1. INTRODUCTION
Periodontal diseases are one of the most prevalent human diseases worldwide, affecting 750,847 million people in 2016. One of the most commonly occurring diseases of the oral cavity is dental caries. If left untreated, dental caries can lead to pain, dental abscesses, destruction of bone, other health problems, and even deaths [1].
Dental caries or tooth decay is a disease that is caused by the metabolism of sugar into organic acid which dissolutes the enamel. Streptococcus mutans is seen as the most common acid producer and is a factor in most cases of caries; therefore, S. mutans is often regarded as a major pathogen of dental caries [1–3]. Streptococcus mutans metabolizes sucrose to form biofilm on the tooth’s surface and consequently produces lactic acid to degrade the tooth’s enamel [4]. To prevent the development of dental caries, the usage of antibacterial agent is used, as it inhibits the growth of the microorganisms and consequently inhibits the formation of the biofilm [5,6].
Guava (Psidium guajava L.) leaves are widely researched for their antimicrobial activities and have diverse applications in curing many diseases [7]. Both Gram-positive and Gram-negative bacteria are inhibited by guava, and this antimicrobial activity comes from the flavonoids; some of them are quercetin and guaijaverin that are present in the leaves [8]. Guaijaverin and quercetin isolated from guava leaves were reported to possess antibacterial activity against S. mutans [9,10]. Extraction of P. guajava L. plant parts (including leaves) using hot water, acetone, methanol, ethyl acetate, and ethanol has good antibacterial activity against S. mutans [11]
Guava leaves extract has been developed into a chewable tablet; however, it has a bitter taste and nonpleasant mouthfeel [12]. Therefore, in this research, guava leaves extract was added to a jelly candy. Jelly candy is a type of confectionery that is produced by using a hydrocolloid base to provide a network to hold relatively high moisture content sugar syrup [13]. Jelly in the recent years has been rising as a novel drug delivery system as it has a rapid onset of action and because of the general preference of jellies by children and other age groups when compared to oral liquid and tablets. Jelly also solves the problem of dose wastage or dose dumping, as jellies have a definite size and shape which is going to hold a definite dose of the medicine [14,15]. Jelly candies are also preferred because they are often flavored with fruit juices and extracts and have sweetness property [16].
To overcome the bitter taste from guava leaves extract, jelly candy was also added with peppermint essential oil. Peppermint as a flavoring agent works by producing impulses for the olfactory receptors in the nose to send a signal to the brain, which can mask unpleasant flavors [17]. Thus, this research aimed to determine the antibacterial activity of guava leaves extract against S. mutans and to apply it in jelly candy making, added with peppermint essential oil as the flavoring agent to improve its sensory properties.
2. MATERIALS AND METHODS
2.1. Materials
The materials used in the research were leaves of red-fleshed guava (P. guajava L.) obtained from Balai Penelitian Tanaman Rempah dan Obat (Balittro), West Java, Indonesia, which are green in color and have a length of 6–14 cm, “Darjeeling” peppermint (Mentha piperita L.) essential oil, S. mutans culture obtained from Lembaga Ilmu Pengetahuan Indonesia Cibinong, Indonesia, demineralized water, “Now Real Food” xylitol, “Misol” 180 Bloom gelatin, “CAD” 41.08 DE glucose syrup, and “cap gajah” citric acid, while the chemical reagents used for the analyses were food-grade hexane, ethyl acetate, ethanol, Folin–Ciocalteu reagent, sodium carbonate, gallic acid, aluminum chloride, potassium acetate, quercetin, Brain Heart Infused Agar (BHIA), and Brain Heart Infused Broth.
2.2. Guava Leaves Extraction
Guava leaves extract was obtained from sequential extraction. The solvents used in extractions (sequentially) were 95% hexane, 99% ethyl acetate, and 96% ethanol. All solvents used were food-grade solvents [18].
Before the extraction of the guava leaves, the leaves were made into powder. Guava leaves were dried in cabinet dryer for 24 hours at 50oC and were further size-reduced using a disc mill and sieved using 60-mesh sieve. 50 g of the leaves powder was first mixed with hexane (nonpolar solvent) with a ratio of 1:5, macerated on a platform shaker (250 revolutions per minute/RPM ), 27oC, 24 hours), and filtered using Whatman no. 1 filter paper. Hexane was then removed using a rotary evaporator. The crude hexanic extract was obtained and analyzed; meanwhile, the residue was mixed with ethyl acetate. A similar procedure was repeated using ethyl acetate and subsequently ethanol as the solvent. In the end, there were three extracts obtained, that is, guava leaves hexanic extract, ethyl acetate extract, and ethanolic extract. Each extract was analyzed for its moisture content [19], total phenolic content [20], total flavonoid content [20], yield [18], and antibacterial activity against S. mutans [21] to find the best extraction solvent.
2.3. Antimicrobial Activity of Guava Leaves Extract
Antibacterial activity of guava leaves extract was determined based on the well-diffusion method [21]. 0.2% of S. mutans (to obtain the number of bacteria of about 106 cfu/ml) was added to the BHIA and then added to a Petri dish and let to set. 6 mm diameter wells were made in the medium and 60 μl of specified concentrations (50,000, 100,000, 150,000, 200,000, and 250,000 ppm) of each extract was put into each well. Solvents of each extract were used as controls. The plates were then incubated at 37oC for 24 hours. The clear zone formed around the wells was measured using Vernier caliper as the inhibition diameter (mm).
The inhibition diameter data obtained from the antibacterial activity analysis were used to obtain the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) [22]. This method plots the ln of the concentration of extract used (in mg/l or part per million/ppm ) as the x-axis against the square value of the inhibition zone (in mm) as the y-axis. Intersection of the linear regression is as follows: Y = aX + b, with X being the Mt value, with MBC (in ppm) being equal to eMt; meanwhile, MIC (in ppm) is 0.25 × MBC.
2.4. Preparation of Jelly Candy Added with Guava Leaves Extract
The best guava leaves extract and peppermint essential oil were added to jelly candy formulation. The concentrations of guava leave extract added were 2 MIC, 3 MIC, 4 MIC, and 5 MIC. Meanwhile, the concentrations of peppermint essential oil added were 0%, 0.25%, and 0.5%. The formulation of the guava leaves jelly candy itself can be seen in Table 1 [23].
Jelly candy was made by dissolving gelatin in water and dissolving xylitol, glucose, and citric acid in water in a separate bowl. These ingredients were then mixed and cooked in the pan until 100oC. The mixture was then cooled down until 40oC, at which then the extract and peppermint essential oil of specified concentrations were added to the mixture. It was then poured into a mold and cooled in the refrigerator for 1 hour. The jelly candy was then analyzed for its antimicrobial activity against S. mutans [21], total phenolic and flavonoid content [20], color [24], texture [25], and sensory properties using hedonic test [26].
2.5. Antimicrobial Activity of Guava Leaves Jelly Candy
Antibacterial activity of jelly candy added with guava leaves extract was determined using the well-diffusion method [21]. Based on its solubility in water, 0.78 grams of jelly candy added with different concentrations of guava leaves extract and peppermint essential oil was dissolved into 1 ml of sterile water. 0.2% of S. mutans was added to the BHIA and then added to a Petri dish and let to be set. 6 mm diameter wells were made in the medium and 60 μl of each jelly candy was put into each well. Jelly candy without any addition of peppermint oil and guava leaves extract was used as a control. The plates were then incubated at 37oC for 24 hours. The clear zone formed around the wells was measured using Vernier caliper as the inhibition diameter (mm).
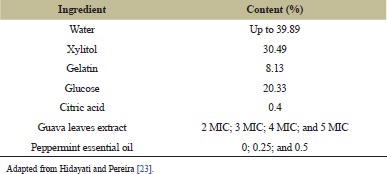 | Table 1: Formulation of guava leaves jelly candy. [Click here to view] |
2.6. Color and Texture Analyses of Jelly Candy
The color analysis of both control and jelly candy added with guava leaves extract was done using the “Konica Minolta” chromameter, using the CIELAB (Commission Internationale de l´Eclairage LAB) color space [24]. The sample was measured in terms of its lightness (L*), redness (a*), and yellowness (b*), and the data obtained were further converted into oHue value. The lightness of the sample ranges from 0 to 100, with 0 being black and 100 being white.
The texture of both control and jelly candy added with guava leaves extract was analyzed using a “Stable Micro Systems TA-XT plus” texture analyzer. The experiment was conducted using the texture profile analysis mode, with a P0.5R cylinder probe, pretest speed of 5 mm/s, test speed of 1 mm/s, posttest speed of 5 mm/s, trigger force 5 g, and distance of 7.5 mm or 75% of the sample’s height. The springiness, gumminess, and chewiness of the products were recorded [25].
2.7. Sensory Evaluation of Guava Leaves Jelly Candy
Sensory analysis of jelly candy added with different concentration of guava leaves extract and peppermint essential oil was done using a hedonic test by 15 semitrained panelists. Hedonic test is a sensory test that is usually performed to determine consumer’s acceptability of a specific product. Therefore, this test is done based on the likeness level of the panelist. In this research, panelists were asked to determine their likeness towards jelly candy, based on several attributes, that is, color, aroma, texture, taste, bitter aftertaste, and overall acceptability of the jelly candies produced. The scales for the hedonic test ranged from 1 to 7 (1 = dislike extremely; 7 = like extremely) [26].
2.8. Statistical Analysis
All data obtained in this research were analyzed statistically with univariate analysis using the Statistical Package for the Social Sciences 25th version software. Duncan post hoc test and significance level of 0.05 were used. All analyses were done with two replications.
3. RESULTS AND DISCUSSION
3.1. Characteristics of Guava Leaves Powder and Extract
The main material used in this research, that is, guava leaves, was made into powder form and then extracted sequentially, using hexane, ethyl acetate, and ethanol. Each of these is analyzed for its moisture content and yield, and these data can be observed in Table 2.
The moisture content of the leaves used in this research was seen to be lower than that in a previous research, which was about 82.5% [27]. The moisture content of the guava leaves powder was also seen to be lower when compared to the moisture content of guava leaf powder in previous research with a value of 6.50% ± 0.04% and 8.67% ± 0.37%, respectively [28]. This might be because the component of the plant might vary, according to the maturity, variety, and geology, and processing conditions of the sample [29].
The yield of ethanolic extract was higher than that of the other extracts, with a value of 60.87% ± 4.89%. Meanwhile, the yield of hexanic extract and ethyl acetate extract was 2.52% ± 0.03% and 2.82% ± 0.31%, respectively. Choice of solvents is important in the extraction of total solids, phytochemical composition, and antioxidant capacity of a sample [30]. This infers that ethanolic extract of P. guajava L. leaves has a higher yield when compared to the other extracts because more solids and phytochemicals are extracted in the ethanolic extracts.
Furthermore, all guava extracts obtained were also analyzed for their total phenolic and flavonoid content. Table 3 shows that both total phenolic content and total flavonoid of ethanolic extract were the highest compared to the other extracts. This shows that the concentration of total phenolic and flavonoids depends on the nature of the solvent used to extract [31]. Furthermore, several researches reported that the amount of phytochemicals (more specifically total phenolic count and total flavonoid count) significantly increased with the increase of the polarity of the extraction solvent and might drop at a high polarity solvent such as water [31–33]. Hence why the extract produced from ethanol extraction which has a polarity index of 5.2 has a higher content of both total phenolic count and total flavonoid count when compared to ethyl acetate (4.4) and hexanic extract (nonpolar) [34].
Total phenolic content obtained from this research is lower compared to a previous research, but higher in terms of total flavonoid content. Dry guava extract obtained from reextraction using ethanol contains phenolic of 766.08 ± 14.52 mg/g and flavonoids of 118.90 ± 5.47 mg/g [35]. Lower phenolic content might be caused by a difference in standard used in measurement, that is, tannic acid; meanwhile, this research used gallic acid as a standard. However, higher flavonoid content suggests that sequential extraction using different solvents in this research gave a better result in extracting flavonoid compounds.
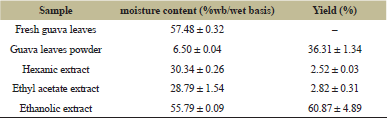 | Table 2: Guava leaves, powder, and extract moisture content and yield. [Click here to view] |
 | Table 3: Total phenolic and flavonoid content of guava leaves extracts. [Click here to view] |
MIC and MBC of guava leave extracts on S. mutans analysis were done to choose which extract had the best antibacterial activity after sequential extraction against the said bacteria.
Results in Table 4 show that hexanic extract has no antibacterial activity towards S. mutans, whereas after the sequential extraction of guava leaves, ethanolic extract shows the best antibacterial activity against S. mutans, indicated by the lowest MIC value. It means ethanolic extract inhibits S. mutans at a lower concentration.
On the other hand, this result is also in accordance with previous research that reported ethanolic and methanolic extracts of guava leaves to have better antibacterial properties against Gram-positive bacteria, one of which was S. mutans, related to the peptidoglycan layer in the cell wall which allows for the permeation of the extracts into the cells of bacteria [21]. Quercetin, which is present in guava leaves, can disrupt the membrane of S. mutans and inactivate the extracellular proteins. Other than that, guaijaverin that is also present acts in in vitro S. mutans inhibition by inhibiting the growth, adherence, and coaggregation of the bacteria [8].
The difference in the antibacterial activity of each extract can be attributed to the difference in chemical compounds present in each extract, as each solvent’s natural properties will significantly affect the extraction of specific lipophilic or hydrophilic compounds [31]. Flavonoids, which have been found to have an in vitro antimicrobial activity against various microorganisms, are found more easily in the polar extract fractions [21,32].
Furthermore, the MIC result (3,758.41 ppm or 3.75 mg/ml) against S. mutans obtained in this research was higher than the MIC of guava leaves reported from a previous research, that is, 2.5 mg/ml [36]. However, the MIC value towards S. mutans obtained in this research is lower compared to some research, that is, black tea extract (7 mg/ml), unfermented cocoa extract (4 mg/ml), and cinnamon extract powder (8 mg/ml) [37]. Other than different plant extract, the difference in MIC might be the result of a difference in the total phenolic content of the leaves, which are attributed to the difference in agroclimatic condition, maturity of the leaves at harvest, and the difference in extraction technique [32].
3.2. Effect of Extract and Peppermint Essential Oil Concentration on the Antibacterial Activity of Guava Leaves Jelly Candy
The ethanolic extract was chosen for jelly candy making because it has the highest antibacterial activity against S. mutans. To ensure that the concentrations added to the jelly candy are adequate, the antibacterial activity of jelly candy after the addition of extract and peppermint essential oil was assessed using the well-diffusion method. Based on the univariate statistical analysis, both the concentration of peppermint (%) and the peppermint essential oil concentration significantly affect the inhibition diameter of the jelly candy towards S. mutans (p ≤ 0.05). However, there was no interaction between the extract and the peppermint essential oil concentration (p > 0.05). Post hoc test results using Duncan can be seen in Tables 5 and 6.
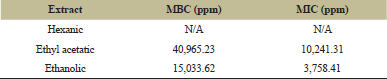 | Table 4: MIC and MBC of guava leaves extracts. [Click here to view] |
Table 5 shows that guava leaves’ jelly candy acts as an effective in vitro antibacterial agent against S. mutans starting from 4 MIC concentration and that the antibacterial activity of the jelly candy significantly increases with the increase of the extract concentration (p ≤ 0.05). A higher concentration of the extract added to the jelly candy results in higher antibacterial activity [12]. However, at a lower concentration of the extracts (2 MIC and 3 MIC), no antibacterial activity was observed. This might be because a certain amount of natural compounds needs to be present to have been effective against microorganisms [38].
Table 6 shows that the addition of peppermint essential oil in different concentrations also affected the antibacterial activity of the jelly candy against S. mutans. This might be caused by the fact that peppermint essential oils are comprised of menthol (30.35%), menthone (21.12%), and trans-carane (10.99%). It also contains isomenthol, isopulegol, and camphor [29]. The peppermint’s antibacterial activity came from the menthol which was reported to be antibacterial [39]. The mechanism of essential oils in inhibiting the growth of bacteria is not fully known as it also depends on the type of microorganisms and their cell wall structure. S. mutans is Gram-positive bacteria; therefore, the possible mechanisms could be that essential oil components destroy the bacterial cell wall and cytoplasmic membrane, which then results in leakage of the cytoplasm. Furthermore, it is also reported that essential oils can inhibit the synthesis of DNA, RNA, protein, and polysaccharides in bacterial cells [40].
 | Table 5: Effect of extract concentration on inhibition diameter against S. mutans in guava leaves jelly candy. [Click here to view] |
 | Table 6: Effect of peppermint essential oil concentration on inhibition diameter against S. mutans in guava leaves jelly candy. [Click here to view] |
3.3. Effect of Extract and Peppermint Essential Oil Concentration on the Color of Guava Leaves Jelly Candy
The color analysis of the jelly candy was done to objectively measure the color of the jelly candy, as it might also affect the acceptability of the jelly candy. Color of guava leaves jelly candy was analyzed for its lightness and oHue value. Based on the univariate statistical analysis, both the extract concentration and peppermint essential concentration significantly affect the lightness of the jelly candy (p ≤ 0.05). Furthermore, there was an interaction between the extract concentration and peppermint essential oil concentration, also significantly affecting the lightness of the candy (p ≤ 0.05), which can be observed in Table 7.
Table 7 shows that generally higher concentration of both extract and peppermint essential oil results in darker color of guava leaves jelly candy. It is because the color of guava leaves extract is dark green, therefore affecting the color of jelly candy. Other than that, the jelly candies’ Lightness value of 29–40 indicates that the candies are relatively dark colored candies. Based on its oHue value, guava leaves’ jelly candy from all treatments had oHue value ranging from 55 to 80, which indicates a yellow-red color [41].
 | Table 7: Effect of extract and peppermint essential oil concentration on the lightness of guava leaves jelly candy. [Click here to view] |
3.4. Effect of Extract and Peppermint Essential Oil Concentration on the Texture of Guava Leaves Jelly Candy
The texture analysis was done to objectively measure the texture of jelly candy, as it might also affect the acceptability of the guava leaves jelly candy. Based on univariate statistical analysis results, there was the interaction between the extract and peppermint essential oil concentration significantly affected the springiness, gumminess, and chewiness of the jelly candies (p ≤ 0.05). These results can be seen in Table 8.
The springiness, also known as the elasticity of a jelly candy, is inversely proportional to the hardness (as firmness increases, elasticity will decrease) [42]. Furthermore, the springiness of jelly candy is more related to the concentration of gelatin used. Thus, as the concentration of the gelatin used in the research is the same throughout (8.13%), a not significantly different springiness throughout the treatments should be expected. However, it was not the case in this experiment, in which Table 8 shows that higher extract concentration decreases springiness, whereas higher concentration of peppermint essential oil increases springiness. However, the values of the springiness were still in accordance with a previous research, with values of 0.945–0.990 [42].
Gumminess and chewiness of a jelly candy are affected by the addition of extract, with the addition of extract reduces gumminess and chewiness of a product, which might be associated with the increase of total acidity as a result of the increase in extract [25,43]. Meanwhile, another research stated that none of textural properties of jelly candies was affected by the addition of rosemary extract [44]. Table 8 also shows a contradictive result in this research, as a higher concentration of guava leaves extract and peppermint essential oil increased the gumminess and chewiness of jelly candy (p ≤ 0.05).
3.5. Effect of Extract and Peppermint Essential Oil Concentration on the Sensory Properties of Guava Leaves Jelly Candy
Sensory analysis was performed using the hedonic test to determine the acceptance of panelists towards guava leaves jelly candy. Statistical analysis using univariate shows that neither the extract concentration nor the peppermint essential oil concentration significantly affected the likeness of the panelists towards the color and texture of the jelly candy. With a mean value of 4.53 ± 1.28, the panelists neither liked nor disliked the color of the jelly candy. Moreover, a texture hedonic value of 4.97 ± 1.47 infers that the panelists tend to slightly like the guava leaves jelly candy.
 | Table 8: Effect of extract and peppermint essential oil concentration interaction on the texture of guava leaves jelly candy. [Click here to view] |
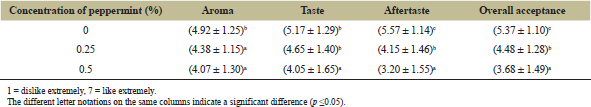 | Table 9: Effect of peppermint essential oil concentration on hedonic value of guava leaves jelly candy. [Click here to view] |
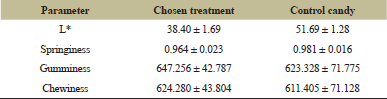 | Table 10: Comparison between chosen guava leaves jelly candy with control jelly candy. [Click here to view] |
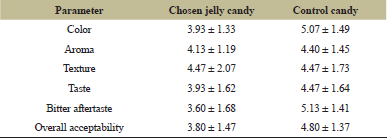 | Table 11: Sensory results comparison between chosen guava leaves jelly candy with control jelly candy. [Click here to view] |
Another interesting finding in this research is that extract concentration did not significantly affect the panelists’ acceptance in terms of aroma, taste, bitter aftertaste, and overall acceptance of guava leaves jelly candy. Statistical analysis shows that only peppermint essential oil concentration significantly contributes to acceptance on those parameters (p ≤ 0.05). Post hoc results using the Duncan test can be observed in Table 9.
The increase of the peppermint essential oil concentration decreases the likeness towards aroma, taste, bitter aftertaste, and overall acceptance of guava leaves jelly candy. In terms of aroma, at the highest concentration in the research (0.5%), aroma hedonic value was 4.07 ± 1.30, which means that the panelists neither like nor dislike the aroma. In terms of taste, at 0.5% concentration of peppermint essential oil addition, the taste hedonic value decreases significantly to 4.05 ± 1.65 from 5.17 ± 1.30, meaning that the panelists neither like nor dislike the candies.
Furthermore, the increase of peppermint essential oil concentration leads to a lower degree of likeness towards the aftertaste of jelly candy. At 0.5% concentration, panelists started to slightly dislike the aftertaste of the candy. Meanwhile, at 0.25% concentration, panelists neither like nor disliked the aftertaste of the candy. This might be contributed by the menthol present in the peppermint essential oil, as menthol is a chemical compound which can be received and stimulate the neurons which normally responds to bitter-tasting substances [45].
Interestingly, even though guava leaves have been seen to lower the sensory scores because of their astringent taste [46] and have been identified to have volatile compounds like terpene hydrocarbons, carbonyls, and ester which contributes to the characteristics odor notes and flavor of guava leaves [47], this effect was not seen in this research. This might mean that the extract concentration was not high enough to be detected by most of the panelists and that the peppermint essential oil could mask the flavor of the guava leaves. The increase in the concentration of peppermint essential oil used results in a significant decrease in the overall acceptability of the jelly candy. Panelists tend to slightly like the jelly candies with 0% concentration of peppermint concentration, tend to neither liked nor disliked the jelly candies with 0.25% concentration of peppermint, and start to slightly dislike the jelly candies with 0.5% concentration of peppermint essential oil.
Therefore, according to the overall acceptability hedonic test results of this research, the maximum concentration of peppermint essential oil that should be added to the jelly candy was 0.25%. In conclusion, to produce the highest antibacterial activity jelly candy against S. mutans with good sensory characteristics, the usage of 5 MIC P. guajava L. leaves ethanolic extract and 0.25% peppermint essential oil concentration should be used.
3.6. Comparison between the Chosen Treatment and the Control Candy
The chosen treatment was compared with the control candy of the research, the candy which was produced without the addition of guava leaves extract and peppermint essential oil. This comparison can be observed in Tables 10 and 11.
Table 10 shows that, in terms of color, the chosen treatment’s lightness is lower than that of the control candy; this is expected, as the presence of tannin and chlorophyll in guava leaves extract might affect the color and darkness of a product [48–50].
Based on Table 10, it is seen that the chosen treatment’s springiness is lower than that of the control candy, with gumminess and chewiness that are higher than that of control candy. Both candies have a relatively low springiness [42]. As gumminess and chewiness are similar, with both increases with the increase in hardness, the chosen treatment is seen to be gummier and chewier, which might affect the sensory evaluation of panelists.
In terms of sensory properties as seen in Table 11, chosen jelly candy has comparable hedonic value in terms of texture and aroma compared to control candy. Meanwhile, it has a lower hedonic value in terms of color, taste, bitter aftertaste, and overall acceptability, which means that panelists still prefer jelly candy without the addition of guava leaves extract and peppermint essential oil. However, the chosen guava leaves jelly candy offers better functional properties, as it has antibacterial activity towards S. mutans with an inhibition diameter of 9.68 ± 0.54 mm, which can be categorized as mild active [51].
4. CONCLUSION
The best extraction solvent for the highest antibacterial activity of P. guajava L. leaves against S. mutans in sequential extraction was ethanol with MIC of 3,758.41 ppm, MBC of 15,033.62 ppm, yield of 60.87% ± 4.89%, total phenolic content of 355.0347 ± 10.8186 mg GAE/g extract, and total flavonoid content of 234.2960 ± 2.5074 mg QE/g extract. Guava leaves with 5 MIC ethanolic extract concentration and 0.25% peppermint essential oil concentration could be a potential antibacterial agent against S. mutans in jelly candy to prevent dental caries. It has an inhibition diameter of 9.68 ± 0.54 mm, with 3.80 ± 1.47 overall acceptability hedonic rating.
5. ACKNOWLEDGMENTS
The authors would like to acknowledge the Microbiology Laboratory and Quality Control Laboratory of Universitas Pelita Harapan for providing the necessary facilities.
6. CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
7. FUNDING
The authors would like to acknowledge the Center of Research and Community Development, Universitas Pelita Harapan, Indonesia, for financially supported this research through Research Project No. P-003-FaST/I/2020.
REFERENCES
1. Zhu B, Macleod LC, Kitten T, Xu P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol 2018;13(8):915–32. CrossRef
2. Nomura R, Matayoshi S, Otsugu M, Kitamura T, Teramoto N, Nakanoa K. Contribution of severe dental caries induced by Streptococcus mutans to the pathogenicity of infective endocarditis. Infect Immun 2020;88(7):1–12. CrossRef
3. Maeda Y, Goldsmith CE, Coulter WA, Mason C, Dooley JSG, Lowery CJ, et al. The viridans group Streptococci. Rev Med Microbiol 2010;21:69–79. CrossRef
4. Garcia SS, Blackledge MS, Michalek S, Su L, Ptacek T, Eipers P, et al. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. J Dent Res 2017;96(7):807–14. CrossRef
5. Scharnow AM, Solinski AE, Wuest WM. Targeting S. mutans biofilms: a perspective on preventing dental caries. Med Chem Commun 2019;10(7):1057–67. CrossRef
6. Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342–9. CrossRef
7. Singh SP. Guava (Psidium guajava L.). In: Yahia EM, (ed.). Postharvest biology and technology of tropical and subtropical fruits. Woodhead Publishing Ltd, Oxford, UK, pp 213–45, 2011. CrossRef
8. Ravi K, Divyashree P. Psidium guajava: a review on its potential as an adjunct in treating periodontal disease. Pharmacogn Rev 2014;8(16):96–100. CrossRef
9. Phaiboon N, Pulbutr P, Sungthong B, Rattanakiat S. Effects of the ethanolic extracts of guava leaves, licorice roots and cloves on the cariogenic properties of Streptococcus mutans. Pharmacognosy J 2019;11(5):1029–36. CrossRef
10. Prabu GR, Gnanamani A, Sadulla S. Guaijaverin – a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J Appl Microbiol 2016;101:487–95. CrossRef
11. Gomashe AV, Sharma AA, Kasulkar A. Investigation of biofilm inhibition activity and antibacterial activity of Psidium guajava plant extracts against Streptococcus mutans causing dental plaque. Int J Curr Microbiol Appl Sci 2014;3(9):335–51.
12. Saraya S, Kanta J, Sarisuta N, Temsiririrkkul R, Suvathi Y, Samranri K, et al. Development of guava extract chewable tablets for anticariogenic activity against Streptococcus mutans. Mahidol Univ J Pharm Sci 2008;35(1–4):18–23.
13. Hartel RW, von Elbe JH, Hofberger R. Confectionery science and technology. 1st edition. Springer International Publishing AG, Cham, Switzerland, 2018. CrossRef
14. Ruheena T, Sirisha M. Soft chewable drug delivery system: oral medicated jelly and soft chew. J Drug Deliv Therap 2018;8(4):65–72. CrossRef
15. Godhwani T, Chhajed M, Chajed A, Tiwari D. Formulation development and evaluation of unit moulded semisolid jelly for oral administration as a calcium supplement. World J Pharm Res 2013;1(3):626–34.
16. Yadav C, Tangri S, Yadav R. A review: recent advancement in formulation of oral medicated jelly. World J Pharm Pharm Sci 2018;7(7):417–26.
17. Abraham J, Mathew F. Taste masking of paediatric formulation: a review on technologies, recent trends and regulatory aspects. Int J Pharm Pharm Sci 2014;6(1):12–9.
18. Uthayarasa K, Pathmanathan K, Jeyadevan JP, Jeyaseelan EC. Antibacterial activity and qualitative phytochemical analysis of medicinal plant extracts obtained by sequential extraction method. Int J Integr Biol 2010;10(2):76–81.
19. Horwitz W, Latimer G. Official methods of analysis of AOAC international. 18th edition, AOAC International, Gaithersburg, MD, 2005.
20. Venkatachalam RN, Singh K, Marar T. Phytochemical screening and in-vitro antioxidant activity of Psidium guajava. Free Radic Antioxid 2012;2(1):31–6. CrossRef
21. Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int J Microbiol 2013;2013:1–7. CrossRef
22. Bloomfield SF. Methods for assessing antimicrobial activity. In: Denyer SP, Hugo WB (eds.). Mechanisms of action of chemical biocides: their study and exploitation. Blackwell Scientific Publication, Oxford, UK, pp 256–8, 1991.
23. Hidayati L, Pereira OC. The quality evaluation of bilimbi jelly candy. Adv Soc Sci Educ Humanit Res 2017;12:89–92.
24. Nielsen S. Food analysis. Springer, London, UK, 2010.
25. Charoen R, Savedboworn W, Phuditcharnchnakun S, Khuntaweetap T. Development of antioxidant gummy jelly candy supplemented with Psidium guajava leaf extract. Int J Appl Sci Technol 2015;8(2):145–51. CrossRef
26. Purba CF, Sinaga H, Nurminah M. Effect of ratio of moringa leaves juice with pineapple juice and arabic gum on the quality of jelly candy. Indones J Agric Res 2018;1(2):162–71. CrossRef
27. Chuku EC. Proximate composition of guava leaves (Psidium guajava L.) and associated field fungi. Niger J Mycology 2009;2(1):65–70.
28. Jassal K, Kaushal S. Phytochemical and antioxidant screening of guava (Psidium guajava) leaf essential oil. J Agric Res 2019;56(3):528–33. CrossRef
29. Tsai ML, Wu CT, Lin TF, Lin WC, Huang YC, Yang CH. Chemical composition and biological properties of essential oils of two mint species. Trop J Pharm Res 2013;12(4):577–82. CrossRef
30. Ngo TV, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Qual 2017;2017:1–8. CrossRef
31. Bae H, Jayaprakasha GK, Crosby K, Jifon JL, Patil BS. Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum Nutr 2012;67:120–8. CrossRef
32. Ashraf A, Sarfraz RA, Rashid MA, Mahmood A, Shahid M, Noor N. Chemical composition, antioxidant, antitumor, anticancer, and cytotoxic effects of Psidium guajava leaf extracts. Pharm Biol 2016;54(10):1971–81. CrossRef
33. Wakeel A, Jan SA, Ullah I, Shinwari ZK, Xu M. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. PeerJ 2019;7:e7857. CrossRef
34. Aftab T, Fereira JFS, Khan MMA, Naeem M. Artemisia annua – pharmacology and biotechnology. Springer-Verlag Berlin, Heidelberg, Germany, 2014. CrossRef
35. Braga T, Dores R, Ramos C, Evangelista F, Tinoco L, Varotti F, et al. Antioxidant, antibacterial and antitumor activity of ethanolic extract of the Psidium guajava leaves. Am J Plant Sci 2014;5(23):3492–500. CrossRef
36. Tiyawanangkul K, Jinda N. Antibacterial activity of the guava leaf extract on dental plaque. Srinakharinwirot Acad 2013;7:277–82.
37. Smullen J, Finney M, Storey DM, Foster HA. Prevention of artificial dental plaque formation in vitro by plant extracts. J Appl Microbiol 2012;113:964–73. CrossRef
38. Gottardi D, Bukvicki D, Prasad S, Tyagi AK. Beneficial effects of spices in food preservation and safety. Front Microbiol 2016;7:1–20. CrossRef
39. Singh R, Shushni MAM, Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arab J Chem 2015;8(3):1–7. CrossRef
40. Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003;10:813–29. CrossRef
41. Yenrina R, Sayuti K, Anggraini T. Effect of natural colorants on color and antioxidant activity of “Kolang Kaling” (sugar palm fruit) jam. Pak J Nutr 2016;15:1061–6. CrossRef
42. Yusof N, Jaswir I, Jamal P, Jami MS. Texture profile analysis (TPA) of the jelly dessert prepared from halal gelatin extracted using high pressure processing (HPP). Malays J Fundam Appl Sci 2019;15(4):604–8. CrossRef
43. Kitpot T, Promjeen K, Chanburee S. Development of gummy jelly mixed with Thunbergia laurifolia Linn. extract for reducing breath alcohol concentration. Food Appl Biosci J 2020;8(3):29–37.
44. Cedeño-Pinos C, Martínez-Tomé M, Murcia MA, Jordán MJ, Bañón S. Assessment of rosemary (Rosmarinus ocinalis L.) extract as antioxidant in jelly candies made with fructan fibres and stevia. Antioxid 2020;9(12):1289. CrossRef
45. Green BG, Schullery MT. Stimulation of bitterness by capcaisin and menthol: differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem Senses 2003;28:45–55. CrossRef
46. Hong CS, Yoon SR, Lee GD, Kim MO, Kim HK, Kwon JH. Quality properties of guava (Psidium guajava L.) leaves processed using different methods. Korean J Food Preserv 2007;14(6):605–10.
47. Lee S, Kim YS, Choi HK, Cho SK. Determination of the volatile components in the fruits and leaves of guava plants (Psidium guajava L.) grown on Jeju Island, South Korea. J Essent Oil Res 2011;23(6):52–6. CrossRef
48. Kamble PN, Giri SP, Mane RS, Tiwana A. Estimation of chlorophyll content in young and adult leaves of some selected plants. Univers J Environ Res Technol 2015;5(6):306–10.
49. Olatunde OO, Benjakul S. Vongkamjan K. Antioxidant and antibacterial properties of guava leaf extracts as affected by solvents used for prior dechlorophyllization. J Food Biochem 2018;42(5):1–11. CrossRef
50. Pardede L, Kusdiyantini E, Budiharjo A. Ekstraksi dan uji stabilitas zat warna daun jambu biji (Psidium guajava L.). J Biol 2014;3(3):9–15.
51. Chandra R, Dwivedy V, Shivam K, Jha AK. Detection of antimicrobial activity of Oscimum sanctum (Tulsi) & Trigonella foenum graecum (Methi) against some selected bacterial & fungal strains. Res J Pharm Biol Chem Sci 2011; 2(4):809–13.