1. INTRODUCTION
Household and personal care products such as toothpaste, deodorants, and soaps frequently use triclosan (TCS), also known as 5-chloro-2-(2,4-dichlorophenoxy) phenol; TCS, as a synthetic antimicrobial agent due to its capacity to prevent the growth of bacteria and fungi [1,2]. The chemical structure of TCS lets it break down membrane function and stop bacterial enoyl-acyl carrier protein reductase from working, which kills bacteria [3]. TCS production peaked at 1500 tonnes/year in 1998 and continues to increase [4]. It is also an endocrine disrupting chemicals that affects multiple signaling pathways (neuroendocrine signaling, the p38/TRHr signaling pathway, the PI3K/Akt/JNK signaling cascade, and Toll-like receptor signaling) in humans [5,6]. Because the structure of the TCS is similar to that of thyroid hormones, it interferes with normal thyroid function and disrupts normal growth and development in humans, particularly in children’s brain development. It also exhibits weak androgenic and oestrogenic activities and is also associated with metabolic disorders, including obesity and Type 2 diabetes, highlighting its disruptive effects on endocrine function [7,8]. Due to its extensive usage, it has been found in significant quantities in our sewage, rivers, ponds, and eventually the oceans. Their persistence and constant increase in abundance in the global waters pose a threat to all types of aquatic flora and fauna. Estimates currently place their environmental concentration in global waters between 0.0001 ng/L and 100 ng/L [4]. Studies have demonstrated that these concentrations significantly affect most aquatic microfauna and cause significant ecotoxicity [4,6,9]. For this reason, it is important to study the effects of this chemical agent on various aquatic flora and fauna to measure and analyze whether it is worth it to continue to use this antimicrobial agent in our everyday products.
Brachionus plicatilis is a euryhaline rotifer in the family Brachionidae that can withstand a wide range of environmental conditions [10]. This ability enables them to thrive in various parts of the world, brackish water, and oceans, indicating their adaptability to varying salinities. Rotifers function as a bridge between the primary producers and primary consumers of the food chain. They are an important part of any food chain and a large percentage of zooplankton [11]. Rotifers are ideal model organisms for ecotoxicological studies because they are the fastest-reproducing metazoans and can be easily cultured in a controlled environment with bladder chemostatic agents [12]. All these reasons together make them a leading candidate as a model organism for studying many exciting ecological and evolutionary phenomena.
Previous studies have shown that rotifers adapt to different environmental conditions. Given the presence of rotifers in both freshwater and oceanic environments, it is reasonable to infer that they have the ability to adjust their body functions and mechanisms to varying salinities [13]. Salinity plays a crucial role in influencing the pharmacokinetics and pharmacodynamics of xenobiotics in aquatic organisms, as it alters their bioavailability in water [14]. It also influences the distribution and maintenance of the organism in aquatic environments [14-16]. This creates a gap in the existing knowledge on the effects of various xenobiotics on rotifers, highlighting the importance of studying the effects of TCS exposure on rotifers at different salinities. This, in turn, will pave the way for further research to investigate the toxic effects of TCS in different animal species under salinity conditions. Thus, the current research focuses on evaluating the acute toxicity of TCS and its impact on the behavior, reproduction, and antioxidant systems of B. plicatilis under varying salinity conditions and using environmentally relevant concentrations.
2. MATERIALS AND METHODS
2.1. Experimental Organism and Culturing Conditions
The marine monogonont rotifer B. plicatilis and unicellular green algae Nannochloropsis sp. were collected from the Central Marine Fisheries Research Institute, Field Lab, Kovalam, Tamil Nadu. Rotifers were cultured in 3 ppt artificial seawater (ASW), and the salinity was gradually increased to 30 ppt to adjust to the required experimental specifications. The green alga Nannochloropsis sp. with a density of ~2 × 105 cells/mL was fed to rotifers every 24 h and kept separately under laboratory conditions with a photoperiod of 16:8 h (light: dark) at 26°C.
2.2. Acute Toxicity Test
The acute toxic effects were carried out following the methods described in ISO 19820: 2016(E). In brief, B. plicatilis neonates were selected within 2 h and inoculated onto 24-well culture plates and exposed to five different concentrations of TCS (100, 200, 300, 400, and 500 μg/L) and controlled for 24 h without feed along with two controls, including a blank control with 15 Practical Salinity Units (psu) ASW [17]. The dead and live rotifers were counted after 24 h of exposure using a stereomicroscope [18-20]. All the exposures were carried out in triplicate. The 24-h LC50 value of TCS for rotifer B. plicatilis was calculated by probit analysis.
2.3. Effect of TCS in different Salinity on the Population Growth
To investigate the impact of TCS exposure in varying salinities on population growth, <12 h after hatching, rotifer B. plicatilis was placed in triplicate onto a 12-well cell culture plate with 4 mL of environmentally relevant concentrations of 100 ng/L and 200 ng/L of TCS at two distinct salinities (3 and 30 psu). The number of rotifers was checked every 24 h for 4 days using a stereomicroscope. During the experiment, we replaced 50% of the test medium (2 mL) with Nannochloropsis sp. with a density of ~2 × 105 cells/ml every 24 h. For each test, the intrinsic rate of population increase (R) was determined by the equation [21,22]:
r = (Ln (Nt.) – Ln (N0))/T
where N0 is the initial population density and Nt is the density of the population after time T (days).
2.5. Effect of TCS in different Salinity on Locomotor Behavior of B. plicatilis
Approximately 1000 rotifers were exposed to the same concentrations of the population growth studies for 4 h to assess the effect of TCS in different salinities on the swimming speed, according to the procedure prescribed by Liang et al. [23]. Briefly, ten adults were picked at random for each test concentration. They were then filmed for one minute under a microscope using an ocular micrometer (magnification ×40) (n = 10) and the free software Virtual Dub 1.9.10 in 30 frames/s, uncompressed AVI format. The open-source program measures each rotifer’s trajectory and produces an output in readable form [24]. After importing the data into Microsoft Excel, we obtained per-frame measurements for each rotifer by converting the pixel into absolute distance. Locomotor behavior (mean swimming speed in mm/s) was calculated for individual rotifers [25].
2.6. Effect of TCS in different Salinity on Antioxidant Enzymes Activity and Lipid Peroxidation in B. plicatilis
For the estimation of antioxidant enzyme activity and lipid peroxidation, the samples were prepared as previously described [26]. In brief, the rotifers were collected and homogenized in 0.1 M phosphate buffer (pH 7.4), and the resultant lysate was centrifuged at 5000 g for 10 min, and then the supernatant was used to measure the antioxidant enzyme activity and lipid peroxidation. The total amount of protein in the sample homogenates was estimated by the Bradford method [27].
2.6.1. Superoxide dismutase (SOD) activity
As previously described [28], SOD activity was estimated by determining the percentage inhibition of epinephrine auto-oxidation. For performing SOD activity analysis, carbonate buffer (0.1 M), epinephrine (1.3 M), and EDTA (0.6 M) were freshly prepared before performing the test. To perform the analysis, we prepared one auto-oxidation control consisting of carbonate buffer (100 μL), distilled water (100 μL), EDTA (50 μL), and epinephrine (50 μL). Another auto-oxidation sample was prepared using sample (10 μL), EDTA (50 μl), distilled water (100 μL), carbonate buffer (100 μL), and epinephrine (50 μL). After preparing both the control and samples without any delay, absorbance at 480 nm was measured. One unit (U) is defined as the amount of protein required for oxidation with 50% inhibition.
2.6.2. Catalase (CAT) activity
After diluting the exposure experiment sample homogenates with 60 mM sodium phosphate buffer (pH 7.4), 65 μM hydrogen peroxide solution was added, and the mixture was incubated for 4 min. ThermoFisher, USA’s Multiskan Microplate Spectrophotometer was used to measure the absorbance of the yellow complex at 405 nm after adding 32.4 mM ammonium molybdate to halt the reactions. CAT activity is expressed as units per milligram of protein. One unit is defined as 1 μmol of H2O2 reduced per minute [29].
2.6.3. Glutathione-S-transferase (GST) activity
The activity of GST was estimated as prescribed by Habig and Jakoby [30]. To sum up, 25 mM 1-chloro-2, 4-dinitrobenzene in 95% ethanol was added after the sample homogenates were diluted with 0.5 M phosphate buffer (pH 6.5). ThermoFisher, USA’s Multiskan Microplate Spectrophotometer was used to measure the absorbance at 340 nm as soon as 20 mM reduced glutathione was added to the reaction mixture. The absorbance was measured every 30 s for 1.5 min, and the activity was given as units per milligram of protein. A unit of measurement was established as the quantity of protein needed to generate one μmol of GS-DNB conjugate/min.
2.6.4. Lipid peroxidation
The lipid peroxidation was estimated in terms of the adduct formation with thiobarbituric acid (TBA) [31]. The sample homogenates were precipitated by adding ice-cold 10% trichloroacetic acid and incubated for 15 min on ice. Equal volumes of 0.67% TBA are added to the supernatants, and they are then incubated in a water bath at 100°C for 10 min. ThermoFisher, USA’s Multiskan Microplate Spectrophotometer was used to measure and record absorbance at 532 nm after the samples had cooled. Malondialdehyde (MDA) equivalents, measured in nmol per mg of protein, were used to express the results.
2.7. Statistical Analysis
To compare the means of the experimental groups with the means of the control group, all the data were analyzed using one-way analysis of variance followed by Dunnett’s multiple comparisons tests. The results are presented as a mean ± standard deviation of the mean (SD) with P < 0.05 designated as the significance level.
3. RESULTS
3.1. Acute Toxicity Test
Figure 1 shows the acute toxicity test of B. plicatilis 24 h-LC50 values exposed to 100–500 μg/L TCS, and by Probit analysis, related confidence intervals of 95% of TCS were determined and listed in Table 1. The 24 h-LC50 of TCS for Brachionus sp. was found to be 271.29 μg/L.
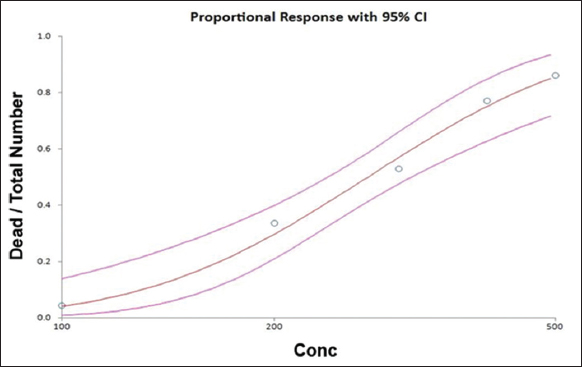 | Figure 1: Acute toxicity test of triclosan in Brachionus plicatilis. [Click here to view] |
Table 1: LC50 Values for TCS in Brachionus plicatilis.
| Time point (h) | LC50 (mg/L) | Confidence interval (mg/L) |
|---|---|---|
| 24 | 271.29 | 214.29–312.61 |
3.2. Effect of TCS in Different Salinity on the Population Growth
A reproductive bioassay was performed to provide the intrinsic rate of the population of rotifers in different salinities with TCS exposure [Figure 2]. The population growth rate shows a significant decrease as the concentration of TCS exposure increases at 3 and 30 psu. The lowest population growth rate was observed in the 200 ng/L at 30 psu TCS exposure group compared to the control.
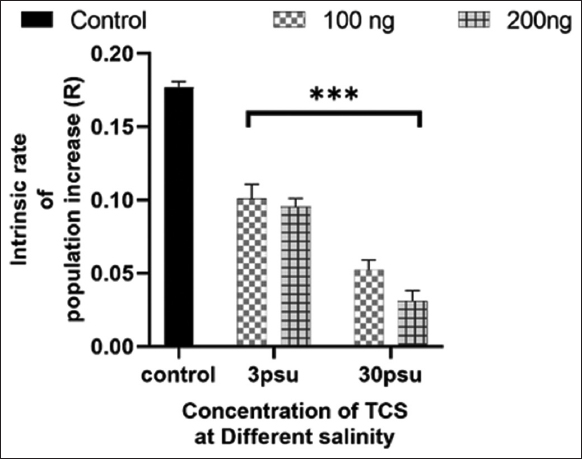 | Figure 2: Effect of Triclosan in different salinities on the population growth. The results were presented as mean ± SD (n = 3). * at P < 0.05; ** at P < 0.01; *** at P < 0.001; and **** at P < 0.0001 levels, the asterisk above the bar indicates a statistically significant difference against the controls. [Click here to view] |
3.3. Effect of TCS in Different Salinity on Locomotor Behavior of B. plicatilis
Figure 3 shows the effect of TCS in different salinity on the locomotor behavior of B. plicatilis. The average speed of rotifers was significant decreased in all the concentration of TCS in both salinities when compared with control. However, the maximum inhibition of rotifer speed was observed in the 100 ng at 30 psu.
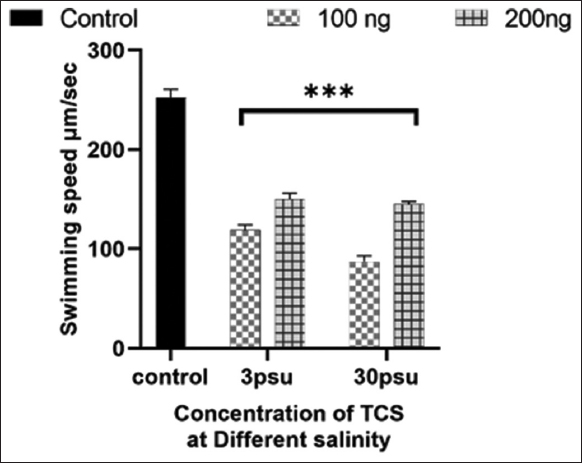 | Figure 3: Effect of Triclosan in different salinity on locomotor behavior of Brachionus plicatilis. The results were presented as mean ± SD (n = 3). * at P < 0.05; ** at P < 0.01; *** at P < 0.001; and **** at P < 0.0001 levels, the asterisk above the bar indicates a statistically significant difference against the controls. [Click here to view] |
3.4. Effect of TCS in Different Salinity on Antioxidant Enzymes Activity and Lipid Peroxidation in B. plicatilis
To examine whether TCS in different salinities induces oxidative stress in B. plicatilis, we measured antioxidant enzymatic activities (CAT, SOD, and GST) and levels of lipid peroxidation after exposure to TCS in different salinities [Figure 4]. The activity of SOD significantly increased in concentration with 100 ng/L of TCS in both salinities. However, the activity of SOD decreases as the concentration of TCS increases in both salinities. The concentration of 200 ng/L at 30 psu showed the maximum reduction in activity [Figure 4a]. The activity of CAT in rotifers exposed to TCS in different salinities shows no significant change in the CAT activity in both concentrations of TCS at 3 psu and 100 ng/L at 30 psu; the activity of CAT is significantly reduced in 200 ng/L at 30 psu [Figure 4b]. GST activity was increased in the 100 ng/L at 3 psu, but activity was reduced as the concentration and salinity increased to 200 ng/L and 30 psu [Figure 4c]. To examine whether TCS in different salinities induces oxidative stress in B. plicatilis, we measured antioxidant enzymatic activities (CAT, SOD, and GST) and levels of lipid peroxidation after exposure to TCS in different salinities [Figure 4]. The activity of SOD significantly increased in concentration with 100 ng/L of TCS in both salinities. However, the activity of SOD decreases as the concentration of TCS increases in both salinities. The concentration of 200 ng/L at 30 psu showed the maximum reduction in activity [Figure 4a]. The activity of CAT in rotifers exposed to TCS in different salinities shows no significant change in the CAT activity in both concentrations of TCS at 3 psu and 100 ng/L at 30 psu; the activity of CAT is significantly reduced in 200 ng/L at 30 psu [Figure 4b]. GST activity was increased in the 100 ng/L at 3 psu, but activity was reduced as the concentration and salinity increased to 200 ng/L and 30 psu [Figure 4c].
 | Figure 4: (a-d) Effect of Triclosan in different salinity on antioxidant enzymes activity and lipid peroxidation in Brachionus plicatilis. The results were presented as mean ± SD (n = 3). * at P < 0.05; ** at P < 0.01; *** at P < 0.001; and **** at P < 0.0001 levels, the asterisk above the bar indicates a statistically significant difference against the controls. [Click here to view] |
Figure 4d shows the lipid peroxidation level because of TCS in rotifers with different salinities. In comparison with Control, MDA concentration significantly increased in a concentration-dependent manner and salinity. The maximum amount of lipid peroxidation was observed at 200 ng/L at 30.
4. DISCUSSION
The survival rate decreased significantly when exposed to TCS. Results from the 24-h acute toxicity test assessed the LC50 value of TCS towards Brachionus sp. to be 271.29 µg/L (95% confidence limits of 214.29–312.61 µg/L) [Table 1]. The maximum concentration of TCS detected in marine water is 100 μg/L. The LC50 value of TCS towards marine rotifers Brachionus koreanus and Brachionus havanaensis was 393 μg/L and 500 μg/L, respectively, within 24 h [18,32]. Lower acute toxicity was found in other multicellular organisms when exposed to TCS at environmental concentrations. For instance, the 24 h-LC50 values for Chironomus tentans, Xiphophorus helleri, Hyalella azteca, and Ceriodaphnia dubia were determined to be 3000 μg/L, 1470 μg/L, and 1000 μg/L, respectively [21]. Similarly, Artemia salina, Thamnocephalus platyurus, Chironomus riparius, and Caenorhabditis elegans have shown 24 h-LC50 of 171, 470, 3428, and 9840 μg/L TCS, respectively [33-35]. The toxicity of TCS to higher trophic level multicellular organisms mentioned above implies that Brachionus sp. is sensitive to low concentrations of TCS, but when compared to C. dubia and A. salina, it is to be noted that they are more sensitive to TCS than Brachionus sp.
The population growth rate was determined for rotifers in both salinities with 100 ng/L and 200 ng/L TCS chronic exposure for 4 days. The population growth significantly slowed down as the concentration of TCS increased at higher salinities. Consistent with our findings, a reduction in survival and reproduction when exposed to toxicants was observed in previous studies with rotifers [36,38,39]. For example, steady growth retardation was observed in B. koreanus at 100 μg/L of four antibiotics exposure [37]. For example, species like B. plicatilis are greatly affected by high levels of TCS in the environment, which changes how they swim and reproduce. Previous studies have shown that exposure to high concentrations impacts population growth and life history parameters by shortening the reproductive period and prolonging the post-reproductive period in B. plicatilis [40]. In a study, the population growth rate was inhibited at concentrations higher than 1.0 μg/L of TCS in Brachionus calyciflorus that were tested for 4 days [21]. There was a decrease in population growth rate due to the exposure of Dichlorvos, Triazophos, and chlorpyrifos at 10 mg/L and 1 mg/L, respectively, in freshwater B. calyciflorus [36]. Studies reveal that the toxicity of ibuprofen alters the population rate in Daphnia magna, in addition to rotifers [41]. Previous studies clearly indicate that toxicants disrupt the population growth rate. Another reason for the declining rate of population growth could be the change in salinity [19]. Rotifers can tolerate varying levels of salinity, but it is limited to a certain range, as changes in salinity could alter energy reallocation to the basal metabolic rate for maintaining homeostasis [19]. We could say that we observed a significant reduction of rotifers in 3 psu salinity due to treatment with TCS in low salinity. With increased salinity, rotifers show more significant reduction in the growth population rate. This suggests that a combination of both salinity and TCS could be the reason for the adverse effects on rotifers, or individual effects are also possible.
Other aspects studied in the work were swimming behavior. Both internal and external factors can influence the swimming behavior of rotifers, which requires a significant amount of energy and impacts their metabolism [42]. In our study, it was observed that swimming speed was reduced significantly by TCS in different concentrations at different salinities. This decrease in swimming behavior is observed in rotifers and is similar to the effect when exposed to other drugs like dimethoate [43]. It is also found that TCS alters the expression of genes related to cilium organization and assembly, which are crucial for swimming, as well as pathways like focal adhesion and hedgehog signaling [40]. Other organisms, such as zebrafish and Daphnia pulex, also exhibit impaired swimming behavior when exposed to toxic drugs or chemicals [26,44,45]. Similarly, in Micropogonias undalatus, there was a reduction in the reflex of the organism when exposed to TCS and two temperatures [46]. Palaemon varians, which was also exposed to different concentrations of TCS, showed a similar reduction in swimming activity [47].
The antioxidant defense system is one of the important roles in eliminating reactive oxygen species (ROS) and maintaining the redox homeostasis within the cells. Therefore, the assessment of antioxidant activity could serve as a valuable biomarker for evaluating the extent of oxidative damage within an organism. Each of the antioxidant enzymes is responsible for eliminating one or more ROS. Many studies show that an increase in ROS activity within the cells causes a change in the intracellular antioxidant activity [19], where an increase in ROS activity due to salinity stress caused a change in antioxidant activity such as SOD, CAT, and GST of monogonont rotifer B. plicatilis. A similar effect was observed in this study, that exposure to TCS at different salinities alters the antioxidant enzyme in rotifers. SOD eliminates radical superoxide anions by converting them into hydrogen, which is then disposed of by other enzymes [26]. It was observed that the activity of SOD was increased in the lower concentration and reduced significantly as the concentration increased [Figure 3a]. This might be due to increased ROS activity within the cells, resulting in the production of more enzymes to counteract the ROS, but at high concentrations, SOD cannot be able to cope with the production of ROS; hence the activity was reduced at high concentrations [19]. Moreover, the reduction in activity could also be due to the interaction between TCS and SOD on a molecular level [48]. A similar effect was observed when zebrafish were exposed to pesticides [26].
CAT enzyme is responsible for converting H2O2 to H2O and molecular oxygen since H2O2 is a ROS that can disrupt the integrity of the cell. Previous studies on rotifers and zebrafish have shown changes in CAT activity when exposed to antimicrobial chemicals or due to stress-inducing factors in extreme salinity or temperature [19,26,49]. It was observed that TCS alters the activity of CAT at different salinities [Figure 3b]. The decrease in the activity at the high concentration of TCS and salinity is due to an increase in the production of H2O2 due to salinity stress or stress caused by TCS; similar results are seen in the work [26]. GST is an important antioxidant enzyme that catalyses the interaction between reduced glutathione and xenobiotic metabolites, allowing for faster xenobiotic elimination. GST, along with reduced glutathione, plays a major role in defense against ROS and lipid peroxidation. In Figure 3c, we can see a reduction in GST activity in both the salinities at higher concentrations; it might be to counter the TCS in the cell, or it might also be reduced due to the induced salinity stress. Previous studies show varying GST activity when exposed to antimicrobial and varying salinity [19,49]. An increase in GST activity is also observed in 0.3% salinity with a 100 ng/L concentration of TCS; this increase might be to reduce the ROS induced by TCS. Zebrafish exhibit similar results when exposed to xenobiotics [45].
ROS also initiates lipid peroxidation reactions and leads to structural and functional alterations in the molecule that has lipid [50-52]. In this study, it was observed that lipid peroxidation in rotifers increased as the concentration of the TCS increased, as the salinity increased [Figure 3d]. ROS induced by TCS showed to damage the integrity of cell membranes by lipid peroxidation [52]. A similar effect was observed when the freshwater rotifer B. calyciflorus was exposed to a binary mixture of microcystin and nitrite [53]. In B. koreanus, exposure to high concentrations of TCS leads to growth retardation and reduced fecundity, attributed to oxidative stress and the modulation of detoxification and antioxidant proteins, such as GST-sigma and Cu/ZnSOD. Increased ROS levels and GST enzymatic activity accompany these changes, indicating a stress response that impacts the rotifers’ lifespan and reproductive capabilities [18]. In addition, the neurotoxic effects of similar compounds, such as TDCPP, further illustrate how chemical exposure can disrupt neural functions and energy metabolism, leading to impaired ciliary movement and swimming behavior in rotifers [54]. Overall, the presence of TCS in aquatic environments poses a significant ecological risk by disrupting the biological functions essential for the survival and reproduction of rotifers.
5. CONCLUSION
Our research concludes that exposure to TCS at varying salinities significantly alters the locomotor behavior and reproductive patterns of B. plicatilis and slows population growth, accompanied by oxidative stress. These changes highlight the ecological and biological consequences of TCS contamination, as altered swimming patterns and impaired reproduction can disrupt the species’ role in aquatic ecosystems. High TCS concentrations may lead to reduced biodiversity and ecosystem stability due to these negative impacts on rotifers, which are crucial for nutrient cycling and food webs. Our study enhances understanding of the mechanical aspects of TCS toxicity across different salinities, revealing potential risks to aquatic life. To mitigate these effects, alternatives to TCS, such as using more environmentally benign compounds or improved waste treatment technologies, should be considered to reduce contamination in aquatic habitats.
6. ACKNOWLEDGMENTS
The authors are thankful to the Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu.
7. AUTHOR’S CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. FUNDING SOURCE
There is no funding to report.
9. CONFLICTS OF INTERESTS
The author reports no financial or any other conflicts of interest in this work.
10. ETHICAL STATEMENT
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
12. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
13. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Srain HS, Beazley KF, Walker TR. Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms. Environ Rev 2021;29:142-81. [CrossRef]
2. Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev 2017;20:447-69. [CrossRef]
3. Heath RJ, Li J, Roland GE, Rock CO. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J Biol Chem 2000;275:4654-9. [CrossRef]
4. Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledón M, Verma M, et al. Triclosan:Current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health 2015;12:5657-84. [CrossRef]
5. Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens:Receptor-based bioassay screens. Environ Health Perspect 2008;116:1203-10. [CrossRef]
6. Wang CF, Tian Y. Reproductive endocrine-disrupting effects of triclosan:Population exposure, present evidence and potential mechanisms. Environ Pollut 2015;206:195-201. [CrossRef]
7. Lee DG. Removal of a synthetic broad-spectrum antimicrobial agent, triclosan, in wastewater treatment systems:A short review. Environ Eng Res 2015;20:111-20. [CrossRef]
8. Sinicropi MS, Iacopetta D, Ceramella J, Catalano A, Mariconda A, Pellegrino M, et al. Triclosan:A small molecule with controversial roles. Antibiotics (Basel) 2022;11:735. [CrossRef]
9. Yueh MF, Tukey RH. Triclosan:A widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol 2016;56:251-72. [CrossRef]
10. Rebecchi L, Boschetti C, Nelson DR. Extreme-tolerance mechanisms in meiofaunal organisms:A case study with tardigrades, rotifers and nematodes. Hydrobiologia 2020;847:2779-99. [CrossRef]
11. Van Der Stap I, Matthijs AE, Ae V, Mooij WM. Inducible defenses and rotifer food chain dynamics. Hydrobiologia 2007;593:103-10. [CrossRef]
12. Rico-Martínez R, Arzate-Cárdenas MA, Robles-Vargas D, Pérez-Legaspi IA, Alvarado-Flores J, Santos-Medrano GE. Rotifers as models in toxicity screening of chemicals and environmental samples. Invert Exp Models Toxic Screen 2016;10:57-99.
13. Mustahal T, Yamasaki S, Hirata H. Salinity adaptability of five different strains of the Rotifer Brachionus plicatilis. Nippon Suisan Gakkaishi 1991;57:1997-2000. [CrossRef]
14. DeLorenzo ME, Wallace SC, Danese LE, Baird TD. Temperature and salinity effects on the toxicity of common pesticides to the grass shrimp, Palaemonetes pugio. J Environ Sci Health B 2009;44:455-60. [CrossRef]
15. Lin Y, Chen J. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J Exp Mar Biol Ecol 2001;259:109-19. [CrossRef]
16. Mehta K. Impact of temperature on contaminants toxicity in fish fauna:A review. Indian J Sci Technol 2017;10:1-6. [CrossRef]
17. Yoo HK, Kim SS, Lee KW, Lee SY, Jung MM, Woo SJ. Determination of the optimal conditions for the mass culture of large-type rotifers (Brachionus plicatilis) at low temperatures. Water (Switzerland) 2023;15:3310. [CrossRef]
18. Han J, Won EJ, Hwang UK, Kim IC, Yim JH, Lee JS. Triclosan (TCS) and Triclocarban (TCC) cause lifespan reduction and reproductive impairment through oxidative stress-mediated expression of the defensome in the monogonont rotifer (Brachionus koreanus). Comp Biochem Physiol C Toxicol Pharmacol 2016;185-6:131-7. [CrossRef]
19. Han J, Lee KW. Influence of salinity on population growth, oxidative stress and antioxidant defense system in the marine monogonont rotifer Brachionus plicatilis. Comp Biochem Physiol B Biochem Mol Biol 2020;250:110487. [CrossRef]
20. Fernández-Casalderry A, Ferrando MD, Andreu-Moliner E. Acute toxicity of several pesticides to rotifer (Brachionus calyciflorus). Bull Environ Contam Toxicol 1992;48:14-7. [CrossRef]
21. Zhang L, Niu J, Wang Y. Full life-cycle toxicity assessment on triclosan using rotifer Brachionus calyciflorus. Ecotoxicol Environ Saf 2016;127:30-5. [CrossRef]
22. Snell TW, Moffat BD. A 2-d life cycle test with the rotifer Brachionus calyciflorus. Environ Toxicol Chem 1992;11:1249-57. [CrossRef]
23. Liang Y, Lu X, Min Y, Liu L, Yang J. Interactive effects of microcystin and ammonia on the reproductive performance and phenotypic traits of the rotifer Brachionus calyciflorus. Ecotoxicol Environ Saf 2018;147:413-22. [CrossRef]
24. Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, de Polavieja GG. idTracker:Tracking individuals in a group by automatic identification of unmarked animals. Nat Methods 2014;11:743-8. [CrossRef]
25. Audira G, Sampurna B, Juniardi S, Liang ST, Lai YH, Hsiao CD. A simple setup to perform 3D locomotion tracking in Zebrafish by using a single camera. Inventions 2018;3:11-20. [CrossRef]
26. Kuppuswamy JM, Seetharaman B. Monocrotophos based pesticide alters the behavior response associated with oxidative indices and transcription of genes related to apoptosis in adult Zebrafish (Danio rerio) brain. Biomed Pharmacol J 2020;13:1291-304. [CrossRef]
27. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54. [CrossRef]
28. Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 1978;90:81-9. [CrossRef]
29. Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 1991;196:143-51. [CrossRef]
30. Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol 1981;77:398-405. [CrossRef]
31. Yagi K. Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol 1998;108:107-10. [CrossRef]
32. González-Pérez BK, Sarma SS, Castellanos-Páez ME, Nandini S. Multigenerational effects of triclosan on the demography of Plationus patulus and Brachionus havanaensis (ROTIFERA). Ecotoxicol Environ Saf 2018;147:275-82. [CrossRef]
33. Xu X, Lu Y, Zhang D, Wang Y, Zhou X, Xu H, et al. Toxic assessment of Triclosan and Triclocarban on Artemia salina. Bull Environ Contam Toxicol 2015;95:728-33. [CrossRef]
34. Khatikarn J, Satapornvanit K, Price OR, Van den Brink PJ. Effects of triclosan on aquatic invertebrates in tropics and the influence of pH on its toxicity on microalgae. Environ Sci Pollut Res Int 2018;25:13244-53. [CrossRef]
35. García-Espiñeira MC, Tejeda-Benítez LP, Olivero-Verbel J. Toxic effects of bisphenol A, propyl paraben, and triclosan on Caenorhabditis elegans. Int J Environ Res Public Health 2018;15:684. [CrossRef]
36. Li-Xia K, Yi-Long X, Chun-Wang Z, Li-Li D. Effects of three organophosphorus pesticides on population growth and sexual reproduction of rotifer Brachionus calyciflorus Pallas. Acta Ecologica Sinica 2009;29:182-5. [CrossRef]
37. Rhee JS, Jeong CB, Kim BM, Lee JS. P-glycoprotein (P-gp) in the monogonont rotifer, Brachionus koreanus:Molecular characterization and expression in response to pharmaceuticals. Aquat Toxicol 2012;114-5:104-18. [CrossRef]
38. Zhang L, Niu J, Li Y, Wang Y, Sun D. Evaluating the sub-lethal toxicity of PFOS and PFOA using rotifer Brachionus calyciflorus. Environ Pollut 2013;180:34-40. [CrossRef]
39. Zhang L, Niu J, Wang Y, Shi J, Huang Q. Chronic effects of PFOA and PFOS on sexual reproduction of freshwater rotifer Brachionus calyciflorus. Chemosphere 2014;114:114-20. [CrossRef]
40. YunhongYang, Mao T, Ding Y, Ge L, Feng L, Cai M, et al. Variations in life history parameters, population dynamics, and transcriptome regulation of Brachionus plicatilis exposed to triclosan. Mar Pollut Bull 2024;199:115918. [CrossRef]
41. Heckmann LH, Callaghan A, Hooper HL, Connon R, Hutchinson TH, Maund SJ, et al. Chronic toxicity of ibuprofen to Daphnia magna:Effects on life history traits and population dynamics. Toxicol Lett 2007;172:137-45. [CrossRef]
42. Epp RW, Lewis WM Jr. Cost and speed of locomotion for rotifers. Oecologia 1984;61:289-92. [CrossRef]
43. Guo R, Ren X, Ren H. Assessment the toxic effects of dimethoate to rotifer using swimming behavior. Bull Environ Contam Toxicol 2012;89:568-71. [CrossRef]
44. Sha J, Wang Y, Lv J, Wang H, Chen H, Qi L, et al. Effects of two polybrominated diphenyl ethers (BDE-47, BDE-209) on the swimming behavior, population growth and reproduction of the rotifer Brachionus plicatilis. J Environ Sci (China) 2015;28:54-63. [CrossRef]
45. Alla LN, Monshi M, Siddiqua Z, Shields J, Alame K, Wahls A, et al. Detection of endocrine disrupting chemicals in Danio rerio and Daphnia pulex:Step-one, behavioral screen. Chemosphere 2021;271:129442. [CrossRef]
46. Hedrick-Hopper TL, Koster LP, Diamond SL. Accumulation of triclosan from diet and its neuroendocrine effects in Atlantic croaker (Micropogonias undulatus) under two temperature regimes. Mar Environ Res 2015;112:52-60. [CrossRef]
47. Araújo CV, Gómez L, Silva DC, Pintado-Herrera MG, Lara-Martín PA, Hampel M, et al. Risk of triclosan based on avoidance by the shrimp Palaemon varians in a heterogeneous contamination scenario:How sensitive is this approach?Chemosphere 2019;235:126-35. [CrossRef]
48. Mi C, Teng Y, Wang X, Yu H, Huang Z, Zong W, et al. Molecular interaction of triclosan with superoxide dismutase (SOD) reveals a potentially toxic mechanism of the antimicrobial agent. Ecotoxicol Environ Saf 2018;153:78-83. [CrossRef]
49. Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol 1994;366:43-58. [CrossRef]
50. Aboul Ezz HS, Khadrawy YA, Mourad IM. The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology 2015;67:145-55. [CrossRef]
51. Knight JA, Pieper RK, McClellan L. Specificity of the thiobarbituric acid reaction:Its use in studies of lipid peroxidation. Clin Chem 1988;34:2433-8. [CrossRef]
52. Xin X, Huang G, An C, Lu C, Xiong W. Exploring the biophysicochemical alteration of green alga Asterococcus superbus interactively affected by nanoparticles, triclosan and illumination. J Hazard Mater 2020;398:122855. [CrossRef]
53. Liang Y, Su Y, Ouyang K, Chen X, Yang J. Effects of microcystin-producing and microcystin-free Microcystis aeruginosa on enzyme activity and nutrient content in the rotifer Brachionus calyciflorus. Environ Sci Pollut Res Int 2017;24:10430-42. [CrossRef]
54. Zhang X, Tong X, Tang X, Yang Y, Zhang L, Zhan X, et al. Behavioral toxicity of TDCPP in marine zooplankton:Evidence from feeding and swimming responses, molecular dynamics and metabolomics of rotifers. Sci Total Environ 2024;921:170864. [CrossRef]