolites [49]. Some of these compounds have identified their health-giving effects as anti-bacterial, anti-viral, anti-malarial, anti-cancer, and immunosuppressive [50]. It is assumed that there is a change in the content of nitrogen-containing secondary metabolites of C. xanthorrhiza through the NPK treatment. However, This hypothesis needs to be proven through different biochemical analyses. Thirdly, environmental factors and post-harvest handling can affect the composition of plant primary and secondary metabolites [51] reported an increase in flavonoids in the leaves of Lithocarpus litseifolius due to the application of light intensity. Meanwhile, the increase in scutellarin, a flavone glycoside, in the leaves of Erigeron breviscapus occurred due to direct exposure to sunlight with high light intensity [52]. Li et al. [53] reported the activation of α-linolenic acid biosynthesis and jasmonic acid and their accumulation during the cold period in Camellia japonica. In addition, various reports agree that drought stress affects the accumulation of plant phenolic [54-56]. In addition to factors that occur in the experimental field, season and harvesting time allow for altering the plant phytochemicals and their pharmacological effects [57,58]. Overall, there are many factors, both endogenously and exogenously, that affect the accumulation of plant metabolites. In addition, One plausible explanation for the decrease in plant metabolite yields resulting from fertilizer treatment is the occurrence of antagonistic relationships between nutrients. Antagonistic effects between nutrients can occur when plant yields are lower than expected due to the combination of the two nutrients. Many studies have stated that the antagonistic effect of nutrients usually occurs in divalent cations. However, in several cases, the interaction between nitrogen (NH4+) and potassium (K+) has been confirmed to have an antagonistic relationship [59]. Antagonistic relationships between nutrients can significantly affect crop yields [60]. Therefore, it is suspected that the low phenolic content due to the application of two nutrients may be caused by the antagonistic effect of nutrients. However, this hypothesis needs to be properly and comprehensively proven in further research.
This study also reveals a theoretical relationship between TPC and TFC [Figure 3]. Pagassini et al. [45] explained that phenolic and flavonoid compounds are present in the same biosynthetic pathway, namely the shikimate pathway and phenylpropanoids. Besides polyphenol content analysis, Four antioxidant analyses were conducted, which included two assays for scavenging activities (DPPH and ABTS) and two assays for reducing power capacities (FRAP and CUPRAC). Most interestingly, the correlation value between TFC and the CUPRAC assay, which is relatively strong, indicates a tremendous contribution of flavonoids as antioxidants with reducing capacities of free radicals. Fundamentally, the CUPRAC and FRAP assays aim to analyze the reducing capacity of antioxidants through electron transfer mechanisms [61,62]. However, in this study, the antioxidant activity based on the CUPRAC assay was more dominant than the FRAP assay. Therefore, the polyphenol of C. xanthorrhiza obtained from methanol extract has a reducing power antioxidant activity. These results correspond to several antioxidant analyses, such as the winged bean [61], Celosia cristata [8,11], Tea [63], Phaseolus lunatus, and Canavalia ensiformis [64].
5. CONCLUSION
The pplication of both single and combined NPK fertilizers leads to a significant increase in the polyphenol content and antioxidant capacity of C. xanthorrhiza. The polyphenol content of C. xanthorrhiza methanol extract has high antioxidant activity, especially with the reduced power capacities.
6. ACKNOWLEDGMENTS
The authors would like to acknowledge the Chemistry Education Study Program at Jambi University and the Departments of Biochemistry and Agronomy and Horticulture at IPB University for their unwavering support in writing this journal article. We would also like to express our gratitude to Jambi University and IPB University Research Collaborations for their financial support (grant numbers 2158/UN21.11/PT.01.05/SPK/2022 and 4014/IT3.L1/PT.01.03/M/T/2022) in funding the project and facilitating manuscript preparation.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICT OF INTEREST
State that the authors have no conflict of interest.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analysed are included within this research article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Nurcholis W, Priosoeryanto BP, Purwakusumah ED, Katayama T, Suzuki T. Antioxidant, cytotoxic activities and total phenolic content of four indonesian medicinal plants. J Kim Valensi 2012;2:501-10. [CrossRef]
2. Fitria R, Seno DS, Priosoeryanto BP, Hartanti, Nurcholis W. Volatile compound profiles and cytotoxicity in essential oils from rhizome of Curcuma aeruginosa and Curcuma zanthorrhiza. Biodiversitas J Biol Divers 2019;20:2943-8. [CrossRef]
3. Sukweenadhi J, Yunita O, Setiawan F, Kartini, Siagian MT, Danduru AP, et al. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas J Biol Divers 2020;21:2062-7. [CrossRef]
4. Rahmat E, Lee J, Kang Y. Javanese turmeric (Curcuma xanthorrhiza Roxb.):Ethnobotany, phytochemistry, biotechnology, and pharmacological activities. Evid Based Complement Altern Med 2021;2021:9960813. [CrossRef]
5. Atun S, Sinardekawati A, Purpratama AC, Aznam N, Sangal A. Curcuminoid nanoemulsion from Curcuma xanthorrhiza extract and its activity as antioxidant, antibacterial and antifungal. Rasayan J Chem 2022;15:907-13. [CrossRef]
6. Tulim S, Nazar K, Margono A, Meidyawati R, Yanti E. Comparison of antifungal effect of Xanthorrhizol (Curcuma xanthorrhiza roxb.) and 2% chlorhexidine against candida albicans American type culture collection 10231 biofilm. Int J Appl Pharm 2020;12:80-4. [CrossRef]
7. Cahyono B, Ariani J, Failasufa H, Suzery M, Susanti S, Hadiyanto H. Extraction of homologous compounds of curcuminoid isolated from temulawak (Curcuma xanthorriza roxb.) plant. Rasayan J Chem 2019;12:7-13. [CrossRef]
8. Aisyah SI, Yudha YS, Sukma D, Nurcholis W. Phenotypic variation and the polyphenols content alteration of Celosia cristata due to chronically induced mutation using ethyl methane sulphonate. J Southwest Jiaotong Univ 2022;57:221-30. [CrossRef]
9. Okello OP, Gweyi JP, Nawiri MP, Musila W. Effects of water stress on phenolic content and antioxidant activity of African nightshades. Biofarmasi J Nat Prod Biochem 2017;15:79-95. [CrossRef]
10. Shi H, Geng B, Zhao Y, Liu Y, Huang R, Zhao P, et al. EMS-induced mutations in common vetch (Vicia sativa L.) and two mutants without anthocyanin accumulation showing increased cold tolerance. Grassl Sci 2021;67:148-55. [CrossRef]
11. Yudha YS, Aisyah S, Sukma D, Nurcholis W. Phenolic, flavonoid and antioxidant capacities evaluation of Celosia cristata resulted from induced mutation using ethyl methane sulphonate. Pak J Biol Sci 2022;25:380-6. [CrossRef]
12. ?miechowska M, Dmowski P, Skowierzak L. Edible flowers'antioxidant properties and polyphenols content reflect their applicability for household and craft tincture production. Appl Sci Switz 2021;11:10095. [CrossRef]
13. Sahid ZD, Syukur M, Maharijaya A, Nurcholis W. Polyphenol content and pharmacological activities of Capsicum frutescens and C. chinense genotypes. Biodiversitas J Biol Divers 2021;22:3838-43. [CrossRef]
14. Hong MJ, Kim DY, Jo YD, Choi HI, Ahn JW, Kwon SJ, et al. Biological effect of gamma rays according to exposure time on germination and plant growth in wheat. Appl Sci 2022;12:3208. [CrossRef]
15. Jorkesh A, Hamidoghli Y, Olfati J, Samizadeh H, Bakhshi D, Palá-Paúl J. Morphological and biochemical variability of Froriepia. Int J Veg Sci 2020;26:262-74. [CrossRef]
16. Ilakkiya V, Dhanalakshmi B, Kaviya V. Effect of physical treatment on the physicochemical, rheological and functional properties of yam meal of the cultivar “Ngumvu“from Dioscorea alata L. of Congo. Int J Recent Sci Res 2021;12:41490-5.
17. Rindita, Anggia V, Rahmaesa E, Devi RK, Alawiyah LF. Exploration, phenolic content determination, and antioxidant activity of dominant pteridophytes in Gunung Malang village, Mount Halimun Salak national park, Indonesia. Biodiversitas J Biol Divers 2020;21:3676-82. [CrossRef]
18. Sidhiq DF, Widiyastuti Y, Subositi D, Pujiasmanto B, Yunus A. Morphological diversity, total phenolic and flavonoid content of Echinacea purpurea cultivated in Karangpandan, central Java, Indonesia. Biodiversitas J Biol Divers 2020;21:1265-71. [CrossRef]
19. Kumar S, Sandhir R, Ojha S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res Notes 2014;7:560. [CrossRef]
20. Šamec D, Karalija E, Šola I, Vuj?i?Bok V, Salopek-Sondi B. The role of polyphenols in abiotic stress response:The influence of molecular structure. Plants 2021;10:118. [CrossRef]
21. Yu D, Huang T, Tian B, Zhan J. Advances in biosynthesis and biological functions of proanthocyanidins in horticultural plants. Foods 2020;9:1774. [CrossRef]
22. Nurcholis W, Ambarsari L, Purwakusumah ED. Curcumin analysis and cytotoxic activities of some Curcuma xanthorrhiza roxb. Accessions. Int J PharmTech Res 2016;9:175-80.
23. Aslam MM, Farhat F, Siddiqui MA, Yasmeen S, Khan MT, Sial MA, et al. Exploration of physiological and biochemical processes of canola with exogenously applied fertilizers and plant growth regulators under drought stress. PLoS One 2021;16:e0260960. [CrossRef]
24. SombiéPA, Sama H, SidibéH, Kiendrébéogo M. Effect of organic (Jatropha cake) and NPK fertilizers on improving biochemical components and antioxidant properties of five cowpea (Vigna unguiculata L. Walp.) genotypes. J Agric Sci 2019;11:48-62. [CrossRef]
25. Ahmad I, Zhu G, Zhou G, Song X, Ibrahim ME, Salih EG. Effect of N on growth, antioxidant capacity, and chlorophyll content of sorghum. Agronomy 2022;12:501. [CrossRef]
26. Marlin M, Simarmata M, Salamah U, Nurcholis W. Effect of nitrogen and potassium application on growth, total phenolic, flavonoid contents, and antioxidant activity of Eleutherine palmifolia. Agric Food 2022;7:580-93. [CrossRef]
27. Xu X, Du X, Wang F, Sha J, Chen Q, Tian G, et al. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front Plant Sci 2020;11:904. [CrossRef]
28. Ahmed A, Sambo B, Arunah U, Odion E. Response of farmyard manure and inorganic fertilizers for sustainable growth of carrot (Daucus carota L.) in northern Nigeria. IOSR J Agric Vet Sci 2014;7:18-25. [CrossRef]
29. Sun J, Luo H, Jiang Y, Wang L, Xiao C, Weng L. Influence of nutrient (NPK) factors on growth, and pharmacodynamic component biosynthesis of Atractylodes chinensis:An insight on Acetyl-CoA Carboxylase (ACC), 3-Hydroxy-3-Methylglutaryl-CoA reductase (HMGR), and Farnesyl Pyrophosphate Synthase (FPPS) Si. Front Plant Sci 2022;13:799201. [CrossRef]
30. Carol MM, Joshua OO, Robert MG. Effect of NPK fertilizer rates on secondary metabolites of pepino melon (Solanum muricatum Aiton). J Hortic For 2021;13:25-34. [CrossRef]
31. Nihayati E, Wardiyati T, Retnowati R, Soemarno S. The curcumin content of Temulawak (Curcuma xanthorriza Roxb.) rhizome as affected by N, K and micronutrients B, Fe, Zn. Agrivita J Agric Sci 2013;35:218-26. [CrossRef]
32. Peng LC, Ng LT. Impacts of nitrogen and phosphorus fertilization on biomass, polyphenol contents, and essential oil yield and composition of Vitex negundo Linn. Agriculture 2022;12:859. [CrossRef]
33. Khumaida N, Syukur M, Bintang M, Nurcholis W. Phenolic and flavonoid content in ethanol extract and agro-morphological diversity of Curcuma aeruginosa accessions growing in West Java, Indonesia. Biodiversitas J Biol Divers 2019;20:656-63. [CrossRef]
34. Nurcholis W, Khumaida N, Syukur M, Bintang M. Evaluation of free radical scavenging activity in ethanolic extract from promising accessions of Curcuma aeruginosa RoxB. Molekul 2017;12:133-8. [CrossRef]
35. Nurcholis W, Putri DN, Husnawati H, Aisyah SI, Priosoeryanto BP. Total flavonoid content and antioxidant activity of ethanol and ethyl acetate extracts from accessions of Amomum compactum fruits. Ann Agric Sci 2021;66:58-62. [CrossRef]
36. Suleria HA, Barrow CJ, Dunshea FR. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020;9:1206. [CrossRef]
37. Zheng J, Yu X, Maninder M, Xu B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int J Food Prop 2018;21:1524-40. [CrossRef]
38. Radušien?J, Marksa M, Ivanauskas L, Jakštas V, Çali?kan Ö, Kurt D, et al. Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Industrial Crops and Products 2019;139:1–9.
39. Xiaohong O, Ye Y, Lanping G, Duanwei Z, Dahui L. Effect of organic-inorganic N sources on growth, NPK nutrients and secondary metabolites of Panax notoginseng (Burk.) F. H. Chen. Emir J Food Agric 2017;29:629-38. [CrossRef]
40. Wang Y, Wang YM, Lu YT, Qiu QL, Fan DM, Wang XC, et al. Influence of different nitrogen sources on carbon and nitrogen metabolism and gene expression in tea plants (Camellia sinensis L.). Plant Physiol Biochem 2021;167:561-6. [CrossRef]
41. Salinas R, Sánchez E, Ruíz JM, Lao MT, Romero L. Phosphorus levels influence plasma membrane H+-ATPase Activity and K+, Ca2+, and Mg2+ assimilation in Green bean. Commun Soil Sci Plant Anal 2013;44:456-64. [CrossRef]
42. Rani P, Saini I, Singh N, Kaushik P, Wijaya L, Al-Barty A, et al. Effect of potassium fertilizer on the growth, physiological parameters, and water status of Brassica juncea cultivars under different irrigation regimes. PLOS One 2021;16:e0257023. [CrossRef]
43. Radušien?J, Marksa M, Ivanauskas L, Jakštas V, Çali?kan Ö, Kurt D, et al. Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Ind Crops Prod 2019;139:111519. [CrossRef]
44. Zhou W, Liang X, Li K, Dai P, Li J, Liang B, et al. Metabolomics analysis reveals potential mechanisms of phenolic accumulation in lettuce (Lactuca sativa L.) induced by low nitrogen supply. Plant Physiol Biochem 2021;158:446-53. [CrossRef]
45. Pagassini JA, de Godoy LJ, Campos FG, Barzotto GR, Vieira MA, Boaro CS. Silicon and mechanical damage increase polyphenols and vitexin in Passiflora incarnata L. Sci Rep 2021;11:22064. [CrossRef]
46. Narvekar AS, Tharayil N. Nitrogen fertilization influences the quantity, composition, and tissue association of foliar phenolics in strawberries. Front Plant Sci 2021;12:613839. [CrossRef]
47. Rivai RR, Wardani FF, Zulkarnaen RN. The effect of NPK fertilizer and planting media on plant growth and saponin content of the medicinal plant Anchomanes difformis. Nusant Biosci 2017;9:141-5. [CrossRef]
48. Shimizu Y, Rai A, Okawa Y, Tomatsu H, Sato M, Kera K, et al. Metabolic diversification of nitrogen-containing metabolites by the expression of a heterologous lysine decarboxylase gene in Arabidopsis. Plant J 2019;100:505-21. [CrossRef]
49. Song C, Jiao C, Jin Q, Chen C, Cai Y, Lin Y. Metabolomics analysis of nitrogen-containing metabolites between two Dendrobium plants. Physiol Mol Biol Plants 2020;26:1425-35. [CrossRef]
50. Puri SK, Habbu PV, Kulkarni PV, Kulkarni VH. Nitrogen containing secondary metabolites from endophytes of medicinal plants and their biological/pharmacological activities-a review. Syst Rev Pharm 2018;9:22-30. [CrossRef]
51. Li A, Li S, Wu X, Zhang J, He A, Zhao G, et al. Effect of light intensity on leaf photosynthetic characteristics and accumulation of flavonoids in Lithocarpus litseifolius (Hance) Chun. (Fagaceae). Open J For 2016;6:445-59. [CrossRef]
52. Zhou R, Su WH, Zhang GF, Zhang YN, Guo XR. Relationship between flavonoids and photoprotection in shade-developed Erigeron breviscapus transferred to sunlight. Photosynthetica 2016;54:201-9. [CrossRef]
53. Li Q, Lei S, Du K, Li L, Pang X, Wang Z, et al. RNA-seq based transcriptomic analysis uncovers a-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci Rep 2016;6:36463. [CrossRef]
54. Chaimala A, Jogloy S, Vorasoot N, Toomsan B, Jongrungklang N, Kesmala T, et al. Responses of total biomass, shoot dry weight, yield and yield components of jerusalem artichoke (Helianthus tuberosus L.) varieties under different terminal drought duration. Agriculture 2020;10:198. [CrossRef]
55. Singh AK, Dhanapal S, Yadav BS. The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol Biol Rep 2020;47:1459-70. [CrossRef]
56. Naoura G, Sawadogo N, Atchozou EA, Emendack Y, Hassan MA, Reoungal D, et al. Assessment of agro-morphological variability of dry-season sorghum cultivars in Chad as novel sources of drought tolerance. Sci Rep 2019;9:19581. [CrossRef]
57. Bansal M, Reddy MS, Kumar A. Seasonal variations in harvest index and bacoside a contents amongst accessions of Bacopa monnieri (L.) Wettst. Collected from wild populations. Physiol Mol Biol Plants 2016;22:407-13. [CrossRef]
58. Vinolina NS, Siregar L, Sirat B, Nurhayati E, Adelina E, Nazirah L. Effect of harvest time on bioactive compounds of field-cultivated Centella asiatica (L) Urban. J Agron 2019;18:55-60. [CrossRef]
59. Coskun D, Britto DT, Kronzucker HJ. The nitrogen-potassium intersection:Membranes, metabolism, and mechanism:The N-K intersection. Plant Cell Environ 2017;40:2029-41. [CrossRef]
60. Rietra RP, Heinen M, Dimkpa CO, Bindraban PS. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun Soil Sci Plant Anal 2017;48:1895-920. [CrossRef]
61. Calvindi J, Syukur M, Nurcholis W. Investigation of biochemical characters and antioxidant properties of different winged bean (Psophocarpus tetragonolobus) genotypes grown in Indonesia. Biodiversitas J Biol Divers 2020;21:2420-4. [CrossRef]
62. Wairata J, Fadlan A, Purnomo AS, Taher M, Ersam T. Total phenolic and flavonoid contents, antioxidant, antidiabetic and antiplasmodial activities of Garcinia forbesii King:A correlation study. Arab J Chem 2022;15:103541. [CrossRef]
63. Bahadori MB, Zengin G, Dinparast L, Eskandani M. The health benefits of three hedgenettle herbal teas (Stachys byzantina, Stachys inflata, and Stachys lavandulifolia)-profiling phenolic and antioxidant activities. Eur J Integr Med 2020;36:101134. [CrossRef]
64. Koley TK, Maurya A, Tripathi A, Singh BK, Singh M, Bhutia TL, et al. Antioxidant potential of commonly consumed underutilized leguminous vegetables. Int J Veg Sci 2019;25:362-72. [CrossRef]