1. INTRODUCTION
Literature reveals that, even today in western medicine and despite progress in synthetic chemistry, plants are the backbone of primary health care and approximately 80% population still relies on plants [1-3]. Approximately 70,000 plant species are used in traditional and modern medicinal methods. Worldwide, various underutilized plant species have been consumed in villages [3]. Detailed investigations of these underutilized plant species are the necessity of time, especially in developing and under developing countries, where health-care sector is strongly relay on traditional drugs [5-7].
Aromatic or medicinal plants are widely used due to high nutrient and medicinal values. The consumption of these plants is increasing day by day due to their antioxidant and medicinal importance [8-10]. These products are working as alternatives of synthetic antioxidants or chemicals [6-10]. The major constituents of plants are polyphenols and flavonoids those having antioxidant and medicinal values [9,10]. These constituents inhibit or suppress the redox reactions of free radicals in biological system [7-10]. It is worthy to say that maximum aromatic plants contained these constituents. Herbal drugs are a mixture of one or more plants or their different parts, and these formulations are more vital with respect to medicinal, nutrient, and antioxidant values.
Celastrus paniculatus (CP) is belonging to the genus Celastrus, and very few reports have been noticed in literature [1-5]. CP is considered as folk medicinal plant and needs to explore its study [1,2,5]. All parts of CP may be utilized with respect to its nutritional and medicinal values. It is noticed that seeds of CP are very helpful for wounds healing, scabies, leukoderma, cough, cardiac debility, asthma, skin disease, amenorrhea, epilepsy, gouts, paralysis, inflammatory, and dysmenorrhea and utilized by tribals in India. Fruits of CP and its oil use to cure diarrhea, skin disease, amenorrhea, and dysentery, whereas roots of CP are useful against scorpion bite [1-4]. Recent in vivo studies revealed that various solvent extracts of the seeds of CP have shown antioxidant and anti-inflammatory potent on mice. Due to high antioxidant potential, seed extracts have shown the strong cognitive enhancing effect on rats [4,5].
To the best of our knowledge, maximum studies were performed on the seeds of CP, and this is the first detailed study on the phytochemical, antimicrobial, protein binding, anti-inflammatory, and antioxidant properties of the hydroalcoholic extract of the whole plant of CP.
2. EXPERIMENTAL
2.1. Material and Reagents
All the chemicals (due to long list, listed in supplementary section S0) used were of AR grade.
2.2. Plant Material Collection
CP whole plant (excluding roots) material was collected from local area, Gwalior district, Madhya Pradesh (India), and dried under shade [Figure 1]. The shade-dried stem sample was powdered and stored in airtight plastic bags for solvent extraction. The dried powder was stored at room temperature (25°C ± 2°C) to be used conveniently in the study.
 | Figure 1: Pictorial view of plant part (excluding roots) of Celastrus paniculatus used for the study [Click here to view] |
2.3. Preparation of Plant Extracts
For the extraction of phytochemicals, the hydroalcoholic solvent system (70:30) was used. About 4 g of the powdered sample was soaked in the conical flask containing solvent (100 mL), wrapped with aluminum foil, shaken for 6 h, keep in the dark for 24 h, and filtered through Whatman No. 1 filter paper. Filtered extract was stored in sterile capped bottles under refrigeration condition (4°C) before the use for further studies.
2.4. Determination of Physicochemical Parameters
Phytochemical analysis for various tests was done as per described in literature [7-10]. Detailed protocols of phytochemical analysis are mentioned in supplementary section (S1).
2.5. Antioxidant, Total Phenolic Content (TPC), Total Flavonoids Content (TFC), Anti-inflammatory, and Protein Binding Assays using UV-visible Spectrophotometric Methods
UV-visible spectrophotometer (Shimadzu-1800) was used to analyze the antioxidant TPC, TFC, and protein binding assays. Quartz cuvettes were used in whole experiment having a capacity of 3 mL. Single antioxidant assay never confirms the antioxidant activity of any nutrient and food [6-10]. Hence, in the present study, the antioxidant activity was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging [9,18,19], metal chelating (MC) [8,9], ferric reducing ability of plasma (FRAP) [10], reducing power [7,8], scavenging activity of superoxide anion [9], and nitric oxide (NO) scavenging activity [6]. Quantitative analysis of TFC and TPC was analyzed as per the protocols explained in literature [11-17]. Protein binding and binding constant calculations of drugs under the study were performed by well-described methods in literature [18-22]. Anti-inflammatory assay was performed by following the bovine serum albumin (BSA) protein method [9-11]. Above mentioned assays were performed according to previously prescribed protocols. The detailed protocols are mentioned in supplementary data under protocol section (S2-S6).
2.6. Antimicrobial Assays
Antimicrobial activities of all the extracts were performed on three fungal and four bacterial strains. The fungal strains (Aspergillus parasiticus NCIM 696, Candida albicans MTCC 183, and Aspergillus niger NCIM 501) and bacterial strains (Streptococcus mutans MTCC 497 [Gram-positive], Staphylococcus aureus MTCC 7443 [Gram-negative], Salmonella enteric MTCC 164 [Gram-negative], and Pseudomonas aeruginosa MTCC 4673 [Gram-negative]) were purchased from the Institute of Mcrobial Technology, Chandigarh, and National Chemical Laboratory with unique code number. An antimicrobial activity of various extracts of small branches and stem bark of DP was analyzed against three bacterial and three fungal strains as protocol previously described and standardized by various authors [18,19]. The detailed steps are mentioned under supplementary data (S7).
2.7. Statistical Analysis
All the quantitative tests were performed in triplicates. Analysis of variance (ANOVA) at P ≤ 0.05 and other statistical analysis were performed using the SPSS version 16.
3. RESULTS AND DISCUSSIONS
3.1. Qualitative Analysis of Phytochemicals
On screening the phytochemical activity of extract, it showed the presence of terpenoids, glycosides, proteins, amino acids, alkaloids, carbohydrates, flavonoids, phenols, saponins, steroid, and tannins [Table 1]. Hydroalcoholic extract of CP has shown a good source of different classes of compounds. This indicated the effectiveness of polar and green solvents to extracts the biological active compounds from CP. Qualitatively, the presence of terpenoids, flavonoids, and phenols was observed in a very good rate in CP [Table 1]. As compared to literature, the minor differences observed can be due to change in geographic location, weather, genetic makeup of the plants, extraction procedure, or their phytochemicals drugs [4-12].
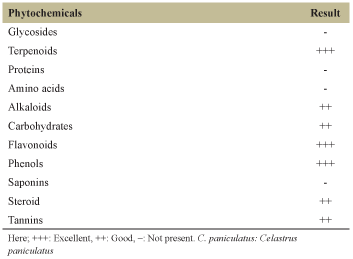 | Table 1: Qualitative analysis of phytochemicals present in the hydroalcoholic extract of C. paniculatus [Click here to view] |
3.2. In Vitro Antioxidant Assays
As single antioxidant assay cannot imitate the total antioxidant capacity, so in the present study, multiple antioxidant assays (DPPH, MC, FRAP, superoxide radical [SA], and NO) have been performed. Table 2 has highlighted the excellent antioxidant properties of CP.
 | Table 2: In vitro antioxidant assays, total phenolics and flavonoids contents, and quantitative analysis of BSA to extracts binding constants of hydroalcoholic extract of CP [Click here to view] |
The bleaching of DPPH absorption by a test sample illustrates its capacity to scavenge free radicals, without any enzyme or transition metal-based system [6,18,19]. The observed antioxidant radical scavenging activity (%) for ascorbic acid (control) was 91.30% ± 4.78%, and for extract of CP was 54.17% ± 2.11%. In recent studies, the observed DPPH bleaching ability of the various extracting solvent of seeds of CP was 99% ± 0.2% (aqueous), 95.2% ± 0.2% (chloroform), and 92% ± 0.3% (methanol) [7-15].
The observed MC power antioxidant activity (%) for ethylenediaminetetraacetic acid (control) was 89% ± 4.84% and for extract of CP was 79.42% ± 3.74%. The observed FRAP antioxidant activity (%) for ferrous sulfate (control) was 82.73% ± 3.42%, and extract of CP was 44.71% ± 2.53%. The observed reducing power antioxidant activity (%) for ascorbic acid (control) was 33.23% ± 1.44% and for extract of CP was 3.13% ± 0.36%. The observed antioxidant SA scavenging activity (%) for ascorbic acid (control) was 64.98% ± 2.38% and for extract of CP was 25.58% ± 1.11%. The observed NO antioxidant radical scavenging activity (%) for ascorbic acid (control) was 29.21% ± 1.15% and for extract of CP was 16.57 ± 1.51. Clearly, extract of CP has significantly different antioxidant property as that of control.
The antioxidant qualities of plants and medicinal values are linked with each other. Herbal drug may contain different ingredients with different antioxidant and medicinal properties [4-7]. Different method to measure the antioxidant capacity covers all the mechanism of antioxidant action [8-10]. Richness of antioxidant quality of any plant or herbal drug is the symbol of valuable food/drug supplement which may control the damage of biological molecules through different mechanisms [12-16]. These antioxidant-rich products/compounds inhibit the free radicals including SAs (O2Ë™), peroxide (ROOË™), and hydroxyl radical (OHË™), in biological system, and consequently rejuvenate the body functions. The antioxidant-rich products/compounds prevent from health issues such as heart disease, fatigues, and cancers. Most importantly, natural antioxidant, either through daily diet of through herbal drugs, may replace synthetic antioxidants including butylated hydroxyl toluene, butylated hydroxyl anisole, and propyl gallate. The synthetic antioxidants have shown damage (external and internal bleeding) in guinea pigs and rats at high dose [6,10]. Hence, a drug with such great antioxidants values may be a panacea for human and society welfare. It is a well-known fact that aromatic plant is rich in glycosides, terpenoids, proteins, amino acids, alkaloids, carbohydrates, flavonoids, phenols, saponins, steroid, and tannins. These constituents have a wide medicinal and nutritional values including antioxidant qualities.
3.3. Total Flavonoids and Phenol Contents and Correlation with Antioxidant Properties
The results of total flavonoids and TPC of herbal drugs under the study are shown in Table 2. Total phenol content was calculated as gallic acid equivalents (GAE) (Y = 0.303X + 0.007), (R2 = 0.999). The observed TPC and TFC of the extract were 8.38±0.15 mg GAE/g and 12.91±0.21 mg quercetin equivalent (QE)/g crude extract [Table 2]. The standard curve used for TFC was Y = 0.040X + 0.012, R2 = 0.999. The observed TPC and TFC values were closed to the study performed in recent past on various extracts by various authors [1-10].
Linear correlation analysis was followed to check the correlation between the antioxidant activities to TFC and TPC of hydroalcoholic extract of C P. Relatively, strong linear positive relationships of extract was observed. Among the DPPH assay, NO radicals scavenging, and reducing power assay, a strong linear correlation has been observed. It indicates the role of the terpenoid, phenol, and flavonoid compounds as reducing agents, thus contributing to antioxidant activity [10-14]. However, a poor correlation among the MC assay, FRAP assay, and superoxide anion free radicals assay of sample to TPC/TFC has been noticed. It might belong to the other diverse mechanisms of action of these assays [12-16]. Moreover, from Table 2, it is apparent that plant extract under the study has mild TPC and high TFC content with very good antioxidant potential.
3.4. Anti-inflammatory and Protein Binding Assay
Denaturation of proteins is the major cause of inflammation. In the current study, as compared to acetylsalicylic acid, extract samples have shown excellent inhibition (95–98%) to the heat-induced recovery of BSA protein. Interaction behavior (in terms of binding constant) of various extract samples or acetylsalicylic acid (control) was observed [Table 2]. In general, strong interactions of any medicine with proteins with binding constant (k) ranging from 106 to 1011/M are not a good thing for the proper drug delivery [7-10]. BSA protein binding to acetylsalicylic acid and extract of CP were performed as described by Abdi et al. 2012 [9]. An increase in the concentrations resulted in an increase in UV light absorption and shifting of BSA band from 280 to 295 nm that can be related to complex formation. The average values of protein binding constants are 2.31 ± 0.05 × 10-5/μM (for acetylsalicylic acid) and 1.11 ± 0.79 × 10-5/μM for sample or extract [Table 2]. All these results suggest a weak interaction of various extract with BSA complex which is a good thing for the proper drug delivery.
Since the medicines form various plants have significant influence on protein-binding properties. The interesting point of the study is that extract under study has shown least binding rate with BSA protein as compared to acetylsalicylic acid, which is the feature of good medicines [21,22]. However, complexation with albumin proteins can stabilize the medicines against aggregation, leading to retention of the antimicrobial activities [19-15].
3.5. Antimicrobial Activities
In the present study, hydroalcoholic extract of CP was active at higher concentration (1000 mg/L), with minor (5–8 mm) antimicrobial effects [Table 3]. Data revealed that maximum inhibition in the growth of antibacterial strains was noticed for P. aeruginosa, (followed by remaining three strains (S. aureus, S. enteric, and S. mutans). Among the three fungal strains, hydroalcoholic extract of CP has shown inhibition in the growth of A. niger and A. parasiticus (6 mm) while inactive against the other one strain (C. albicans). In recent study, ethyl alcohol, methanol, and aqueous extracts were found active against S. mutans and P. aeruginosa, which was very close to the current study [1-5].
 | Table 3: Antibacterial and antifungal activities of hydroalcoholic extract of CP [Click here to view] |
Literature revealed that antimicrobial activities of the plant extracts are due to the presence of potent compounds such as alkaloids, flavonoids, tannins, etc. [1-5]. Moreover, the absence of activities in extracts does not signify lack of bioactive compounds but indicate the presence of lower amount of these compounds or their actions were antagonized due to the presence of other compounds [2-4].
4. CONCLUSION
The obtained results provide the supporting evidence for the effective use of selected plant extract. Natural plants possess diverse therapeutic agents, but its properties depend on the nature of the plant, procedure used to isolate these agents, and method used to assess the particular character. In the current study, hydroalcoholic extract of CP exhibited better antioxidant and protein binding activities and poor antimicrobial activities. A significant relationship between the phenolic/flavonoid compounds and antioxidant capacities indicated that these are the major contributors of antioxidant capacities of these plants. Further, there is a need to explore the studies, namely, isolation of specific compound with maximum therapeutic activities including antimicrobial and antioxidant properties.
5. ACKNOWLEDGMENTS
We are also thankful to CCRAS, New Delhi and RARIDD, Gwalior for library and laboratory facilities.
7. REFERENCES
1. Kalaskar MG, Saner SY, Pawar MV, Rokade DL, Surana SJ. Pharmacognostical investigation and physicochemical analysis of Celastrus paniculatus willd. leaves, Asian Pac J Trop Biomed 2012;12:1232-6.
2. Hemanth KK, Venuprasad MP, Ajay P, Farhath K. Phytochemical analysis and exercise enhancing effects of hydroalcoholic extract of Celastrus paniculatus Willd, Ind. Crops Prod 2014;55:217-24. Crossref
3. Neha A, Shashi PR. GC–MS analysis of the essential oil of Celastrus paniculatus Willd. seeds and antioxidant, anti-inflammatory study of its various solventextracts, Ind. Crops Prod 2014;61:345-51. Crossref
4. Jing RW, Ming H Y, Wei YL. Cytotoxic constituents from Celastrus paniculatus induce apoptosis and autophagy in breast cancer cells. Photochemistry 2013;94:211-9. Crossref
5. Yen G, Chen H. Antioxidant activity of various tea extract in relation to their antimutagenicity. J Agric Food Chem 1995;43:7-32. Crossref
6. Maity S, Chatterjee S, Variyar PS, Sharma A, Adhikari S, Mazumder S. Evaluation of antioxidant activity and characterization of phenolic constituents of Phyllanthus amarus root. J Agric Food Chem 2013;61:3443-50. Crossref
7. Prasad R, Upadhyay N, Kumar V. Simultaneous determination of seven carbamate pesticide residues in gram wheat lentil soybean fenugreek leaves and apple matrices. Microchem J 2013;111:91-7. Crossref
8. Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 2000;48:3396-402. Crossref
9. Abdi K, Nafisi Sh, Manouchehri F, Bonsaii M, Khalaj A. Interaction of 5-fluorouracil and its derivatives with bovine serum albumin. J Photochem Photobiol B 2012;107:20-6. Crossref
10. Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 1968;20:169-73. Crossref
11. Kumar V, Singh S, Singh J, Upadhyay N. Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol 2015;94:807-14. Crossref
12. Kaur S, Kumar V, Chawla M, Cavallo L, Poater A, Upadhyay N, et al. Pesticides curbing soil fertility: Effect of complexation of free metal ions. Front Chem 2017;5:43. Crossref
13. Kumar V, Singh S, Singh R, Upadhyay N, Singh J. Design, synthesis, and characterization of 2,2-bis(2,4-dinitrophenyl)-2-(phosphonatomethylamino)acetate as a herbicidal and biological active agent. J Chem Biol 2017;10:179-90. Crossref
14. Kumar V, Kaur S, Singh S, Upadhyay N. Unexpected formation of N’-phenyl-thiophosphorohydrazidic acid O,S-dimethyl ester from acephate: Chemical, biotechnical and computational study 3 Biotech 2016;6:1.
15. Kumar V, Upadhyay N, Kumar V, Sharma S. A review on sample preparation and chromatographic determination of acephate and methamidophos in different samples. Arabian J Chem 2015;8:624-31. Crossref
16. Kumar V, Singh S, Singh R, Upadhyay N, Singh J, Pant P, Singh R, Shrivastava B, Singh A, Subhose V. Spectral, structural and energetic study of acephate, glyphosate, monocrotophos and phorate: An experimental and computational approach. Journal of Taibah University for Science, 2018;12:69-78. Crossref
17. Kumar V, Upadhyay N, Manhas A. Designing syntheses characterization computational study and biological activities of silver-phenothiazine metal complex. J Mol Str 2015;1099:135-40. Crossref
18. Kumar V, Kumar V, Upadhyay N, Sharma S. Interactions of atrazine with transition metal ions in aqueous media: Experimental and computational approach 3 Biotech 2015;5:791-8.
19. Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition M27-A3, Vol. 0 No. 0, Replaces M27-A2, Vol. 22 No. 15; 2007.
20. Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, et al. Toxicity monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett 2016;14:317-29. Crossref
21. Singh S, Kumar V, Upadhyay N, Singh J, Singla S, Datta S, et al. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and fe(III)], and humic acid. 3 Biotech 2017;7:262. Crossref
22. Kumar V, Chawla M, Cavallo L, Wani AB, Manhas A, Kaur S, et al. Complexation of trichlorosalicylic acid with alkaline and first row transition metals as a switch for their antibacterial activity. Inorganica Chim Acta 2018;469:379-86. Crossref
SUPPLEMENTARY DATA
S0. MATERIAL AND REAGENTS
Folin–Ciocalteu reagent, sodium carbonate, sodium nitrite, methanol, aluminum chloride, potassium acetate, bovine serum albumin (BSA), acetyl salicylic acid, gallic acid, quercetrin, 1,1-diphenyl picrylhyrazyl (DPPH), potassium ferricyanide, trichloroacetic acid (TCA), ferric chloride, nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT), phenazine methosulfate (PMS), sodium phosphate, ammonium molybdate, sulfanillic acid (SA), ascorbic acid, ethylenediaminetetraacetic acid (EDTA), ferrozine, sodium nitroprusside (SNP), napthylethylenediamine dichloride (NED), Griess reagent, ascorbic acid, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), tris-HCl, sodium acetate, and ferrous chloride were purchased from Merck. Ethanol, petroleum ether, methanol, hydrochloric acid (HCl), sulfuric acid (H2SO4), chloroform, ammonia, glacial acetic acid, sodium hydroxide, disodium phosphate, phosphoric acid, and acetone were of AR grade purchased from Loba Chemie.
S1. QUALITATIVE ANALYSIS OF PHYTOCHEMICALS
Small branches and stem bark extracts (petroleum ether, acetone, and methanol) of DP were analyzed for the presence of various phytochemicals using the respective chemical tests as follows.
S1.1. Test for Glycosides
0.5 mL extract was taken in a test tube, 0.2 mL of 10% ferric chloride solution, and 50% glacial acetic acid added. Few drops of concentrated sulfuric acid were added. A blue color production shows the presence of glycosides.
S1.2. Tests for Terpenoids
Extract was mixed with chloroform and a few drops of concentrated H2SO4 were added, shaken well, and allowed to stand for some time. Red color appeared at the lower layer indicated the presence of steroids and formation of yellow-colored layer indicated the presence of terpenoids.
S1.3. Test for Proteins
An aliquot of 2 mL of extract was treated with one drop of 2% copper sulfate solution. To this, 1 mL of ethanol (90%) was added, followed by an excess of potassium hydroxide pellets. Pink color in ethanol layer indicated the presence of proteins.
S1.4. Test for Amino Acids
Two drops of ninhydrin (5%) were added to 1 mL of extract. A characteristic purple color indicated the presence of amino acids.
S1.5. Test for Alkaloids
Two milliliters of 1% HCl was mixed with 0.1 g of crude extract and heated slightly. After cooling, Wagner’s reagent and Mayer’s reagent were added to it. The presence of buff-colored precipitate indicated the presence of alkaloids.
S1.6. Test for Carbohydrates
Benedict’s reagents were mixed with the 1 mL of crude extract and slightly boiled, and the appearance of reddish-brown precipitate indicated the presence of the carbohydrates.
S1.7. Test for Flavonoids
The appearance of pink scarlet color when 1 mL of crude extract was mixed with few drops of concentrated HCl and Mg pellets indicated the presence of flavonoids.
S1.8. Test for Phenols
2 mL of 2% ferric chloride was mixed with the 1 mL of crude extract and the presence of blue-green or black coloration indicated the presence of phenols.
S1.9. Test for Saponins
1 mL of crude extract was mixed well in water and shaken; the appearance of foam indicated the preliminary evidence for the presence of saponins.
S1.10. Test for Steroid
2 mL of extract was mixed with 2 mL H2SO4 and slowly added to 2 mL of acetic anhydride. The color change from violet to green or blue indicated the presence of steroids.
S1.11. Test for Tannins
1 mL of extract mixed in distilled water and filtered. Few drops of ferric chloride solution were added to the filtrate. The green or blue-green precipitate indicated the presence of tannins.
S2. ANTIOXIDANT ASSAYS OF THE VARIOUS EXTRACTS
S2.1. DPPH Radical Scavenging Assay
100 μL of 0.01 mM solutions of different extracts and ascorbic acid were added to 2.0 mL of 0.01 mM solution of DPPH (prepared in ethanol) in 5.0 mL amber color volumetric flasks. After 5 min, the absorbance at 517 nm was recorded. Pure DPPH solution was used as a control. The radical scavenging ability was calculated according to the formula: Radical scavenging activity % = (A0-AT/A0 ) ×100, where A0 = absorbance of pure DPPH solution and AT = absorbance of (DPPH + 100 μL extract or ascorbic acid).
S2.2. MC Activity
200 μL of the extract or control was incubated for 10 min together with 100 μL of FeSO4 (2 mM) and 400 μL ferrozine (5 mM). After 5 min, 2 mL methanol was added and incubated at 37°C for 15 min. The absorbance was measured at 560 nm. The % chelating activity was calculated by the following formula: MC ability (%) = [(A0-A1)/ A0]×100, where A0 = absorbance of the control and A1 = absorbance of the sample/standard. EDTA was taken as a positive control.
S2.3. Ferric Reducing Ability of Plasma (FRAP) Assay
The antioxidant capacity of extracts was estimated according to the procedure given below. FRAP reagent (900 μL), prepared freshly and incubated at 37°C, was mixed with 90 μL of distilled water and 30 μL of test sample (for the reagent blank). The test samples and reagent blank were incubated at 37°C for 30 min in a water bath. The FRAP reagent contained 2.5 mL of 20 mM TPTZ solution in 40 mM HCl plus 2.5 mL of 20 mM FeCl3.6H2O and 25 mL of 0.3 M acetate buffer, pH 3.6. At the end of the incubation period, the absorbance readings were recorded immediately at 593 nm using a spectrophotometer. Percentage inhibition of FRAP activity was calculated as follows: % Inhibition = ((A0-A1)/A0)×100, where A0 is the absorbance of the control (Fe(II)) and A1 is the absorbance of the sample.
S2.4. Ferric Reducing Power
100 μL of extract or control was added to 2.5 mL of potassium ferricyanide (1% prepared in phosphate buffer pH 6.6) and incubated at 50°C for 20 min. After incubation, 2.5 mL of 10% trichloroacetic acid was added to the reaction mixture and centrifuged at 1000 r/min for 8 min. About 5 mL of supernatant was extracted and made up to 10 mL with deionized water. To this solution, 0.5 mL of 0.01% ferric chloride was added and absorbance was measured at 700 nm. Ascorbic acid was used as a standard.
S2.5. Scavenging Activity of Superoxide Anion
This activity was carried out by mixing the 100 μL of extract(s) or control to 1 mL of NBT solution (156 μM prepared in Tris-HCl buffer), 1 ml of NADH solution (468 μM prepared in Tris-HCl buffer), 700 μL Tris-HCl buffer (pH 8.0), and 0.1 mL of phenazine methosulphate solution (60 μM prepared in Tris - HCl buffer) and incubated at 25°C for 5 min. After 5 min, the absorbance was read at 560 nm. Ascorbic acid was used as a positive control. Percentage inhibition of scavenging activity of superoxide anion was calculated as follows: % Inhibition = ((A0-A1)/A0 )×100, where A0 is the absorbance of the control and A1 is the absorbance of the sample.
S2.6. Nitric Oxide Scavenging Activity
Nitric oxide was generated from sodium nitroprusside, and its quantity was determined using Griess reagent. 100 μL of extract(s) and control were mixed with 1 mL of sodium nitroprusside solution (10 mM, prepared in phosphate buffer pH 7.2) and incubated at 37°C for 120 min. 1 mL of sulfanilic acid (1%, prepared in 2% H3PO4) was added and incubated at 37°C for 5 min. 0.5 mL of naphthylethylenediamine dihydrochloride (0.1%) was added and incubated at 37°C for 20 min. The absorbance was measured at 540 nm. The percentage of nitric oxide scavenging by the following formula: Nitric oxide scavenging (%) = [(A0-A1 )/A0]×100, where A0 = absorbance of the control and A1 = absorbance of the sample/standard. Ascorbic acid was used as the standard.
S3. TOTAL PHENOLIC CONTENT
Total phenolic content of the extract was analyzed using Folin-Ciocalteu reagent. Briefly, 1 mL of sample of varying concentrations was incubated in 5 mL of Folin-Ciocalteu reagent and 4 mL of 1 mol/L Na2CO3. After 15 min of incubation, absorbance was measured at 765 nm by spectrophotometer. Gallic acid dissolved in ethanol was used as standard. The total phenol content was reported in terms of mg of GAE/g of dry eight GAEs/g DW. All the tests were performed in triplicates.
S4. TOTAL FLAVONOIDS CONTENT
The total flavonoid content was estimated by taking 0.3 mL of 5% (w/v) NaNO2 to every 4 mL of sample of varying concentrations. After 5 min of incubation, 0.3 mL of 10% (w/v) AlCl3 was added. After 6 min, 2 mL of 1 mol/L NaOH was added. The total volume was made up to 10 mL with distilled water. After vortex shaking for 1 min, the absorbance was noted at 510 nm. The total flavonoid content was reported in terms of mg of quercetin equivalents/g of dry weight of drug (QRE/g DW). All the tests were performed in triplicates.
S5. PROTEIN (BSA) BINDING ASSAY
Aspirin (control) (of concentrations 50 μL, 100 μL, 150 μL, 200 μL, and 250 μL) was added to 2 mL of BSA solution (5 mM) (prepared in phosphate buffer of pH 7.2). Final volume was make up to 5 mL. Absorbance was noticed at 280 nm. An increase in the concentrations resulted in an increase in UV light absorption and shifting of BSA band from 280 to 272 nm that can be related to complex formation. The binding constants of the complexes are determined using UV-vis spectroscopic method. The double reciprocal plot of 1/(A-A0) versus 1/(ligand concentration) is found linear, and the binding constant (k) can be estimated from the ratio of the intercept to the slope. The same was repeated with various extracts of DP. All the tests were performed in triplicates.
S6. ANTI-INFLAMMATORY ACTIVITY OF THE EXTRACTS
Inhibition of protein denaturation was evaluated by the method given below. 2 mL of 1% BSA was added to 200 μL of plant extract. This mixture was kept at room temperature for 10 min, followed by heating at 51°C for 20 min. The resulting solution was cooled down to room temperature, and absorbance was recorded at 660 nm. Acetylsalicylic acid was taken as a positive control. The experiment was carried out in triplicates, and percentage inhibition for protein denaturation was calculated using the following formula: % Inhibition = 100-((A1-A2)/A0)×100), where A1 is the absorbance of the sample, A2 is the absorbance of the product control, and A0 is the absorbance of the positive control. All the tests were performed in triplicates.
S7. ANTIMICROBIAL ACTIVITIES OF THE EXTRACTS
Antimicrobial activities of all the extracts were performed on three fungal and four bacterial strains. The fungal strains (Aspergillus parasiticus NCIM 696, Candida albicans MTCC 183, and Aspergillus niger NCIM 501) and bacterial strains (Streptococcus mutans MTCC 497 [Gram-positive], Salmonella enteric MTCC 164 [Gram-negative], Staphylococcus aureus MTCC 7443 [Gram-positive], and Pseudomonas aeruginosa MTCC 4673 [Gram-negative]) were purchased from the Institute of Microbial Technology, Chandigarh, and National Chemical Laboratory with unique code number. Antimicrobial activities of the various extracts of small branches and stem bark of DP were analyzed against three bacterial and three fungal strains as protocol given below.
S7.1. Antibacterial Activities
In vitro antibacterial studies were carried out by agar disc diffusion method against test organisms. Nutrient broth (NB) plates were swabbed with 24 h old broth culture (100 ml) of test bacteria. Sterile paper discs (5 mm) were put into each Petri plate. Different concentrations of various extracts (250, 500, and 1000 mg/L) were added into the discs by dipping individual disc into solution containing test tubes. In our experiment, solvent(s) acted as negative control and chloramphenicol as positive control. The plates were incubated at 37°C for 24 h. After appropriate incubation, the diameter of zone of inhibition of each disc was measured. All the tests were performed in triplicates.
S7.2. Antifungal Activities
In vitro antifungal studies were carried out by potato dextrose agar medium disc diffusion method against test organisms. All fungi were cultured in potato dextrose agar medium. For this purpose, potato dextrose agar medium was poured in the sterilized Petri dishes and allowed to solidify, where dishes were inoculated with a spore suspension of (106 spores/mL of medium). Test crude extracts (250, 500, and 1000 mg/L) and solvent soaked filter paper discs were placed on the already seeded plates and incubated at 37°C for 96 h.. After 96 h, inhibition zone appeared around the discs in each plate was measured. In this experiment, solvent acted as negative control and ketoconazole as positive control. All the tests were performed in triplicates.