1. INTRODUCTION
Recently, many researchers have focused on finding biomaterials that have high bioactivity and environmentally friendly. One material that has caught the attention of researchers is chitosan. Chitosan is a derivative form of a naturally occurred cationic polymer, chitin, or N-acetyl D-glucosamine. The physicochemical properties of chitosan are the key reason for its versatility in wide range of applications [1]. Chitosan molecules have various functional groups that can interact with other molecules. The molecular weight and deacetylation degree (DD) of chitosan greatly determine its bioactivity [2] particularly antibacterial, antioxidant, anti-inflammatory, anticancer activity, and hemocompatibility [2,3]. Lately, chitosan has been used in a wide variety of fields, such as agriculture, food, medicine, as well as for wastewater treatment [4]. Chitosan is also attracting special attention in the medical field due to its biocompatibility, non-toxicity, and biodegradability [5].
Commercial chitosan is commonly obtained from crustacean by-products, namely, crab and shrimp’s unused shell due to its abundance and high chitin content. Nevertheless, there are still other sources of chitin discovered in nature particularly the cell wall of fungi and exoskeleton of insects that are mainly composed of chitin [6,7]. Therefore, recently chitosan from fungi and insects has gained more attention due to the increasing demand for this polymer which leads to the sought of non-crustacean alternative sources of chitosan [8].
Fungi known to contain chitin in their cell walls is Mucorales the largest order of Zygomycetes that naturally produce chitosan, such as Mucor spp., Absidia spp., and Rhizopus spp. Aspergillus niger and M. rouxii have been reported to produce chitin [9]. Mucor spp. and R. oryzae are commonly used in the fermentation process due to their fast-growing ability and have a wide range of cultivation temperatures [10-12]. Chitosan yields from A. niger and R. oryzae which have been reported to be 11% and 14%, respectively [13]. Just like fungi, the main component of their cell wall is chitin; insects also have chitin in an important part of their body, which is contained in the exoskeleton.
Chitin in insects is known as the most abundant aminopolysaccharide polymer as a building material that strengthen the exoskeleton of an insect [14]. One of the insects that have chitin in its exoskeleton and are interesting to study is Hermetia illucens or black soldier fly (BSF). Recently, BSF larvae (BSFL) have attracted numerous attentions due to its potential as an organic waste conversion agent as well as a great source of protein, lipid as well as chitin and chitosan. The chitin content of BSF lies in the range of 8–24% depending on its lifecycle [15]. Chitin from the BSFL can be converted into chitosan by removal of acetyl group through enzymatic or chemical deacetylation process [14,16,17]. At present, production of high yield and physicochemical properties of chitosan from fungi and insect is still being studied. Hence, this study aims to investigate the synthesis chitosan from insect biomass of BSFL, and fungal biomass of R. oryzae and Mucor spp., as well as their antibacterial activity which may provide further information about the potential application of chitosan in the field of food, feed, and medicine.
2. MATERIALS AND METHODS
2.1. Materials
2.1.1. Chemicals
HCl, NaOH, n-hexane, distilled water, acetic acid, ethanol 96%, acetone, nutrient agar (NA; peptone 5 g/L, HM peptone 1.5 g/L, yeast extract 1.5 g/L, sodium chloride 5 g/L, agar 15 g/L), potato dextrose agar (PDA; potato infusion 200 g/L, dextrose 20 g/L, and agar 15 g/L), MgSO4, (NH4)2SO4, BaCl2, H2SO4, yeast extract, and NaCl were obtained from School of Life Science and Technology, Institut Teknologi Bandung, Indonesia. Other chemicals such as Muller Hinton broth (MHB; infusion from beef 300 g/L, casein acid hydrolase 17.5 g/L, and starch 1.5 g/L) and agar (MHA; infusion B from HM 300 g/L, acicase 17.5 g/L, starch 1.5 g/L, and agar 17 g/L), chloramphenicol, commercial shrimp chitin, and chitosan were obtained from local chemical suppliers in Bandung, Indonesia.
2.1.2. Black Soldier Fly Larvae
Dried BSFL biomass used in this study was obtained from biorefinery society, a local startup company in Bandung, Indonesia. The larvae were cultivated with a mixture of dried coconut endosperm waste and mixture of agriculture residue every 3 days. The cultivation was carried out in a dedicated place at Bandung with a temperature range of 21–31°C. After cultivation for 2 weeks, the BSFL were harvested and inactivated in a microwave at 800 watt for 6 min.
2.1.3. Microbes
The fungal culture of Mucor spp. InaCC no. F07 and R. oryzae InaCC no. F149 used in this study was obtained from Indonesian Culture Center of Indonesia Institute of Science, Cibinong, Indonesia. The fungal cultures were grown in PDA medium until further use. The bacterial culture of Escherichia coli and Staphylococcus aureus was obtained from Microbial Collection of School of Life Sciences and Technology, Institut Teknologi Bandung, Indonesia. The bacterial cultures were grown in commercial NA medium until further use.
2.2. Cultivation of Mucor spp. and R. oryzae
Cultivation of Mucor spp. and R. oryzae to to produce chitosan was based on the previous study by [18]. Mucor spp. and R. oryzae were grown in a tailor-made medium which consists of sugarcane juice, yeast extract 3 g/L, MgSO4 0.6 g/L, and (NH4)2SO4 1.4 g/L dissolved in distilled water until it reaches 250 mL in total volume. To produce maximum biomass, Mucor spp. was cultivated in 25% sugarcane juice, C: N ratio of 19.5, incubated at 28°C 150 rpm agitation speed for 4 days while R. oryzae was cultivated in 75% sugarcane juice 25%, C: N ratio of 21.5, incubated at 28°C 150 rpm agitation speed for 6 days before being harvested [18].
2.3. Defatting of Dried BSFL
Before any further treatment, the dried BSFL were subjected to a defatting process using a Soxhlet method procedures [19]. Briefly, 50 g of the dried BSFL were crushed using an electrical blender. After that, the crushed sample was refluxed in a Soxhlet apparatus using 250 mL technical grade n-hexane at 70°C for 4 h until most of the fat was separated from the sample.
2.4. Preparation of Chitosan from BSFL and Fungi
Chitosan from the biomass of Mucor spp., R. oryzae, and defatted BSFL was prepared by removing protein content and extracted using acetic acid [20]. The dried samples were deproteinized using 2% NaOH solution with a ratio of 30:1 volume to weight (v/w) with respect to the samples at 90°C for 2 h. The mixture was then separated using centrifugation at 4000 g for 15 min. After that, the pellet was washed using neutralized water (pH 7) to stabilize the pH. Chitosan extraction was carried out by refluxing the sample with 10% acetic acid (ratio of 40:1 v/w to the sample) at 60°C for 6 h followed by separation with centrifugation at 4000 g for 15 min. Two fractions were obtained, chitosan in the supernatant and the remaining pellet was chitin rich fraction. The chitosan solution was precipitated using 30% NaOH solution at room temperature (25°C) and then separated using centrifugation at 4000 g for 15 min. The precipitated phase was being washed using three solutions, respectively, (1) water, (2) ethanol with a ratio of 20:1 v/w to the sample for 3 times, and (3) acetone with a ratio of 20:1 v/w to the sample for 3 times to minimize impurities. Finally, the chitosan obtained from the previous step was dried inside an oven at 30°C.
2.5. Characterization of Chitosan
Physicochemical analysis of chitosan produced from fungi and insects was carried out by observing the functional groups of chitosan with Fourier-transformation infrared spectroscopy (FT-IR). Determination of the degree of deacetylation (DD) in this study was also based on functional groups detected at certain wavelengths. This value can provide an overview of the purity of the chitosan extract produced and determine its correlation with bioactivity. Structural analysis of chitin rich pellet, crude chitosan from BSFL, Mucor spp. and R. oryzae, as well as commercial chitin and chitosan shrimp was determined using a FT-IR Prestige 21 Shimadzu at Laboratory of Analytical Chemistry at Faculty of Mathematic and Natural Sciences, Institute Technology Bandung. DD of the chitosan was determined using Equation (1) [21].
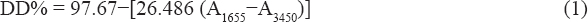 |
Where DD is DD (%), A1655 is the absorption value at a wavelength of 1655 cm-1, and A3450 is the absorption value at a wavelength of 3450 cm-1.
2.6. Antibacterial Activity Assay of Chitosan
Antibacterial activity of chitosan was determined using a disk diffusion test procedure [22]. In this study, S. aureus was used as a sample for Gram-positive bacteria whereas E. coli was used to represent the Gram-negative bacteria. The diffusion disks were immersed in the chitosan obtained from fungi and BSFL dissolved in 0.2% v/v acetic acid as a negative reference and 30 mg/mL of commercial antibiotic chloramphenicol as a positive reference. The soaked disks were arranged and placed on the spread Petri dish. After that, the samples were incubated flipped side at 37°C for 2 days. The diameter of the inhibition formed on the MHA inside the Petri dish was then measured. The measurements were carried out twice and the average values were reported.
3. RESULTS AND DISCUSSION
3.1. Chitosan from Mucor spp., Rhizopus. Oryzae and Hermetia illucens
Chitosan from fungal and BSFL biomass obtained in this study has distinctive appearance in color. Chitosan from R. oryzae has a dark brown color, while Mucor spp. and BSFL appear in brown to white color. The mycelia of R. oryzae culture on PDA was initially white and cottony and then became brown to gray color. The dark color appears due to the presence of sporangia containing more spores as it grows. The R. oryzae colonies grown on PDA were white cottony at first and then become brownish-black with the appearance of sporangia that contain thousands of brownish-black streaked sporangiospore [23]. The brown to white color also appears in Mucor spp. and BSFL chitosan. Mycelia of Mucor spp. culture produce white to grey color, not as dark as R. oryzae culture. The amount of pigment contained in fungi results in a variety of chitosan colors.
The chitosan from all samples appears brown color because they still contain unextracted pigments. One of the pigments that can be found in fungi and insect is melanin. Melanin is a natural pigment produced by a variety of fungus in the conidia and hyphae [24]. Nitrogen-containing pigments melanin is a bioactive molecule synthesized by insect as part of the cuticle, hemolymph, complex eye, intestine, fat body, and determining its color [25]. The amount of melanin content of organism may vary depending on many factors. The higher the melanin content, the darker the color will appear. One of melanin which may give the fungi black or dark brown color found in nature is eumelanins and for yellow or red color are pheomelanins [26]. To produce high purity chitosan, melanin pigment can be removed by decolorization step.
3.2. Chitin, Chitosan, and Other Byproducts Recovery from R. oryzae, Mucor spp., and BSFL
The recovery of chitosan from selected fungi and insects is presented in Table 1 and the schematic diagram of chitin and chitosan production from Mucor spp. R. oryzae, and BSFL is shown in Figure 1. Chitosan from Mucor spp. biomass has the highest recovery (157.3 mg/g biomass) while chitosan from defatted BSFL has the lowest recovery (4.282 mg/g biomass). The reason behind the high chitosan recovery in fungi, especially Mucoraceae family may be due to the relatively high chitin content that can contribute up to 50% w of its total biomass composition [27]. In addition, chitosan from R. oryzae also shows a promising result which yielded up to 133.1 mg/g biomass. Chitosan in fungal cells are not only bound to glucan in membrane materials but also as free chitosan [28] During exponential growth phase, the amount of free chitosan molecules is relatively high due to active formation of the building blocks of cells [29]. Therefore, the formation of more cell walls results to the higher amount of chitin and chitosan. When the yield of biomass and chitosan is compared, the yield of R. oryzae biomass (1.307 g/100 mL) is higher than the Mucor spp. (0.554 g/100 mL), but the chitosan yield is the opposite. Extractable chitin and chitosan yield depends on species, culture condition, age or cultivation time, and extraction method [29, 30].
Table 1: Chitosan recovery form fungal and defatted BSFL biomass.
| Sample | Method | Chitin content (%) of biomass | Chitosan content (%) of biomass | Deacetylation degree (%) | Reference |
|---|---|---|---|---|---|
| Mucor spp. | Defatting: n-hexane, deproteination: 2% NaOH 90°C, deacetylation: 10% acetic acid 60°C, precipitation: alkali | 50.94 | 15.73 | 80.09 | This study |
| R. oryzae | 48.99 | 13.31 | 80.92 | ||
| F. pinicola | Acid pretreatment: 2 M HCl, demineralization: distilled water, deproteination: 2 M NaOH, decolorization: chloroform, methanol, water (1:2:4) | 30.11 | 21.60 | 73.1 | [7] |
| C. elegans R. arrhizus | Deproteination: 1 M NaOH, chitosan extraction: 2% acetic acid, precipitation chitosan: alkali | 7.229 8.32 | 3.31 4.93 | 25 82 | [27] |
| M. circinelloides | Deproteination: 2% NaOH, chitosan extraction: 10% acetic acid, precipitation: 4 M NaOH, washing: distilled water, ethanol, acetone | 50.0 | 6.4 | 83 | [29] |
| Defatted BSFL | Defatting: n-hexane, deproteination: 2% NaOH 90°C, deacetylation: 10% acetic acid 60°C, precipitation: alkali | 3.59 | 0.43 | 84.18 | This study |
| B. portentosus | Deproteination: 1 M NaOH, demineralization: 1 g/L oxalic acid, decolorization: 1% NaClO. deacetylation: 50% NaOH | 4.3–7.1 | 2.4–5.8 | 80.05 | [34] |
| Hermetia illucens | Defatting: CHCl3:CH3OH 20°C, demineralization: 2% HCl 20°C, deproteinization: 5% NaOH 50°C, deacetylation: 50% NaOH 100°C, purification: 1% CH3COOH, precipitation: 1 M NaOH, dialysis MWCO membrane | 7% | 0.26 | 90 | [31] |
| BSFL | Demineralization: 1 M HCl 70°C, deproteinization: 1 M NaOH 80°C, decolorization: acetone (100 mg/mL), deacetylation: 50% NaOH 65°C, purification: 0.1 M acetic acid 60°C | 9.2 | 8.5 | 82.05 | [37] |
| BSFL exoskeleton | Demineralization: 0.5 M formic acid, deproteinization: 2 M NaOH 80°C, deacetylation: 10 M NaOH 4°C | 28 | 14–16 | 34–72 | [47] |
BSFL: Black soldier fly larvae, R. oryzae: Rhizopus oryzae, F. pinicola: Fomitopsis pinicola, C. elegans: Cunninghamella elegans, R. arrhizus: Rhizopus arrhizus, M. circinelloides: Mucor circinelloides, B. portentosus: Brachytrupes portentosus.
 | Figure 1: Schematic diagram of chitin and chitosan production from Mucor spp. (a), Rhizopus oryzae (b), and black soldier fly larvae (c). [Click here to view] |
The yield of chitosan from BSFL is relatively higher than the reported value 0.26% [31]. The small recovery of chitosan from BSFL in this study may be due to small amount of insoluble dry matter from the deproteinization process (19.48% w) that still contains other components, such as unreacted chitin and ashes as impurities. From 21.64 g defatted sample that was deproteinated, only 5.24 g of pellet chitin rich was produced. Thus, the losses of chitosan recovery during the BSFL processing are relatively high with more than 50% of the component not being recovered. Chitin in insects binds with other molecules such as melanin, minerals, and others to form complex molecules which hinder the extraction and purification processes [32]. Recovery of chitin and chitosan may be different depending on sources, species, cultivation condition, time, and the method of extraction, isolation, and purification [33,34].
3.3. Physicochemical Properties of Chitin
Chitin obtained BSFL was subjected to FT-IR analysis and compared with crude chitin obtained from the deproteinization process as well with commercial chitin derived from shrimp [Figure 2]. The IR spectra from chitin BSFL, chitin shrimp, and deproteinized BSFL are almost identical which confirms that the product has a high purity. The functional group of the samples was compared with the peaks [Table 2]. All samples have two visible peaks in the range of 2000–1500 cm-1, more precisely at 1657 and 1564 cm-1 for deproteinized BSFL, 1659 and 1560 cm-1 for chitin derived from BSFL, and 1657 and 1557 cm-1 for chitin derived from shrimp. The peaks near 1650, 1620, and 1550 cm-1 correspond to C = O amide band of the a-chitin [35].
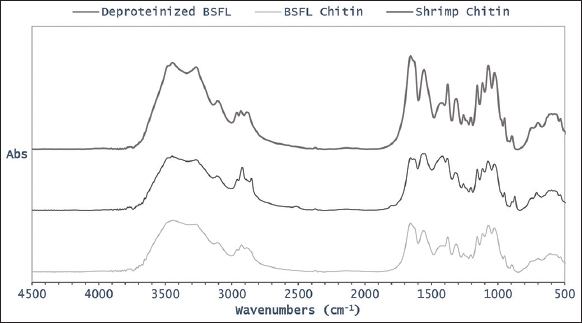 | Figure 2: Fourier-transformation infrared spectra of deproteinized black soldier fly larvae (BSFL), chitin derived from BSFL, and commercial chitin derived from shrimp. [Click here to view] |
Table 2: Fourier-transformation infrared peaks of deproteinized BSFL, chitin derived from BSFL, and commercial chitin derived from shrimp.
| Functional group and vibration modes | Wavenumber (cm-1) | ||||
|---|---|---|---|---|---|
| Deproteinized BSFLa | Chitin BSFLa | Chitin Shrimpa | Chitin BSFLb | Chitin B. portentosusc | |
| O-H stretching | 3447 | 3447 | 3445 | 3443 | 3433 |
| N-H stretching | 3269–3111 | 3271–3111 | 3269–3107 | 3269–3107 | 3103–3257 |
| CH3 sym. and CH2 asym. Stretching (Aliphatic compounds) | 2922 | 2926 | 2932 | 2926 | 2881 |
| C-H asym. Stretching (Aliphatic compounds) | 2852 | - | 2887 | 2891 | - |
| C=O secondary amide stretching (Amide I) | 1657 | 1659 | 1657 | 1659 | 1653 |
| C=O secondary amide stretching (Amide I) | - | - | - | 1626 | 1622 |
| N-H bending and C-N stretching (Amide II) | 1564 | 1560 | 1557 | 1559 | 1554 |
| CH2 bending and CH3 deformation | 1418 | - | 1420 | 1430 | 1423 |
| C-H bending and sym. CH3 deformation | 1381 | 1379 | 1377 | 1378 | 1375 |
| CH2 wagging (Amide II, components of protein) | 1321 | 1315 | 1314 | 1315 | 1311 |
| Asym. bridge oxygen stretching | 1157 | 1157 | 1157 | 1158 | 1153 |
| Asym. in-plane ring stretching | 1117 | 1115 | 1117 | 1116 | 1112 |
| C-O-C asym. stretching in phase ring (Saccharide rings) | 1076 | 1072 | 1074 | 1073 | 1066 |
| C-O asym. in phase ring | 1028 | 1028 | 1028 | 1028 | 1014 |
| CH3 wagging (Along chain) | 953 | 951 | 951 | 953 | 952 |
| CH ring stretching (Saccharide rings) | 874 | 897 | 897 | 896 | 896 |
*References:
a Current study,
In this study, a sharp amide peak is observed at a wavenumber near 1650 cm 1. This result agrees with the previous study that observed a peak at 1660 cm-1 for β-chitin [36]. The other significant peaks observed for distinguishing the α- and β-chitin are N-H bending and C-N stretching, N-H stretching, O-H stretching, asymmetric CH3 stretching, C-H stretching, C-O-C asymmetric stretching, and C-O-C symmetric stretching [35, 37]. The peak at 896–897 cm-1 is a sign of β-glycosidic bond presence which can be used as a reference for determining α-chitin [38]. The peaks are found in deproteinized BSFL, chitin derived from BSFL, and chitin derived from shrimp, as shown in Table 2. It was found that the peaks of chitin derived from BSFL, and shrimp examined in this study resembles the spectra of a α-chitin as reported in the previous studies [31, 34-37, 39, 40].
Fungal chitin also forms α-crystal as indicated by the peaks around 1650, 1620, and 1550 cm-1 [7]. The IR spectrum of chitin from R. arrhizus showed the presence of two amide I peaks (1652 and 1654 cm-1) and amide II peaks (1546 and 1564 cm-1). Other major peaks in the IR spectra are CH2 (1311 and 1313 cm-1), amide II (1146 and 1171 cm-1), C-O stretching of the -CH2-OH (1371 and 1377 cm-1), axial deformation of amide C-N (1411 and 1453 cm-1), C-H stretching (2933 and 2917 cm-1), and axial deformation of O-H (3441 and 3430 cm-1). The presence of these peaks suggests that the chitin examined in this study belongs to the α-form [27]. In another study, the chitin derived from Mucor circinelloides showed the presence of peaks in the amide I region at 1665, 1555, and 1313 cm-1 that may corresponds to the C = O stretching, N-H deformation in CONH plane and CN bond stretching, and CH2 wagging [29].
3.4. Physicochemical Properties of Fungal and Insect Chitosan
The IR spectra of chitosan derived from Mucor spp., R. oryzae, and BSFL are compared to the commercial chitosan derived from shrimp, as shown in Figure 3. All samples reveal sharp peak at 3431, 3431, 3412, and 3447 cm-1, respectively, due to vibration of NH2 in the primary amines association with vibration of OH in pyranose ring [Figure 3 and Table 3]. Amide I peak is present in all samples at 1639, 1637, 1655, and 1659 cm-1, respectively [Table 3]. However, amide II peak is present at 1564, 1574, and 1595 cm-1 only for chitosan derived from Mucor spp., R. oryzae, and shrimp. The presence of these peaks indicates the characteristic functional group of chitosan. However, only amide I was found at 1655 cm-1 for chitosan derived from BSFL. Other chitosan samples derived from R. arrhizus also show similar peaks at 1312–1311, 1453–1408 cm-1, with a sharp peak at 1654–1655 cm-1 and disappears at 1550 cm-1 due to the N-deacetylation process [27]. Similar findings have also been reported that sharp peaks were observed at 1658 and 1597 cm-1 for chitosan derived whereas amide peaks were observed at 1620 and 1556 cm-1 for chitosan derived from Brachytrupes portentosus [34, 37]. The IR spectrum of fungal-based chitosan with amide peaks shows that the chitin was not completely deacetylated to chitosan [27].
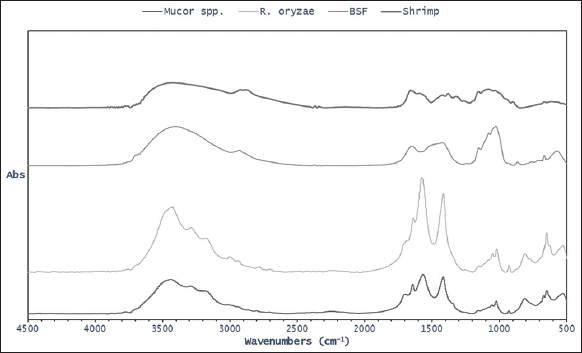 | Figure 3: Fourier-transformation infrared spectra of chitosan derived from Mucor spp., Rhizopus oryzae, and black soldier fly larvae and commercial shrimp. [Click here to view] |
Table 3: FT-IR peaks of chitosan derived from BSFL, and commercial chitosan derived from shrimp.
| Vibration modes | Wavenumber (cm-1) | |||||
|---|---|---|---|---|---|---|
| Chitosan Mucor spp.a | Chitosan R. oryzaea | Chitosan BSFLa | Chitosan Shrimpa | Chitosan BSFLb | Chitosan B. portentosusc | |
| v (NH2) assoc. in primary amines and v (OH) assoc. in pyranose ring | 3431 | 3431 | 3412 | 3447 | 3441 | 3263–3421 |
| vas (CH2) in CH2OH group | - | 2937 | 2930 | 2920 | - | 2920 |
| v (C-H) in pyranose ring | - | 2785 | - | 2881 | 2852 | 2885 |
| v (C=O) in NHCOCH3 group (Amide I) | 1639 | 1637 | 1655 | 1659 | 1658 | 1620 |
| v (NH2) in NHCOCH3 group (Amide II) | 1564 | 1574 | - | 1595 | 1597 | 1556 |
| δ(CH2) in CH2OH group | 1413 | 1414 | 1417 | 1422 | 1422 | 1425 |
| δs (CH3) in NHCOCH3 group | - | - | - | 1379 | 1380 | 1375 |
| δ(C-H) in pyranose group | - | - | - | - | 1323 | 1303 |
| vs (C-O-C) glycosidic linkage | 1151 | 1152 | 1153 | 1153 | 1155 | 1153 |
| vas (C-O-C) glycosidic linkage | - | 1045 | 1078 | 1080 | 1090 | 1066 |
| v (C-O) in secondary OH group | 1020 | 1016 | 1026 | 1032 | - | 1016 |
| v (C-O) in primary OH group | 925 | 925 | - | - | - | 900 |
| Pyranose skeletal ring vibrations | 806 | 808 | 866 | 897 | 895 | 830 |
*References:
a Current study,
One of the most important physicochemical properties of chitosan is its DD that contributes to its bioactivity. The value of DD can be determined by IR spectroscopy analysis to confirm the purity [31]. The percentage of free amine group (-NH2) in chitosan based on the relationship between absorbance values at 1655 and 3450 cm-1 represents amine and hydroxyl groups. The chitosan derived from BSFL (84.18%) has the highest DD value compared to other samples. The chitosan derived from R. oryzae and Mucor spp. also has high DD values of 80.92 % and 80.09%, respectively. The DD values were slightly higher compared to the chitosan derived from commercial shrimp analyzed in this study (79.14%). Those results are comparable with the previous DD value of chitosan derived from M. rouxii (80,3%) [41]. Another study reported that the DD value of chitosan derived from fungi lies in the range of 65–95% [42].
3.5. Antibacterial Activity of Chitosan
Antibacterial activity assay of chitosan derived from fungal and BSFL biomass was determined using disk diffusion test and the diameter of the inhibition zone is shown in Table 4. All samples can inhibit both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria as shown by the clear area around the disks which indicate that there was no colony-forming activity within the magnitude.
Table 4: Inhibition zone diameter of chitosan derived from fungal and BSFL biomass.
| Source of chitosan | Inhibition zone diameter (mm) | |
|---|---|---|
| S. aureus | E. coli | |
| Mucor spp. | 16.8±0.4 | 12.8±0.5 |
| R. oryzae | 20.9±0.1 | 14.9±0.5 |
| Defatted BSFL (30 mg/mL)* | 28.5±2.8 | 25.2±1.9 |
| (a) Chloramphenicol (30 mg/mL) | 28.8±0.3 | 20.3±0.4 |
| (b) Chloramphenicol (30 mg/mL) | 38.5±4.0 | 40.5±1.8 |
| Acetic acid (0.2%) | - | - |
* Same batch alongside antibacterial activity assay of chitosan from fungal biomass (a), and BSFL biomass (b), BSFL: Black soldier fly larvae, S. aureus: Staphylococcus aureus, E. coli: Escherichia coli, R. oryzae: Rhizopus oryzae.
The diameter of the inhibition zone varies among all the chitosan samples. The chitosan derived from BSFL shows the highest diameter of inhibition zone among all samples, particularly 28.5 mm against S. aureus and 25.2 mm against E. coli. This diameter size is nearly comparable to that commercial antibiotic, chloramphenicol, with the same concentration inhibited 28.8–38.5 mm against S. aureus and 20.3–40.5 mm against E. coli as reported by [43]. The larger diameter size of inhibition zone is not always linearly correlated to the higher activity of antibacterial agent. The inhibition zone does not indicate that the chitosan has killed the bacteria but only prevent their growth. The previous study also reported that 20% chitosan resulted in 3 mm diameter of inhibition zone against both of S. aureus and E. coli, but the percentage of bacterial reduction was lower for E. coli (88.84%) as compared to S. aureus (95.75%) [43]. Nevertheless, the antibacterial activity demonstrated by the samples indicates the capability of inhibiting bacterial grow.
The chitosan has positive charges that bind to the negative charges on cell membrane and causes bacterial lysis. The density of positive charge is associated with DD% of chitosan or its derivative [44]. Therefore, the higher percentage of DD as well as concentration of chitosan will lead to more positive charges that can bind to the negative charges of the cell membrane and inhibit bacterial growth. In this study, the diameter of the inhibition zone for chitosan derived from BSFL is larger than the Mucor spp. and R. oryzae which may be due to the higher percentage of DD. The chitosan can act as a chelating agent to bind metal ions and inhibit microorganism growth and its product formation [45]. In addition, at lower concentration, chitosan is polycationic that can bind the negative charge on bacterial surface, causing lysis, while at higher concentration; a large amount chitosan forms a net of positive charge to trap microbes [46].
4. CONCLUSION
In brief, this study successfully determined chemical characteristic and antimicrobial activity of chitosan obtained from fungi Mucor spp., R. Oryzae, and H. illucens. The chitosan derived from Mucor spp. biomass has the highest recovery (157.3 mg/g biomass), followed by R. oryzae (133.1 mg/g biomass) and H. illucens (4.28 mg/g biomass). However, the chitosan derived from H. illucens has the highest DD (84.18%) followed by R. oryzae (80.92%) and Mucor spp (80.09%). All the chitosan samples can inhibit the Gram-negative (S. aureus) and Gram-positive (E. coli) bacteria.
6. FUNDING
Research grant from Institut Teknologi Bandung (Grant number: PPMI SITH 2021).
6. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
7. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
Additional data is available upon request.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Arbia W, Arbia L, Adour L, Amrane A. Chitin extraction from crustacean shells using biological methods-a review. Food Technol Biotechnol 2013;51:12-25.
2. Kim S. Competitive biological activities of chitosan and its derivatives:Antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int J Polym Sci 2018;2018:1-13. [CrossRef]
3. Ali SS, Kenawy ER, Sonbol FI, Sun J, Al-Etewy M, Ali A, et al. Pharmaceutical potential of a novel chitosan derivative Schiff base with special reference to antibacterial, anti-biofilm, antioxidant, anti-inflammatory, hemocompatibility and cytotoxic activities. Pharm Res 2019;36:5. [CrossRef]
4. Gortari MC, Hours RA. Biotechnological processes for chitin recovery out of crustacean waste:A mini review. Electron J Biotechnol 2013;16:14. [CrossRef]
5. Madni A, Kousar R, Naeem N, Wahid F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J Bioresour Bioprod 2021;6:11-25. [CrossRef]
6. Pusztahelyi T. Chitin and chitin-related compounds in plant-fungal interactions. Mycology 2018;9:189-201. [CrossRef]
7. Kaya M, Akata I, Baran T, Mente?A. Physicochemical properties of chitin and chitosan produced from medicinal fungus (Fomitopsis pinicola). Food Biophys 2015;10:162-8. [CrossRef]
8. Kaya M, Baran T. Description of a new surface morphology for chitin extracted from wings of cockroach (Periplaneta americana). Int J Biol Macromol 2015;75:7-12. [CrossRef]
9. Wu T, Zivanovic S, Draughon FA, Conway WS, Sams CE. Physicochemical properties and bioactivity of fungal chitin and chitosan. J Agric Food Chem 2005;53:3888-94. [CrossRef]
10. Bullerman LB. Encyclopedia of Food Sciences and Nutrition. 2nd ed. United States:Academic Press;2003.
11. Botha A, du Preez JC. Encyclopedia of Food Microbiology. Netherland:Elsevier;1999.
12. Cantabrana I, Perise R, Hernández I. Uses of Rhizopus oryzae in the kitchen. Int J Gastron Food Sci 2015;2:103-11. [CrossRef]
13. Pochanavanich P, Suntornsuk W. Fungal chitosan production and its characterization. Lett Appl Microbiol 2002;35:17-21. [CrossRef]
14. Elieh-Ali-Komi D, Hamblin MR. Chitin and chitosan:Production and application of versatile biomedical nanomaterials. Int J Adv Res (Indore) 2016;4:411-27.
15. Soetemans L, Uyttebroek M, Bastiaens L. Characteristics of chitin extracted from black soldier fly in different life stages. Int J Biol Macromol 2020;165:3206-14. [CrossRef]
16. Pokhrel S, Yadav PN, Adhikari R. Applications of chitin and chitosan in industry and medical science:A review. Nepal J Sci Technol 2015;16:99-104. [CrossRef]
17. Yeul VS, Rayalu SS. Unprecedented chitin and chitosan:A chemical overview. J Polym Environ 2013;21:606-614. [CrossRef]
18. Jamilah M. Bioconversion of Sugar Cane Juice (Saccharum Officinarum) into Biomass of Mucorales Fungi to Produce Chitosan as a Natural Preservative. Thesis. Indonesia:Institut Teknologi Bandung;2017.
19. Firmansyah M, Abduh MY. Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 2019;5:e02005. [CrossRef]
20. Synowiecki J, Al-Khateeb NA. Mycelia of mucor rouxii as a source of chitin and chitosan. Food Chem 1997;60:605-10. [CrossRef]
21. Monteiro OA Jr., Airoldi C. The influence of chitosans with defined degrees of acetylation on the thermodynamic data for copper coordination. J Colloid Interface Sci 2005;282:32-7. [CrossRef]
22. Cavalieri SJ. Manual of Antimicrobial Susceptibility Testing. Washington, DC:American Society for Microbiology;2005.
23. Kwon JH, Kang SW, Kim JS, Park CS. Rhizopus soft rot on cherry tomato caused by Rhizopus stolonifer in Korea. Mycobiology 2001;29:176-8. [CrossRef]
24. Chongkae S, Nosanchuk JD, Pruksaphon K, Laliam A, Pornsuwan S, Youngchim S. Production of melanin pigments in saprophytic fungi in vitro and during infection. J Basic Microbiol 2019;59:1092-104. [CrossRef]
25. Ushakova N, Dontsov A, Sakina N, Bastrakov A, Ostrovsky M. Antioxidative properties of melanins and ommochromes from black soldier fly Hermetia illucens. Biomolecules 2019;9:408. [CrossRef]
26. Mérillon JM, Ramawat KG. Fungal Metabolites. 3rd ed. Germany:Springer;2017. [CrossRef]
27. Berger LR, Stamford TC, Stamford-Arnaud TM, de Alcantara SR, daSilva AC, da Silva AM, et al. Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopus arrhizus and Cunninghamella elegans strains. Int J Mol Sci 2014;15:9082-102. [CrossRef]
28. Nwe N, Furuike T, Osaka I, Fujimori H, Kawasaki H, Ryuichi A, et al. Laboratory scale production of 13C labeled chitosan by fungi Absidia coerulea and Gongronella butleri grown in solid substrate and submerged fermentation. Carbohydr Polym 2011;84:743-50. [CrossRef]
29. Fai AE, Stamford TC, Stamford-Arnaud TM, Santa-Cruz PD, da Silva MC, Campos-Takaki GM, et al. Physico-chemical characteristics and functional properties of chitin and chitosan produced by Mucor circinelloides using yam bean as substrate. Molecules 2011;16:7143-54. [CrossRef]
30. Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl Biochem Biotechnol 2017;181:1314-37. [CrossRef]
31. Khayrova A, Lopatin S, Varlamov V. black soldier fly Hermetia illucens as a novel source of chitin and chitosan. Int J Sci 2019;8:81-6. [CrossRef]
32. Nwe N, Uike TF, Tamura H. Production, properties and applications of fungal cell wall polysaccharides:Chitosan and glucan. Adv Polym Sci 2011;244:187-208. [CrossRef]
33. Mitra D, Rasmussen ML, Chand P, Chintareddy VR, Yao L, Grewell D, et al. Value-added oil and animal feed production from corn-ethanol stillage using the oleaginous fungus Mucor circinelloides. Bioresour Technol 2012;107:368-75. [CrossRef]
34. Ibitoye EB, Lokman IH, Hezmee MN, Goh YM, Zuki AB, Jimoh AA. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed Mater 2018;13:025009. [CrossRef]
35. Purkayastha D, Sarkar S. Physicochemical structure analysis of chitin extracted from pupa exuviae and dead imago of wild black soldier fly (Hermetia illucens). J Polym Environ 2020;28:445-57. [CrossRef]
36. Kaya M, Baublys V, Can E, Šatkauskien?I, Bitim B, Tubelyt?V, et al. Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology 2014;133:285-93. [CrossRef]
37. Leke-Aladekoba AA. Comparison of Extraction Methods and Characterisation of Chitin and Chitosan with Antimicrobial and Antioxidant Properties from black soldier fly (Hermetia illucens) Meal. Master of Science. Thesis. Halifax, Novia Scotia:Dalhousie University;2018.
38. Wysokowski M, Bazhenov VV, Tsurkan MV, Galli R, Stelling AL, Stöcker H, et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int J Biol Macromol 2013;62:94-100. [CrossRef]
39. Kanto DA, Permana AD, Hertadi R. Extraction and characterization of chitin and chitosan from black soldier fly (Hermetia illucens). J Ilmiah Farmako Bahari 2019;10:23-32. [CrossRef]
40. Ravi HK, Degrou A, Costil J, Trespeuch C, Chemat F, Vian MA. Effect of devitalization techniques on the lipid, protein, antioxidant, and chitin fractions of black soldier fly (Hermetia illucens) larvae. Eur Food Res Technol 2020;246:2549-68. [CrossRef]
41. Gharieb MM, El-Sabbagh SM, Shalaby MA, Darwesh OM. Production of chitosan from different species of zygomycetes and its antimicrobial activity. Int J Sci Eng Res 2015;6:124-30.
42. Shimahara K, Takiguchi Y, Kobayashi T, Uda K, Sannan T. Screening of Mucoraceae strains suitable for chitosan production. In:Chitin and Chitosan. New York:Elsevier Applied Science;1989. 171-8.
43. Teli MD, Sheikh J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. Int J Biol Macromol 2012;50:1195-200. [CrossRef]
44. Atay HY. Antibacterial activity of chitosan-based systems. In:Functional Chitosan. Singapore:Springer;2019. 457-89. [CrossRef]
45. Cuero RG, Osuji G, Washington A. N-carboxymethylchitosan inhibition of aflatoxin production:Role of zinc. Biotechnol Lett 1991;13:441-4. [CrossRef]
46. Sudharshan NR, Hoover DG, Knorr D. Antibacterial action of chitosan. Food Biotechnol 1992;6:257-72. [CrossRef]
47. Hahn T, Roth A, Ji R, Schmitt E, Zibek S. Chitosan production with larval exoskeletons derived from the insect protein production. J Biotechnol 2019;310:62-7. [CrossRef]