1. INTRODUCTION
Nerium odorum Linn., commonly identified as rose ray, is an evergreen shrub, used as ornamental plant belongs to family Apocynaceae, omnipresent in temperate and subtropical regions of Africa, Europe, and South East Asia. The plant is widely grown in Indian gardens and found in humid and coastal areas including Assam, Arunachal Pradesh, Himachal Pradesh, Rajasthan, Karnataka, and in all parts of India. N. odorum plant is one of the most toxic plants of this family and it is effective in snakebite cure. The crushed leaves, twig, root, and bark are used as a rat poison and as an insecticide too. The root is suggested for external appliance to skin diseases, and the plant is popular medication for mental or venereal diseases as abortifacient in traditional medicine. The oil extracted from the root bark is suggested for medication against leprosy, epilepsy, and scaly nature of skin/skin diseases. The N. odorum leaves consist of minute quantity of latex which is used to make rubber [1].
N. odorum produces several secondary metabolites among these alkaloids are having a number of pharmacological attention and even primary compounds such as cardenolides, flavonoids, and terpenes have their own attention. Oleandrin has been recognized as effective antitumor compound; in addition, it is used indigenously as a cardiac tonic and diuretic. N. odorum is recognized to be toxic against extensive range of tumor cells, and this plant also recognized as antioxidants, the leaves of N. odorum contain two novel cytotoxic pentacyclic and trans-Karenin and potential secondary metabolites [2]. Similar reports are also indicated the occurrence of oleandrin, folineriin, adynerin, and digitoxigenin cardiac glycosides in N. odorum [3]. The plant seeds consist of about 12% of 9-hydroxy-isoricinoleic acid. Methanolic extract of the leaves was found to be anticonvulsant, central nervous system depressant, and analgesic. N. odorum extracts have been reported to for oleanolic acid. Lipid peroxidation inhibitory activity from flowers of N. odorum was reported earlier [4]. N. odorum plants with milky sap have a high medicinal value; the enlarge demands of N. odorum plant for industrial and commercial exploitation require a substitute rate of proliferation.
During these decades, in vitro practice of callus culture is being extensively useful to produce indistinguishable quality of callus and disease-free plants. In observation of this evidence, the study was conducted for in vitro propagation of N. odorum. The cultured plant callus is extensively accepted as capable alternative for the production of secondary metabolites [5-7]. The opportunity of housing cell and tissue cultures for secondary metabolites production has been investigated, but the yield has not been optimized to reach higher levels for commercial application [8,9].
In the literature, no reports are available on the effect of growth hormones and their interactions toward secondary metabolites production, antioxidant, and antibacterial activities in callus cultures of N. odorum. In this connection, the present study aims for to compare the effects of different growth hormones, on the production of total phenols, antioxidant, and antimicrobial activities of N. odorum callus.
2. MATERIALS AND METHODS
2.1. Collection of Explants, Implantation, and Culture Conditions
The N. odorum, plant materials, was brought from plants grown in the Botanical Garden of University of Mysore, Mysore, Karnataka, India. An authentic sample was identified by taxonomist and the voucher specimen was submitted to the herbarium of the Department of Studies in Biotechnology, University of Mysore, Mysore. Leaves of 8-12 cm length were collected from N. odorum plants growing at an altitude of 2400 m at location 12°18’29.45”N and 76°38’18.83”E in the area of Mysore region, Karnataka, in June 2015 and were used for callus initiation.
2.2. Initiation of Callus Cultures
The newly formed young leaves excised from N. odorum were further sterilized in 0.5% (w/v) mercuric chloride used for 15 min and washed for 5 times for 5 min each in sterile H2O. N. odorum leaves were cut into small pieces and inoculated to a sterile Murashige and Skoog’s (MS) medium for callus initiation [10], supplemented with 8% agar, along with 2,4-dichlorophenoxyacetic acid (2,4-D), indole- 3-acetic acid (IAA), 6-benzylaminopurine (BAP), kinetin (Kin), and 1-naphthaleneacetic acid (NAA) (Hi-Media, Bangalore, India) at different concentration of 0.5-5 mg/L. The sucrose was used as a single carbon source; the pH optimized to 5.8 before autoclaving with 1N NaOH and HCL. The explants on the medium were incubated at 24°C ± 2°C under diffuse fluorescent light (72/71 intensity) in an 18-h photoperiod. Calli were maintained with regular subculturing on a monthly basis which was originated from the explants within 21 days.
2.3. Determination of Callus Fresh/Dry Weight (FW/DW) and Callus Moisture Content
The callus was collected from tissue culture laboratory after 40 days of inoculation, and its media was completely washed with sterile distilled water. The calluses were placed beneath a fan (on blotting paper) to remove water and the weight of callus was weighed in a balance. Subsequently, the calli were positioned on Petri dishes and heated in an oven for 10 min at 65°C for drying. DW of callus was determined in an electronic balance. The moisture content was determined using the FW and DW of callus by the following procedures of Rashmi and Trivedi [11].
A=Weight of empty Petri dish
B=Weight of Petri dish with fresh calli
C=Weight of Petri dish with dried calli
Moisture content % = (B-A)-(C-A)/(B-A) x 100
2.4. Extract Preparation
Calli on diverse growth hormones were harvested, 200 mg of DW in each callus was successively extracted successively utilizing 500 ml of non-polar, moderately polar, and polar solvents (Merck, Bangalore, India) in increasing polarity (hexane < ethyl acetate < methanol < water) using a Soxhlet apparatus by continuous hot percolation (boiling point, 52-62°C) until the solvent became colorless. The resultant solvent extracts were concentrated in a rotary evaporator (Thermo Scientific, Germany) under controlled pressure. For the studies undertaken, the required amount of extract was weighed and solubilized in dimethyl sulfoxide (1 mg/ml) and was further diluted as indicated in the sections below.
2.5. Determination of Antioxidant Activity
2.5.1. Total phenolic content, 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay, and 2,2’-azinobis-(3- ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical cation decolorization assay
The total polyphenols of the Soxhlet extracts were determined by Folin-Ciocalteu method as described by Pasko et al. [12]. The free radical scavenging capacity of Soxhlet extract was determined by DPPH and ABTs method following the procedures of Brand-Williams et al. [13] and Re et al. [14]. The experiments consisted of three replicates and were repeated 3 times.2.6. Antibacterial Activity
The Gram-negative bacteria, Escherichia coli, Klebsiella pneumonia, Salmonella, and Shigella and the Gram-positive bacteria, Staphylococcus aureus and Bacillus subtilis were obtained from stock culture unit of the Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore, Karnataka, India, and used for the assay. The extracts were screened for antibacterial activity using well diffusion method. The Soxhlet extracts from callus (100 μg/ml) were added to wells seeded agar plates. The plates were incubated at 37°C ± 2°C for 16 h. Chloramphenicol and methanol were used as positive and negative controls, respectively.
2.7. Statistical Analysis
All experiments including DPPH, ABTS, measurements of total phenolic content, and antimicrobial activity assay were conducted in triplicates and repeated for 3 times. The reported value for each sample was calculated as the mean and standard deviation of three independent experiments.
3. RESULTS
3.1. Effects of Hormonal Treatments on Callus Induction
Plant growth regulators are synthetic molecules used in plant tissue culture and supplemented at relatively low concentrations to work as signaling compounds for plant growth and development. In the present study, stem (nodal) and leaf were used as explants, in which leaf part of explants appear to be the best for callus induction which represents the results in accordance with the earlier reports Rashmi and Trivedi [11]. The MS medium, without any growth hormones, was unable to induce callus. Among all the growth hormones, IAA and 2,4-D hormones exhibited more competence in callus induction in individual along with their different combinations which correlate the earlier reports [11,15]. The MS medium supplemented with different combination of enzyme, with different concentration varying from 0.5 to 5 mg/L of IAA, NAA, 2,4- D, BAP, and Kin exhibited stimulation and induction of callus. The highest maximum callusing retort of 89 % was observed at the 2.0 mg/L of IAA [Figure 1]. At 0.5 mg/L of IAA, the induction of callus response was very less; simultaneously, on increase in the hormonal concentration up to 2 mg/L, the induction of callus response was very good. Interestingly, at 2.5 mg/L, and onward, callusing response was reduced at 5 mg/L, no callusing or growth was observed.
 | Figure 1: Callus induction of MS fortified with growth hormones. A; 2,4-D (2.5 mg/L), B; IAA (2.0 mg/L), C; Combination of 2,4-D and IAA at concentration 2.0 mg/L respectively. [Click here to view] |
 | Table 1: Callus induction on leaf explants on MS medium supplemented with different concentrations and combinations of growth hormones (mg/L) in Nerium odorum. [Click here to view] |
MS media supplemented with varying concentration of 0.5-2.0 mg/L of NAA and BAP exhibited significant effect on callus induction. The maximum highest callusing response (25%) was recorded at 2.5 and 1 mg/L for BAP and NAA. Different concentrations of NAA and Kin did not show any stimulatory effects on callus induction. Maximum callusing response (17%) was recorded at 1 and 1.5 mg/L for Kin and BAP. At 2.5-5 mg/L of Kin and BAP, no callusing or growth was observed. In the present work, BAP, NAA, and Kin either individually or in combination could not induce callus significantly. In further experiments, BAP and NAA were supplemented to the MS media in combination with auxins (2,4- D and IAA). The optimum treatment for callus induction in this study was identified in MS medium supplemented with 2.0 mg/L D and 2.0 mg/L IAA combination. The findings revealed that the supplementation of auxins at an optimum concentration and combination with 2,4-D and IAA is required to produce calli with the desirable morphology. The hormonal combination of 2,4-D and IAA was found effective in producing optimum callus induction in several plant species. The FW and DWs and moisture content showed good growth of callus on 2,4-D and IAA along with their combinations [11,15,16].
3.2. Effects of Growth Hormones on Nature and Moisture Content of Callus
The FW and DW of the callus was measured after various periods, the significant difference in FW and DW (P = 0.01) was observed in the value of weight from callus initiation up to 6 months, whereas no significant differences were found at 6-9 months. The time involved of callus growth in its optimization of media and a uniform callus was obtained after 5 months with frequent subculturing. The values for FW and DW after 1 month were 256 and 53 mg, respectively. These FW and DWs increased rapidly to 3622 and 198, respectively, after 6 months by frequent subculturing. The FW and DW of callus from 6 to 9 months exhibited 268 mg of callus increased to an average weight of 357 mg every month in FW. Figure 1 depicts the callus cultures of N. odorum. Leaf callus was loose in texture and friable. It was white and yellow in color with different growth hormone treated with combinations of 2,4-D. Callus was compact and non-friable, light yellow to light green in color with different growth hormone in combinations of IAA [Table 1]. The moisture content varied in the callus derived from explants under the influence of various 2,4-D and IAA. It was observed that moisture content varied from 69% to 80% which supports good growth of callus.
3.3. In Vitro Antioxidant Assays
Three in vitro tests (total phenolic content, DPPH, and ABTS) were used to characterize antioxidant property of an isolated callus of N. odorum. The phenolic content of N. odorum callus cultures under different growth hormonal conditions is presented in Figure 2. The results show that the production of polyphenols was affected by the type of growth hormone used during the growth of callus. The total phenolic content of ethyl acetate extract was between 37.81 and 23.96 mg GA/g DW for IAA and 2,4-D, respectively, except these for the all other growth hormone (BAP and NAA) the phenolic content have no significant effect. Interestingly, when comparing with the combination of IAA and 2,4-D, the production of polyphenols by callus of N. odorum is superior to the production of polyphenols by individual growth hormones IAA and 2,4-D, which exhibited a total phenolic content of 92.14 mg GA/g DW [Figure 2].
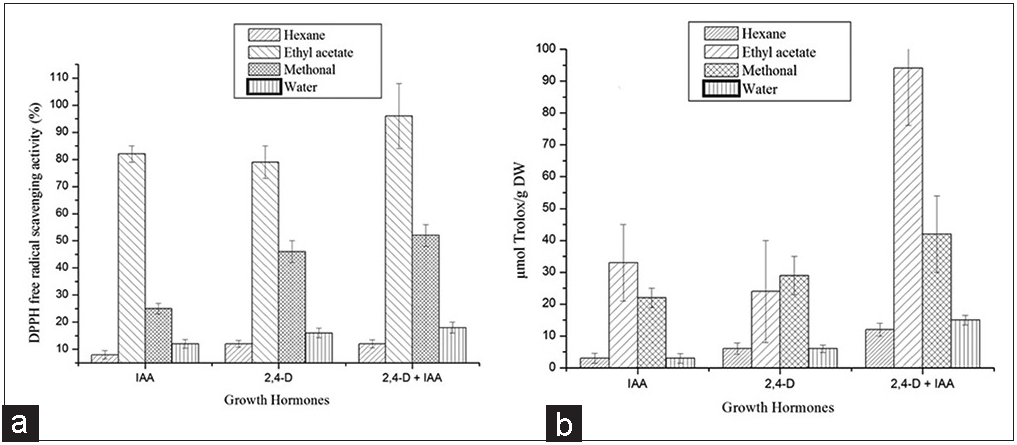 | Figure 2: Total phenolic content in callus cultures of Nerium odorum under different growth hormonal conditions in different solvent extraction. The values are means of three individual experiments with three replicates. Bars indicate standard errors. [Click here to view] |
 | Figure 3: The DPPH and ABTS capacity of callus cultures of Nerium odorum under different growth hormonal conditions in different solvent extraction. The values are means of three individual experiments with three replicates. Bars indicate standard errors. [Click here to view] |
The ethyl acetate extracts of callus from N. odorum were highly inhibiting the growth of both Gram-positive and Gram-negative bacteria. The variation with respect to concentration of extract, the zone of inhibition was observed from 10 to 16 mm from the extracts of IAA and 2,4-D growth hormones [Table 2]. The extracts of 2,4- D were most effective, exhibiting a zone of inhibition ranged from 19 to 24 mm for B. subtilis, K. pneumonia, and S. aureus, whereas the inhibition zone for E. coli ranged from 13 to 17 mm. The extract of IAA showed zone of inhibition ranging from 8 to 16 mm against K. pneumonia, B. subtilis, and E. coli [Table 2]. The antibacterial activity of the tuber may be due to the presence of phenolic active compounds in N. odorum. Antibacterial effect against Gram-negative and Gram-positive bacteria could be as natural source for producing pharmacological products [19,20]. The results of the current study supported the traditional treatment by medicinal plants and proposed antibacterial agents from plant extracts with antibacterial properties. The maximum activity was observed against Gram-negative and Gram-positive bacteria from ethyl acetate extract grown on 2,4- D compared with IAA and along with the combination of IAA and 2,4- D, respectively. Antimicrobial properties of medicinal plants are being increasingly stated from various parts of the world. Based on the World Health Organization report, the plant active constituents are used as folk medicine in traditional therapies of 80% of the world’s population [20,21]. In this study, the extracts obtained showed strong activity against most of the tested bacterial strains. The results were compared with standard drug. The effect of antibacterial in medicinal plants varies intensely depending on the phytochemical features of plant [19,20].
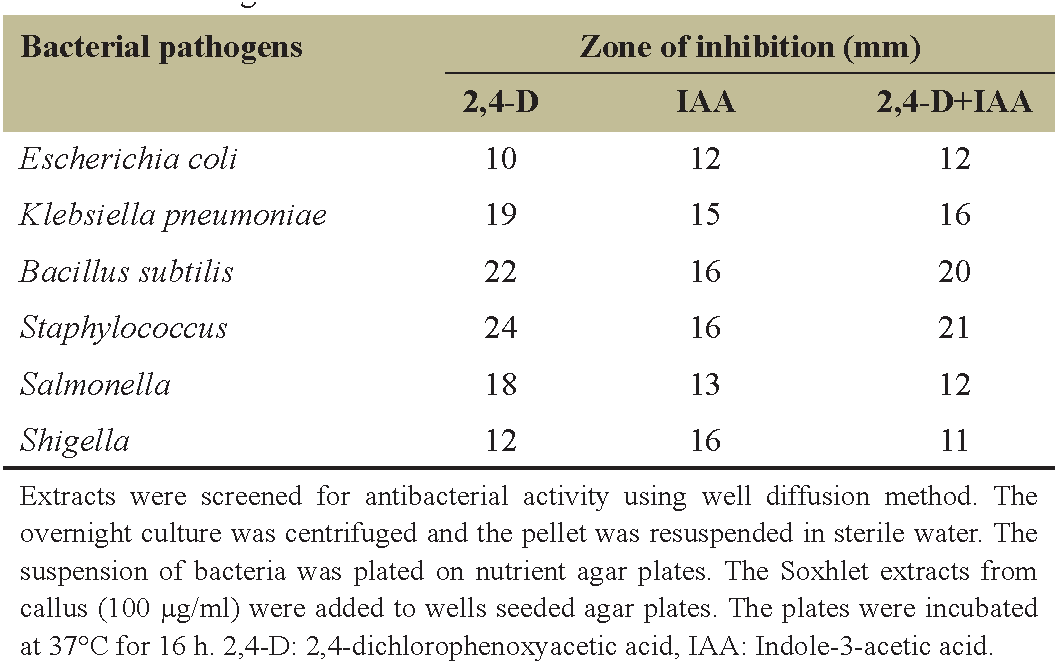 | Table 2: Antimicrobial activity and the zone of inhibition of the ethyl acetate extracts from callus supplemented with different concentrations and combinations of growth hormones. [Click here to view] |
4. CONCLUSION
Finally, it can be concluded that combinations of IAA and 2,4-D in MS media exhibited the highest calli formation from leaf explants of N. odorum. Our data revealed that calli subculture on IAA and 2,4-D was effective in increasing phenolic content and antioxidant property of IAA and 2,4-D exudation. This study also showed that ethyl acetate extracts from in vivo gave higher results for antioxidant activity and inhibition zone against Gram-negative and Gram-positive bacteria compared to the same concentration of callus extract from individual growth hormones (IAA/2,4-D). Further and more specific studies toward characterization of compounds, in vivo or in vitro, are recommended to determine the characteristics of secondary metabolites.
5. ACKNOWLEDGMENTS
This work was supported by the grants from DBT-MRP [BT/PR10406/ SPD/9/1232/2014]. Authors are grateful to IOE, University of Mysore, for spectral characterization and Chairman, DOS in Biotechnology, University of Mysore, Mysore-570006, for their infrastructure facilities.
6. REFERENCES
1. Zibbu G, Batra A. A review on chemistry and pharmacological activity of Nerium oleander L. J Chem Pharm Res 2010;2:351-8.
2. Siddiqui BS, Khatoon N, Begum S, Farooq AD, Qamar K, Bhatti HA, et al. Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity. Phytochemistry 2012;77:238-44.
3. Bandara V, Weinstein SA, White J, Eddleston M. A review of the natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon 2010;56:273-81.
4. Dey P. Chaudhuri TK. Pharmacological aspects of Nerium indicum Mill: A comprehensive review. Pharm Rev 2014;8:156.
5. Lipsky KA. Problems of optimization of plant cell culture processes. J Biotechnol 1992;54:93-108.
6. McDonald KA, Jackman AP, Thorup JE, Dandekar AM. Plant callus as a source for biochemicals. Appl Biochem Biotechnol 1997;54:89-108.
7. Dix PJ. Plant Cell Line Selection. Weinheim: VCH; 1990. p. 4-17, 30-7, 187-211.
8. Yeoman MM, Yeomen CL. Manipulating secondary metabolism in cultured plant cells. Plant Cell Rep 1996;134:553-69.
9. Profumo P, Gastado AM, Riboldi CU. Formation of cardiac glycosides in calli from leaf explants of Nerium oleander L. Plant Med Phytother 1993;26:346-9.
10. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 1962;15:473-97.
11. Rashmi R, Trivedi MP. Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Appl Biochem Biotechnol 2014;172:2562-70.
12. Pasko P, Barton H, Zagrodzki P, Gorinstein S, Folta M, Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem 2009;115:994-8.
13. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 1995;28:25-30.
14. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231-7.
15. Sehrawat AR, Sanjogta U, Chowdhury JB. Establishment of plantlets and evaluation of differentiated roots for alkaloids in Rauwolfia serpentina. J Plant Biochem Biotechnol 2002;11:105-8.
16. Phelan S, Hunter A, Douglas GC. Effect of explants source on shoot proliferation and adventitious regeneration in 10 Buddleia cultivars. Sci Hortic 2009;120:518-24.
17. Chidambaram U, Pachamuthu V, Natarajan S, Elango B, Suriyanarayanan, Ramkumar KM, et al. In vitro evaluation of free radical scavenging activity of Codariocalyx motorius root extract. Asian Pac J Trop Med 2013;6:188-94.
18. Farzinebrahimi R, Mat Taha R, Rashid KA, Ali Ahmed B, Danaee M, Rozali SE. Preliminary screening of antioxidant and antibacterial activities and establishment of an efficient callus induction in Curculigo latifolia Dryand (Lemba). Evid Based Complement Altern Med ECAM 2016;2016:6429652.
19. Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two Gramnegative and Gram-positive bacteria. Int J Microbiol 2013;2013:746165.
20. Oroojalian F, Kasra-Kermanshahi R, Azizi M, Bassami MR. Phytochemical composition of the essential oils from three Apiaceae species and their antibacterial effects on food-borne pathogens. Food Chem 2010;120:765-70.
21. Prakasha A, Grice ID, Kumar KV, Sadashiva MP, Shankar HN, Umesha S. Extracellular polysaccharide from Ralstonia solanacearum; A strong inducer of eggplant defense against bacterial wilt. Biol Control 2017;110:107-16.