1. INTRODUCTION
Selenium is an essential trace element that plays a crucial role in various biological functions in both humans and animals. Though required in small amounts, it is indispensable for maintaining overall health and preventing disease. Selenium is incorporated into selenoproteins, vital for antioxidant defense, regulation of redox processes, and metabolism of thyroid hormones. These functions underscore the importance of selenium in biological systems and highlight its significance as a micronutrient essential for optimal health [1,2]. Its biological significance is highlighted through its role in crucial enzymes like glutathione peroxidase, which shields cells from oxidative harm by lowering hydrogen peroxide and organic hydroperoxides. Furthermore, selenium plays a pivotal role in thioredoxin reductase, aiding in the preservation of the cell’s reducing environment and participating in DNA synthesis and repair processes [3,4]. Selenium deficiency poses significant health risks, including the development of Keshan disease, a cardiomyopathy prevalent in regions low in selenium, and Kashin-Beck disease, characterized by osteoarthropathy that affects bone and joints. These conditions underscore the critical role of selenium in maintaining overall health and preventing specific diseases associated with its deficiency [5]. However, selenium’s role as an antioxidant has spurred investigations into its potential to mitigate the risk of chronic conditions such as cancer, cardiovascular disease, and thyroid dysfunction [6]. Selenoproteins, including glutathione peroxidase and thioredoxin reductase, play crucial roles in cellular antioxidant defense, safeguarding cells from oxidative stress and ensuring cellular balance [7]. These enzymes are involved in detoxification of reactive oxygen species and modulation of thyroid hormone metabolism, indicating selenium’s role in metabolic regulation [1].
Selenium-enriched probiotics can thus bolster body’s antioxidant defense, reducing oxidative stress and its associated risks, such as chronic inflammation and cancer. The synergy of selenium and probiotics can therefore provide a robust boost to the immune system, potentially lowering the risk of infections and autoimmune diseases [8]. Probiotics are extensively used to promote gastrointestinal health by balancing the gut microbiota, preventing the growth of harmful bacteria, and enhancing the gut barrier function. Selenium-enriched probiotics can further support gastrointestinal health by reducing inflammation and oxidative damage in the gut, thereby improving overall gut function and preventing gastrointestinal diseases [9]. The combination of selenium and probiotics can thus provide synergistic benefits, enhancing immune function more effectively than either component alone. Selenium-enriched probiotics have been shown to improve the immune response in both animal models and human studies, indicating their potential in boosting immune health and reducing infection risk [10].
Spore-forming probiotics, particularly from Bacillus, are commonly isolated from fermented cereals. Studies in isolated Bacillus subtilis and Bacillus coagulants from various fermented foods have noted their high resilience and ability to survive harsh conditions such as those in the gastrointestinal tract. These strains are identified through advanced techniques such as high-throughput sequencing and metagenomics, which help in understanding their composition and functional potential [11,12]. Isolated strains from fermented cereals exhibit various probiotic properties, including antimicrobial activity, the ability to modulate the gut microbiota, and immune-enhancing effects. Recent research evidences prove that Bacillus strains isolated from fermented cereals have shown potential in improving gut health and protecting against gastrointestinal pathogens [13]. In India, the consumption of naturally fermented foods varies across regions and communities, reflecting diverse cultural practices. Fermentation, a traditional global practice, enhances taste and nutritional value, offers health benefits, and extends food shelf life by acting as a natural preservative [14-16]. Koozh is a delicious, naturally fermented cereal-based beverage consumed regularly in the rural areas of Tamil Nadu, India. It is a significant staple meal and offers nourishment and energy to millions of people in India with low and medium incomes. It is made by cooking presoaked millet or rice, fermenting it overnight, and then blending with or without curd for breakfast [14]. Research indicates that selenium-enriched probiotics can improve metabolic health by modulating lipid metabolism and enhancing insulin sensitivity. This is particularly beneficial for conditions such as non-alcoholic fatty liver disease and obesity induced by high-fat diets [17,18].
Despite their potential, there are challenges in the consistent isolation and cultivation of these probiotics. Factors such as the medium composition, sporulation conditions, and recovery techniques are critical for optimizing the yield and viability of spore-forming probiotics. Future research is directed toward optimizing these conditions and exploring the synergistic effects of different probiotic strains in fermented cereal matrices [19]. Selenium-enriched bacterial strains can biotransform inorganic selenium into organic selenium. Thus, it has been demonstrated that selenium-enriched strains can meet the recommended dietary allowance of populations, prevent diseases, and improve immune function and gastrointestinal health [20].
The bioavailability of selenium (Se) is affected by its chemical form and the microbial carrier. Probiotic bacteria, including Lactobacillus and Bifidobacterium, have been identified to transform inorganic selenium (e.g., selenite) into organic forms such as selenomethionine and selenium-enriched proteins, which exhibit greater bioavailability and reduced toxicity to humans [21]. Furthermore, selenium-enriched probiotics provide dual benefits by supplying essential trace elements and exhibiting probiotic properties. A comparative study revealed that various bacterial species exhibit considerable differences in their capacity to accumulate and convert selenium into bioavailable forms, highlighting the significance of strain selection in selenium enrichment strategies [22]. Recent investigations indicate that selenium-enriched probiotics maintain their functional properties throughout the processing and storage in food applications. A study demonstrated that selenium-enriched Lactobacillus plantarum integrated into yogurt preserved selenium stability and probiotic viability for 21 days under cold storage, suggesting its applicability in commercial food products [23].
While extensive research exists on the bioaccumulation and biotransformation capabilities of selenium-enriched species, studies focusing on the probiotic potential of selenium-enriched strains isolated from fermented South Indian rice samples remain limited. This study aims to isolate probiotic strains from various traditionally fermented rice samples. The objectives include optimizing isolation techniques to enhance selenium uptake by probiotics, characterizing the isolated strains for their selenium content, evaluating their probiotic potential, and exploring their application in developing selenium-enriched functional foods. This study is one of the few recent investigations into the possibility of selenium-enriched probiotics to enhance immune function. It highlights a promising approach in improving gut health and combating oxidative stress by isolating and optimizing bacterial strains from fermented cereal-based products.
2. MATERIALS AND METHODS
The study focused on preparing koozh using two different cereals: Bamboo Rice and Karuppu Kavuni Rice. The methodology involved the following: 500 g of each grain was weighed, thoroughly rinsed with deionized water, and soaked in deionized water for 12 h at room temperature. After soaking, the grains were cooked in boiling water for 30 min and then drained and allowed to cool to room temperature. The cooled grains were transferred to containers covered with breathable fabric to facilitate natural fermentation. The samples were left to ferment at ambient temperature (25–30°C) for 5–7 days, during which signs of fermentation–such as bubbling and development of a sour odor–were monitored.
The mixture was homogenized using a stomacher or blender for 2 min to release the microorganisms. Serial dilutions of the homogenized sample were performed in sterile saline solution (e.g., 10−1–10−6). A known volume of 0.1 mL of each dilution was plated onto de Man, Rogosa, and Sharpe (MRS) agar plates to isolate lactic acid bacteria, and the plates were incubated anaerobically at 37°C for 48–72 h. The plates were then examined for distinct colonies exhibiting probiotic characteristics such as smooth, white, or creamy colonies. Representative colonies were selected and streaked onto fresh MRS agar plates to obtain pure cultures, which were incubated anaerobically at 37°C for 24–48 h [24].
2.1. Strain Identification by 16S ribosomal RNA (16S rRNA) Sequencing
Genomic DNA was isolated using the Qiagen DNeasy Blood and Tissue Kit, according to the manufacturer’s protocol, with slight modifications to enhance bacterial DNA yield. The quality and quantity of the DNA were assessed using a Nanodrop spectrophotometer. The V3–V4 region of the 16S rRNA gene, commonly used for identifying lactic acid bacteria, was amplified with the primer pair 341F (5’-CCTACGGGNGGCWGCAG-3’) and 805R (5’-GACTACHVGGGTATCTAATCC-3’). Polymerase chain reaction (PCR) amplification was carried out in a 25 μL reaction mixture, consisting of 12.5 μL of 2× KAPA HiFi HotStart ReadyMix, 0.2 μM of each primer, and approximately 10 ng of template DNA. The thermal cycling conditions were as follows: initial denaturation at 95°C for 3 min, 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, followed by a final extension at 72°C for 5 min. PCR products were purified using AMPure XP beads as per the manufacturer’s guidelines. The concentration and purity of the products were verified through gel electrophoresis and a Qubit fluorometer. Sequencing libraries were created using the Nextera XT DNA Library Preparation Kit and sequenced on an Illumina MiSeq platform with 2 × 300 bp paired-end reads. Raw sequencing reads underwent quality control with Trimmomatic [13]. High-quality reads were further analyzed using the DADA2 pipeline in QIIME2 [17] for sequence denoising, chimera removal, and identification of amplicon sequence variants. Thereafter, the phylogenetic tree was constructed by sequencing outcome of amplicon by the neighbor-joining method using MEGA software.
2.2. Carbohydrate Fermentation Test
A series of carbohydrate broths, each containing a specific carbohydrate (glucose, lactose, sucrose, or mannitol), were prepared with phenol red as a pH indicator. A Durham tube was placed in each broth to capture the gas produced during fermentation. Pure cultures of the probiotic candidates were inoculated into these sterile broths and incubated at 37°C for 24–48 h. During incubation, bacterial metabolism of the carbohydrates produces organic acids and/or gases. The production of acids lowered the pH, causing the phenol red to change from red to yellow. Gas production was indicated by bubbles in the Durham tube. After incubation, results were interpreted by observing color changes and gas production [25].
2.3. Evaluation of Tolerance to Artificial Gastric and Intestinal Fluids
Initially, 1 mL of bacterial suspension of each strain was mixed with 9 mL of artificial gastric fluid, comprising 0.2 g sodium chloride and 1 g pepsin dissolved in 100 mL of HCl solution adjusted to pH 2.0. These samples were incubated statically at 37°C for 0, 30, 60, 90, and 120 min. At each time point, samples were withdrawn to determine the viable bacterial count in CFU/mL. Subsequently, 1 mL of the bacterial suspension in artificial gastric fluid was mixed with 9 mL of artificial intestinal fluid, containing 0.68 g potassium dihydrogen phosphate and 1.0 g trypsin dissolved in 100 mL distilled water, adjusted to pH 7.0 using NaOH solution. This mixture was similarly incubated at 37°C for 150, 180, 210, and 240 min, with samples taken at each interval for CFU/mL determination. The survival rates were calculated based on the initial and final viable bacterial counts, providing insights into the ability of the probiotic strains to withstand the harsh conditions of the stomach and intestine. The survival rate of the bacteria was calculated using the formula: Survival rate (%) = (Final CFU/mL/Initial CFU/mL) × 100. Statistical analysis was conducted to assess the reliability of the results across experimental replicates. This methodology enabled the evaluation of probiotic strains’ ability to withstand gastrointestinal conditions, crucial for assessing their potential health benefits and viability as probiotic supplements.
2.4. Determination of Bile Salt Tolerance
Bacterial suspensions of each strain were prepared, and their initial viable counts (CFU/mL) were determined by plating serial dilutions on agar plates. Subsequently, the suspensions were separately inoculated into media containing different concentrations of oxgall: 0% (control), 0.3%, and 0.5% (w/v). The cultures were then incubated at 37°C for 24 h. After incubation, final bacterial counts were determined by plating and counting CFUs. The results were analyzed to assess the tolerance of each strain to bile salts at varying concentrations. For each strain, the survival rate (%) was calculated using the formula: Final CFU/mL after exposure/Initial CFU/mL × 100. This calculation yielded the percentage of viable bacteria that survived exposure to bile salts at each concentration.
2.5. Selenium-enriched Bacterial Cultivation and Selenium Quantification
Isolated pure cultures were utilized for preparing selenium-enriched MRS broth by adding a selenium solution, specifically sodium selenite, to attain a final concentration of 0.5 mg/L. The pure cultures were initially streaked on MRS agar plates to confirm purity and were then transferred to fresh MRS broth. A 1% (v/v) inoculum of these pure cultures was introduced into the selenium-enriched MRS broth. The inoculated broth was subsequently incubated under anaerobic conditions at a constant temperature of 37°C for 24–48 h to facilitate optimal bacterial growth and selenium assimilation. Following the incubation period, the cultures were subjected to centrifugation at 5000 rpm for 10 min to separate the bacterial cells from the growth medium. The resulting bacterial pellet was carefully collected and washed twice with sterile saline solution (0.85% NaCl) to eliminate any residual selenium that might remain in the medium. Subsequently, the strains were analyzed for their capacity to withstand selenium.
The concentrations of selenium in bacterial biomasses were measured using ultraviolet (UV)–vis molecular absorption spectroscopy [18]. For the calibration curve, aliquots ranging from 0.025 to 0.250 mg/L of Se were taken from a stock solution and placed into a series of 10-mL volumetric flasks. Each flask received 1 mL of 2% (mass/vol.) KI and 1 mL of 2 M HCl. The mixture was gently stirred until a yellow color appeared, indicating the release of iodine. Then, 200 μL of 1% (mass/vol.) starch solution was added, and the final volume was brought up with ultrapure water [19]. The absorbance of the solutions was measured with a UV–vis molecular absorption spectrophotometer at 589 nm. All samples underwent the same procedure, and the measurements were performed in triplicate. Statistical analysis was conducted using t test, and all analyses were performed using SPSS version 23.0.
3. RESULTS AND DISCUSSION
3.1. Colony Morphology
The colony morphology of probiotics isolated from fermented samples was characterized and shown in Table 1. On MRS media, colonies appeared with various characteristics: irregular shapes with a rough surface and convex elevation, creamy white, and size ranging from 2 mm to 4 mm. Other colonies were circular, smooth, and raised, appearing creamy white and measuring 1–2 mm. Some colonies were also circular and raised, showing a creamy white to pale yellow color, approximately 1–2 mm in diameter. In addition, colonies displayed irregular shapes with a rough surface, often with a flat to slightly raised elevation, creamy white, and typically sized between 3 mm and 5 mm. Furthermore, the total bacterial count for isolates 1 and 2 was 1.8 × 108 CFU/mL and 1.7 × 108 CFU/mL, respectively.
Table 1: Colony morphology of the selenium-enriched probiotics.
| Isolate number | Colony shape | Surface texture | Elevation | Color | Size (mm) | Total bacterial count (CFU/mL) |
|---|---|---|---|---|---|---|
| Bacillus siamensis | Circular | Smooth | Raised | Creamy white | 1–2 mm | 1.8×108 |
| Staphylococcus arlettae | Circular | Smooth | Raised | Creamy white to pale yellow | 1–2 mm | 1.7×108 |
3.2. Strain Identification by 16S rRNA Sequencing
The 16S rRNA sequencing analysis of selenium-enriched strains isolated from fermented samples revealed the taxonomic composition of the microbial community. Of the 20 strains isolated, only 2 were capable of withstanding selenium uptake. Each sample analysis identified a single bacterial strain, and the findings are summarized in Table 2. Three morphologically distinct colonies subjected to 16S rRNA sequencing were identified as representatives of the genera Bacillus and Staphylococcus. These results underscore the diverse microbial population present in the fermentation ecosystem of the samples.
Table 2: Identification of samples from the fermented samples.
| Type of cereals | Code | Identified bacterial strains |
|---|---|---|
| Bamboo rice | BR | Bacillus siamensis |
| Purple rice | PR | Staphylococcus arlettae |
The isolates were identified as Bacillus siamensis and Staphylococcus arlettae, showing 100% sequence homology. The sequencing results of these bacterial strains are shown in Figures 1 and 2. Comprehensive characterization tests were performed for both the bacterial isolates, B. siamensis and S. arlettae.
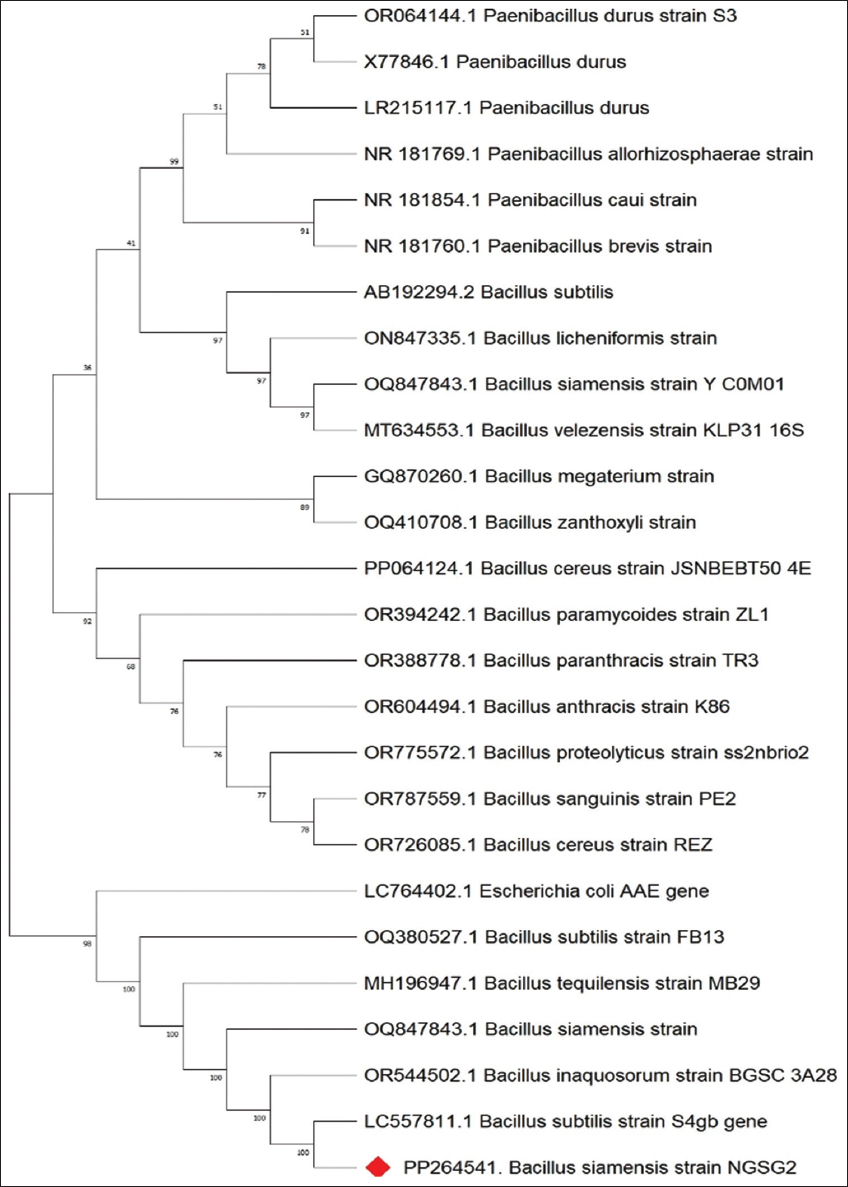 | Figure 1: 16S ribosomal RNA sequencing result of Bacillus siamensis. [Click here to view] |
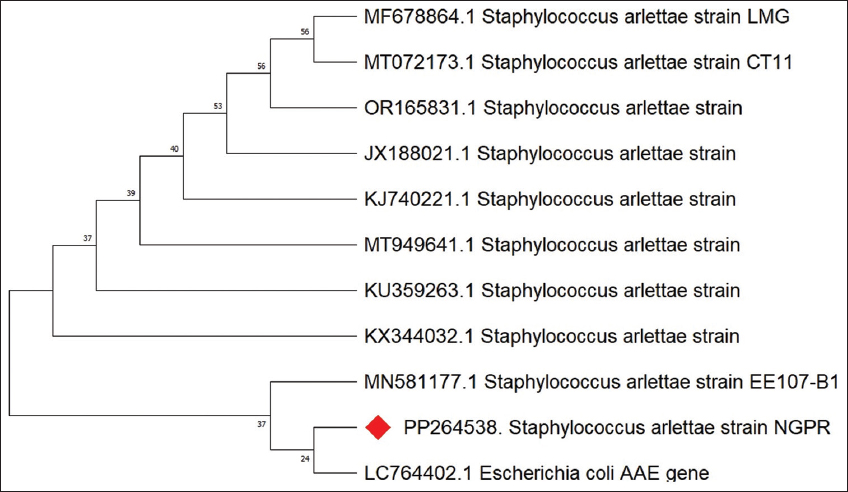 | Figure 2: 16S ribosomal RNA sequencing result of Staphylococcus arlettae. [Click here to view] |
3.3. Carbohydrate Fermentation Test
The carbohydrate fermentation test was employed to identify probiotic strains by assessing their ability to ferment various sugars, which supports their metabolic and functional potential in gut health. The results of the carbohydrate fermentation tests conducted on the isolates from fermented samples are summarized in Table 3. Positive results, indicated by a color change from red to yellow in the phenol red broth, were observed for all tested carbohydrates across isolates from isolated samples. This indicates the robust carbohydrate fermentation capabilities of the isolates across various carbohydrate substrates commonly found in fermented cereals. These results demonstrate the carbohydrate fermentation abilities of probiotics isolated from the fermented samples, highlighting their capacity to utilize various carbohydrates for metabolic activity.
Table 3: Carbohydrate fermentation test.
| Bacteria | Glucose | Lactose | Sucrose | Mannitol | Maltose |
|---|---|---|---|---|---|
| Bacillus siamensis | + | + | - | + | + |
| Staphylococcus arlettae | + | + | - | - | - |
3.4. Assessment of Resistance to Gastric and Intestinal Fluids
The assessment of resistance to gastric and intestinal fluids evaluates a probiotic strain’s ability to survive harsh gastrointestinal conditions, including acidic pH and digestive enzymes. This test is crucial for determining the strain’s potential efficacy in reaching the gut alive, where it can exert its beneficial effects. The bacteria were assessed for their tolerance to artificial gastric and intestinal fluids at intervals of 0, 30, 60, 90, and 120 min. Initial observations showed varied responses among the strains. For 0–30 min, these strains maintained stability, with minimal changes noted in their viability. At 60 min, one strain began to exhibit decreased viability in the gastric fluid environment, while the other remained resilient. This trend continued at 90 and 120 min, where significant reductions in viability were observed, particularly in the acidic gastric environment. The evaluation of tolerance to artificial gastric and intestinal fluids is shown in Table 4.
Table 4: Assessment of resistance to gastric and intestinal fluids.
| Colony | Time (min) | CFU/mL in gastric fluid | CFU/mL in intestinal fluid | P-value |
|---|---|---|---|---|
| Bacillus siamensis | 0 | 1.5×108 | 1.5×108 | 0.08 |
| 30 | 1.4×108 | 1.5×108 | ||
| 60 | 1.2×108 | 1.4×108 | ||
| 90 | 1.0×108 | 1.3×108 | ||
| 120 | 5.8×107 | 6.2×107 | ||
| Staphylococcus arlettae | 0 | 1.5×108 | 1.5×108 | 0.31 |
| 30 | 1.3×108 | 1.4×108 | ||
| 60 | 1.0×108 | 1.2×108 | ||
| 90 | 8.0×107 | 1.0×108 | ||
| 120 | 3.2×107 | 4.5×107 |
For the survival rates based on the data, initial counts of bacterial populations were determined as 1.5 × 108 CFU/mL in both artificial gastric and intestinal fluids. For B. siamensis after 120 min of exposure, the final counts in gastric fluid were measured at 5.8 × 107 CFU/mL, while in intestinal fluid, they were 6.2 × 107 CFU/mL. However, for S. arlettae, the counts were 3.2 × 107 CFU/mL and 4.5 × 107 CFU/mL in intestinal fluid.
3.5. Bile Salt Tolerance Determination
The survival and growth of strains were evaluated after 24 h in media containing varying concentrations of oxgall (0%, 0.3%, and 0.5%). Initial colony-forming units per milliliter (CFU/mL) for each strain were consistent across all conditions: B. siamensis started at 1.5 × 108 CFU/mL, but S. arlettae at 8.5 × 108 CFU/mL. After exposure to 0% oxgall, the final CFU/mL remained unchanged for all strains. In 0.3% oxgall, Strain A reduced to 1.0 × 108 CFU/mL, but Strain B to 6.5 × 107 CFU/mL. The highest concentration tested, 0.5% oxgall, further showed 7.3 × 107 CFU/mL for Strain A and 4.8 × 107 CFU/mL for Strain B. These results demonstrate varying degrees of susceptibility among the strains to oxgall, with higher concentrations correlating with decreased bacterial survival and growth after 24 h. The overall results of the bile tolerance test are shown in Table 5. The bile tolerance test assesses a probiotic strain’s ability to withstand bile salts, simulating the conditions of the small intestine, which is essential for determining its survival and potential for gut colonization. The outcome of this study demonstrated that these three isolated organisms indicate their ability to survive in the small intestine. The P-value suggested that no statistical significance was found in bacterial counts between gastric fluid and intestinal fluid among two isolates, indicating our isolated culture can survive under different harsh environmental conditions. Therefore, these isolates are more suitable for selenium enrichment.
Table 5: Bile tolerance determination of isolated probiotics.
| Strain | Initial CFU/mL | Final CFU/mL after 24 h in 0% oxgall | Final CFU/mL after 24 h in 0.3% oxgall | Final CFU/mL after 24 h in 0.5% oxgall |
|---|---|---|---|---|
| Bacillus siamensis | 1.5×108 | 1.5×108 | 1.0×108 | 7.3×107 |
| Staphylococcus arlettae | 8.5×107 | 8.5×107 | 6.5×107 | 4.8×107 |
3.6. Selenium Quantification
The quantification of selenium in bacteria isolated from fermented samples highlights significant variations in selenium uptake across different species. B. siamensis exhibited a selenium concentration of 200 μg/g dry weight and an absorbance of 0.600 AU, suggesting a greater capability for selenium uptake. The most efficient selenium uptake was observed in S. arlettae, which recorded the highest selenium concentration at 220 μg/g dry weight and absorbance of 0.660 AU. The results of the selenium utilization are shown in Table 6.
Table 6: Selenium uptake by bacteria.
| Bacterial species | Absorbance (AU, Mean±SEM) | Selenium concentration in biomass (mg/g dry weight) |
|---|---|---|
| Bacillus siamensis | 0.598±0.007 | 200 |
| Staphylococcus arlettae | 0.605±0.004 | 220 |
This study isolated and identified selenium-enriched strains of B. siamensis and S. arlettae from fermented cereals consumed by the South Indian population, highlighting their potential in enhancing the nutritional value of foods through selenium bioaccumulation. Using selective culture techniques and 16S rRNA gene sequencing, the researchers accurately identified these bacterial species. The strains demonstrated significant selenium uptake in selenium-supplemented media, indicating their bioaccumulation capabilities. B. siamensis is robust and has potential in agricultural and environmental applications, while S. arlettae, found in fermented foods, also showed notable selenium accumulation. The selenium enrichment suggests promising applications in producing selenium-fortified fermented foods, addressing selenium deficiency, and providing antioxidant benefits. This study opens new avenues for using these bacterial strains to create functional foods aimed at improving nutritional health and dietary supplementation strategies.
The research conducted by Dong et al. demonstrated the isolation of B. siamensis from various fermented food products, highlighting its significant presence in such environments [26]. In addition, Heo et al. identified B. siamensis in fermented kimchi, further corroborating its prevalence in fermented foods [27]. These studies collectively underscore the ubiquitous nature of B. siamensis in fermented food matrices, suggesting its integral role in the fermentation process and its potential probiotic applications. This study on the isolation of probiotics from fermented Bamboo Rice koozh revealed the presence of B. siamensis, consistent with the previous researches by Dong et al. [26] and Heo et al. [27]. These consistent findings across different studies reinforce the established presence of B. siamensis in fermented foods.
The study conducted by Fowoyo and Ogunbanwo specifically isolated probiotic strains, emphasizing the prevalence of Staphylococcus species within fermented foods [28]. This research sheds light on the potential probiotic properties of Staphylococcus strains found in traditional fermented food sources. The research conducted by Jia et al unveiled the isolation of probiotic strains, with a particular emphasis on Staphylococcus species derived from fermented foods [29]. This work on isolating probiotics from fermented Kavuni Rice koozh revealed the presence of S. arlettae. This observation corresponds with prior research emphasizing the presence of Staphylococcus species in fermented foods, indicating their potential as probiotics within these traditional food contexts.
Research on the carbohydrate fermentation tests of probiotics such as Staphylococcus and Bacillus species has demonstrated varied abilities to ferment different carbohydrates. A study on Bacillus species by Simon et al. revealed its capability to ferment 11 out of 49 tested carbohydrates, including D-ribose, L-arabinose, D-xylose, D-glucose, D-fructose, and D-mannitol. This was confirmed through in vitro genomic analysis identifying related metabolic genes [30]. Similarly, the research by Nithya et al. revealed that the fermentation abilities of Bacillus species isolated from different food sources, demonstrating their potential probiotic characteristics. These species showed significant fermentation activities, which are critical for their probiotic functionality and contribution to gut health [31]. The results of this investigation corroborate that probiotics isolated from fermented cereals possess the ability to ferment a diverse array of carbohydrates. These results are consistent with the previous studies conducted on probiotics derived from other fermented foods, which have also demonstrated similar carbohydrate fermentation capabilities.
Research has shown that probiotics such as Staphylococcus and Bacillus species exhibit resistance to gastric and intestinal fluids, making them effective in surviving the harsh conditions of the gastrointestinal tract. According to several research evidences by Khatri et al. and Lee et al., probiotics possess mechanisms that enable them to withstand the acidic conditions of the stomach and the bile salts in the intestines [32,33]. This includes their ability to form spores, which are highly resistant to extreme conditions. These research results also confirm that the probiotics isolated from fermented cereals exhibit significant resistance to gastric and intestinal fluids. This observation aligns with the existing literature indicating that probiotics must withstand the acidic conditions of the stomach and the presence of bile salts in the intestines to be effective.
Research evidence indicates that probiotics such as Staphylococcus and Bacillus species exhibit notable tolerance to bile salts, a critical trait for their survival and functionality within the gastrointestinal tract. Bacillus species isolated from various food sources were tested for bile tolerance by assessing their growth at different bile concentrations and determining the time lag to reach the logarithmic phase [34,35]. This study confirms that probiotics isolated from fermented cereals have significant resistance to bile salts. This finding underscores their robust survivability under conditions mimicking the gastrointestinal environment, which is crucial for their potential application as probiotic supplements or functional foods.
The quantification of selenium in bacteria isolated from fermented cereals reveals notable differences in selenium uptake among the species studied, with B. siamensis and S. arlettae displaying varying efficiencies in selenium assimilation. Previous research supports these findings, highlighting the potential for certain bacterial species to accumulate selenium. For instance, Mörschbächer et al. demonstrated that bacterial strains Enterococcus faecalis, Lactobacillus parabuchneri, Lactobacillus paracasei, and L. plantarum could utilize the selenite to give elemental selenium, accumulating significant amounts of selenium within the bacterial cells [36]. This study underscores the ability of certain bacteria to transform selenium into less toxic forms and store it intracellularly, which aligns with the high selenium concentrations observed in our study. Selenium is a key trace element known to influence microbial metabolism and stress resistance when incorporated in bioavailable forms. In the context of probiotics, selenium enrichment has been reported to enhance not only bacterial survivability under oxidative and gastrointestinal stress, but also to boost health-promoting functions such as antioxidant activity and host adhesion [37,38]. Although S. arlettae showed the highest selenium accumulation, further validation is needed to confirm its functional benefits. Future studies will include antioxidant (DPPH/ABTS), aggregation, and adhesion assays using intestinal cell lines (e.g., Caco-2) to assess the probiotic potential. In addition, selenium-related enzyme activity and gene expression (selD, selA, selB) will be analyzed to clarify the mechanisms of selenium assimilation and its impact on probiotic efficacy. Though the selenium intake method in S. arlettae is not completely elucidated yet, few studies have proposed the selenium uptake mechanism in Staphylococcus spp., which entails the movement of selenium ions, usually as selenite or selenate, via membrane-associated transporter proteins. Upon entering the cell, selenium either gets reduced to its elemental state or integrated into selenoproteins by enzymatic activities [39-41].
This research intended to isolate selenium-enriched probiotics from fermented cereals, specifically targeting Staphylococcus and Bacillus species. 16S rRNA analysis, carbohydrate fermentation assays, and assessments of resistance to stomach and intestinal fluids, as well as bile salt tolerance, completely support the presence of selenium enrichment in the isolated strains. Genetic analysis identified them as Staphylococcus and Bacillus species. These probiotics demonstrated robust carbohydrate fermentation capabilities and significant tolerance to gastrointestinal conditions, highlighting their potential health benefits. However, further research is needed to assess their long-term efficacy and safety as probiotic supplements or functional food ingredients in promoting health. The future direction of this study involves exploring the long-term effects and safety of selenium-enriched probiotics in human health, particularly their role in immune function and oxidative stress reduction. This research lies in the identification of specific Staphylococcus and Bacillus species from fermented cereals, offering a unique approach to developing functional foods with enhanced antioxidant and probiotic benefits.
4. CONCLUSION
The present study effectively isolated and characterized selenium-enriched Bacillus siamensis and Staphylococcus arlettae from koozh, emphasizing their probiotic potential and selenium absorption. Their resistance under gastrointestinal circumstances renders them a potential option for the production of functional foods. Furthermore, subsequent investigations should examine their in vivo efficacy and safety to facilitate extensive implementation in food fortification..
5. ACKNOWLEDGMENTS
The authors are grateful to the management of SRMIST for providing the facilities for conducting this research. Special thanks to Dr. Alagesan M., Assistant Professor, Department of English and Foreign Languages, Faculty of Engineering & Technology, SRM Institute of Science and Technology, for editing and verifying the language of the manuscript.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
The data supporting the findings of this study are available with all the authors and will be provided upon reasonable request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Genchi G, Lauria G, Catalano A, Sinicropi MS, Carocci A. Biological activity of selenium and its impact on human health. Int J Mol Sci. 2023;24(3):2633. [CrossRef]
2. Schomburg L. Dietary selenium and human health. Nutrients. 2016;9(1):22. [CrossRef]
3. FlohéL, Toppo S, Orian L. The glutathione peroxidase family:Discoveries and mechanism. Free Radic Biol Med. 2022;187:113-22. [CrossRef]
4. Huang JQ, Zhou JC, Wu YY, Ren FZ, Lei XG. Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic Biol Med. 2018;127:108-15. [CrossRef]
5. Cardoso BR, Cominetti C, Seale LA. Editorial:Selenium, human health and chronic disease. Front Nutr. 2022;8:827759. [CrossRef]
6. Bjørklund G, Shanaida M, Lysiuk R, Antonyak H, Klishch I, Shanaida V, et al. Selenium:An antioxidant with a critical role in anti-aging. Molecules. 2022;27(19):6613. [CrossRef]
7. Hatfield DL, Tsuji PA, Carlson BA, Gladyshev V. Selenium and selenocysteine:Roles in cancer, health, and development. Trends Biochem Sci. 2014;39:112-20. [CrossRef]
8. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506-14. [CrossRef]
9. Deng S, Hu S, Xue J, Yang K, Zhuo R, Xiao Y, et al. Productive performance, serum antioxidant status, tissue selenium deposition, and gut health analysis of broiler chickens supplemented with selenium and probiotics-a pilot study. Animals (Basel). 2022;12(9):1086. [CrossRef]
10. Xiao D, Li T, Huang X, Zhu K, Li Z, Dong Y, et al. Advances in the study of selenium-enriched probiotics:From the inorganic se into se nanoparticles. Mol Nutr FoodRes. 2023;67(23):2300432. [CrossRef]
11. Caetano RG, Xavier IB, Feldmann V, Lacerda IC. Traditional fermented products:Potential origin for probiotic strains. Curr Food Sci Technol Rep. 2024;2(2):201-11. [CrossRef]
12. Guo X, Li D, Lu W, Piao X, Chen X. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie van Leeuwenhoek. 2006;90:139-46. [CrossRef]
13. Mirmajidi SH, Irajie C, Savardashtaki A, Nezafat N, Morowvat MH, Ghasemi Y. Optimization of spore production in Bacillus coagulans using response surface methodology approach. Appl Biochem Biotechnol. 2024;196:7557-69. [CrossRef]
14. Ilango S, Antony U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci Technol. 2021;118:617-38. [CrossRef]
15. Ray M, Ghosh K, Singh S, Mondal KC. Folk to functional:An explorative overview of rice-based fermented foods and beverages in India. J Ethnic Foods. 2016;3(1):5-18. [CrossRef]
16. Nath S, Roy M, Sikidar J, Deb B, Sharma I, Guha A. Characterization and in-vitro screening of probiotic potential of novel Weissella confusa strain GCC 19R1 isolated from fermented sour rice. Curr Res Biotechnol. 2021;3:99-108. [CrossRef]
17. Ibrahim HA, Zhu Y, Wu C, Lu C, Ezekwe MO, Liao SF, et al. Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. Biol Trace Elem Res. 2012;147:251-60. [CrossRef]
18. Pant R, Sharma N, Kabeer SW, Sharma S, Tikoo K. Selenium-enriched probiotic alleviates western diet-induced non-alcoholic fatty liver disease in rats via modulation of autophagy through AMPK/SIRT-1 pathway. Biol Trace Elem Res. 2023;201(3):1344-57. [CrossRef]
19. Kumari K, Kashyap P, Chakrabarti P. Germination and probiotic fermentation:A way to enhance nutritional and biochemical properties of cereals and millets. Food Sci Biotechnol. 2024;33(3):505-18. [CrossRef]
20. Yang J, Wang J, Yang K, Liu M, Qi Y, Zhang T, et al. Antibacterial activity of selenium-enriched lactic acid bacteria against common food-borne pathogens in vitro. J Dairy Sci. 2018;101(3):1930-42. [CrossRef]
21. Huang J, Liu J, Wang X, Zhang Z. Comparative analysis of selenium accumulation and transformation by probiotic strains:Implications for functional food development. J Funct Foods. 2021;87:104743. [CrossRef]
22. Luo Y, Zhang X, Li Y, Wang Q. Selenium-enriched probiotics:Production, bioavailability, and potential health benefits. Trends Food Sci Technol. 2023;133:214-23. [CrossRef]
23. Shang X, Geng L, Zhao Z, Luo L, Shi X, Zhang Q, et al. Transcriptomics reveals the mechanism of selenium-enriched Lactobacillus plantarum alleviating brain oxidative stress under cadmium stress in Luciobarbus capito. Ecotoxicol Environ Saf. 2022;242:113890. [CrossRef]
24. Meena KK, Taneja NK, Jain D, Ojha A, Kumawat D, Mishra V. In vitro assessment of probiotic and technological properties of lactic acid bacteria isolated from indigenously fermented cereal-based food products. Fermentation. 2022;8(10):529. [CrossRef]
25. Gunkova PI, Buchilina AS, Maksimiuk NN, Bazarnova YG, Girel KS. Carbohydrate fermentation test of lactic acid starter cultures. IOP Conf Ser Earth Environ Sci. 2021;852(1):012035. [CrossRef]
26. Dong Q, Liu Q, Goodwin PH, Deng X, Xu W, Xia M, et al. Isolation and genome-based characterization of biocontrol potential of Bacillus siamensis YB-1631 against wheat crown rot caused by Fusarium pseudograminearum. J Fungi (Basel). 2023;9(5):547. [CrossRef]
27. Heo S, Kim JH, Kwak MS, Jeong DW, Sung MH. Functional genomic insights into probiotic Bacillus siamensis strain B28 from traditional Korean fermented Kimchi. Foods. 2021;10(8):1906. [CrossRef]
28. Fowoyo PT, Ogunbanwo ST. Antimicrobial resistance in coagulase-negative staphylococci from Nigerian traditional fermented foods. Ann Clin Microbiol Antimicrob. 2017;16:1-7. [CrossRef]
29. Jia Y, Niu CT, Xu X, Zheng FY, Liu CF, Wang JJ, et al. Metabolic potential of microbial community and distribution mechanism of Staphylococcus species during broad bean paste fermentation. Food Res Int. 2021;148:110533. [CrossRef]
30. Simon A, Colom J, Mazhar S, Khokhlova E, Deaton J, Rea K. Bacillus megaterium Renuspore®as a potential probiotic for gut health and detoxification of unwanted dietary contaminants. Front Microbiol. 2023;14:1125616. [CrossRef]
31. Nithya V, Halami PM. Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann Microbiol. 2013;63:129-37. [CrossRef]
32. Khatri I, Sharma G, Subramanian S. Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina®, and insights into its probiotic properties. BMC Microbiol. 2019;19:1-15. [CrossRef]
33. Lee NK, Kim WS, Paik HD. Bacillus strains as human probiotics:Characterization, safety, microbiome, and probiotic carrier. Food Sci Biotechnol. 2019;8:1297-1305. [CrossRef]
34. Chen C, Yu L, Tian F, Zhao J, Zhai Q. Identification of novel bile salt-tolerant genes in Lactobacillus using comparative genomics and its application in the rapid screening of tolerant strains. Microorganisms. 2022;10(12):2371. [CrossRef]
35. Patel AK, Singhania RR, Pandey A, Chincholkar SB. Probiotic bile salt hydrolase:Current developments and perspectives. Appl Biochem Biotechnol. 2010;162:166-80. [CrossRef]
36. Mörschbächer AP, Dullius A, Dullius CH, Bandt CR, Kuhn D, Brietzke DT, et al. Assessment of selenium bioaccumulation in lactic acid bacteria. J Dairy Sci. 2018;101(12):10626-35. [CrossRef]
37. Liu Y, Zhang H, Wang Y, Wang T, Guo X. Effects of selenium enrichment on growth and functional characteristics of probiotics: A review. J Funct Foods. 2021;86:104713. [CrossRef]
38. Wang R, Wang T, Chen Y, Liu Y. Enhancement of antioxidant and gastrointestinal resistance properties of Lactobacillus plantarum via selenium biotransformation. Food Biosci. 2023;52:102322.
39. Kessi J, Turner RJ, Zannoni D, Kessi J, Turner RJ, Zannoni D. Tellurite and selenite:How can these two oxyanions be chemically different yet so similar in the way they are transformed to their metal forms by bacteria?Biol Res. 2022;55:17. [CrossRef]
40. The Role of Microbial Sulfur Metabolism in Biogeochemical Cycling of Tellurium and Selenium ProQuest;n.d. Available from: https://www.proquest.com/openview/3a64fa212ec2ec49d31d5c9ce56fc2da/1?cbl=18750 [Last accessed on 2025 May 25].
41. Zannoni D, Borsetti F, Harrison JJ, Turner RJ. The Bacterial Response to the Chalcogen Metalloids Se and Te. In:Poole RK, editor. Advances in Microbial Physiology. Vol. 53. Academic Press;2007. pp. 1-312. [CrossRef]