1. INTRODUCTION
Pulses are the potential sources of vegetable proteins in the human diet. India is the world’s leading producer of pulses. It is cultivated in several parts of the world and does not require much water as it is considered a drought-resistant crop. These have a protein level of 20–25% by weight, which is twice that of wheat and three times that of rice. Pigeon pea (Cajanus cajan L.) is extensively utilized in the form of a pulse and is considered an inexpensive source of proteins. Besides, it is a vital source of nutraceutical and bioactive components. The bioactive components of pigeon pea were examined for their role in increasing the anti-carcinogenic and antioxidant effects, as well as these, have been reported to play a crucial role in modulating the gut microbiota [1]. It is a great source of B-complex vitamins, carbohydrates, and minerals. Pigeon pea when supplemented with other cereals provides a well-balanced diet with all essential amino acids and is equivalent to other protein-rich sources such as soybean and whey [2]. Due to the existence of various flavonoids and polyphenolic compounds in pigeon pea, it has several nutraceutical characteristics in addition to its high nutritional value. Several studies have shown that consuming pigeon pea reduces the risk of various lifestyle diseases such as diabetes, obesity, cancer, and cardiovascular disorders [3]. Pigeon pea is a dense source of nutrients, but some anti-nutrients such as phytic acid, tannins, and trypsin inhibitors bind with its nutritional elements making them unavailable to our body. Phytic acid binds with dietary minerals, such as iron, calcium, zinc, etc., tannins bind with proteins preventing their absorption, and trypsin inhibitors bind with the enzyme trypsin, thereby reducing its biological activity. Soaking is a conventional method used for hydrating the grains in the water [4] and proved useful for the reduction as well as the elimination of the anti-nutrients existing in the food grains [5]. It has been reported from various studies that soaking of food grains for 12–18 h is the best effective processing treatment to decrease the level of anti-nutrients such as trypsin inhibitors, phytic acid, etc. which are wholly or partially soluble in water [4,6]. Germination is a commonly used conventional technique that enhances the digestibility of nutrients, improves bioactive components, and reduces some anti-nutritional components in pulses. It also enhances the concentration of bioactive compounds such as total phenolic components, reducing power, metal chelating activity, and flavonoids. and these changes can differ depending on the variety of the seeds and the germination conditions. Stachyose and raffinose, which are generally assumed to be responsible for flatulence, get decreased during germination [7]. Soaking and germination also increase the bioavailability of minerals by reducing the anti-nutritional components such as phytic acid, tannins, and saponins that are responsible for the binding of macro and micronutrients which are not absorbed by our body [8]. Keeping in view the above benefits of soaking and germination treatments, the present research was planned to study the influence of these treatments on the nutritional, anti-nutritional and bioactive potential of pigeon pea grains to improve the nutritional quality of functional food products to be prepared from these underutilized pulses. This study will also help in the promotion of traditional processing techniques in enhancing the utilization of underutilized pulses with increased nutritional value and bioavailability of micronutrients due to reduced anti-nutritional components.
2. MATERIALS AND METHODS
2.1. Materials
The pigeon pea (AL801 cultivar) used in the present study was procured from Punjab Agriculture University, Ludhiana. The chemicals and reagents of ultrapure grade were used in the present study. These were obtained from the standard companies of chemicals such as Qualigens, Hi-Media, Merck India, and Sigma-Aldrich.
2.2. Physico-chemical Evaluation
The physicochemical evaluation of raw, soaked and germinated pigeon pea was carried out at the laboratories of Eternal University, Sirmour, Himachal Pradesh, India.
2.2.1. Physical and functional characteristics
The pigeon pea grains were evaluated for their physical and functional characteristics. Physical parameters such as length, breadth, as well as thickness were determined with the help of the Vernier caliper. The thousand-grain weight (TGW) was determined by measuring the weight of thousand grains of soybean and expressed in g [9]. The bulk density (BD) was evaluated as per the methodology expressed by Huang et al. [10]. The tap density (TD) was estimated as per the procedure described by Jones et al. [11], and the water absorption capacity (WAC) was estimated by the method specified by Sosulski [12], with minor modification (centrifugation was done at 5100 rpm for 20 min). The water solubility index (WSI) was estimated as per the method of Stojceska et al. [13]. The oil absorption capacity (OAC) of grains was estimated as per the method given by Kaur et al. [14] and the swelling capacity of grains was estimated as per the method of William et al. [15].
2.2.2.Chemical properties
Moisture content (%) of grains was estimated by following the hot air-oven method [16]. The equipment Fibroplus FBS 08P (Pelican Inc.) was used to determine the crude fiber, Soxoplus SPS 06 AS (Pelican Inc.) was used for crude fat, and Kjelodist CAS VA (Pelican Inc.), was used to estimate the crude proteins. The ash contents were determined as per the methods defined by Ranganna [17]. The total carbohydrate contents were assessed by deducting the measured moisture, crude protein, ash, crude fat, and crude fiber from 100. The calorific value (kcal/100 g) was determined by using the factors of 4.0, 9.1, and 4.2 kcal/g for crude protein (Nx6.25), fats, and carbohydrates, respectively [18]. The mineral components such as iron, zinc, manganese, and copper were assessed using Atomic Absorption Spectrometer (AA240FS, Agilent Technology, CA, USA) [16] and were expressed in ppm. Tannins (%) were determined using the technique evaluated by Saxena et al. [19] and the antioxidant activity (%) was estimated (DPPH radical scavenging activity) as per the methodology stated by Bouaziz et al. [20]. Total phenolic contents (TPC) were determined using the Folin-Ciocalteu reagent by following the method of Ainsworth and Gillespie [21] and were expressed as mg GAE/100g [22]. The extraction and quantification of phytic acid in pigeon pea was done as per the method described by Gao et al. [23]. The in-vitro protein digestibility (%) was estimated as per the method of Sharma et al. [24]. The flavonoid contents were estimated as mg/100g as per the method described by Lahlou et al. [25].
2.3. Processing Treatments
The processing treatments such as soaking and germination of pigeon pea grains were conducted by following the methods described by Egli et al. [21] with minor modifications. Grains were cleaned by removing the foreign impurities and soaking of grains was conducted in the distilled water in the ratio of 1:5. The seeds were soaked for 12 and 24 h at room temperature conditions and then dried in the hot-air oven at 40°C for 24 h. The grains were stored at 4°C after packaging in air-tight pouches for further analysis. The seeds after the steeping process were drained off and then covered with the wet muslin cloth. The process of germination was conducted in the incubator for 0 (control), 24, 48, and 72 h at a temperature of 25°C. During germination treatment, the water was sprinkled intermittently over the muslin cloth to keep it moist. After each germination time treatment, the seeds were dried at 40°C for 24 h in a hot air oven and converted to a fine flour using a laboratory flour mill (SANCO). The germinated pigeon pea flour was then stored at 4°C till further analysis [Figure 1].
2.4. Statistical Analysis
Data obtained during the research were analyzed using one-way analysis of variance using SPSS Statistical software. Values in tables are presented as mean ± standard deviation of three replicates and differences at the level of P ≤ 0.05 were considered significant.
3. RESULTS AND DISCUSSION
3.1. Physical, Functional, and Nutritional Characteristics of Raw Pigeon Pea Grains
3.1.1. Physical properties
The length, thickness, and width in raw grains (RG) observed in the present study were 4.69, 4.13, and 4.39 mm, respectively. Baryeh and Mangope [26] reported 5.81, 4.28, and 5.13 mm as length, thickness, and width of pigeon pea grains. The TGW of pigeon pea was observed as 99.25 g. Sangani and Davara [27] found the TGW of pigeon pea as 97.8 g [Table 1].
3.1.2. Functional properties
Data on the functional properties of raw pigeon pea grains is depicted in Table 1. The WAC and OAC of pigeon pea flour were observed as 2.78 and 1.75 ml/g, respectively. The difference in the protein concentration, their degree of interaction with water and oil may cause the differences in water and OAC. Oyewole et al. [28] found the WAC and OAC of pigeon pea flour as 2.60 and 1.66 ml/g, respectively, which is equivalent to the values reported in the present investigation. The value for WSI observed in the current study was 12.70% which is analogous with the outcomes of Maninder et al. [29], who stated a WSI of 14.5% in pigeon pea flour. The SC of pigeon pea grains was estimated as 120.55%. Oyewole et al. [28] reported a swelling capacity of 129% in pigeon pea grains. The BD and TD of pigeon pea seed flours observed were 0.86 and 0.94 g/cm3, respectively, and were found similar to the results obtained by Oyewole et al. [28].
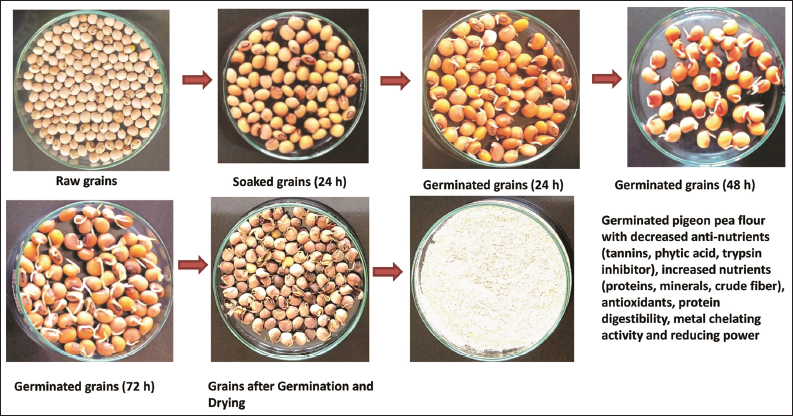 | Figure 1: Steps involved in preparation of germinated pigeon pea flour. [Click here to view] |
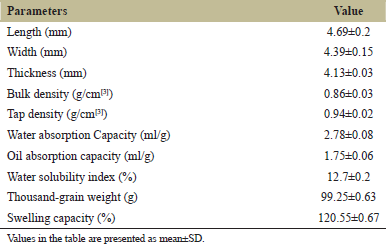 | Table 1: Physical and functional characteristics of raw pigeon pea grains. [Click here to view] |
3.1.3. Nutritional characteristics
The nutritional characteristics of raw pigeon pea grains are presented in Table 2. The moisture, fat, fiber, and ash contents observed in RG were 8.17, 1.64, 4.75, and 3.23%. The results are similar to the findings of Igbedioh et al. [30] where moisture, fat, fiber, and ash content in pigeon pea grains were observed as 11.05, 1.51, 4.27, and 3.62%, respectively. The protein content as determined in RG was 19.53%. Uwaegbute et al. [31] found a protein content of 24.2% in raw pigeon pea. The in-vitro protein digestibility observed in RG was 65.35%. Sharma et al. [24] found in-vitro protein digestibility of 69.86% in RG of pigeon pea.
The antioxidant content and reducing capacity observed in RG were 20.12 and 23.19%, respectively. James et al. [32] reported 32.95 and 26.10% of anti-oxidant and reducing capacity, respectively, whereas, Sharma et al. [24] found an antioxidant activity of 21.57% in raw pigeon pea grains. The metal chelating activity observed in RG was 38.98% and similar findings were witnessed by Sharma et al. [24] where the metal chelating activity in RG of pigeon pea was found as 42.02%. The phenolic contents of RG observed in the present study were 196.34 mg GAE/100g and flavonoid contents as 12.57 mg/100g. Similar results were found by James et al. [32] where phenolic and flavonoid content were found as 196.33 and 11.51 mg/100g, respectively. The levels of phytic acid and tannin contents observed in RG were 8.40 and 8.50 mg/100g, respectively. Sangronis and Machado [7] reported a phytic acid content of 7.34 mg/100g and James et al. [25] found tannin contents of 7.01 mg/100g in RG of pigeon pea and results are comparable with the findings in the current study.
The copper and zinc contents were observed as 0.52 and 5.85 ppm, respectively. Sangronis and Machado [7] reported copper and zinc content of 0.9 and 6.1 ppm, respectively in RG of pigeon pea. The iron and manganese contents were observed as 3.35 and 2.04 ppm in raw pigeon pea grains. Oloyo [33] found 7.18 and 3.16 ppm of iron and manganese, respectively in pigeon pea grains.
3.2. Changes in Nutritional, Anti-nutritional, and Bioactive Components during Processing Treatments
Changes in nutritional, anti-nutritional, and bioactive components as well as the antioxidant activity, reducing power, and metal chelating activity during the soaking as well germination treatments of pigeon pea grains are illustrated under the following sub-headings.
3.2.1. Nutritional components
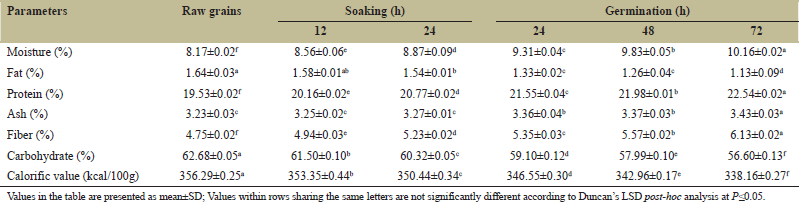
The changes in nutritional components during the soaking and germination of pigeon pea are described in Table 3. Moisture content in pigeon pea grains augmented significantly (P ≤ 0.05) from 8.17% to 10.16%. The highest contents were detected during germination for 72 h (G72) (10.16%) and lowest in RG (8.17%). The rise in moisture content can be due to the imbibition of water into legumes by simple diffusion. There was a 24.35% increase in germinated and oven-dried grains as compared to RG and an 8.56% increase in moisture content during soaking, similar findings were observed by Devi et al. [34] who found a 22.86% increase in moisture content in germinated pigeon pea grains. Fat content in pigeon pea grains reduced significantly (P ≤ 0.05) from 1.64 (RG) to 1.13% (G72). There was a 31.48% and 6.09% of reduction in the fat content of germinated and soaked grains. Devi et al. [34] and Igbedioh et al. [30] informed a 24.33 and 8% decline in fat content in germinated and soaked pigeon pea. During germination, the decrease in fat content can be due to the breakdown of fat into fatty acid and glycerol and its subsequent oxidation for energy production. The reduction in fat content could also be attributed to the reduction of stored fat, due to the high catabolic activities in seeds during germination [35].
Protein content in pigeon pea enhanced significantly (P ≤ 0.05) from 19.53 (RG) to 22.54% (grains germinated for 72 h). The protein content augmented to 15.41 and 6.34% during the sprouting and soaking process of grains. Devi et al. [34] and Igbedioh et al. [30] described a 9.46 and 5.23% increase in protein content during the sprouting and soaking of cowpea and Bambara groundnut. The rise in proteins may be attributed to several factors like the synthesis of enzymes responsible for the manufacture of some amino acids during protein synthesis [31]. The ash content increased from 3.23 (RG) to 3.43% (grains germinated for 72 h) contributing to a 6.19% rise in ash content and a 1.23% increase in ash content during soaking. It can be due to an upsurge in the activity of the phytase enzyme during sprouting. The increased activity of enzyme phytase caused the hydrolysis of phytic acid making the minerals free, and increasing the minerals as well as ash contents of germinated grains [36]. Devi et al. [34] informed a 4.50% increase in ash content in germinated cowpea grains and Abd El-Hady and Habiba [37] found a 1.58% increase in ash content in soaked kidney beans.
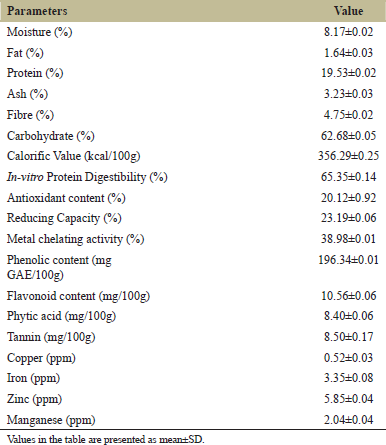 | Table 2: Nutritional characteristics of raw pigeon pea grains. [Click here to view] |
The fiber contents in pigeon pea enhanced significantly (P ≤ 0.05) from 4.75 (RG) to 6.13% (G72) contributing to a 29.05% rise in fiber content during germination and a 10.10% increase during soaking. The results are analogous to the findings of Devi et al. [34] who described a 30.46% rise in fiber content in germinated cowpea. The carbohydrate contents and calorific values decreased by 9.70 and 5.08%, respectively during germination for 72 h. The carbohydrate and calorific values during soaking decreased to 3.76% and 1.64%. Uppal and Bains [38] found a 5.6% reduction in carbohydrate contents after 24 h of germination in cowpea and Igbedioh et al. [30] found an 8.75% reduction in carbohydrate during soaking. The decline in carbohydrate content during germination might be due to the activity of alpha-amylase where complex carbohydrates are converted into absorbable sugars that are further used by the growing seedling during the early stages of sprouting [34]. The reduction in the level of carbohydrates resulted ultimately in decreasing the calorific value of germinated grains.
The changes in antioxidant activity, reducing power, in-vitro protein digestibility, and metal-chelating activity during the soaking and germination of pigeon pea are presented in Figure 2. The antioxidant activity in pigeon pea grains enhanced significantly (P ≤ 0.05) from 20.12% (RG) to 35.44% (grains germinated for 72 h). There was a 76.14 and 47.31% increment in antioxidant activity during germination and soaking. James et al. [32] and Sharma et al. [24] observed a 75.53% and 56.74% increase in antioxidant activity, respectively in germinated pigeon pea grains. The antioxidant activity was found to get increased in white cowpeas, mung beans, black beans, peanuts, soybeans, and adzuki beans through germination for 5 days [38]. According to Khang et al. [39], the phenolic contents get augmented in sprouted legumes because of the presence of various hydroxyl groups that acted as a free radical scavenger and resulted in an upsurge in the antioxidant activity of germinated grains. Whereas, Uchegbu and Ishiwu [40] stated that there was an increase in the concentration of various bioactive components such as vitamins and carotenoids in legumes which acted as additional antioxidants and enhanced the antioxidant activity of the legumes during sprouting.
 | Table 3: Changes in nutritional characteristics during the soaking and germination of pigeon pea. [Click here to view] |
Reducing power in pigeon pea augmented significantly (P ≤ 0.05) from 23.19 (RG) to 44.40% (grains germinated for 72 h). There was a 91.46 and 28.84% increase in reducing power during germination for 72 h and soaking treatments. Sharma et al. [24] and James et al. [32] found a 74.09% and 88.16% increase in reducing capacity, respectively, in germinated pigeon pea grains. Similarly, Liu et al. [41] observed an increase in the reducing capacity of the sesame sprouts after the germination period of 5 days as compared to RG.
In-vitro protein digestibility in pigeon pea enhanced significantly (P ≤ 0.05) from 67.35% (RG) to 86.44% (grains germinated for 72 h). During germination, the in-vitro protein digestibility increased by 28.34% and during soaking, it increased to 11.64%. The results obtained are equivalent to the findings of Sharma et al. [24] and Abd El-Hady and Habiba [37] who observed a 25.05 and 12.88% rise in in-vitro protein digestibility in germinated pigeon pea grains and soaked kidney beans. According to Khatoon and Prakash [42], there was activation of several hydrolytic enzymes such as proteases which facilitated the degradation and modification of storage proteins and encouraged the structural and metabolic transformation of the proteins by the production of free amino acids and shorter polypeptides chains. The enzymatic action on protein also resulted in chain flexibility, which enhanced their susceptibility to be acted upon by the enzymes such as proteases, thereby improving the in-vitro protein digestibility in sprouted grains.
 | Figure 2: Changes in antioxidant activity, reducing power, in-vitro protein digestibility, and metal-chelating activity during the soaking and germination of pigeon pea (RG-Raw grains, S 12-Soaking for 12 h; S24- Soaking for 24 h, G24- Germination for 24 h, G48- Germination for 48 h; G72- Germination for 72 h). [Click here to view] |
The metal-chelating activity of pigeon pea enhanced significantly (P ≤ 0.05) from 38.98 (RG) to 63.99% (grains germinated for 72 h). There was a 64.16 and 12.18% increase in metal chelating activity during the germination and soaking process. Similar outcomes were observed by Sharma et al. [24] who observed a 62.97 and 5.09% increase in metal chelating activity in germinated and soaked pigeon pea. ?wieca et al. [43] observed that the metal-chelating activity of Lens culinaris sprouts enhanced significantly with germination time. During germination, modification of phenolic structure and conversion of phenolic components into different products which in turn acted as antioxidants [44] and behaved as metal chelators thereby are enhancing the metal-chelating action of germinated pigeon peas. During the drying of sprouted grains, the production of various Maillard reaction products such as melanoidin (produced by the carbohydrates and proteins interaction) also contributed towards increasing the metal chelating activity [45].
The phenolic contents in pigeon pea increased from 196.34 (RG) to 206.90 mg GAE/100g (grains germinated for 72 h) [Table 4]. The phenolic content increased by 5.3 and 0.97% during the germination and soaking process. James et al. [32] and Sharma et al. [24] reported a 2.2 and 4.7% increase in phenolic content, respectively, in germinated pigeon pea grains. During sprouting, the biosynthesis, and bioaccumulation of phenolic contents occurred in legumes with the conversion of insoluble polymers into soluble ones, synthesis of free polyphenols, hydrolysis, and oxidation of glycosylated flavonoids resulted in increasing the TPC of sprouted grains [36]. Furthermore, the components of the cell walls get disrupted and released more phenolic acids after the breakdown of the insoluble phenolic components, and other cellular constituents resulting in enhancement of total phenolic components in germinated grains [46].
Flavonoid contents in pigeon pea enhanced significantly (P ≤ 0.05) from 12.57 (RG) to 20.60 mg/100 g (grains germinated for 72 h). It gets increased by 63.88 and 11.13% during germination and soaking processes. The results obtained are similar to the findings of Sharma et al. [24] who reported a 60.14% increase in flavonoid contents in germinated pigeon pea grains. Fouad et al. [36] and Khole et al. [47] observed a significant increase in the total flavonoid content of germinated seeds. During sprouting, the phenylpropanoid metabolic pathway gets activated resulting in the production of acetyl coenzyme A esters that are transformed to flavonoids thereby increasing the total flavonoid contents of germinated grains [48].
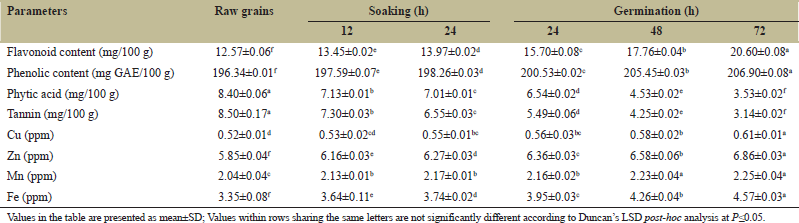 | Table 4: Changes in total phenolic, flavonoid, anti?nutritional and mineral contents during the soaking and germination of pigeon pea. [Click here to view] |
3.2.2. Anti-nutritional components
The phytic acid content in pigeon pea declined significantly (P ≤ 0.05) from 8.40 (RG) to 3.53 mg/100g (grains germinated for 72 h). There was a 57.97 and 16.54% reduction of phytic acid in germinated and soaked grains and similar results were observed by Sangronis and Machado [7] and Igbedioh et al. [30] who reported a 41.14 and 18.18% reduction in phytic acid content in soaked and germinated pigeon pea. Similarly, the tannin content in pigeon pea reduced significantly (P ≤ 0.05) from 8.50 (RG) to 3.14 mg/100 g (grains germinated for 72 h). The tannin content decreased by 22.94 and 63.05% during soaking and germination treatments, respectively. The reduction in phytic content during soaking may be due to the leaching of phytate ions in water due to diffusion [49]. The results are similar to the findings observed by Onwuka [50] and James et al. [25] who observed 25 and 50.49% decreases in the tannins of soaked and germinated pigeon pea. The decline in tannins with increasing germination time can be attributed to several factors. Saharan et al. [51] stated that this decline in tannin contents can be caused due to the formation of tannin-enzyme and tannin-protein complexes in the plant matrix. Furthermore, the decline in tannin content could be due to the binding and leaching of tannins contents with other organic compounds like carbohydrates or proteins. The overall reduction may be attributed to the combined effects of the soaking and germinating treatments of pigeon pea grains [7].
3.2.3. Mineral components
The mineral contents of pigeon pea grains increased significantly after the germination and soaking treatment of pigeon pea grains. The copper content increased from 0.52 (RG) to 0.61 ppm (grains germinated for 72 h) resulting in a 17.30 and 5.76% increment during germination and soaking. Sangronis et al. [7] reported a 22.22% increase in copper content in germinated black beans.
Similarly, the zinc content increased from 5.85 (RG) to 6.86 ppm (grains germinated for 72 h). There were 17.66 and 7.17% augmentation in zinc contents during the germination and soaking process. Sangronis and Machado [7] and Obizoba [48] reported a 37.70% and 10.53% increase in zinc content, respectively, in germinated pigeon pea. Duhan et al. [52] also observed 4.39% increases in zinc content in soaked pigeon pea. The manganese content increased from 2.04 (RG) to 2.25 ppm (grains germinated for 72 h) resulting in a 10.29 and 6.37% increase in the manganese content during germination and soaking of pigeon pea grains. Furthermore, the content of iron in germinated grains augmented significantly (P ≤ 0.05) from 3.35 (RG) to 4.57 ppm (grains germinated for 72 h). There were a 36.41 and 11.64% increase in iron content during the germination and soaking treatment. Oloyo [33] observed an increase of 7.14% and 30.07% in manganese and iron contents, respectively in germinated pigeon pea and Duhan et al. [49] observed 4.13% increase of iron content during soaking of pigeon pea. The increase in minerals such as Cu, Zn, Mn, and Fe content after sprouting could be attributed due to destruction or partial elimination or both antinutritional factors (phytic acid and tannin contents) which released more iron from its organically bound complexes in the dry seeds [53].
4. CONCLUSIONS
The present investigation was planned with the objective to study the impact of processing treatments such as soaking and germination on the nutritional composition, anti-nutritional factors, and bioactive components of pigeon pea. The processing techniques were found to decrease the anti-nutritional components such as phytic acid and tannin contents in processed grains. There was an increase in the antioxidant activity and the TPC of germinated pigeon pea grains. The mineral contents were found to increase significantly after processing treatments. Hence, the processing techniques caused a signi?cant change in the biochemical compositions of germinated grains, and there was an improvement in the overall nutritional value of germinated grains. The flour obtained after milling of germinated pigeon pea grains can be utilized with wheat flour for the preparation of innovative bakery products. This study will help in the promotion of traditional processing techniques in enhancing the utilization of underutilized pigeon pea by incorporating them for the development of functional food products with high nutritional value, lower anti-nutritional components, and better bioavailability of micronutrients.
5. ACKNOWLEDGMENTS
The financial support provided by the Ministry of Food Processing Industries (MoFPI) Govt. of India grant (F. No. 5-11/2010-HRD) for the development of infrastructural capability at the University is duly acknowledged.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors requirements/guidelines.
7. FUNDING
This research is supported by Ministry of Food Processing Industries (MoFPI) Govt. of India grant (F. No. 5-11/2010-HRD) for the development of infrastructural facilities at the Department of Food Technology, Eternal University, Baru Sahib.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
There is no involvement of experiments on animals or human beings.
10. DATA AVAILABILITY
Not applicable.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Talari A, Shakappa D. Role of pigeon pea (Cajanus cajan L.) in human nutrition and health: A review. Asian J Dairy Food Res 2018;37:212-20. CrossRef
2. Akporhonor E, Egwaikhide P, Eguavoen I. Effect of Sprouting on in vitro digestibility of some locally consumed leguminous seeds. J Appl Sci Environ Manag 2006;10:55-8. CrossRef
3. Singh J, Basu PS. Non-Nutritive Bioactive Compounds in Pulses and Their Impact on Human Health: An Overview; 2012. CrossRef
4. Embaby HE. Effect of heat treatments on certain antinutrients and in vitro protein digestibility of peanut and sesame seeds. Food Sci Technol Res 2010;17:31-8. CrossRef
5. Singh B, Singh JP, Shevkani K, Singh N, Kaur A. Bioactive constituents in pulses and their health benefits. J Food Sci Technol 2017;54:858-70. CrossRef
6. Kajihausa O, Fasasi R, Atolagbe Y. Effect of different soaking time and boiling on the proximate composition and functional properties of sprouted sesame seed flour. Niger Food J 2014;32:8-15. CrossRef
7. Sangronis E, Machado C. Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. LWT Food Sci Technol 2007;40:116-20. CrossRef
8. Thakur P, Kumar K, Dhaliwal HS. Nutritional facts, bio-active components and processing aspects of pseudocereals: A comprehensive review. Food Biosci 2021;42:101170. CrossRef
9. AACC. Approved Methods of the American Association of Cereal Chemists: Amer Assn of Cereal Chemists. Washington DC, United States: AACC; 2000.
10. Huang S, Shiau C, Liu T, Chu C, Hwang DF. Effects of rice bran on sensory and physico-chemical properties of emulsified pork meatballs. Meat Sci. 2005;70:613-9. CrossRef
11. Jones D, Chinnaswamy R, Tan Y, Hanna M. Physiochemical properties of ready-to-eat breakfast cereals. Cereal Foods World 2000;45:164-8.
12. Sosulski F. The centrifuge method for determining flour absorption in hard red spring wheats. Cereal Chem 1962;39:344-50.
13. Stojceska V, Ainsworth P, Plunkett A, ?bano?lu E, ?bano?lu ?. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J Food Eng 2008;87:554-63. CrossRef
14. Kaur M, Sandhu KS, Arora A, Sharma A. Gluten free biscuits prepared from buckwheat flour by incorporation of various gums: Physicochemical and sensory properties. LWT Food Sci Technol 2015;62:628-32. CrossRef
15. William P, Nakoul H, Singh K. Relationship between cooking time and some physical characteristics in chick pea (Cicer arietinum L.). J Sci Food Agric 1983;34:492-6. CrossRef
16. AOAC. Official Methods of Analysis. Washington, DC: AOAC; 1990. p. 684.
17. Ranganna S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products. New York, United States: Tata McGraw-Hill Education; 1986.
18. WHO. Energy and Protein Requirements. WHO Technical Report Series No. 522 (and FAO Nutrition Meeting Report Series No. 52). Geneva: World Health Organization; 1973.
19. Saxena V, Mishra G, Saxena A, Vishwakarma K. A comparative study on quantitative estimation of tannins in Terminalia chebula, Terminalia belerica, Terminalia arjuna and Saraca indica using spectrophotometer. Asian J Pharm Clin Res 2013;6:148-9.
20. Bouaziz M, Fki I, Jemai H, Ayadi M, Sayadi S. Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem 2008;108:253-62. CrossRef
21. Egli I, Davidsson L, Juillerat M, Barclay D, Hurrell R. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feedin. J Food Sci 2002;67:3484-8. CrossRef
22. Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2007;2:875-7. CrossRef
23. Gao Y, Shang C, Maroof M, Biyashev R, Grabau E, Kwanyuen P, et al. A modified colorimetric method for phytic acid analysis in soybean. Crop Sci 2007;47:1797-803. CrossRef
24. Lahlou FA, Hmimid F, Loutfi M, Bourhim N. Antioxidant activity and quantification of phenolic compounds of euphorbia echinus. Int J Pharm Pharm Sci 2014;6:6-9.
25. Baryeh EA, Mangope B. Some physical properties of QP-38 variety pigeon pea. J Food Eng 2003;56:59-65. CrossRef
26. Sangani V, Davara P. Moisture dependent physical properties of pigeon pea grains. Int J Postharvest Technol 2013;3:51-62. CrossRef
27. Oyewole AA, Abu T, Enujiugha V. Emulsification and foaming properties of locust bean (Parkiabi globosa) and pigeon pea (Cajanus cajan) seed flours and their protein isolates. Nutr Food Sci Int J 2017;2:1-9. CrossRef
28. Maninder K, Sandhu KS, Singh N. Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars. Food Chem 2007;104:259-67. CrossRef
29. Igbedioh SO, Olugbemi KT, Akpapunam MA. Effects of processing methods on phytic acid level and some constituents in bambara groundnut (Vigna subterranea) and pigeon pea (Cajanus cajan). Food Chem 1994;50:147-51. CrossRef
30. Uwaegbute A, Iroegbu C, Eke O. Chemical and sensory evaluation of germinated cowpeas (Vigna unguiculata) and their products. Food Chem 2000;68:141-6. CrossRef
31. Sharma S, Singh A, Singh B. Characterization of in vitro antioxidant activity, bioactive components, and nutrient digestibility in pigeon pea (Cajanus cajan) as influenced by germination time and temperature. J Food Biochem 2019;43:e12706. CrossRef
32. James S, Nwabueze TU, Ndife J, Onwuka GI, Usman MA. Influence of fermentation and germination on some bioactive components of selected lesser legumes indigenous to Nigeria. J Agric Food Res 2020;2:100086. CrossRef
33. Oloyo RA. Chemical and nutritional quality changes in germinating seeds of Cajanus cajan L. Food Chem 2004;85:497-502. CrossRef
34. Devi CB, Kushwaha A, Kumar A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J Food Sci Technol 2015;52:6821-7. CrossRef
35. Onimawo I, Asugo S. Effects of germination on the nutrient content and functional properties of pigeon pea flour. J Food Sci Tech Mys 2004;41:170-4.
36. Fouad AA, Rehab F. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Sci Pol Technol Aliment 2015;14:233-46. CrossRef
37. Abd El-Hady E, Habiba R. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci Technol 2003;36:285-93. CrossRef
38. Uppal V, Bains K. Effect of germination periods and hydrothermal treatments on in vitro protein and starch digestibility of germinated legumes. J Food Sci Technol 2012;49:184-91. CrossRef
39. Khang DT, Dung TN, Elzaawely AA, Xuan TD. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016;5:27. CrossRef
40. Uchegbu NN, Ishiwu CN. Germinated pigeon pea (Cajanus cajan): A novel diet for lowering oxidative stress and hyperglycemia. Food Sci Nutr 2016;4:772-7. CrossRef
41. Liu B, Guo X, Zhu K, Liu Y. Nutritional evaluation and antioxidant activity of sesame sprouts. Food Chem 2011;129:799-803. CrossRef
42. Khatoon N, Prakash J. Nutrient retention in microwave cooked germinated legumes. Food Chem 2006;97:115-21. CrossRef
43. ?wieca M, Gawlik-Dziki U, Kowalczyk D, Z?otek U. Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of Lens culinaris sprouts. Sci Hortic 2012;140:87-95. CrossRef
44. Randhir R, Kwon YI, Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innov Food Sci Emerg Technol 2008;9:355-64. CrossRef
45. Gujral HS, Sharma P, Gupta N, Wani AA. Antioxidant properties of legumes and their morphological fractions as affected by cooking. Food Sci Biotechnol 2013;22:187-94. CrossRef
46. Boateng J, Verghese M, Walker L, Ogutu S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT Food Sci Technol 2008;41:1541-7. CrossRef
47. Khole S, Chatterjee S, Variyar P, Sharma A, Devasagayam T, Ghaskadbi S. Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J Funct Foods 2014;6:270-9. CrossRef
48. Singh A, Sharma S. Bioactive components and functional properties of biologically activated cereal grains: A bibliographic review. Crit Rev Food Sci Nutr 2017;57:3051-71. CrossRef
49. Duhan A, Khetarpaul N, Bishnoi S. Changes in phytates and HCl extractability of calcium, phosphorus, and iron of soaked, dehulled, cooked, and sprouted pigeon pea cultivar (UPAS-120). Plant Foods Hum Nutr 2002;57:275-84. CrossRef
50. Onwuka G. Soaking, boiling and antinutritional factors in pigeon peas (Cajanus cajan) and cowpeas (Vigna unguiculata). J Food Process Preserv 2006;30:616-30. CrossRef
51. Saharan K, Khetarpaul N, Bishnoi S. Antinutrients and protein digestibility of fababean and ricebean as affected by soaking, dehulling and germination. J Food Sci Technol 2002;39:418-22.
52. Duhan A, Khetarpaul N, Bishnoi S. HCl-extractability of zinc and copper as affected by soaking, dehulling, cooking and germination of high yielding pigeon pea cultivars. J Food Compost Anal 2004;17:597-604. CrossRef
53. Obizoba IC. Effect of sprouting on the nitrogenous constituents and mineral composition of pigeon pea (Cajanus cajan) seeds. Plant Foods Hum Nutr 1991;41:21-6. CrossRef