1. INTRODUCTION
Fruits and vegetables are regarded as medicinal plants, which play important roles in human nutrition and community health because they are rich in secondary metabolites and dietary fibers. [1,2]. Many chemical compounds are found in these plants, including alkaloids, flavonoids, glycosides, saponins, resins, oleoresins, sesquiterpene, phenolic compounds, fats, and oils [3,4]. Citrus fruits are one of the most abundant and widely available fruit crops worldwide, due to their high productivity, low cost, nutritive values, sweet taste, flavor, and healthy dietary properties [4-6]. Citrus fruits belong to Rutaceae family which composes about 1300 species and the commonly cultivated citrus fruits include Lemon (Citrus limon), Orange (Citrus sinensis), Grapefruit (Citrus paradisi), Lime (Citrus latifolia), and Mandarin/Tangerine (Citrus reticulate) [4,7]. Oil extract of lemon leaf has been reported to inhibit the growth of bacteria due to monoterpenes and alkaloids in it. Furthermore, lemon juice plus hot water has been described as the best antibacterial gargle [8].
Several studies have documented global production of citrus fruit to be of about 160 million tons per annum, with 75% being freshly consumed while the remaining are processed into other products. About 50–60% of waste can be generated from the fresh fruit mass which are used as livestock feeds or discarded on an open land thereby causing environmental pollution, underutilization of its high-value application potentials, and reduction in the nation’s economy [7-10].
Citrus fruit wastes especially the peels, pomace, and seeds are part of the important agro-wastes generated from crops [11,12]. Documented evidence shows that bio-based polymers were first generated from plant-based biopolymers especially polymers obtained from agriculture wastes [13]. Moreover, agro-wastes like citrus fruit wastes possess a significant number of compounds such as fibers (cellulose, hemicelluloses, lignin, and pectin), lipids, carbohydrates, peptides, carotenes, essential oils, phenols, some bioactive components such terpenoids, carotenoids, coumarins, limonoids and vitamins, and so on, that are bioactive and of multi-functional values [4,14,15]. They have a lot of high-value application potentials and are therefore used after conversion or recycling as ingredients incorporated into some food products to aid their nutritional and antimicrobial properties, shelf lives, and other qualities [2].
Several studies have reported citrus fruit wastes as an important source of bioactive compounds and antioxidants such as ascorbic acid, flavonoids phenolic compounds, and pectins, which are essential in human nutrition. Eriocitrin, Narirutin, Naringin, and Hesperidine, are main flavonoids found in citrus species and its by-products or wastes. For instance, the Citrus wastes possess naturally occurring flavonoids especially the peel, which has the highest concentration of flavonoids such as flavanones and polymethoxylated flavones, compared to other plant where they are scarcely found [4,16]. Despite the development of various antibiotics by the pharmaceutical industry, bacterial populations are rapidly developing antibacterial resistance, posing a serious threat to the success of infectious disease treatment. On the other hand, commercial antibiotic-related side effects are frequently mentioned, therefore, new antimicrobial compounds need to be discovered and researched frequently. Plant-based bioactive components are important source for the discovery of novel and natural antimicrobial and antioxidant potentials and they have demonstrated ability to prevent enzymatic activity, interact with proteins, boost immune system, and hinder pathogenesis of metabolic-related human disease condition by harming microbial cells [17,18].
The essential oils obtained from citrus fruit peels comprise many volatile compounds, mainly aldehydes, ketones, esters, alcohols, and terpenes, which give it characteristic aromas and flavors. Citrus essential oils are greatly utilized as flavoring agent in the food and beverage industries, and as fragrance materials in the perfumery, toiletries, fine chemicals, and cosmetic products. They can also be used to some extent as a traditional medicine [19]. Attention has been drawn to the application of agricultural and food processing wastes as one of the major sources of bioactive compounds for their natural antibacterial, antifungal, anticancer, antidiabetic, and antiviral activities which will consequently enhance the nation’s health systems and economy, the prevent environmental pollution and sustains the ecosystem [20]. However, a few information has been reported on the bioactive components of citrus fruit waste extracts and essential oils to establish the facts about their biological significance and potential health effects. Hence, this study aimed to investigate the bioactive properties of essential oils and ethanol extracts of two citrus fruits (lemon and lime) wastes.
2. MATERIALS AND METHODS
2.1. Collection of Plant Materials
Lemon (Citrus limon) and lime (Citrus aurantifolia) fruits were obtained from a local market in Ado-Ekiti, Ekiti State, Nigeria. A voucher sample was deposited and authenticated at the Department of Biotechnology and Plant Science, University of Ado-Ekiti, Ekiti State Nigeria, Herbarium numbers: UHAE2023003 (Lemon) and UHAE2023002 (Lime) were thereafter provided. A total of six bacterial and five fungal pathogens were used for antibacterial and antifungal assay, respectively. The bacterial species were Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, and S. aureus while the fungal cultures were, Aspergillus niger, Alternaria sp., Corynespora sp., Fusarium sp., and Rhizopus sp. All the bacterial and fungal pathogens were obtained from the Microbiology Laboratory in the Department of Biological Sciences, Afe Babalola University, Ado-Ekiti, Nigeria. Before use, the bacterial and fungal pathogens were sub cultured on nutrient agar and sabouraud dextrose agar plates, respectively, to ascertain their purity.
2.2. Preparation of Plant Extracts
This study employed two citrus fruits: Lemon (Citrus limon) and lime (Citrus aurantifolia). Peels, pomace, seeds, and essential oils were used for each of the fruits. The fruit samples were washed several times with distilled water before use to remove sand and other debris or dirt. To obtain the pomace and seeds, the samples were peeled, cut, and the juice was squeezed out. Each fruit part was then air-dried for 14 days before being pulverized with a sterile electric blender and weighed. 200 g of each of the pulverized peels, pomace, and seed were soaked in 400 mL of absolute ethanol for 24 h for extraction. The solution was then filtered using Whatman No. 1 filter paper and transferred to a rotatory evaporator for concentration of the extracts.
2.3. Preparation of the Essential Oils
The essential oils were prepared using the method described by the Association of Official Analytical Chemist (AOAC) 920.85 with Soxhlet apparatus [21]. 100 g of the air-dried respective sample was wrapped with a filter paper and placed into the thimble of the Soxhlet extractor. The material sample was continuously extracted for 6 h using n-hexane as the solvent. At the end of the extraction, the sample was removed from the thimble and the solvent distilled off. The oil was poured into a sample bottle and kept for further analysis.
2.4. Antimicrobial Assay
In vitro, antibacterial and antifungal activity was evaluated by the agar-well diffusion methods. The respective peel, pomace and seed extracts, and essential oils were tested against all the bacterial and fungal species. For stock extract solution, 1 g of the respective extracts were dissolved in 20 mL of dimethylsulfoxide. Antibacterial assay of the extracts and essential oils was carried out as described elsewhere [22]. For antifungal assay, mycelial discs of young actively growing respective fungal cultures were cut separately with a sterile cork borer and inoculated at the center of already prepared plates containing the different extracts and the control plates (without extracts) and incubated at 28 ± 2°C for 3 d.
The mycelial growth diameter (cm) of each pathogen was measured and the percentage of growth inhibition was calculated according to [23] as follows:
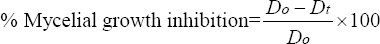 |
Where Do = Diameter of mycelial growth of fungal pathogen in the control plates; Dt = Diameter of mycelial growth of fungal pathogen in the treatment plates.
2.5. Qualitative Phytochemical and In Vitro Antioxidant Assays
The qualitative phytochemical screening of the respective extracts was determined as described [24]. The DPPH (Sigma–Aldrich, Sternheim, Germany) free radical scavenging activity assay of the extracts was carried out according to the method described [25]. A properly diluted portion (1 mL) was combined with an equal volume of a methanol-based 0.4 mM DPPH solution. The mixture was measured at 516 nm for absorbance after 30 min of dark incubation, using Quercetin as a standard. The DPPH free radical scavenging activity was reported as a percentage (%) control inhibition.
2.6. Gas Chromatography-Mass Spectrophotometric (GS-MS) Analysis
GC-MS analysis of the respective samples was carried using a Varian 3800/4000 gas chromatograph mass spectrometer equipped with an Agilent equipped with a BP5 (30 m × 0.25 mm × 0.25 microns) capillary column.
2.6.1. Identification of compounds
Organic compounds in the samples were identified in Wiley’s NIST 08 Mass Spectral Library to obtained comparison scores that were higher than 95%. Otherwise, fragmentation peaks of the compounds were evaluated, and the compounds were identified using the memory background for the identification of the compounds that appeared in GCMS chromatograms.
3. RESULTS
3.1. Antibacterial Properties of the Extracts and Essential Oil
Antibacterial assay of the lemon extracts and essential oil revealed sensitivity of all the bacterial species to the seed extracts. However, B. subtilis, P. aeruginosa, and S. aureus were observed to be resistant to the pomace, peel extracts, and essential oil, respectively. In general, highest zone of inhibition (16 mm) was recorded against Klebsiella pneumonia, for the peel extract. None of the lime extracts and essential oil inhibited the growth of P. aeruginosa. B. subtilis and Salmonella typhi were observed to be resistant to the pomace extract while all the seed extract did not inhibit the growth of any of the bacterial species [Table 1].
Table 1: Antibacterial potential of the extracts and essential oil.
| Extracts | Zone of inhibition (mm) | Salmonella typhi | ||||
|---|---|---|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Klebsiella pneumoniae | Bacillus subtilis | ||
| Lemon extracts | ||||||
| Peel | 5 | 8 | 14 | 16 | Resistant | 13 |
| Pomace | 10 | Resistant | 10 | 11 | 8 | 10 |
| Seed | 8 | 8 | 10 | 13 | 8 | 10 |
| Oil | 7 | 5 | Resistant | 15 | 9 | 8 |
| Lime extracts | ||||||
| Peel | 9 | Resistant | 8 | 28 | Resistant | Resistant |
| Pomace | 11 | Resistant | 11 | 28 | 8 | 13 |
| Seed | Resistant | Resistant | Resistant | Resistant | Resistant | Resistant |
| Oil | 5 | R | 11 | 9 | 15 | 5 |
3.2. Fungal Inhibitory Potential of the Extracts and Essential Oil
In general, all the extracts and essential oils showed mycelia inhibition against the test fungal pathogens. The highest mycelial inhibition of 84, 71, 78, and 67% was observed against Corynespora sp. when treated with the lemon peel, pomace and seed extracts and essential oil, respectively. In the lime extracts and essential oil, the highest mycelial inhibition of 62% (peel and pomace), 73% (seed), and 67 (essential oil) was observed against Corynespora sp. [Table 2].
Table 2: Fungal inhibitory potential of the extracts and essential oil.
| Extracts | Percentage mycelial inhibition | ||||
|---|---|---|---|---|---|
| Aspergillus niger | Alternaria sp. | Corynespora sp. | Fusarium sp. | Rhizopus sp. | |
| Lemon extracts | |||||
| Peel | 35 | 44 | 84 | 25 | 25 |
| Pomace | 31 | 29 | 71 | 22 | 10 |
| Seed | 38 | 49 | 78 | 13 | 65 |
| Oil | 22 | 60 | 67 | 37 | 65 |
| Lime extracts | |||||
| Peel | 38 | 40 | 62 | 50 | 5 |
| Pomace | 18 | 56 | 62 | 25 | 10 |
| Seed | 38 | 40 | 73 | 50 | 65 |
| Oil | 18 | 60 | 67 | 37 | 60 |
3.3. Phytochemical Components of the Extracts
Phytochemical screening of the extracts revealed the presence of alkaloids, saponins, carbohydrates, reducing sugars, flavonoids, terpenoids, phenols, and tannins in the lemon peel, and seed extracts. Reducing sugars were however absent in the lemon pomace. In the case of the lime peel and pomace, terpenoids were observed to be absent, while flavonoids, phenols, and tannins were absent in the lime seed extract [Table 3].
Table 3: Phytochemical composition of the extracts.
| Extracts | Alkaloids | Saponins | Carbohydrates | Reducing sugars | Flavonoids | Terpenoids | Phenols | Tannins |
|---|---|---|---|---|---|---|---|---|
| Lemon | ||||||||
| Peel | + | + | + | + | + | + | + | + |
| Pomace | + | + | + | − | + | + | + | + |
| Seed | + | + | + | + | + | + | + | + |
| Lime | ||||||||
| Peel | + | + | + | + | + | − | + | + |
| Pomace | + | + | + | + | + | − | + | + |
| Seed | + | + | + | + | − | + | − | − |
“+” and “−” represent present and absent, respectively,
3.4. DPPH Scavenging Activity of the Extracts
The antioxidant potential of the extracts revealed DPPH free radical inhibiting ability in a concentration-dependent manner. At the concentrations studied, all the lemon and lime extracts (peel, pomace, and seed) showed higher DPPH scavenging ability than Quercetin [Figure 1].
 | Figure 1: 2,2-diphenylpicrylhydrazyl scavenging activity of the extracts at different concentrations. [Click here to view] |
3.5. Compounds Detected in the Lemon Extracts and Essential Oils
A total of 15 compounds were detected from each of the extracts and essential oils. The major compounds identified in the lemon extracts and essential oil were tetradecanoic acid, 1,4-butanediol, 2-(1-ethoxyethoxy)-3-methyl-, n-hexadecanoic acid, 1,2-benzenedicarboxylic acid, diisooctyl ester, stigmasterol, spathulenol, octadecane and diethyl phthalate, and 3,7,11,15-tetramethyl-2-hexadecen-1-ol. The highest detection of tetradecanoic acid (20.36%) was detected in the peel extract [Figure 2]. In the lime extracts and essential oil, the dominant compounds detected were n-hexadecanoic acid, 1,2-benzenedicarboxylic acid, diisooctyl ester, tetradecanoic acid, 9,12,15-octadecatrienoic acid, methyl ester, stigmasterol, spathulenol, benzoic acid, 4-hydroxy-3,5-dimethoxy-, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, and 9,12-octadecadienoic acid (Z, Z)-. The highest detection of n-hexadecenoic acid (18.90%) was observed in the pomace extract [Figure 3].
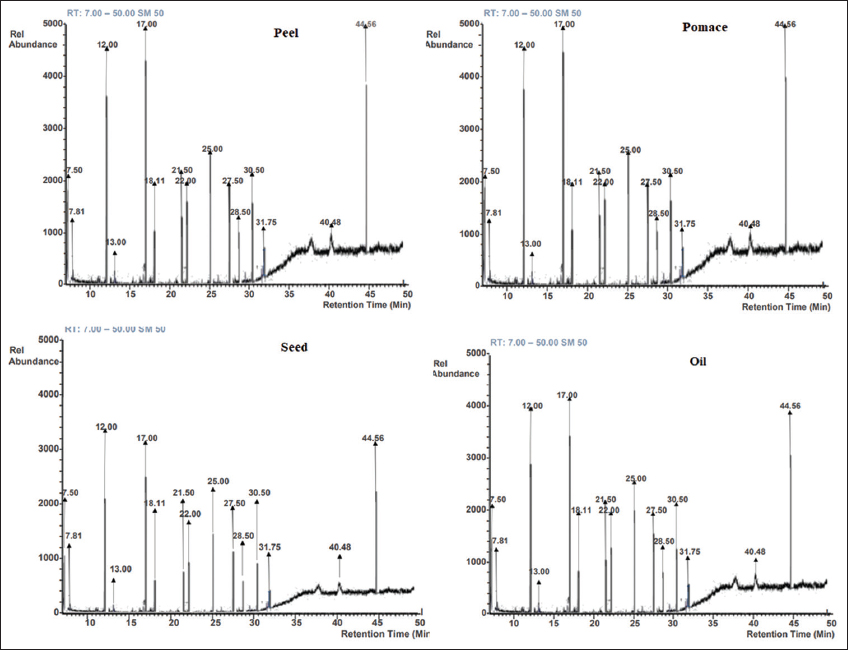 | Figure 2: Gas chromatograms of the lemon extracts and essential oil. [Click here to view] |
 | Figure 3: Gas chromatograms of the lime extracts and essential oil. [Click here to view] |
4. DISCUSSION
The results in this study revealed the susceptibility of all the microorganisms tested against the lemon seed extracts. This is consistent with the findings [22] and [24] that reported a remarkable bioactivity of lemon extract against most of the tested bacterial species in this study. Furthermore, S. typhi, among the bacterial isolates tested, was most vulnerable to the essential oil of citrus species. This antibacterial activity of lemon extracts and essential oil could be due to the synergistic effect of the available bioactive constituents. These compounds could also be responsible for the inhibitory action against the tested species by the interaction of the phytochemicals with the microbes’ intracellular sites through the reported formation of hydrogen bond which alters their cellular protein structures and consequently results to microbial death [18,22,27].
Similarly, all the extracts and essential oils showed mycelia inhibition of the tested fungal pathogens and the highest mycelial inhibition was demonstrated against Corynespora sp. when treated with the lemon peel, pomace, seed extracts, and essential oils. This observation corroborates several other studies that reported inhibitory effect of citrus extracts against the growth of different fungal species [23,28,29].
The presence of alkaloids, saponins, carbohydrates, reducing sugars, flavonoids, terpenoids, phenols, and tannins was confirmed by phytochemical screening of the lemon peel and seed extracts. However, reports of previous studies have linked the antimicrobial activity of some of these metabolites to the presence of oxygenated terpenes and phenolic groups in particular, as well as the synergetic effects of all the chemical constituents in the extracts and essential oils [22,30]. Moreover, therapeutic properties (antidiabetic, anticarcinogenic, antithrombotic, stimulatory, antibacterial, antifungal, and antiviral) of citrus extracts and essential oils have been related to the presence of some bioactive components such as flavonoids, carotenoids, and alkaloids. [15,18,19,31].
In addition, at the various concentrations investigated, all the lemon and lime extracts (peel, pomace, and seed) showed higher antioxidant capacity through their DPPH free radical scavenging ability in a favorable competitive manner with quercetin. This effect could possibly be linked to the ability of lemon and lime extracts to release hydrogen atoms (H+) and consequently neutralize the effects of unstable electrophiles/free radicals [18]. The antioxidant activity of lemon and lime extracts could also be due to the presence of detected bioactive compounds as revealed in the result of GC-MS analysis
A total of 15 compounds were detected from each of the extracts and essential oils which are in consistent with past report [32]. Moreover, the highest level or concentration of tetradecanoic acid was confirmed in the peel extract while n-hexadecenoic acid in the pomace extract. Several studies have reported citrus extracts especially tetradecanoic acid detected in lemon peel and n-Hexadecanoic acid in lime pomace to possess or exhibit l antibacterial, anti-inflammatory, antioxidant, and antifungal effects when used alone or combined [33]. The differences in the n-hexadecanoic acid (lemon, 16.33%; lime, 13.12%), 1,2-benzenedicarboxylic acid, diisooctyl ester (lemon, 15.33%; lime, 10.33%) and octadecane (lemon, 10.75%; lime, 5.08%) could also be another factor which made lemon extracts to be more effective on all the bacterial and fungal isolates compared to lime extracts.
5. CONCLUSION
The findings of this work revealed that all lemon extracts and essential oil possessed higher antimicrobial activity against many of the bacterial and fungal isolates tested, most especially K. pneumoniae and salmonella typhi. Whereas, lemon seed and peel demonstrated a higher antioxidant activity and percentage composition of tetradecanoic acid. Therefore, it could be suggested that lemon extracts and its essential oil possess antimicrobial activity than lime extract and essential oil, an observation that could possibly be linked to the activities of available phytochemicals (secondary metabolites). The findings of this study revealed that extracts and essential oils of lemon and lime could be explored for their possible bioactivities and potential health benefits.
6. ACKNOWLEDGMENTS
The authors are grateful to Afe Babalola University for providing facilities for the study.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Khan B, Niazi MB, Samin G, Jahan Z. Thermoplastic starch:A possible biodegradable food packaging material-A review. J. Food Process Eng 2017;40:e12447. [CrossRef]
2. Lemes AC, Egea MB, Filho JG, Gautério GV, Ribeiro BD, Coelho MA. Biological approaches for extraction of bioactive compounds from agro-industrial by-products:A review. Front Bioeng Biotechnol 2022;9:802543. [CrossRef]
3. Salem MZ, Mervat EH, Ali HM, Abdel-Megeed A, El-Settawy AA, Böhm M, et al. Plants-derived bioactives:Novel utilization as antimicrobial, antioxidant, and phytoreducing agents for the biosynthesis of metallic nanoparticles. Microb Pathog 2021;158:105107. [CrossRef]
4. Amutha R, Kavusik T, Sudha A. Analysis of bioactive compounds in citrus fruit peels. Int J Sci Res Rev 2017;6:19-27.
5. Yun D, Liu J. Recent advances on the development of food packaging films based on Citrus processing wastes:A review. J Sci Food Agric 2022;9:100316. [CrossRef]
6. Iqbal A, Schulz P, Rizvi SS. Valorization of bioactive compounds in fruit pomace from agro-fruit industries:Present insights and future challenges. Food Biosci 2021;44:101384. [CrossRef]
7. Andrade MA, Barbosa CH, Shah MA, Ahmad N, Vilarinho F, Khwaldia K, et al. Citrus by-products:Valuable source of bioactive compounds for food applications. Antioxidants 2022;12:38. [CrossRef]
8. Chaturvedi D, Suhane N, Shrivastava RR. Basketful benefit of Citrus limon. Int Res J Pharm 2016;7:1-4. [CrossRef]
9. Rai P, Mehrotra S, Priya S, Gnansounou E, Sharma SK. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour Technol 2021;325:124739. [CrossRef]
10. Chama MA, Onyame HA, Fleischer C, Osei-Safo D, Waibel R, Otchere J, et al. In vitro activities of crude extracts and triterpenoid constituents of Dichapetalum crassifolium Chodat against clinical isolates of Schistosoma haematobium. Heliyon 2020;6:e04460. [CrossRef]
11. Dwivedi S, Tanveer A, Yadav S, Anand G, Yadav D. Agro-wastes for cost effective production of industrially important microbial enzymes:An overview. In:Microbial Biotechnology:Role in Ecological Sustainability and Research. Vol. 26. United States:Wiley;2022. 435-60. [CrossRef]
12. Jena S, Singh R. Agricultural crop waste materials- A potential reservoir of molecules. Environ Res 2022;206:112284. [CrossRef]
13. Chan JX, Wong JF, Hassan A, Zakaria Z. Bioplastics from agricultural waste. In:Biopolymers and Biocomposites from Agro-waste for Packaging Applications. United Kingdom:Woodhead Publishing;2021. 141-69. [CrossRef]
14. Naz S, Ahmad N, Akhtar J, Ahmad NM, Ali A, Zia M. Management of citrus waste by switching in the production of nanocellulose. IET Nanobiotechnol 2016;10:395-9. [CrossRef]
15. Mahcene Z, Khelil A, Hasni S, Akman PK, Bozkurt F, Birech K, et al. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int J Biol Macromol 2020;145:124-32. [CrossRef]
16. Bayram B, Ozkan G, Kostka T, Capanoglu E, Esatbeyoglu T. Valorization and application of fruit and vegetable wastes and by-products for food packaging materials. Molecules 2021;26:4031. [CrossRef]
17. Ponnusha BS, Subramaniyam S, Pasupathi P. Antioxidant and antimicrobial properties of glycine max-a review. Int J Curr Biol Med Sci 2011;1:49-62.
18. Olasehinde OR, Afolabi OB, Owolabi OV, Akawa AB, Omiyale OB. GC-MS analysis of phytochemical constituents of methanolic fraction of Annona muricata leaf and its inhibition against two key enzymes linked to Type II diabetes. Sci Afr 2022;16:e01178. [CrossRef]
19. Zhang H, Xie Y, Liu C, Chen S, Hu S, Xie Z, et al. Comprehensive comparative analysis of volatile compounds in Citrus fruits of different species. Food Chem 2017;230:316-26. [CrossRef]
20. Zannini D, Dal Poggetto G, Malinconico M, Santagata G, Immirzi B. Citrus pomace biomass as a source of pectin and lignocellulose fibers:From waste to upgraded biocomposites for mulching applications. Polymers (Basel) 2021;13:1280. [CrossRef]
21. Association of Official Analytical Chemists, Association of Official Agricultural Chemists (US). Maryland:Association of Official Analytical Chemists;2010.
22. Javed S, Javaid A, Mahmood Z, Javaid A, Nasim F. Biocidal activity of Citrus peel essential oils against some food spoilage bacteria. J Med Plant Res 2011;5:3697-701.
23. Odebode AC, Mdachi SJ, Joseph CC, Irungu BN. Antibacterial activities of constituents from Isolona cauliflora and Cleistochlamys kirkii. Tanz J Sci 2003;29:19-26. [CrossRef]
24. Ferdous N, Rahman M, Alamgir AN. Investigation on phytochemical, cytotoxic and antimicrobial properties of ethanolic extracts of Centella asiatica (L.) Urban. J Med Plant Res 2017;5:187-8.
25. Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana:Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol 1999;32:661-7. [CrossRef]
26. Hasija S, Ibrahim G, Wadia A. Antimicrobial activity of Citrus sinensis (orange), Citrus limetta (sweet lime) and Citrus limon (lemon) peel oil on selected food borne pathogens. Int J Life Sci Res 2015;3:35-9.
27. Edogbanya P, Suleiman MO, Olorunmola JB, Oijagbe IJ. Comparative study on the antimicrobial effects of essential oils from peels of three Citrus fruits. MOJ Biol Med 2019;4:49-54. [CrossRef]
28. Dosoky NS, Setzer WN. Biological activities and safety of Citrus spp. essential oils. Int J Mol Sci 2018;19:1966. [CrossRef]
29. Fagodia SK, Singh HP, Batish DR, Kohli RK. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents:Limonene and citral. Ind Crops Prod 2017;108:708-15. [CrossRef]
30. Sokovic M, Marin PD, Brkic D, van Griensven LJ. Chemical composition and antibacterial activity of essential oils against human pathogenic bacteria. Food 2008;1:220-6.
31. Ali J, Das B, Saikia TR. Antimicrobial activity of lemon peel (Citrus limon) extract. Int J Curr Med Pharm Res 2017;9:79-82. [CrossRef]
32. Cholke PB, Bhor AK, Shete AM, Sonawane RK. Extraction and GC-MS analysis of orange (Citrus sinensis) peel oil. Res J Pharm Biol Chem Sci 2017;2:41-51.
33. Visakh NU, Pathrose B, Narayanankutty A, Alfarhan A, Ramesh V. Utilization of pomelo (Citrus maxima) peel waste into bioactive essential oils:Chemical composition and insecticidal properties. Insects 2022;13:480. [CrossRef]