ARTICLE HIGHLIGHTS
Breast cancer remains a global health concern, prompting continuous research into novel therapeutic options. In this context, the article presents a comprehensive investigation into the potential of Opuntia elatior Mill. as an alternative treatment for breast cancer. The study combines laboratory experimentation and computational analysis to evaluate the phytochemical composition and antiproliferative effects of Opuntia elatior Mill. extract on the breast cancer cell line MCF-7.
The phytochemical analysis revealed a rich composition of bioactive compounds in Opuntia elatior Mill., including flavonoids, alkaloids, and polyphenols, known for their potential health benefits. Furthermore, the study delved into the extract’s antiproliferative activity, demonstrating its ability to inhibit the growth of MCF-7 breast cancer cells and the data was supported by its anti-migratory activity and long survival of cells in the presence of extract. Molecular docking simulations provided data on the binding affinities and potential modes of action, shedding light on the extract’s mechanistic basis for its antiproliferative activity. Opuntia elatior Mill. emerges as a promising candidate for further investigation and potential drug development against breast cancer.
The integration of experimental and computational methods highlights the power of interdisciplinary research in unravelling the complex interactions between natural compounds and cancer cells. As researchers continue to explore alternative treatments, this paper underscores the importance of harnessing the therapeutic potential of botanical sources in the battle against breast cancer.
1. INTRODUCTION
Cancer is a global health concern and is the leading cause of death worldwide. The most widely used chemotherapeutic medications are antimetabolites, such as methotrexate; DNA-interactive medications, such as cisplatin and doxorubicin; anti-tubulin medications, such as taxanes; hormones; and molecular targeting medications [1]. The main drawbacks of chemotherapy are cancer recurrence, drug resistance, and harmful effects on tissues that are not being treated, which might limit the use of anticancer medications and reduce patient quality of life [2]. There is a constant effort being made for identifying novel, promising anticancer drugs from natural sources to address the concerns arising from the current therapies. Opuntia elatior is a xerophytic shrub that belongs to the dicotyledonous angiosperm Cactaceae family, of which over 1500 species are known [3]. O. elatior is abundant in the semi-arid belts of Saurashtra and parts of North Gujarat in the Indian subcontinent [Figure 1]. This particular species drew attention due to its use by the locals of Saurashtra wherein the local community used it as a juice with medicinal value for maintaining healthy blood physiology. Locally, it is referred to as “Hathlo Thor” [4]. The flat, edible stems of most Opuntioideae cacti are known as cladodes, paddles, nopales, or stalks. Young cladodes, also known as nopales, are typically used as a vegetable in salads, and their fruits, also known as tunas, are frequently consumed as fresh seasonal fruit [5]. Oval berries, known as “prickly pears,” have many seeds dispersed throughout the pulp and a thorny, semi-hard skin. In folklore, Opuntia is used for diabetes, anemia, diphtheria, burning sensation in the stomach, and obesity.
 | Figure 1: Opuntia elatior Mill parts (a) Cladode and (b) Fruit. [Click here to view] |
The antiproliferative effect of Opuntia ficus-indica prickly pear juice on MCF-7 human breast cancer cells showed that the juice inhibited the growth of the cancer cells and induced apoptosis, indicating its potential use as a natural anti-cancer agent [6,7]. The researchers suggested that this effect was due to the presence of betalains, which are pigments found in some cactus species that have been shown to have anticancer properties [8]. It is therefore scientifically important to investigate the potential capabilities of O. elatior for its anti-cancer capabilities. Between wild and domesticated species, there are differences in the phytochemical makeup of their plant components (fruits, cladode, roots, flowers, seeds, and stems), altering their nutritional value and consequently, their functional and/or medicinal characteristics. Opuntia species are globally widespread, containing amino acids, carotenoids, and antioxidant compounds such as betalains and flavonoids [9]. However, it is important to note that their phytoconstituents can vary based on their environmental conditions leading to some unique secondary metabolites that could be of importance to human health. The other phytochemical groups known are alkaloids, phenolic acids, coumarins, and terpenoids, which have several health benefits. An array of flavonoids, such as quercetin, kaempferol, and isorhamnetin glycosylated derivatives, were identified through High-Performance Liquid Chromatography-photo diode array-electrospray ionization (ESI)-mass spectrometry (MS) in O. ficus-indica [10,11]. The ethanolic fruit extracts were tested for their antiproliferative activity against the human K-526 cell line, which showed cell cycle arrest at G2/M and S phases [12]. It is important to note here that most of the scientific data available in the literature are for O. ficus-indica while scientific understanding of O. elatior is lacking due to its regional-specific availability. Furthermore, most studies done are inconclusive and lack clarity regarding their applicative in-depth understanding of O. elatior as a phytotherapeutics agent. There are also reports of mucilage and phylloclade being used for recipes, and it will be very interesting to know if phylloclade also has some bio-actives for future use. The literature suggests that the fruits of O. elatior serve as an analgesic due to the presence of betanin [13].
Furthermore, there is astonishingly little evidence to back up the belief that herbal medicines are generally safe since they are “natural,” despite the fact that the general population and some healthcare professionals hold this belief. In times, when existing medication has posed limitations for further use one needs to go back to nature to find solutions to modern-day medication. The present study is one of the pioneering attempts to evaluate the plant’s anti-breast cancer properties, which is the genus of the plant consumed by humans and considered safe. Scientific insight into this plant is important for drug discovery and opens avenues for further repurposing.
2. METHODS
2.1. Plant Material
The cladode and fruits of O. elatior, commonly known as Hathala-Thor, were collected in November 2020 in Jamkhambhaliya (22°09’58” N 70°07’53” E), Gujarat, India. Taxonomist Dr. Karan Rana, from the School of Science, identified and authenticated the whole plant. The voucher specimen (FP1) is deposited in the museum of Navrachana University. The plant material was washed, cut into pieces, and then dried in a hot air oven at 40°C. The dried plant material was coarsely powdered using a mechanical grinder and stored in an airtight container at room temperature for further use.
2.2. Preparation of O. elatior Extract
The powdered cladode and fruit material were subjected to extraction. The soxhlet extraction method was used to prepare the methanolic extract by subjecting 30 g cladode and fruit powders each to extraction with 200 mL of methanol, while the reflux extraction method was used to prepare an aqueous extract in similar proportions. The solvent was evaporated, and the extract was cooled down and stored at –20°C until analysis. Cladode aqueous, cladode methanol, fruit aqueous, and fruit methanol extraction yields were 19.75%, 12.75%, 36.4%, and 38.5%, respectively [14].
2.3. Determination of Total Flavonoid Content
For all investigated plant extracts, 1 mg of extract was dissolved in 1 mL methanol and then mixed with 60 μL 5% NaNO2, 60 μL 10% AlCl3, incubated for 5 min at room temperature, followed by the addition of 0.2 mL of 1 mM NaOH, diluted with 1 mL of distilled water. The absorbance of the reaction mixture was measured at 510 nm with a double-beam Ultraviolet/Visible spectrophotometer. The standard curve was prepared by employing quercetin as a reference standard in the concentration range of 200–1000 μg/mL [15].
2.4. Determination of Total Phenolic Content
The phenolic contents in the plant extracts were determined using the Folin-Ciocalteu method. 1 mL of plant extract (1 mg/mL) was diluted with 3 mL of distilled water, mixed with 0.5 mL Folin-Ciocalteu reagent, and incubated for 3 min. Subsequently, 2 mL of 20% (w/v) sodium carbonate was added, mixed thoroughly, and kept in boiling water for 1 min. The blue color was read at 650 nm against a methanol reagent blank. Total phenolic content was expressed as milligram gallic acid equivalent per gram of the sample [16].
2.5. Determination of Antioxidant Activity using 2, 2-Diphenyl-1-Picryl-Hydrazine-Hydrate (DPPH) Assay
The assay was performed in the sample concentration range of 100–1000 μg/mL with the addition of 2.85 mL of DPPH solution prepared in methanol. The reaction mixture was incubated for 30 min in the dark. The decrease in absorbance was recorded at 517 nm against methanol as a blank [17]. The antioxidant activity was calculated as the percentage of inhibition of the DPPH-free radical with respect to gallic acid standard and determined by the following equation:
% DPPH radical scavenging activity = (1−sample OD/control OD) × 100
2.6. Determination of Antioxidant Activity using Ferric Reducing Antioxidant Power (FRAP) Assay
The antioxidant activity of the plant extracts was estimated by the tripyridyl triazine (TPTZ) method. The reduction of Fe3+ TPTZ complex (colorless complex) to Fe2+ TPTZ (blue-colored complex) formed at acidic pH by donating an electron was recorded spectrophotometrically. The fresh FRAP reagent was prepared by mixing 125 mL acetate buffer (0.3 M), 12.5 mL of TPTZ (10 mM), and 12.5 mL of FeCl3.6H2O (20 mM) at 37°C. To each aliquot (200 μL) of plant extract, 2.85 mL of FRAP reagent was added and incubated for 30 min in the dark. The reaction of plant extract with FRAP reagent formed an intense blue color complex whose absorbance was recorded at 593 nm against the reagent blank. The FRAP values obtained were expressed as milligram gallic acid equivalent per gram of sample [18].
2.7. Identification of Bioactive by High Resolution-Liquid Chromatography-Mass Spectroscopy (HR-LCMS)
The plant extracts were subjected to HRLC-MS analysis (Agilent 6550 iFunnel Quadrupole Time-of-Flight [Q-TOF]’). Chromatographic separation was done using a 1290 infinity Ultra Performance liquid chromatography system fitted with a Hypersil gold column (C18 × 2.1 mm-3 micron) with the help of an autosampler at a flow rate of 0.3 mL/min and 20 μL injection volume. A solution of 0.1% formic acid in water formed solvent system A, while a combination of 0.1% formic acid, 10% water, and 90% acetonitrile formed solvent system B. The chromatographic elution was performed by following the gradients system which was as follows: 0–1 min of 95% (A) and 5% (B), 1–20 min of 100% solvent (B), 20–25 min of 100% solvent (B), 25–26 min of 95% (A) and 5% (B), and 26–30 min of 95% (A) and 5% (B). The mobile phase consisted of 10% acetonitrile, and 10% water (A) and (B). The eluted compounds were ionized using the ESI technique, equipped with a QTOF analyzer (Agilent Technologies, CA, USA). The flow of gas was set at 13 L/min at 250°C temperature, while the capillary tension was set at 3500 V. The sheath gas flow was set at 11 L/min at 300°C, while the pressure of the nebulizer gas was 35 psi. The mass data were recorded on positive and negative ionization modes across a spectral range of m/z 150–1000. The data acquisition and evaluation of MS were carried out using the Agilent Metlin database [19].
2.8. Antiproliferative Activities
2.8.1. Cell viability assay
The antiproliferative activity of crude plant extracts was investigated on human breast adenocarcinoma cancer cell line MCF-7 procured from National Centre for Cell Science (NCSS), Pune, Maharashtra. The cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% heat-inactivated fetal bovine serum, 100U/mL penicillin-streptomycin solution at 37°C and in a humidified atmosphere of 5% CO2 in the air. The cells were seeded at 104 cells/well density in 96 well plates to obtain 80–90% confluency. The cell viability study was performed from a concentration range of 0.5–100 μg/mL using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method. The well plates were incubated for 24, 48, and 72 h with the indicated concentration of plant extracts, positive control (Doxorubicin), and negative control (Dimethyl sulfoxide [DMSO] 0.2%). Then 10 μL MTT (5 mg/mL stock) was added to the well plates. After 4-h incubation, MTT was eliminated, and 100 μL DMSO was added to solubilize the formazan crystals. The concentration was quantified at 490nm in a microplate reader, and the results were shown as percentage inhibition (100% inhibition) [20].
2.8.2. Toxicity studies
The safety of all four extracts was investigated on a human embryonic kidney cell line (HEK-293) normal cell line procured from NCSS, Pune, Maharashtra. HEK-293 is believed to be derived from embryonic kidney cells using adenoviral DNA and is a widely used standard for normal cells. The cells were cultured in minimum essential media supplemented with 10% heat-inactivated fetal bovine serum, 100U/mL penicillin-streptomycin solution at 37°C, and in a humidified atmosphere of 5% CO2 in the air. The cells were seeded at 104 cells/well density in 96 well plates to obtain 80–90% confluency. The cell viability study was performed from a concentration range of 0.5–100 μg/mL using the MTT colorimetric method. The well plates were incubated with the indicated concentration of plant extracts for 24, 48, and 72 h. Then, 10 μL MTT (5 mg/mL stock) was added to the well plates. After 4-h incubation, MTT was eliminated, and 100 μL DMSO was added to solubilize the formazan crystals. The concentration was quantified at 490 nm in a microplate reader, and the results were shown as percentage inhibition (100% inhibition) [20].
2.8.3. Scratch assay (wound healing assay)
The wound-healing assay was carried out according to a previously reported protocol. The MCF-7 at density 104/well were seeded in 24 well microplates. After 80%–90% confluency, a scratch was made in the monolayer using a sterile 200 μL pipette tip. The floating cells and debris were removed using sterile 1× phosphate-buffered saline. The fresh complete growth medium and plant extracts at a half-maximal inhibitory concentration 50% (IC50) concentration were added to the cells. The wells containing cells with medium without extract were used as control. The cells were incubated for 24 h at 37°C in 5% CO2. Images of gap closure were taken at 0, 12, and 24 h using an inverted microscope. The area enclosed between the scratch boundary was analyzed using Image J bundled with 64-bit Java 8. The percentage of wound closure was calculated to the initial gap area at 0 h [21].
2.8.4. Clonogenic assay
The colony formation assay was carried out according to a previously reported protocol with modification. Approximately 200 cells were seeded in 6 well plates and allowed to grow for 24 h at 37°C. The cells were then treated with IC50 concentration of plant extracts for 24 h at 37°C. After this, cells were washed with 1× sterile phosphate buffer saline and added to a fresh medium. The cells were then kept in a fresh medium and incubated for a period of 10 days. Following this, cells were fixed with 37% formaldehyde, stained with 0.5% crystal violet in 100% methanol for 30 min at room temperature, and washed with tap water, and the colonies were analyzed using ImageJ bundled with 64-bit Java 8 [22].
2.9. Preparation of Protein receptors
The 3D X-ray crystallography structure of Bcl-2 (protein data bank [PDB] ID:1G5M), Bax (PDB ID: 4S0O), Caspase-9 (PDB ID: 1NW9), Cyclin D1complexed with CDK4 (PDB ID: 2W99) was downloaded from PDB, removed heteroatoms and added polar hydrogen atoms using Biovia Discovery Studio Visualizer version 4.5 (Dassault Systemes) and saved for docking analysis.
2.10. Molecular Docking
Molecular docking of receptors (macromolecule) and ligand was done on PyRx version 0.9 through the Vina wizard program. Based on the compound’s absorption, distribution, metabolism, excretion, and toxicity (ADMET), which play key roles in drug discovery and development ADMET analysis, three flavonoids (isorhamnetin, herbacetin, and phloretin) were selected. The 3D structure in SDF format for isorhamnetin, phloretin, and herbacetin was downloaded from PubChem and converted to pdbqt format using the extension Openbabel available in PyRx for molecular docking. Hence, after the prediction of the binding site and the docked complexes were visualized through Biovia Discovery Studio Visualizer version 4.5 (Dassault Systemes).
2.11. Statistical Analysis
Results have been expressed as the mean ± standard error means of triplicate analysis. Statistical comparisons were performed using the one-way analysis of variance, and Tukey’s multiple comparisons were used to determine the difference between the means of various samples in GraphPad Prism version 8.4.2 (GraphPad Software, La Jolla, California, USA, http://www.graphpad.com/). Differences were considered significant at *P < 0.05, **P < 0.01 ***P < 0.001. The correlation coefficient (r2) between the parameters tested was established by regression analysis.
3. RESULTS AND DISCUSSION
3.1. Quantitative Phytochemical and Antioxidant Assay
A total of four extracts have been evaluated in this study. Table 1 indicates the values of the total phytochemical analysis of these extracts. Flavonoid content is higher in cladode methanolic extract (226.09 ± 0.15 μg/quercetin equivalent/mg) P < 0.001, while fruit aqueous has more flavonoid content than its methanolic fraction. Total phenols were also higher in cladode methanolic extract (289 ± 0.05 μg/gallic acid equivalent/mg), P < 0.001 while phenolic content was greater in fruit methanolic than in aqueous extract. The DPPH assay was used to calculate the extracts’ capacity to scavenge-free radicals. The assay for determining the antioxidant capacity of plant extracts and chemicals is particularly quick and sensitive. DPPH is a nitrogen-centered, dark violet-colored powder that is stable and undergoes a color change to yellow upon reduction [23]. As the antioxidant quenches, the DPPH radicals by donating hydrogen, the decrease in the intensity of color depends on the antioxidant’s ability to scavenge free radicals. The presence of phenolics and flavonoids endows the plant with the ability to scavenge free radicals [24]. In the current study, fruit methanolic extract (IC50 205.9 μg/mL) followed by methanolic cladode extract (IC50 260.8 μg/mL) showed good scavenging activities against DPPH. The potassium ferricyanide reduction method was used to evaluate the reducing power of plant extract. By donating hydrogen, an antioxidant in the test sample converts iron (Fe+3) to ferrous (Fe+2), and the yellow color of the reaction mixture turns green [25]. The reducing power was higher in aqueous extracts of both the plant parts [Figure 2]. Overall results indicate good antioxidant potential and a good indication of its possible effects on the body. There have been reports of high flavonoid and phenolic contents in seeds, cladode, and other plant parts in O. ficus-indica, Opuntia albicarpa, Opuntia stricta, and others [26-28]. However, this study is the first report on the phytochemical understanding of O. elatior. Also, fewer studies have evaluated the fruit content, and with O. elatior, the main focus is an in-depth understanding of the fruit and cladode phytochemical analysis.
Table 1: TFC, TPC, DPPH scavenging activity, FRAP activity of parts of Opuntia elatior plant.
| Samples | TFC±SEM (µg Qtn/mg) | TPC±SEM (µg GAE/mg) | DPPH IC50( µg/mL) | FRAP (µg GAE/mg) |
|---|---|---|---|---|
| Fruit methanolic extract | 87.7±0.02 | 182±0.21 | 205.9 | 234.2±0.09 |
| Cladode methanolic extract | 226.09±0.15 | 289±0.05 | 260.8 | 105±0.1 |
| Fruit aqueous extract | 97.7±0.15 | 134±0.0 | 377.2 | 80.5±0.05 |
| Cladode aqueous extract | 102.45±0.02 | 169±0.03 | 575.6 | 71.13±0.08 |
Data are presented as mean±SEM, with n=3. SEM: Standard error mean, TFC: Total flavonoid content, TPC: Total phenolic content; DPPH: 2,2-diphenyl-1-picrylhydrazyl, FRAP: Ferric reducing antioxidant power, GAE: Gallic acid equivalent, Qtn: Quercetin equivalent.
 | Figure 2: Quantitative estimation of phytoconstituents. Data are presented as mean ± standard error mean, with n = 3. (a) Total phenol content, (b) Total flavonoid content, (c) DPPH assay, (d) FRAP assay. % Free radical scavenging activity was measured at a concentration of 100–1000 μg/mL. DPPH: 2,2-diphenyl-1-picrylhydrazyl, FRAP: Ferric reducing antioxidant potential, GAE: Gallic acid equivalent, Qtn: Quercetin. Equivalent. Statistical difference was analyzed using the one-way analysis of variance. *P < 0.05, **P < 0.01 ***P < 0.001, ns: Non-significant. [Click here to view] |
3.2. Identification of Bioactive by HR-LCMS
To characterize the secondary metabolites, present in all four extracts, HR-LCMS analysis was carried out, and the results have been indicated in positive and negative ionization modes in Tables 2-5. Each table consists of a list of phyto molecules identified by this analysis in the spectrum of alkaloids, flavonoids, phenolic, glycoside, and terpenoid classes. It is very interesting to note that in methanolic fruit extract, the analysis revealed the presence of 17 novel compounds identified and reported for the 1st time. There are a total of 18 phytocompounds in cladode methanolic and 4 in cladode aqueous extract that have been reported in this study for the first time. Over and above these novel listings of phyto molecules, we have also found some compounds similar to those in O. ficus-indica, such as epicatechin and neogrifolin. Compounds such as quercetin, apigenin, isorhamnetin, and myricetin show similar expressions to that observed in O. ficus-indica [29,30]. There are some reports on the presence of phenolic acids such as luteolin and kaempferol in the fruits of Opuntia humifusa [31]. However, O. elatior has a different and novel accumulation being reported here, and this characterization could be an outcome of its environmental interaction and specific geographical location. There is often a lack of standardization in phytotherapeutics studies, including variations in the composition and purity of plant extracts, making it challenging to compare results between different studies. While some phytotherapeutics compounds have shown promise in preclinical studies, there is limited clinical data on their safety and efficacy in humans. This makes it challenging to translate these findings into clinical practice. The unidentified phytochemicals from O. elatior that were cataloged for their potential bioactivity represent innovative natural therapies that could be used to address different health issues.
Table 2: High-performance liquid chromatography with tandem mass spectrometry (MS/MS) analysis of fruit methanolic extract.
| Fruit methanolic extract | ||||
|---|---|---|---|---|
| Identified compound name | Class of Compound | Molecular weight | Room temperature (min) | m/z |
| Retronecine^ | Pyrrolizidine alkaloid | 155.0932 | 1.178 | 156.1005 |
| beta-Solamarine^ | Steroid alkaloids | 867.4946 | 7.673 | 868.5019 |
| Quercetin* | Flavonols | 302.0405 | 5.29 | 303.0477 |
| (+)-Sophorol^ | Iso flavanones | 300.0613 | 9.107 | 301.0686 |
| Glyurallin B^ | Iso flavanones | 422.1711 | 4.653 | 421.1645 |
| Vicinin 2^ | Flavones | 626.1505 | 5.215 | 625.1432 |
| Myricitrin*^ | Flavonols | 464.097 | 5.928 | 463.0897 |
| Phloretin*^ | Dihydrochalcones (Flavonoid) | 274.0846 | 10.457 | 297.074 |
| Cynaroside^ | Flavones | 448.1024 | 6.42 | 447.0948 |
| Herbacetin^ | Flavonols | 302.0438 | 7.862 | 301.0365 |
| Isorhamnetin*^ | Flavonols | 316.0592 | 8.176 | 315.0518 |
| Luteolin*^ | Flavones | 286.0485 | 8.812 | 285.0413 |
| Isokaempferide*(Apigenin)^ | Trihydroxy flavone | 300.0643 | 9.14 | 299.0571 |
| Paratrifluoromethylphenol^ | Phenol | 162.03 | 4.168 | 163.0372 |
| 5-Hydroxyferulate*#^ | Phenylpropanoids | 210.0532 | 2.829 | 255.0511 |
| Chlorogenic acid*#^ | Phenylpropanoids | 354.0958 | 4.136 | 353.0884 |
| Idoxanthin^ | Carotenoid | 598.4004 | 20.062 | 597.3953 |
| 3-Hydroxy-b, e-caroten-3’- one^ | Carotenoid | 566.4108 | 20.654 | 611.4112 |
| Vernolepin^ | Sesquiterpenoids | 276.0999 | 9.27 | 299.0891 |
| Hemigossypolone^ | Monoterpenoid | 274.0844 | 10.48 | 297.0736 |
| Euphornin^ | Diterpenoids | 584.2999 | 19.174 | 607.2893 |
| Veranisatin C^ | Terpene lactone | 372.1065 | 5.297 | 371.0991 |
| Dihydrocaffeic acid 3-O-glucuronide*#^ | Phenolic glycoside | 358.0882 | 1.078 | 381.0775 |
| Osmaronin^ | Cyanogenic glycosides | 259.1039 | 1.18 | 260.1112 |
| Lotaustralin^ | Cyanogenic glycosides | 261.1196 | 1.18 | 262.1267 |
| Isorhamnetin 3-rutinoside 4’- rhamnoside^ | Glycoside | 770.2297 | 5.606 | 769.2225 |
| D-Linalool 3-glucoside^ | Glycoside | 316.1899 | 6.84 | 361.1882 |
* Compounds also reported in Opuntia ficus-indica fruits.
# Compounds also reported in Opuntia humifusa. ^Compounds reported in Opuntia elatior Mill. for the 1st time.
Table 3: High-performance liquid chromatography and MS/MS analysis of CM extract.
| CM extract | ||||
|---|---|---|---|---|
| Identified compound name | Class of compound | Molecular weight | Room temperature (min) | m/z |
| Retronecine^ | Pyrrolizidine alkaloid | 155.0932 | 1.152 | 156.101 |
| Bellendine^ | Tropane | 205.109 | 3.898 | 206.116 |
| Baptifoline^ | Quinolizidine | 260.1508 | 5.059 | 261.158 |
| Irinotecan^ | Quinoline alkaloids | 586.2788 | 16.984 | 609.268 |
| Senampeline A^ | Pyrrolizidine alkaloid | 473.2084 | 5.8 | 532.224 |
| Ritterazine A^ | Steroidal alkaloid marine | 912.5407 | 20.31 | 971.5569 |
| 4’-O-methyl-(-)-epicatechin-3’-O-beta-glucuronide*#^ | Flavanols | 494.1445 | 6.755 | 493.137 |
| Isorhamnetin*^ | Flavanols | 316.0594 | 8.219 | 315.052 |
| Luteolin*^ | Flavones | 286.0485 | 8.85 | 285.041 |
| Catechin*#^ | Flavan 3-ols | 290.0796 | 8.936 | 313.069 |
| 5-Hydroxyferulate*#^ | Phenolic | 210.0531 | 2.114 | 255.051 |
| 3-cis-Hydroxy-b, e-Caroten-3’- one^ | Carotenoid | 550.4139 | 19.676 | 551.422 |
| Hemigossypolone^ | monoterpenoid | 274.0843 | 10.827 | 297.074 |
| Isotenulin^ | Sesquiterpenoids | 306.1472 | 11.816 | 329.137 |
| Nigakilactone B^ | Triterpenoids | 392.2199 | 11.881 | 415.209 |
| Euphornin^ | Diterpenoids | 584.2993 | 19.309 | 607.289 |
| Microlenin^ | Sesquiterpenoids | 494.2306 | 6.287 | 539.229 |
| Cucurbitacin P^ | Triterpenoids | 520.3273 | 7.842 | 519.32 |
| Geranylfarnesyl diphosphate^ | Polyterpenoids | 518.2574 | 15.96 | 577.272 |
| Methyl salicylate O-(rhamnosyl-[1->6]-glucoside)^ | Glycoside | 460.1595 | 4.859 | 459.152 |
| D-Linalool 3-glucoside^ | Glycoside | 316.19 | 6.823 | 361.188 |
CM: Cladode methanolic,
* Compounds also reported in Opuntia ficus-indica fruits.
# Compounds also reported in Opuntia humifusa. ^Compounds reported in Opuntia elatior Mill. for the 1st time.
Table 4: High-performance liquid chromatography and MS/MS analysis of FA extract.
| FA extract | ||||
|---|---|---|---|---|
| Identified compound name | Class of compound | Molecular weight | Room temperature (min) | m/z |
| Ibutilide^ | Amino compound | 384.2479 | 4.581 | 407.237 |
| Nigakilactone B^ | Quassinoid | 392.2207 | 11.877 | 415.21 |
| Militarinone A^ | Pyridine alkaloid | 459.26 | 11.895 | 460.267 |
| Euphornin^ | Diterpenoid | 584.2996 | 18.434 | 607.289 |
| Oryzanol^ | Triterpenoid | 402.3138 | 19.041 | 405.302 |
| Leonoside A^ | Glycoside | 770.2535 | 5.635 | 769.246 |
| Hydroxy-24-epi-brassinolide^ | Steroid | 496.3341 | 7.846 | 541.334 |
| 3-O-Methylniveusin A^ | Polyphenol | 408.1797 | 12.564 | 407.177 |
| Ajmaline^ | Indole alkaloid | 326.2041 | 13.307 | 325.197 |
FA: Fruit aqueous, ^ Compounds reported in Opuntia elatior Mill. for the 1st time.
Table 5: High-performance liquid chromatography and MS/MS analysis of CA extract.
| CA extract | ||||
|---|---|---|---|---|
| Identified compound name | Class of compound | Molecular weight | Room temperature (min) | m/z |
| Epicatechin 3’-O-glucuronide^ | Flavanols | 466.109 | 3.094 | 489.08 |
| Diferuloylspermidine^ | Cinnamic acid derivative | 497.250 | 9.344 | 498.5 |
| Hypericin^ | Anthraquinone | 504.084 | 1.68 | 549.0 |
| Neogrifolin^ | Sesquiterpenoid | 328.237 | 7.847 | 327.2 |
CA: Cladode aqueous, ^Compounds reported in Opuntia elatior Mill. for the 1st time.
3.3. O. elatior Extracts Alter the Cell Viability and Growth Kinetics of the MCF-7 Cell Line
MTT assay and dose-dependent analysis were performed to evaluate this plant’s antiproliferative potential. The dose-based assay was done for three periods at 24, 48, and 72 h, respectively. The plant extracts have been evaluated on human breast cancer cell line MCF-7. The dose-time assay showed the most impressive results for aqueous fruit extract at 24 h. There is also a clear inhibition of cells with decreasing cell survival on dose increase. At 48 and 72 h, all four extracts show good control over the proliferation of cells. The results of the MTT assay have been indicated in terms of their IC50 values generated from their respective graphs [Figure 3]. At 24 h, both aqueous and methanolic fruit extracts indicate potency at lower doses, which advocates for their safe use in drug formulation.
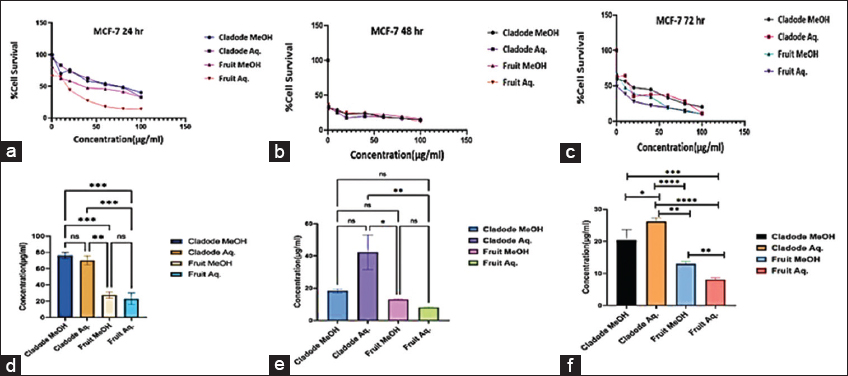 | Figure 3: Antiproliferative effect of plant extracts on human breast cancer cell line MCF-7. (a-c) Dose-response curve of plant extract treatment on MCF-7 cell line at the indicated concentration (0.5–100 μg/mL) for 24, 48, and 72 h respectively. (d-f) IC50 concentration was derived from the graph of growth inhibition against concentration (μg/mL) from the MTT assay. Statistical difference was analyzed using the one-way analysis of variance. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns: Non-significant. [Click here to view] |
Similarly, at 48 and 72 h, a similar trend of lower dose efficacy is seen. As shown in Figure 3, fruit aqueous extract shows cytotoxicity at lower concentrations after 72 h of incubation as compared to fruit methanolic extract (P < 0.01). The cladode methanolic extract exhibited cytotoxicity at a lower concentration after 72 h of incubation as compared to the cladode aqueous extract (P < 0.05). Interestingly, the normal cell line was not affected by extracts [Figure 4]. These results are the first reports; encouraging in-depth evaluation in future studies. To date, there are reports of antiproliferative activity on leukemic cell lines only [12]. Both fruit and cladode have been reported to be effective for species other than O. elatior. However, these studies support the current findings and pave the way for future experimentation. To further investigate the anticancer potential, scratch assay, and colony formation assays were performed. Scratch assay helps evaluate the anti-migratory action in vitro in real time and serves as an important characterization of plant extract for its anticancer capabilities. The wound healing (scratch) assay results as given in Figures 5 and 6, clearly support the anti-migration capability of fruit and cladode extracts. It is observable that the wound is almost healed at 72 h in Doxorubicin-treated cells, while the wound healing is even faster in the untreated control group. Compared to these groups, the most impressive outcomes are observable for fruit methanolic extract and the extract of cladode. The wound is not healed even at 72 h, suggesting their anti-migration capabilities. For the colony formation assay, the ability to colonize and grow has been investigated by incubating the cells with different plant extracts. Interestingly, all four extracts have shown better control when compared to Doxorubicin treated and untreated groups. Out of all the four extracts, the most significant action (P < 0.001) is observed for cladode aqueous extract when compared to untreated cells [Figure 7]. These results are proof of the anticancer potential of O. elatior, and there is a need to evaluate them in greater depth to find newer therapeutic options for breast cancer and metastatic progression.
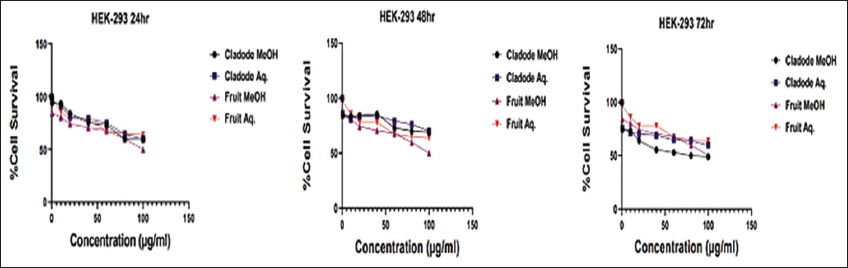 | Figure 4: Safety of plant extracts on normal cell line HEK-293. Dose-response curve of plant extract treatment on HEK-293 cell line at the indicated concentration (0.5–100 μg/mL) for 24, 48, and 72 h, respectively. [Click here to view] |
 | Figure 5: Anti-migratory effect of plant extracts on human breast cancer cell line MCF-7. Representative images were taken by inverted microscope for 0, 6, and 12 h at ×40 magnification. (a) Fruit aqueous extract – 8.1 μg/mL, (b) Fruit methanol extract – 12.9 μg/mL, (c) Cladode aqueous extract – 26.2 μg/mL, (d) Cladode methanol – 20.4 μg/mL, (e) Doxorubicin treated cells, (f) Untreated cells. [Click here to view] |
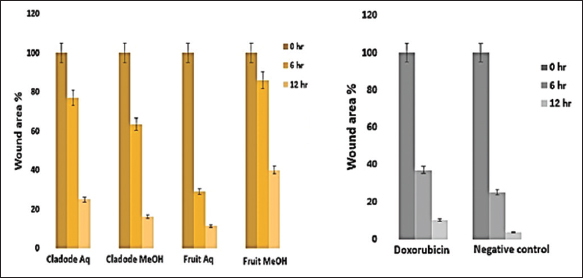 | Figure 6: Effect of plant extracts on wound area percentage. Representative images show wound area percentage at 0, 6, and 24 h for the plant extract analyzed using ImageJ software. Doxorubicin is positive control and 0.2% dimethyl sulfoxide treated cell is negative control. The data are presented as mean ± standard error mean with n = 3. [Click here to view] |
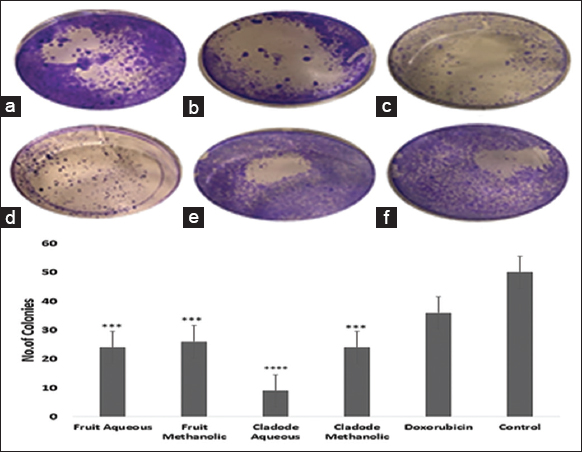 | Figure 7: Effect of plant extracts on MCF-7 cell line. The cells were grown in 6-well plates and treated with IC50 concentration of the plant extracts. (a) Fruit methanolic extract, (b) Fruit aqueous extract, (c) Cladode methanolic extract, (d) Cladode aqueous extract, (e) Dox orubicin, (f) Control. The number of colonies was calculated using imageJ. ***P < 0.05, ****P < 0.001. [Click here to view] |
3.4. Molecular Docking
Based on the promising activity of all four extracts in vitro assays, further investigation was done by detailing phyto molecular understanding using in silico techniques. To decrease the probability of choosing a false positive compound, drug likeliness properties were determined using the Lipinski filter, taking into account the compound’s adsorption, distribution, metabolism, excretion, and toxicity. When logA exceeds 5, molecular weight exceeds 500, the number of N, O (hydrogen bond receptors) exceeds 10, the number of −OH and −NH (hydrogen bond donors) exceeds 5, and the number of rotatable bonds (rotb) exceeds 15, Lipinski’s rule of five is violated [32]. Three important flavonoids isorhamnetin, phloretin, and herbacetin demonstrated zero violations of the range’s physiochemical parameters and successfully complied with Lipinski’s rule of five as stated in [Table 6]. This prediction was based on the findings of medicinal plant lead prediction through the SWISSADME tool [Table 7]. These three lead molecules were then subjected to molecular docking for their binding capabilities of Bcl-2, Bax, Caspase 9, and Cyclin D1 complexed with CDK4. The results of these docking analyses are presented in Table 8 and Figures 8-11. The best docking results of all three isorhamnetin, phloretin, and herbacetin were towards the Bax with a strong binding affinity of −7.8, −7.3 and −7.8, respectively. This suggests that the phytomolecules could be a potential inhibitor, which would lead to the apoptotic process in breast cancer and need for further investigation to evaluate the outcomes of these studies for future drug designing. Such studies have not been reported for the O. elatior species.
Table 6: The pharmacokinetic properties of the selected phytocompounds were performed using SwissADME online tool.
| Compound name | Intestinal absorption (human) (% absorbed) | BBB permeability (log BB) | CYP2D6 substrate (Yes/No) | CYP2D6 inhibitor | Total clearance (log mL/min/kg) | AMES toxicity (Yes/No) | Oral rat acute toxicity (LD50) (mol/kg) | Oral rat chronic toxicity (LOAEL) (log mg/kg bw/day) | Hepatotoxicity (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|
| Isorhamnetin | 76.014 | −1.135 | No | No | 0.508 | No | 2.407 | 2.499 | No |
| Phloretin | 60.5 | −0.927 | No | No | 0.213 | No | 2.381 | 3.318 | No |
| Herbacetin | 87.188 | −1.006 | No | No | 0.232 | No | 2.585 | 1.89 | No |
Table 7: Drug-likeness prediction for flavonoid of Opuntia elatior Mill. fruits by SwissADME.
| Compound name | Molecular weight | Log P | Rotatable bonds | Acceptors | Donors | Surface area |
|---|---|---|---|---|---|---|
| Isorhamnetin | 316.265 | 2.291 | 2 | 7 | 4 | 128.792 |
| Phloretin | 274.272 | 2.3245 | 4 | 5 | 4 | 114.922 |
| Herbacetin | 422.477 | 5.2998 | 5 | 6 | 4 | 179.584 |
Table 8: Binding affinity energy of phytocompounds on target.
| Ligand | Target | Binding affinity energy (Kcal/mol) | H-Bond interactions |
|---|---|---|---|
| Isorhamnetin | Bcl-2 | −7.4 | GLU A: 14, LYS A: 17, HIS A: 20 |
| Bax | −7.8 | ALA A: 42, ARG A: 134 | |
| Caspase 9 | −7.3 | GLY A: 306, TYR A: 324 | |
| Cyclin D1 | −7 | ARG A: 87, LEU A: 148 | |
| Phloretin | Bcl-2 | −6.9 | ASP A: 10 |
| Bax | −7.3 | PRO A: 130, ALA A: 35 | |
| Caspase 9 | −6.3 | GLY A: 306 | |
| Cyclin D1 | −6.6 | No hydrogen bonds | |
| Herbacetin | Bcl-2 | −7.5 | ASP A: 196 |
| Bax | −7.8 | GLN A: 32, LEU A: 47, ALA A: 42 | |
| Caspase 9 | −7.3 | TYR A: 324, GLY A: 306 | |
| Cyclin D1 | −6.9 | THR A: 120 |
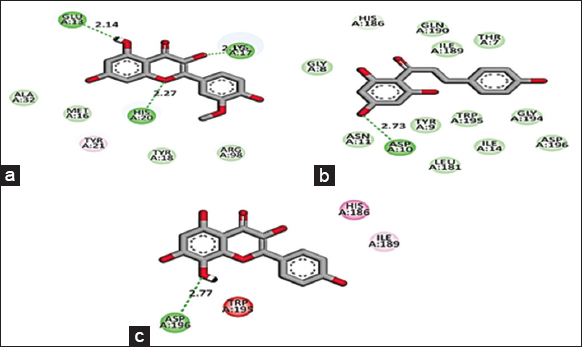 | Figure 8: 2D Molecular interaction of Bcl-2 with (a) Isorhamnetin, (b) Phloretin, (c) Herbacetin. The green colour interactions represent covalent hydrogen bonds with amino acids of receptors. [Click here to view] |
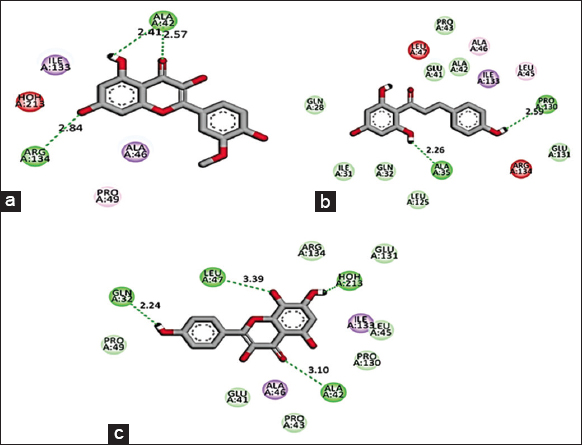 | Figure 9: 2D Molecular interaction of Bax with (a) Isorhamnetin, (b) Phloretin, (c) Herbacetin. The green colour interactions represent covalent hydrogen bonds with amino acids of receptors. [Click here to view] |
 | Figure 10: 2D Molecular interaction of Caspase 9 with (a) Isorhamnetin, (b) Phloretin, (c) Herbacetin. The green colour interactions represent covalent hydrogen bonds with amino acids of receptors. [Click here to view] |
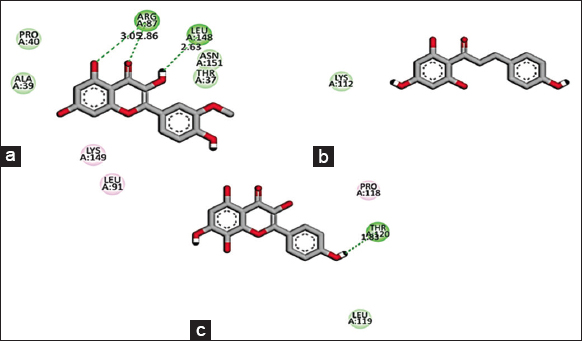 | Figure 11: 2D Molecular interaction of Cyclin D1 complexed with CDK4 with (a) Isorhamnetin, (b) Phloretin, (c) Herbacetin. The green colour interactions represent covalent hydrogen bonds with amino acids of receptors. [Click here to view] |
4. CONCLUSION
This study has presented some novel findings for O. elatior through a systematic in-depth analysis of its phytochemistry, in vitro capabilities of antiproliferative, anti-migratory, and anticancer capabilities. More than 20 phytocompounds are reported for the 1st time, and flavonoid fraction has been a good line for future studies based on the in silico findings presented herein. Further, the in vitro assays of all four extracts on the MCF-7 human breast cancer cell line have shown promising and significant antiproliferative action that makes this plant and its phytoconstituents an important candidate for drug discovery as well as adjuvant therapy for existing anticancer drugs for breast cancer. Another significant observation is the potent efficacy of aqueous fruit extract, which makes it a suitable candidate for anticancer formulations and use. There is also an indication of cell cycle and anti-apoptotic pathway regulation, as revealed by the in silico studies. This will be further investigated in our upcoming studies. Overall, this plant needs to be considered for its anticancer candidature, and the present study presents the first evidence in that direction.
5. ACKNOWLEDGMENT
The author(s) would like to thank the School of Science, Navrachana University, Vadodara, for providing Instrumentation facilities. We thank Sophisticated Analytical Instrument Facility SAIF (IIT Bombay) for providing the HR-LCMS analysis report.
6. AUTHORS’ CONTRIBUTIONS
FP contributed to the plant material collection, extraction, in vitro and in silico assay. KU participated in an in-vitro assay. DM participated in the HRLCMS/MS analyses and interpretation of the data. ER participated in the formal analysis. AVR reviewed the draft and DB designed the study and finalized the draft. FP wrote the first draft of the manuscript, and all authors commented on this version. All authors have read the final manuscript and approved the submission.
7. FUNDING
There is no funding for the article.
8. CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVAL
This study does not involve any human or animal experimental subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included in this research article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021;127:3029-30. [CrossRef]
2. Chari RV. Targeted cancer therapy:Conferring specificity to cytotoxic drugs. Acc Chem Res 2008;41:98-107. [CrossRef]
3. Itankar PR, Sontakke VA, Tauqeer M, Charde SS. Antioxidant potential and its relationship with polyphenol content and degree of polymerization in Opuntia elatior Mill. fruits. Ayu 2014;35:423-7. [CrossRef]
4. Butera D, Tesoriere L, Di Gaudio F, Bongiorno A, Allegra M, Pintaudi AM, et al. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains:Betanin and Indica xanthin. J Agric Food Chem 2002;50:6895-901. [CrossRef]
5. Bhatt MR, Nagar PS. Evaluation of physicochemical property and fatty acid composition of Opuntia elatior seed oil. J Prof Assoc Cactus Dev 2013;15:13-9.
6. Abbas EY, Ezzat MI, El Hefnawy HM, Abdel-Sattar E. An overview and update on the chemical composition and potential health benefits of Opuntia ficus-indica (L.) Miller. J Food Biochem 2022;46:e4310. [CrossRef]
7. Patel S. Opuntia cladodes (nopal):Emerging functional food and dietary supplement. Med J Nutr Metab 2014;7:11-9. [CrossRef]
8. Park E, Edirisinghe I. In vitro antiproliferative and antioxidant properties of indigenous plant extracts from the Midwestern United States. J Agric Food Chem 2011;59:10008-15.
9. Prajapati S, Harisha CR, Acharya RN. Pharmacognostic evaluation of stem of Opuntia elatior Mill. (Nagaphani). Eur J Biomed Pharm Sci 2015;2:351-7.
10. De Leo M, De Abreu MB, Pawlowska AM, Cioni PL, Braca A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC-PDA-ESI-MS and GC/EIMS analyses. Phytochem Lett 2010;3:48-52. [CrossRef]
11. Tomas-Barberan FA, Andres-Lacueva C. Polyphenols and health:Current state and progress. J Agric Food Chem 2012;60:≅-5. [CrossRef]
12. Narayankar CU, Mane M, Patil R, Satpute AM, Gaikwad S, Gaikwad DK. Inhibition of proliferation of K-562 human blood cancer cell due to Opuntia elatior fruit extract. J Sci Technol 2021;6:2456-660.
13. Chauhan SP, Sheth NR, Suhagia BN. Analgesic and anti-inflammatory action of Opuntia elatior mill fruits. J Ayurveda Integr Med 2010;6:75-81. [CrossRef]
14. Daniel M, Mammen D. Analytical Methods for Medicinal Plants and Economic Botany. India:Scientific Publishers;2016.
15. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999;64:555-9. [CrossRef]
16. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144-58. [CrossRef]
17. Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995;28:25-30. [CrossRef]
18. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power“:The FRAP assay. Anal Biochem 1996;239:70-6. [CrossRef]
19. Sowmya TN, Raveesha KA. Polyphenol-rich purified bioactive fraction isolated from Terminalia catappa L.:UHPLC-MS/MS-based metabolite identification and evaluation of their antimicrobial potential. Coatings 2021;11:1210. [CrossRef]
20. Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 1991;3:207-12. [CrossRef]
21. Liang CC, Park AY, Guan JL. In vitro scratch assay:A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2:329-33. [CrossRef]
22. Franken NA, Rodermond HM, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315-9. [CrossRef]
23. Chanda S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties:An overview. Afr J Microbiol Res 2009;3:981-96.
24. Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 2013;21:143-52. [CrossRef]
25. Wang J, Hu S, Nie S, Yu Q, Xie M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev 2016;2016:5692852. [CrossRef]
26. Zeghbib W, Boudjouan F, Vasconcelos V, Lopes G. Phenolic compounds'occurrence in Opuntia species and their role in the inflammatory process:A review. Molecules 2022;27:4763. [CrossRef]
27. del Socorro Santos Díaz M, de la Rosa AP, Héliès-Toussaint C, Guéraud F, Nègre-Salvayre A. Opuntia spp.:Characterization and benefits in chronic diseases. Oxid Med Cell Longev 2017;2017:?249.
28. Kunyanga CN, Vellingiri V, Imungi KJ. Nutritional quality, phytochemical composition and health protective effects of an under-utilized prickly cactus fruit (Opuntia stricta Haw.) collected from Kenya. Afr J Food Agric Nutr Dev 2014;14:9561-77. [CrossRef]
29. El-Hawary SS, Sobeh M, Badr WK, Abdelfattah MA, Ali ZY, El-Tantawy ME, et al. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J Biol Sci 2020;27:2829-38. [CrossRef]
30. Slimen IB, Mabrouk M, Hanène C, Najar T, Abderrabba M. LC-MS analysis of phenolic acids, flavonoids and betanin from spineless Opuntia ficus-indica fruits. Cell Biol 2017;5:17-28. [CrossRef]
31. Cha MN, Jun HI, Lee WJ, Kim MJ, Kim MK, Kim YS. Chemical composition and antioxidant activity of Korean cactus (Opuntia humifusa) fruit. Food Sci Biotechnol 2013;22:523-9. [CrossRef]
32. Mishra H, Singh N, Lahiri T, Misra K. A comparative study on the molecular descriptors for predicting drug-likeness of small molecules. Bioinformation 2009;3:384-8. [CrossRef]