1. INTRODUCTION
The demand for medicinal plants has increased recently because they play an important role in ecosystems. Medicinal plants contain different compounds such as phenols, antioxidants, and other secondary metabolites, and therefore have long been used in alternative treatments for modern medicines [1]. Antioxidants are chemicals or substances that scavenge free radicals and protect cells from their harmful effects [2]. Superoxide radicals, hydrogen peroxide, and hydroxyl radicals are examples of free radicals that have adverse effects on almost all biochemical compounds such as proteins, DNA, and carbohydrates [2,3]. Antioxidant compounds are becoming increasingly important in medicinal chemistry because they perform a protective role by scavenging free radicals and inhibiting the oxidation of molecules [4]. Some fruits and vegetables contain antioxidant compounds, vitamin E, vitamin C, and carotenoid [5]. Phenols and flavonoids are the most common antioxidant compounds found in plants [6]. Phenolic compounds are biological molecules that have an essential role in plant growth, development, and defense. Structurally, it consists of a hydroxyl functional group with a ring structure like benzene [7]. The redox properties of phenolic molecules enhance the antioxidant activity and can play a crucial role in neutralizing free radicals and decomposing peroxides [8].
Heavy metals (HM) are divided into two categories: essential and nonessential. Fe, Mo, and Mn are critical micronutrients among these metals. While Hg, Ag, Sb, Pb, and others have no known nutritional value and may be hazardous to plants and microorganisms [9], they may be toxic to plants and microorganisms. Most HM, especially Cu and Zn, either serve as cofactor and enzyme reaction activators or exert catalytic properties such as the prosthetic group in metalloproteinase [10]. Zn is a vital micronutrient affecting several plant metabolic processes. Zn’s phytotoxicity manifests as a restriction in growth, development, and metabolism, as well as increase in oxidative damage in a variety of plants [11]. Cu is an essential micronutrient that plays a vital role in CO2 assimilation and ATP synthesis. Cu can inhibit respiration, adversely affects nitrogen and protein metabolism, decreases chlorophyll content, and inhibits photosynthetic activity in leaves [12]. Cu can catalyze the production of hydroxyl radicals from nonenzymatic reactions and alleviate oxidation stress and may enhance the antioxidant response in plants [12].
Portulaca oleracea L. is a herbaceous succulent plant that grows in temperate and tropical climates around the world and is used to treat bug bites, dermatitis, and ulcers in traditional Chinese medicine [11,13]. It has the advantage of containing a high amount of ascorbic acid, α-linolenic acid, β-carotene, proteins, carbohydrates, and minerals such as Na, Ca, Zn, and K [13,14]. The leaves and stems are among the edible parts, as they have a slightly sour taste similar to the taste of spinach. Portulaca oleracea is an abundant source of omega-3 fatty acids and antioxidant compounds, particularly flavonoids [15]. This plant is used in many countries as a diuretic, febrifuge, antiseptic, antispasmodic, and vermifuge [11,14].
The enzyme polyphenol oxidase (PPO) is considered a copper-containing enzyme that oxidizes phenolic molecules to o-quinine [16,17]. Tyrosinase, catechol oxidase, and laccase are the three primary forms of PPO found in plants, and they are categorized based on their method of action and substrate specificity [17].
Because of contamination of the environment by some HM and their accumulation in all parts of plants and the cause of harmful effects on plant growth and development, it is necessary to characterize some plant extracts and study the effects of some HMs on some enzyme kinetic parameters. Therefore, the main aim of this study was to measure and analyze the total amounts of phenols and proteins in the crude purslane extract and evaluate its antioxidant activity and to investigate the browning intensity and Zn and Cu’s effects on the enzyme kinetics (Vmax and Km) of PPO in purslane extract.
2. MATERIALS AND METHODS
2.1. Preparation of Crude Plant Extract
The plant samples of purslane were obtained from Mutah city markets (Al-Karak, Jordan) between February and July 2020. Fifty grams of fresh aerial parts of the plant was homogenized with the addition of phosphate buffer (pH 7.0) solution in an electronic blinder machine for 3–5 minutes. The obtained solution was primarily purified by filtration using cloth sheets and filter papers. The solution was homogenized by centrifugation at 1,500 rpm for 10–15 minutes (Jepson Bolton Co. Ltd., UK). Then, the upper layer (supernatant) was collected and kept at 4°C as crude purslane extract [18].
2.2. Total Proteins
The total proteins in the crude extract of purslane were determined using the Lowry method. The calibration standard curve was created using gradient concentrations (0.07–2.4 mg/ml) of the standard bovine serum albumin [19].
2.3. Determination of Total Phenols
The total amount of phenols was analyzed by using the Folin–Ciocalteu method [20]. Gradient concentrations of the gallic acid (as standard) from 0.5 to 4.0 mM were used to obtain the calibration standard curve. Briefly, the mixture contains 200 μl of 10% of Folin–Ciocalteu reagent, 100 ml of crude extract of purslane in phosphate buffer (pH 7.0), and 800 ml of 700 mM Na2CO3. The mixture was incubated for 30 minutes at room temperature. Then, the optical densities were read at 760 nm (Biotech Engineering Management Co. Ltd., UK). The total phenolic amounts were expressed as equivalents of the gallic acid in milligram per gram of the sample extract.
2.4. Determination of the Antioxidant Activity
The colorimetric method of Bal et al. [21] was used to explore the efficiency of the purslane extract to reduce the radical of 2,2-diphenyl-1-picrylhydrazyl (DPPH). The reaction mixture consists of 2.0 ml of DPPH solution (60 μM in methanol) and 50 μl of purslane extract. After incubation at room temperature for 30 minutes, the optical densities were read against methanol solution as a blank sample at 520 nm. The positive control used was gallic acid. Antioxidant activity (%) = (Ac − As) × 100%, where Ac is the control and As is the sample.
2.5. Browning Intensities
For each sample, a cuvette was filled with 1.5 ml of freshly prepared enzyme solution. The optical density (absorbance) was measured at a wavelength of 410 nm by a spectrophotometer at intervals of 20 for 100 minutes. The absorbance was taken as the browning intensity [18].
2.6. The Effects of Substrate Concentrations on PPO Activity
The PPO activity was measured using different concentrations (0.01–0.2 M) of the substrate catechol at pH 6.0 and temperature 40°C. The sample tube contained 2.0 ml of desired concentration of catechol (0.01–0.2 M), 0.1 ml of enzyme solution, and 0.9 ml of 0.2 M phosphate buffer. The absorbance was read at 410 nm against a blank tube containing only the catechol and buffer. One unit of PPO activity was defined as the change in absorbance of 0.001 minute−1 [22].
2.7. The pH and Temperature Effect of pH on PPO Activity
The influence of pH on PPO activity was tested using acetate and phosphate buffer at varying pH values (3, 4, 5, 6, 7, 8, and 9). The effects of temperature in the range of 25°C to 100°C (25°C, 40°C, 60°C, 80°C, and 100°C) were utilized to establish the optimum temperature under standard conditions of catechol concentration and optimum pH value [18,22]. These enzymes’ reaction rates were measured, and the relative activity (%) was calculated.
2.8. Enzyme Kinetics
The kinetic parameters values (Km and Vmax) of PPO were analyzed and determined at different concentrations of catechol. The PPO activity was monitored at 410 nm, and the Km and Vmax values were obtained from the Lineweaver-Burk plot.
Fresh stock solutions of Cu sulfate and Zn sulfate (0.1 and 0.3 M) were prepared. The reaction mixture contained 2.0 ml of catechol (20 mM), 0.4 ml of phosphate buffer, 0.5 ml of heavy metal solution, and 0.1 ml of enzyme solution [23].
2.9. Statistical Analysis
The experimental results were obtained in triplicate. Microsoft Excel 2016 software was used to express the data as mean ± SD, n = 3.
3. RESULTS
3.1. Total Proteins and Total Phenolic Compounds
Purslane’s crude leaves extract was tested to determine the amount of proteins in the sample. The crude extract has a concentration of of 0.847 ± 0.021 mg/ml, according to the data. The phenol content of the crude extracts of leaves of purslane was measured using gallic acid as the standard. The results showed that the crude extract of purslane has a considerable amount of phenols compared to the gallic acid as the control (107.88 mg/ml).
3.2. DPPH Radical Scavenging
The crude leaves extract of purslane was found to possess 48.8% of antioxidant power by using the DPPH radical scavenging method.
3.3. Browning Intensity
This study showed a relationship between the time and browning intensity of the crude extract of purslane. High browning intensities were found (3.0 ± 0.1) after 60 minutes of incubation, and it was turned out that the browning intensities increased over time, as illustrated in Figure 1.
3.4. Temperature and pH Effects on PPO Activity
The influence of pH on PPO activity was investigated by measuring its activity at various pH levels (3.0 to 9.0). The enzyme’s optimum activity of pH in the crude extract of purslane was seen at pH 4.0 and 6.0 (Fig. 2). The relative activities of PPO at pH 4.0 and 6.0 were 90% and 100%, respectively. The optimal temperature value of the PPO activity in the crude extract of purslane was 40°C (Fig. 3).
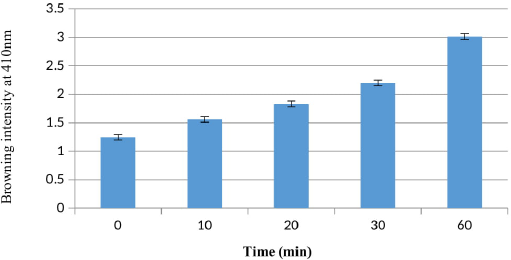 | Figure 1: Browning intensity (optical density) of crude extract of P. oleracea with time (minute) at 410 nm. Mean ± SD, n = 3. [Click here to view] |
 | Figure 2: Relative activity (%) of PPO in the crude extract of P. oleracea at different pH values. Mean ± SD; n = 3. [Click here to view] |
 | Figure 3: Relative activity (%) of PPO in the crude extract of P. oleracea at different temperature values (°C). Mean ± SD; n = 3. [Click here to view] |
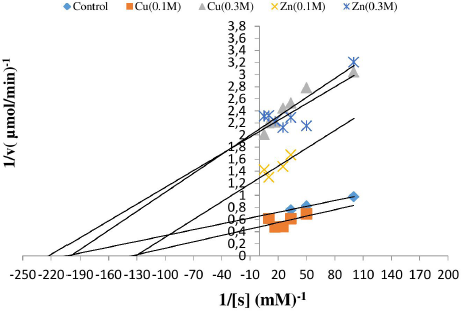 | Figure 4: Lineweaver-Burk reciprocal plot for the determination of Km and Vmax values for PPO in the crude extract of P. oleracea in the presence and absence of HM (Cu 0.1 M, Cu 0.3 M, Zn 0.1 M, and Zn 0.3 M) using catechol as a substrate. Mean ± SD, n = 3. [Click here to view] |
3.5. Analysis of Enzyme Kinetics
The effects of the presence and absence of Cu and Zn metals on PPO kinetic parameters were determined using the initial reaction rates at varying concentrations of the substrate catechol (0.01–0.2 M). The values of Km and Vmax were obtained by the Lineweaver-Burk reciprocal plot (Fig. 4). The results of Km and Vmax values of the enzyme are summarized in Table 1.
4. DISCUSSION
4.1. Characterization of the Crude Extract of Purslane
Characterization of the crude extract of purslane according to the amount of proteins was revealed to contain a low amount of proteins. In the literature, the amount of protein varies from plant to plant, with Cowpea containing 0.23 to 0.28 mg/ml [24] and Ocimum canum containing 10.59 mg/ml [25]. In addition, the results showed that the crude extract of leaves of purslane has a considerable amount of phenols compared to other plant extracts. Previous studies have shown that the amount of phenols varied between plants, such as Melilotus officinalis 289.5 mg/ml, Urtica dioica 24.1 mg/ml, and green tea 31.95 mg/ml [25].
The DPPH technique was used to evaluate the antioxidant activity of the crude leaves extract of purslane. It was shown to exhibit a moderate percentage of DPPH radical scavenging activities compared to the gallic acid as the standard. DPPH assays are the traditional assays for evaluating the antioxidant activity of food products. Literature survey indicated that the DPPH radical scavenging activities varied between plant extracts; for example, it was 31.5% in Styrax formosana 31.5% and 54.74% in Artocarpus lacucha Buch., while it was 33.03% in Hopea odorata. The amount of total phenolic compounds in the plant could explain the differential in antioxidant action. Flavor, color, and shape are three characteristics that consumers consider when purchasing vegetables and fruits. These characteristics are used to assess the quality and suitability of food. The color may be influenced by natural pigments, such as chlorophylls and carotenoids in food, or by pigments resulting from enzymatic or nonenzymatic reactions. Enzymatic browning results from PPO catalyzed oxidation of mono- and diphenols to o-quinines [4].
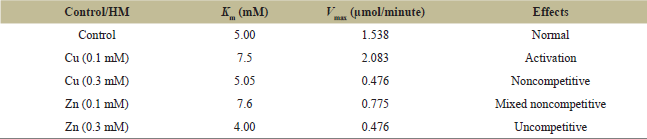 | Table 1: Kinetic values for PPO in the crude extract of P. oleracea in the presence and absence of Cu and Zn using catechol as a substrate at 40°C and pH 6. [Click here to view] |
This study showed a relationship between the time and browning intensity of the selected plant’s crude extract. High browning intensities were found in purslane after 6.0 hours of incubation (data not shown). It was illustrated that the browning intensities increased over time (60 minutes). These results could be demonstrated by the level of PPO activity and the amount of phenolic compounds that can vary between plants. The findings were consistent with those by González et al. [26], Atrooz et al. [1], and Qi et al. [27], who reported that PPO activity and phenolic content are the most important determinants in browning reactions.
4.2. Enzyme Activity and Kinetics
The enzyme’s optimum activity in the crude extract of purslane was seen at pH 4.0 and 6.0. The relative activities of PPO at pH 4.0 and 6.0 were 90% and 100%, respectively. The optimum pH depends on enzyme source and purity, substrate, and buffer system used.
In many vegetables and fruits, PPO enzyme has one optimum pH value, such as Rumex obtusifolius L. 7.0 [28] and Banana fruit 6.5 [29], but in some plants has two optimum pH values, such as lentil 4.5–5.5 [30]. Some plants have two optimum pH values due to the presence of isoenzymes [30].
It was illustrated that the optimal temperature for the PPO activity in the crude extract of purslane was 40°C. At 25°C, the relative activity was 71.8%. Raising the temperature above 45°C caused a remarkable decrease in the action due to the effect of denaturation. Thyme had an exact optimal temperature of 40°C [31], while apple juice had a higher optimum temperature of 50°C and 60°C [32], but raisins had a lower optimum temperature of 25°C [33], and eggplant fruit had a lower optimum temperature of 30°C [34]. The HM Cu and Zn’s criteria on PPO kinetic parameters of Vmax and Km values in the crude plant extract of purslane (treated extract) illustrate different values from untreated extract (control). When compared to the control (Vmax 1.538 mol/minute), only Cu at a low concentration (0.1 M) has an active effect on PPO activity (Vmax 2.083 mol/minute). Other HMs exhibited an inhibition effect on PPO activity (Vmax) in the following order: Cu (0.3 M) = Zn (0.3 M) ? Zn (0.1 M) ? control. The type of inhibition is different. For example, Cu (0.3 M) exhibits a noncompetitive, and Zn (0.1 M) exhibits a mixed noncompetitive inhibition. Because Vmax in both was decreased, Km values may be the same or increased, respectively, while Zn (0.3 M) exhibited uncompetitive inhibition due to a decreased Vmax (0.476 μmol/minute) and Km (4.0 mM) in contrast with control 1.538 μmol/minute and 5.0 mM, respectively.
HM in high concentrations may exert toxicity in plant leaves or reduce metabolic activity or growth. For example, Zn toxicity causes chlorosis in younger leaves, inhibits plant growth, reduces metabolic activity, and causes oxidative damage to some plants [9]. Cu toxicity affects photosynthetic activity and nitrogen and protein metabolism [12].
5. CONCLUSION
The experiments were conducted to characterize the crude leaves extract of purslane and to analyze the Cu and Zn metals’ effects on both PPO activity and kinetics in the extract. It was found that proteins’ amounts were low, while the total phenols in purslane have a considerable amount, which would affect the antioxidant activity. When compared to the control (Vmax 1.538 mol/minute), only Cu at a low concentration (0.1 M) has an active effect on PPO activity (Vmax 2.083 mol/minute). Interestingly, the results of browning intensity and DPPH-scavenging activity in purslane correlated with total phenols. The presence of Cu and Zn metals causes inhibition effects (noncompetitive and uncompetitive) on the PPO activity depending on the type and concentration of Cu and Zn. Only Cu at a low concentration (0.1 M) has an activation effect. Therefore, further testing of the effects of other HMs on PPO kinetic parameters is recommended.
6. CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
7. AUTHORS’ CONTRIBUTIONS
Part of this research was based on master thesis work of Shada Al-Maitah. Prof. Omar M. Atrooz was the supervisor, writer, and editor of this paper.
8. FUNDING
There is no funding to report.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Atrooz OM, AlKhamaisa N, AlRawashdeh I. Determination of the activity and kinetic parameters of polyphenol oxidase enzyme in crude extracts of some Jordanian plants. J Appl Biol Biotechnol 2020;8:69–74.
2. Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MR. The role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Controlled Release 2006;113:189–207. CrossRef
3. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004;36:1–9. CrossRef
4. Aghraz A, Gonçalves S, Rodríguez-Solana R, Dra LA, Di Stefano V, Dugo G, et al. Antioxidant activity and enzyme inhibitory properties of several extracts from two Moroccan Asteraceae species. South Afr J Bot 2018;118:58–64. CrossRef
5. Grzesik M, Napar?o K, Bartosz G, Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem 2018;241:480–92. CrossRef
6. S?czyk ?, ?wieca M, Kapusta I, Gawlik-Dziki U. Protein–phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019;24:408. CrossRef
7. Enache TA, Oliveira-Brett AM. Phenol and para-substituted phenols electrochemical oxidation pathways. J Electroanal Chem 2011;655:9–16. CrossRef
8. Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001;49:5165–70. CrossRef
9. Naz A, Khan S, Qasim M, Khalid S, Muhammad S, Tariq M. Metals toxicity and its bioaccumulation in purslane seedlings grown in a controlled environment. Nat Sci 2013;5:573–9. CrossRef
10. Ebbs SD, Kochian LV. Toxicity of zinc and copper to Brassica species: implications for phytoremediation. J Environ Qual 1997;26:776–81. CrossRef
11. Iziy E, Majd A, Vaezi-Kakhki MR, Nejadsattari T, Kazemi NS. Effects of zinc oxide nanoparticles on enzymatic and nonenzymatic antioxidant content, germination, and biochemical and ultrastructural cell characteristics of Portulaca oleracea L. Acta Soc Bot Pol 2019;88:3639. CrossRef
12. Yruela I. Copper in plants. Brazil J Plant Physiol 2005;17:145–56. CrossRef
13. Lim YY, Quah EP. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem 2007;103:734–40. CrossRef
14. Masoodi MH, Ahmad B, Mir SR, Zargar BA, Tabasum N. Portulaca oleracea L. A review. J Pharm Res 2011;4:3044–8.
15. Erkan N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem 2012;133:775–81. CrossRef
16. Taranto F, Pasqualone A, Mangin G, Tripodi P, Miazzi MM, Pava S, et al. Polyphenol oxidases in crops: biochemical, physiological and genetic aspects. Int J Mol Sci 2017;18:377–93. CrossRef
17. Iqbal A, Murtaza A, Hu W, Ahmad I, Ahmed A, Xu X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod Process 2019;117:170–82. CrossRef
18. Mostafa MM. Gender differences in Egyptian consumers’ green purchase behavior: the effects of environmental knowledge, concern, and attitude. Int J Consumer Stud 2007;31:220–9. CrossRef
19. Nowotny A. Protein determination by the Biuret method. In: Basic experiment in immunochemistry, Springer, Berlin, Heidelberg, Germany, pp 168–9, 1979. CrossRef
20. Tayyebe H, Sayyed AE, Sayyed SM, Mahdi M. Total phenolic contents, antioxidant activities of different extracts, and fractions from the aerial parts of Artemisia biennis Willd. Iran J Pharm Res 2014;13:551–9.
21. Bal A, Pati SG, Panda F, Paital B. Modification of the time of incubation in a colorimetric method for accurate determination of the total antioxidants capacity using 2,2-diphenyl-1-picrylhydrazyl stable free radical. J App Biol Biotech 2021;9(4):156–61.
22. Mishra BB, Gautam S. Polyphenol oxidases: biochemical and molecular characterization, distribution, role, and its control. Enzyme Eng 2016;5:1–9.
23. Güray U, Elif A, Muhammet Ö, Ferhat K, Mehmet SÖ. Determination of heavy metal pollution in Zonguldak (Turkey) by moss analysis (Hypnum cupressiforme). Environ Eng Sci 2009;26:183–94. CrossRef
24. Gerrano AS, Jansen WS, Venter SL, Shargie NG, Amelework BA, Shimelis HA, et al. Selection of cowpea genotypes based on grain mineral and total protein content. Acta Agric Scand B Soil Plant Sci 2019;69:155–66. CrossRef
25. Sarkar S, Mondal M, Ghosh P, Saha M, Chatterjee S. Quantification of total protein content from some traditionally used edible plant leaves a comparative study. J Med Plants Stud 2020;8:166–70. CrossRef
26. González MN, Massa GA, Andersson M, Turesson H, Olsson N, Fält AS, et al. Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complex delivery of the CRISPR/Cas9 system. Front Plant Sci 2020;10:1649–61. CrossRef
27. Qi Y, Liu J, Liu Y, Yan D, Wu H, Li R, et al. Polyphenol oxidase plays a critical role in melanin formation in the fruit skin of persimmon. Food Chem 2020;330:127253. CrossRef
28. Alici EH, Arabaci G. Determination of SOD, POD, PPO, and CAT enzyme activities in Rumexo btusifolius L. Ann Res Rev Biol 2016;11:1–7. CrossRef
29. Yang CP, Fujita S, Kohno K, Kusubayashi A, Ashrafuzzaman MD, Hayashi N. Partial purification and characterization of polyphenol oxidase from banana (Musa sapientum L.) peel. J Agric Food Chem 2001;49:1446–9. CrossRef
30. Sikora M, ?wieca M, Franczyk M, Jakubczyk A, Bochnak J, Z?otek U. Biochemical properties of polyphenol oxidases from ready-to-eat lentil (Lens culinaris Medik.) sprouts and factors affecting their activities: a search for potent tools limiting enzymatic browning. Foods 2019;8:154–64. CrossRef
31. Do?an S, Turan P, Do?an M. Some kinetic properties of polyphenol oxidase from Thymbra spicata L. var. spicata. Process Biochem 2006;41:2379–85. CrossRef
32. Ba?lar M, Ertugay M. The effect of ultrasound and photo sonication treatment on polyphenol oxidase (PPO) activity, total phenolic component, and color of apple juice. Int J Food Sci Technol 2013;48:886–92. CrossRef
33. Ranveer RC, Pawar VN, Sakhale BK, Sahoo AK. Effect of storage conditions on the residual polyphenol oxidase (PPO) activity of raisins. Int J Agric Sci 2010;6:61–4.
34. Concellón A, Añón MC, Chaves AR. Characterization and changes in polyphenol oxidase from eggplant fruit (Solanum melongena L.) during storage at low temperature. Food Chem 2004;88:17–24. CrossRef