1. INTRODUCTION
One of the major traits of cancers in human is unresolved inflammation, which often occurs before the onset of the disease and orchestrates a favorable microenvironment for tumor growth. Over the years, several population-based epidemiological and experimental animal model systems and clinical reports emphasized a major rise in the prevalence of the disease leading up to 70% of cancer deaths globally [1]. Recent studies revealed that the mortality rate of cancer worldwide is increasing every year [2]. Medicinal plants have taken a crucial role in life and have been used by mankind since time immemorial for primary healthcare. They are still vital for treating several disorders, and numerous systems of medicine are especially dedicated to the application of medicinal plants for health care [3]. It is assumed that herbal-based products are nontoxic, safe, and even more reliable than the synthetic compounds used for health care [4]. At present, over 60% of anticancer agents are collected from their natural sources such as plants, microorganisms, and marine organisms [5]. The World Health Organization projected that majority of the population rely on herbal preparations [6].
The production of reactive oxygen species is associated with the activation of RAGE in a variety of cells. RAGE gene has a large extracellular part, a transmembrane domain with 43 amino acid long cytoplasmic tail [7], having a cell surface receptor and is a part of the immunoglobulin superfamily, involved in the pathogenesis of cancer advancement and metastasis [8]. Cumulative evidence supports the use of RAGE level as clinical biomarkers in different types of cancers, such as breast, lung, colorectal, and prostate cancer [9]. The expression of the RAGE gene increases at the site of inflammation, mainly on inflammatory, endothelial, and epithelial cells, and elevates the expression of both cell surfaces [10]. Subsequently, the inflammatory cytokines expression increases, resulting in an inflammatory response with associated cellular migration and proliferation [11].
Mikania micrantha (L.) Kunth. is an exotic, neotropical perennial herbaceous vine belonging to the family Asteraceae. M. micrantha is also known as Mile-a-minute vine. It grows well in humid and fertile soil, though it can survive in less fertile soils. It is widely distributed in the Pacific, Southeast Asia, and so on. In India, it is found in West Bengal, Orissa, Andaman, Nicobar Islands, and some parts of North East India [12]. M. micrantha is a plant that has been used for skin diseases, wound dressings, chickenpox, and so on and as folk medicine in Africa, Jamaica, and Guyana Patanoma [13]. In India, the plant is commonly used by various indigenous communities as medication for several ailments such as itches, body sprain, gout, snake bites, dysentery, diabetes gout, rheumatism, and cancer [14].
Ethnic people of Mizoram apply the juice of M. micrantha to cuts and wounds for clotting the blood as first aid [15]. The leaves decoction is given to the patients suffering from dysentery, as hemostatic [16]. As ethnoveterinary medicine in India, M. micrantha is used for treating diarrhea of veterinary animals and repelling body lice of poultry birds [17].
The present work aims to carry out a preliminary study on the phytochemicals, antioxidant, antiproliferative, and regulation of the RAGE gene of the crude leaf extracts of M. micrantha.
2. MATERIALS AND METHODS
2.1. Collection, Identification, Authentication, and Preparation of Sample
M. micrantha (L.) Kunth. sample was collected from Aizawl, Mizoram, during the month of November 2016 and identified by Dr. Sherry, Department of Plant Biology and Plant Biotechnology, Women’s Christian College, Chennai, Tamil Nadu, and the herbarium was deposited in the Institutional Herbarium, Mizoram University, Aizawl, with voucher 00345. The leaf samples were washed with clean water, air-dried, and made into uniformly fine powder. The powdered sample was subjected to sequential extraction using three solvents based on increasing polarity such as hexane, ethyl acetate, and methanol for 72 h. The extracts were filtered and dried using a rotary evaporator. The extracts obtained were stored at −20°C until further used.
2.2. Qualitative Phytochemical Tests
The preliminary phytochemical analysis of the different extracts of M. micrantha was performed in standard procedures [18].
2.3. Quantitative Phytochemical Analysis
Phenols and flavonoids are the most important groups of secondary products and bioactive compounds in plants [19]. Phenols and flavonoids possess various biological activities, cardioprotective [20], antidiabetic [21], antioxidant [22], cytotoxic, and antitumor [23], in addition to the ability to modify gene expressions [24].
The selective quantitative analysis was carried out for phenol [25]. Different concentrations (100, 200, and 300 mg) of the samples were tested, and a calibration curve for catechol was obtained. The results were compared to a catechol calibration curve, and the total phenol content of the sample was expressed as mg of catechol equivalents per gram of extract.
For flavonoid content [26], the results were expressed as quercetin equivalents (mg quercetin/g dried extract):
Amount TFC = Sample OD/Standard OD × Respective Amount of extract
2.4. Induction of Chromosomal Aberration
2.4.1. Method of treatment
Commercially available onions were used as the test material in the present study. The cytotoxicity and genotoxicity potential of the methanol extract of M. micrantha against Allium cepa was carried out according to Levan [27]. The roots of onions were treated with the plant infusion for three exposures of 1, 6, and 24 h duration, and the concentration was 100 ppm. The treated roots were fixed in freshly prepared acetic acid: ethanol (1:3).
2.4.2. Slide preparation
Each treated root sample was washed in distilled water and then hydrolyzed for 15 min at RT in 1 N HCl. About 4% ferric ammonium sulfate was prepared, and the roots were mordanted for 15–10 min. The roots were stained for 10–12 min using 0.5% hematoxylin after washing with distilled water. The roots were finally squashed on a slide using a drop of 45% acetic acid. DPX was used for sealing the slide.
2.4.3. Scoring
Around 2000 nuclei were recorded from the treated as well as the control samples for calculating the mitotic indices [28].
The incidence of the chromosomal aberrations present in the treated and normal root tips was calculated and classified according to Buckton and Evans [29]. Six root tips were collected from each bulb and used for the experimental point. The mitotic irregularities such as metaphasic clump, anaphasic bridge, prophasic clump, and lagging chromosome were considered.
2.5. DPPH Free Radical Scavenging Assay
The antioxidant activity of the leaf extracts of M. micrantha was carried out according to the method described by Shimamura et al. [30] with slight modifications. The antioxidant capacity was presented as a percentage scavenging effect and calculated using the following equation:
 |
Inhibitory concentration (IC50) was calculated using a free online version of GraphPad Prism.
2.6. Cytotoxicity
The cytotoxicity was assessed according to Mosmann [31]. RAW 264.7 leukemia cell lines (NCCS, Pune) were cultured in DMEM medium added with 10% FBS at 37°C with 5% CO2. The cells were allowed to attach overnight at 37°C after plating in 96-well plates at a density of 1.2 × 104 cells/well. Different concentrations (25, 50, 75, 100, and 150 mg) of the samples were taken. Then, the medium was replaced, and the cells were incubated.
The media were discarded after 24 h of incubating. A 100 ml fresh medium and 10 ml of MTT (5 mg/ml) were added. The media were then discarded after 3–4 h, and 150 ml of DMSO was added for dissolving the formazan crystals. The absorbance was read at 570 nm in a microplate reader.
The positive control used was cyclophosphamide. Cell survival was calculated by the following formula:
Viability (%) = Test OD
Control OD
 |
Cytotoxicity (%) = 100−Viability (%)
2.7. Reverse Transcription Polymerase Chain Reaction (PCR) Analysis
Approximately 1 × 106 cells/ml were seeded into 6-well plates, allowed to adhere, and the ethyl acetate extract was added followed by 24 h incubation.
Tri-RNA reagent (Favorgen Biotech Corp, Taiwan) was used for extracting total RNA from the cells followed by the treatment of the RNA with deoxyribonuclease I (DNase I; Promega, USA). DNase I-treated RNA was reverse transcribed using EasyScript Plus™ Reverse Transcriptase. cDNA was amplified by PCR using these RAGE gene-specific pair primers.
Primer details are as follows:
RAGE F: 5’-GTGGGGACATGTGTGTCAGAGGGAA-3’
RAGE R: 5’-TGAGGAGAGGGCTGGGCAGGGACT-3’
b-Actin F: 5’-ACGGGTCACCACACTGTGC-3’
b-Actin R: 5’-CTAGAAGCATTTGCGGTGGACGATG-3’
Full-length cDNA amplification using Eppendorf Personnel Mastercycler, Germany, for RAGE gene was 35 cycles of 94°C for 30 s, 65°C for 1 min, and 72°C for 7 min, and for b-actin gene was 35 cycles of 94°C for 30 s, 58°C for 1 min, and 72°C for 7 min.
The amplified gene was separated on a 2% agarose gel, stained with ethidium bromide, and visualized with a gel documentation system (SynGene, UK).
2.8. Statistical Analysis
The data were presented as mean ± SEM. Statistical analysis was performed using Excel 2010, and differences were determined by one-way ANOVA. Differences were considered statistically significant at P < 0.01.
3. RESULTS
The qualitative phytochemical analysis showed the presence of carbohydrates, flavonoids, quinones, terpenoids, phenols, and coumarins [Table 1].
Table 1: Phytochemical analysis of leaf extracts of Mikania micrantha in vitro.
| Test | Extract | ||
|---|---|---|---|
| Hexane | Ethyl acetate | Methanol | |
| Carbohydrate | ++ | +++ | +++ |
| Tannins | − | − | + |
| Flavonoids | + | + | ++ |
| Alkaloids | − | + | ++ |
| Quinones | + | ++ | ++ |
| Glycosides | − | + | + |
| Cardiac glycosides | − | + | + |
| Terpenoid | + | +++ | +++ |
| Phenols | + | +++ | +++ |
| Coumarins | + | + | + |
| Steroids | − | ++ | ++ |
| Phylobatannins | − | − | − |
| Anthraquinone | − | − | − |
+++: Strongly present, ++: Moderately present, +: Present in trace amount, −: Absent.
The quantitative estimation for phenols in hexane, ethyl acetate, and methanol extracts was 259.88 mg, 87.67 mg, and 104.70 mg catechol equivalent/g of dried sample [Table 2a], whereas the flavonoid content was 156.55 mg, ethyl acetate 106.73 mg, and hexane 87.35 mg quercetin equivalent/g of dried sample, respectively [Table 2b].
Table 2: Quantification of secondary metabolites content of the leaf extract of Mikania micrantha.
| a. Total phenol content | |||||||
|---|---|---|---|---|---|---|---|
| Concentration (µg) | Sample (OD) hexane | Sample (OD) ethyl acetate | Sample (OD) methanol | Catechol (OD) | Amount of phenol hexane (mg equiv/g) | Amount of phenol ethyl acetate (mg equiv/g) | Amount of phenol methanol (mg equiv/g) |
| 100 | 0.107 | 0.206 | 0.328 | 0.457 | 23.551 | 45.090 | 71.747 |
| 200 | 0.298 | 0.409 | 0.697 | 0.891 | 66.988 | 91.831 | 156.552 |
| 300 | 0.485 | 0.592 | 0.863 | 1.665 | 87.355 | 106.736 | 155.475 |
| b. Total flavonoid content | |||||||
| Concentration (µg) | Sample (OD) hexane | Sample (OD) ethyl acetate | Sample (OD) methanol | Quercetin (OD) | Amount of flavonoid hexane (mg equiv/g) | Amount of flavonoid ethyl acetate (mg equiv/g) | Amount of flavonoid methanol (mg equiv/g) |
| 100 | 0.097 | 0.214 | 0.38 | 0.514 | 18.860 | 41.609 | 73.886 |
| 200 | 0.153 | 0.261 | 0.669 | 0.796 | 38.413 | 65.528 | 167.963 |
| 300 | 0.332 | 0.278 | 0.824 | 0.951 | 104.709 | 87.678 | 259.882 |
The antioxidant capacity of the different extracts showed an increase in concentration manner. The highest activity was observed at 100 mg/ml. The IC50 value for DPPH assay for leaf extracts of hexane, ethyl acetate, and methanol was 71.23 mg/ml, 40.34 mg/ml, and 52.45 mg/ml, respectively, with respect to the positive control (ascorbic acid) with IC50 value of 39.92 mg/ml [Table 3]. The above results show a profound antioxidant activity in the ethyl acetate extract of M. micrantha.
Table 3: Antioxidant analysis of the leaf extracts of Mikania micrantha (DPPH radical scavenging assay).
| Sample | Conc. (µg) | % inhibition | IC50 value |
|---|---|---|---|
| Ascorbic acid | 25 | 36.95 | 39.92 |
| 50 | 63.12 | ||
| 100 | 84.53 | ||
| Hexane | 25 | 38.69 | 71.23 |
| 50 | 44.24 | ||
| 100 | 57.39 | ||
| Ethyl acetate | 25 | 44.57 | 40.34 |
| 50 | 53.47 | ||
| 100 | 70.78 | ||
| Methanol | 25 | 45.26 | 52.45 |
| 50 | 48.23 | ||
| 100 | 60.17 |
The cytotoxicity of M. micrantha extract against RAW 264.7 murine leukemia cell lines showed increased toxicity with an increase in concentration, and its IC50 was hexane (226.5 mg/ml), ethyl acetate (47.68 mg/ml), and methanol (212.3 mg/ml) [Table 4 and Figure 1].
Table 4: Cytotoxicity analysis of leaf extracts of Mikania micrantha on RAW 264.7 murine leukemia cell lines.
| Sample | Conc. (in µg) | % viability | % toxicity | IC50 value |
|---|---|---|---|---|
| Cyclophosphamide | 5 | 7.47 | 92.53 | 90 |
| Hexane | 25 | 73.14 | 26.86 | 226.5 |
| 50 | 68.22 | 31.78 | ||
| 100 | 62.04 | 37.96 | ||
| 125 | 59.27 | 40.73 | ||
| 150 | 51.13 | 48.87 | ||
| Ethyl acetate | 25 | 57.02 | 42.98 | 47.68 |
| 50 | 50.18 | 49.82 | ||
| 100 | 44.04 | 55.96 | ||
| 125 | 36.83 | 63.17 | ||
| 150 | 32.80 | 67.20 | ||
| Methanol | 25 | 71.87 | 28.13 | 212.3 |
| 50 | 68.49 | 31.51 | ||
| 100 | 64.03 | 35.97 | ||
| 125 | 58.37 | 41.63 | ||
| 150 | 48.57 | 51.43 |
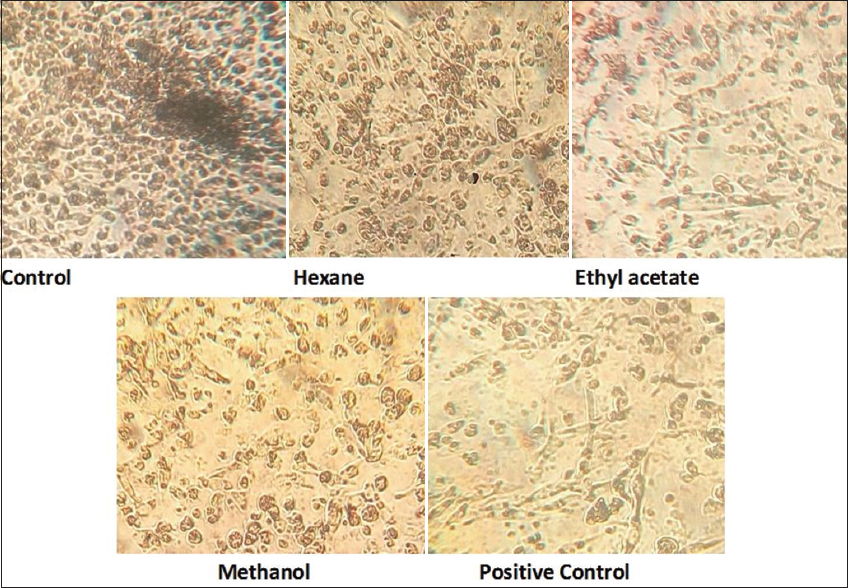 | Figure 1: The cytotoxicity of different leaf extracts of M. micrantha against RAW 264.7 leukemia cell line. [Click here to view] |
The mitotic indices of root meristem treated with leaves extract showed values of 4.12%, 4.7%, and 1.41% in 1 h, 6 h, and 24 h, respectively. The growing root tips exposed to the leaf extracts for 24 h lost their turgidity. The treated cells showed a considerable reduction in the frequency of division when compared to control (9.01%). The mitotic indices in the root tip cells treated for 1 h and 6 h brought about a similar effect [Table 5 and Figures 2a and c].
Table 5: Induction of chromosomal anomalies and mitotic indices in the root meristems of Allium cepa by fresh leaf extract of Mikania micrantha.
| Treatment time (hra) | Cells count (N) | Dividing cells (n) | Mitotic index (n/N×100±SE) | Anomalies (r.) | Mitotic anomalies observed | % anomalies r/n×100 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC | MC | AB | AL | TC | ||||||
| Control | 2235.00±6.48 | 217.16±5.84 | 9.44±0.69 | 1.00±0.05 | - | - | - | - | - | 01.20±0.04 |
| 1 | 2143.33±4.45 | 88.16±2.51 | 4.13±0.54 | 25.83±1.98 | 7 | 15 | 6 | - | - | 29.51±2.99 |
| 6 | 2042.50±7.13 | 89.66±1.75 | 4.80±1.01 | 30.83±1.59 | 14 | 7 | 5 | 3 | 3 | 33.78±2.51 |
| 24 | 2035.66±4.95 | 26.66±2.15 | 1.30±0.42 | 13.00±1.31 | 5 | 4 | 1 | 2 | 4 | 55.29±2.57 |
PC: Prophasic clump, MC: Metaphasic clump, AB: Anaphasic bridge, AL: Anaphasic lag, TC: Telophasic clump. The results are presented as mean±SE, n=5. The t-test was performed to test the significant level between the control and treatment groups at a significant level of
<0.05,
<0.01, and
<0.001.
 | Figure 2: Induction of chromosomal anomalies and mitotic indices in the root meristems of Allium cepa by leaf extract of Mikania micrantha. (a) The total number of cell counts. (b) The total number of dividing cells. (c). Control: 1: Prophase, 2: Metaphase, 3: Anaphase, and 4: Telophase. (d) Treated: 1: Prophasic clump, 2: Metaphasic clump, 3: Anaphasic lag, and 4: Anaphasic bridge. The results are presented as mean±SE, n=5. The t-test was performed to test the significant level between the control and treatment groups at a significant level of *<0.05, @<0.01, and #<0.001. [Click here to view] |
Infusion of leaves extracts of M. micrantha induced chromosomal damage and mitotic anomalies at various periods of exposure in onion root. Observations on the frequencies of aberration are presented in Table 5 and Figure 2b, where the leaves extract of M. micrantha increased the incidence of anomalous mitosis during all three durations of exposure. Toxicity of leaves extract on the induction of mitotic anomalies on the root meristems of onion was duration dependent. Cells treated for 24 h (55%) showed a significantly high increase in mitotic anomalies. An increase in chromosomal anomalies during 1 h (31.1%) exposure showed that the leaves extracts of M. micrantha had an immediate toxic effect on the root meristem. The types of chromosomal aberrations observed in the root meristems with leaves extracts were prophasic clump, metaphasic clump, anaphasic bridge, anaphasic lag, and telophasic clump [Table 5 and Figure 2d]. Prophasic clumps, metaphasic clump, and anaphasic bridge were found to be common during all three durations. They were significantly higher in cells exposed to leaves extracts for 1 h and 6 h. The percentage of anaphasic lag and telophasic clump was found to be of rare occurrence during the three durations [Table 5 and Figure 2d].
The IC50 concentration of the ethyl acetate extracts of M. micrantha was treated, and the RAGE gene expression was determined. The treated showed suppression of RAGE gene compared with untreated control about 2-fold [Figure 3].
 | Figure 3: The effect of ethyl acetate leaf extracts of Mikania micrantha on RAGE gene expression. Expression ratio analyzed by ImageJ software. Lane 1, 100 bp marker; lane 2, untreated/control; and lane 3, treated, 47.68 μg/ml. The result presented as mean±SE, n=5. [Click here to view] |
4. DISCUSSION
Phytochemicals are present naturally in plants and are biologically active chemical compounds. They provide multiple benefits for human health which macronutrients and micronutrients do not possess. They are involved in the inhibition of cancer cell proliferation and protection from oxidative stress that can cause aging, cancer, cardiovascular disease, neurodegenerative diseases, metabolic disorders, and so on and can also inhibit and induce enzymes and gene expression [32,33]. The present phytochemical results are akin to the findings of Dev et al., Lalrinzuali et al., and Borkataky et al. [34-36] who reported the presence of different phytochemicals in the plant powder as well as different extracts of the M. micrantha leaves. A. cepa test performed in this study is used as an indicator of adequate cell proliferation by calculating the mitotic index and replication index [37]. Several other plants such as Calotropis procera, Achyrocline satureioides, Paxia myriantha, and Paxia leiocarpa have been demonstrated to inhibit cell division of A. cepa [38,39]. The present study of the crude leaves extract-treated cells revealed that the plant extract poses toxic effects on the dividing cells by reducing the mitotic index during 1 h exposure, and this observation is in agreement with the reports made on Mikania cordifolia infusions on the cell cycle of A. cepa. Results indicated that two populations of M. cordifolia exhibited a reduction of the mitotic index in all the treatments when compared with the negative control. In both populations, a decrease in the percentage of the mitotic index with increasing concentration of the infusions was observed [40]. The results of the present study demonstrate that root tip cells treated with fresh leaves extract of M. micrantha brought about cell death after 24 h of treatment. The genotoxicity study of M. micrantha extracts using root meristems of onion showed that the extract is toxic to the genetic material by inducing non-clastogenic chromosomal aberrations such as prophasic clump, metaphasic clump, anaphasic bridges, anaphasic lag, and telophasic clump. Prophasic clump, metaphasic clump, and anaphasic bridge were of common occurrence. Chromosome stickiness plate may be due to the action of the extract on the protein leading to the partial dissolution of nucleoprotein, which forms an integral part of the chromosome [41]. Spanish Jasmine extracts also induced mitotic anomalies such as sticky chromosome and C-mitosis on Allium root meristem [42].
In this study, the ethyl acetate and methanol extracts of M. micrantha leaves when tested for antioxidant activities using the in vitro system showed significant DPPH scavenging ability and antioxidant potential with an IC50 value of 40.34 mg/ml and 52.45 mg/ml, respectively, when compared to the positive control 39.92 mg/ml. The different extracts as well as whole plants of M. micrantha have been shown to possess antioxidant activity in the previous reports [43]. The phenolic compounds were prominently found in this extract and could be attributed to the observed antiradical properties. The compounds isolariciresinol, caffeic acid, ethyl protocatechuate, and protocatechuic aldehyde isolated from M. micrantha plant have been shown to have higher DPPH scavenging activity than L-ascorbic acid, which is a standard reference compound in an earlier study [44]. The present study on anticancer activity of hexane, ethyl acetate, and methanol leaf extracts of M. micrantha on Raw 264.7 murine leukemia cell lines suggests that the ethyl acetate extract of the leaves of M. micrantha possesses prominently high cytotoxic activity as the IC50 value achieved was 59.14 mg/ml. The previous studies have shown the anticancer activity of aqueous extract of M. micrantha leaves against K562 and HeLa human cancer cell lines [45]. Different extracts of other species of Mikania have also been reported to show cytotoxic activities against HepG-2 and HeLa cell lines [46]. The ethanolic extracts of M. cordata leaves have also shown cytotoxic activity using Brine Shrimp Lethality Assay [47]. The essential oils extracted from M. micrantha plant, which contain mostly terpenoids, terpenes, and sesquiterpenes, have been shown to possess anticancer activity against the ovarian, pancreatic, and cervical cancer cell lines [48,49]. The ethanol extract of Fimbristylis ovata (Burm.f.) has also been shown to have significant inhibition of RAGE gene expression in human lung adenocarcinoma epithelial cell line [50].
The results from the study showed that the polarity of the solvent used is responsible for the variations in the flavonoid and phenol content of the different extracts. In addition, there was a correlation between the antioxidant capacity and the flavonoid and phenol content. The study revealed the downregulation of RAGE gene expression by M. micrantha leaf extracts. RAGE gene activation results in the transduction of the cell surface signals to various inflammatory pathways, including PI3K-Akt, MAPKs, and NF-kB [51]. Further studies can be done to investigate the inhibition of RAGE gene and the intracellular pathways involved in the regulation.
5. CONCLUSION
This study revealed that the crude extract of M. micrantha leaves is rich in phenol and flavonoid. The ethyl acetate leaf extract exhibited significant in vitro antioxidant and cytotoxic activities compared to hexane and methanol leaf extracts. The ethyl acetate extract suppresses the expression of the RAGE gene in the RAW 264.7 murine leukemia cell line. Our result suggested that the antioxidant property of M. micrantha could be involved in its inflammation-related inhibitory action through the RAGE gene signaling pathway. This is the first report on the suppression of the RAGE gene expression of M. micrantha.
6. ACKNOWLEDGMENT
The authors direct their gratitude to CSIR-UGC, New Delhi, for the financial assistance to Alex Zohmachhuana, Dept. of Botany, to carry out his Ph.D. work in Mizoram University, Aizawl. The authors would also like to express their special thanks to Hema, Madras Christian College for her contributions in the study.
7. CONFLICTS OF INTEREST
Authors declared that they do not have any conflicts of interest.
8. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. A Cancer J Clin 2020;70:7-30. [CrossRef]
2. Zhang Z, Zhou L, Xie N, Nice EC, Zhang T, Cui Y, et al. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther 2020;5:113. [CrossRef]
3. Cordell GA. Sustainable medicines and global health care. Planta Med 2011;77:1129-38. [CrossRef]
4. Moreira DL, Teixeira SS, Monteiro MH, De-Oliveira AC, Paumgartten FJ. Traditional use and safety of herbal medicines. Rev Bras Farmacogn 2014;24:248-57. [CrossRef]
5. Newman DJ, Cragg GM, Sander KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 2003;66:1022-37. [CrossRef]
6. Qazi MA, Molvi K. Herbal medicine:A comprehensive review. J Pharm Res 2016;8:1-5.
7. Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. Fed Am Soc Exp Biol 2009;23:1766-74. [CrossRef]
8. Tesarova P, Kalousova M, Jachymova M, Mestek O, Petruzelka L, Zima T. Receptor for advanced glycation end products (RAGE)--soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Investig 2007;25:720-5. [CrossRef]
9. Zhang S, Hou X, Zi S, Wang Y, Chen L, Kong B. Polymorphisms of receptor for advanced glycation end products and risk of epithelial ovarian cancer in Chinese patients. Cell Physiol Biochem 2013;31:525-31. [CrossRef]
10. Riehl A, Nemeth J, Angel P, Hess J. The receptor RAGE:Bridging inflammation and cancer. Cell Commun Signal 2009;7:12. [CrossRef]
11. Hofmann M, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis:A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999;97:889-901. [CrossRef]
12. Swamy PS, Ramakrishnan PS. Weed potential of Mikania micrantha H.B.K. and its control in fallows after shifting agriculture (Jhum) in North-East India. Agric Ecosyst Environ 1987;18:195-204. [CrossRef]
13. Facey PC, Pascoe KO, Porter RB, Jones AD. Investigation of plants used in Jamaican folk medicine for anti-bacterial activity. J Pharm Pharmacol 1999;51:1455-60. [CrossRef]
14. Borah S, Das AK, Saikia D, Borah J. A note on the use of ethno medicine in treatment of diabetes by Mishing Communities in Assam, India. Ethnobot leaflets. Int J Ethnobot Res 2009;13:1348-52.
15. Lalramghinglova H, Rai PK. Ethnomedicinal plant resources of Mizoram, India:Implication of traditional knowledge in health care system. Ethnobot Leaflets 2010;14:274-305.
16. Bhardwaj S, Gakhar SK. Ethnomedicinal plants used by the tribals of Mizoram to cure cuts and wounds. Indian J Tradit Knowl 2004;4:75-80.
17. Saha SS, Kalyan MS, Rahaman CH. Anato-pharmacogonistic studies of Mikania micrantha Kunth:A promising medicinal climber of the family Asteraceae. Int J Res Ayurveda Pharm 2015;6:773-80. [CrossRef]
18. Krishnaiah D, Devi T, Bono A, Sarbatly R. Studies on phytochemical constituents of six Malaysian medicinal plants. J Med Plants Res 2009;3:67-72.
19. Kim D, Jeond S, Lee C. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 2003;81:321-6. [CrossRef]
20. Nardini M, Natella F, Scaccini C. Role of dietary polyphenols in platelet aggregation. A review of the supplementation studies. Platelets 2007;18:224-43. [CrossRef]
21. Ibrahim Rizvi S, Abu Zaid M. Impairment of sodium pump and Na/H exchanger in erythrocytes from non-insulin dependent diabetes mellitus patients:Effect of tea catechins. Int J Clin Chem Diagn Lab Med 2005;354:59-67. [CrossRef]
22. Rasouli H, Farzaei MH, Khodarahmi R. Polyphenols and their benefits:A review. Int J Food Properties 2017;20:1700-41. [CrossRef]
23. Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann Rev Nutr 2001;21:381-406. [CrossRef]
24. Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Chem Technol Metallurg 2005;40:255-60.
25. Slinkard K, Singleton VL. Total phenol analysis:Automation and comparison with manual methods. Am J Enol Vitic 1977;28:49-55.
26. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999;64:555-9. [CrossRef]
27. Levan A. The effect of colchicines on root mitoses in Allium. Hereditas 1938;24:471-86. [CrossRef]
28. Grant WF. Chromosome aberration assays in Allium:A report of the U.S. environmental protection agency gene-tox program. Mutat Res 1982;99:273-91. [CrossRef]
29. Buckton KE, Evans HJ. Methods for the Analysis of Human Chromosome Aberrations. Switzerland:World Health Organization;1972. 21-2.
30. Shimamura T, Sumikura Y, Yamazaki T, Tada A, Kashiwagi T, Ishikawa H, et al. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives inter-laboratory evaluation study. Anal Sci 2014;30:717-21. [CrossRef]
31. Mosmann T. Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J Immunol Methods 1982;65:55-63. [CrossRef]
32. Carbonell-Capella JM, Buniowska M, Esteve MJ, Frigola A. Effect of Stevia rebaudiana addition on bioaccessibility of bioactive compounds and antioxidant activity of beverages based on exotic fruits mixed with oat following simulated human digestion. Food Chem 2015;184:122-30. [CrossRef]
33. Velmurugan BK, Rathinasamy B, Lohanathan BP, Thiyagarajan V, Weng CF. Neuroprotective role of phytochemicals. Molecules 2018;23:2485. [CrossRef]
34. Dev UK, Hossain MT, Islam MZ. Phytochemical investigation, antioxidant activity and antihelminthic activity of Mikania micrantha leaves. World J Pharm Res 2015;5:121-33.
35. Lalrinzuali K, Vabeiryureilai M, Jagieta GC. Ethnomedicinal use and phytochemical analysis of selected medicinal plants of Mizoram, India. Trends Green Chem 2015;1:18. [CrossRef]
36. Borkataky M, Kakoty BB, Saikia LR. Antimicrobial activity and phytochemical screening of some common weeds of Asteraceae family. Int J Pharm Sci Rev Res 2013;23:116-20.
37. Gadano A, Gurni A, Lopez P, Ferraro GM, Carballo M. In vitro genotoxic evaluation of the medicinal plants Chenopodium ambrosoides L. J Ethnopharmacol 2002;81:11-6. [CrossRef]
38. Fachinetto JM, Bagatini MD, Silva AC, Tedesco SB. Efeito anti-proliferativo das infusões de Achyrocline satureioides DC (Asteraceae) sobre o ciclo celular de Allium cepa. Rev Bras Farmacogn 2007;17:49-54. [CrossRef]
39. Lubini G, Fachinetto JM, Laughinghouse HD 4th, Paranhos JT, Silva AC, Tedesco SB. Extracts affecting mitotic division in root-tip meristematic cells. Biologia 2008;63:647-51. [CrossRef]
40. Dias MG, Canto-Dorow TS, Coelho AP, Tedesco SB. Efeito genotóxico e antiproliferativo de Mikania cordifolia (L.F.) Willd. (Asteraceae) sobre o ciclo celular de Allium cepa L. Rev Bras Plant Med 2014;16:202-8. [CrossRef]
41. El-Sadek LM. The effect of TCA and its herbicidal forms on Faba vulgaris root meristems. Egypt J Genet Cytol 1972;1:280-7.
42. Teerarak M, Laosinwattana C, Charoenying P. Evaluation of allelopathic, decomposition and cytogenetic activities of Jasminum officinale L. f. var grandiflorum (L.) Kob. On bioassay plants. Bioresour Technol 2010;101:5677-84. [CrossRef]
43. Chethan J, Sampath KK, Sekhar S, Prakash HS. Antioxidant, antibacterial and DNA protecting activity of selected medicinally important Asteraceae plants. Int J Pharm Pharm Sci 2012;4:257-61.
44. Dong LM, Jia XC, Luo QW, Zhang Q, Luo B, Liu WB, et al. Phenolics from Mikania micrantha and their antioxidant activity. Molecules 2017;22:1140. [CrossRef]
45. Dou X, Yu Z, Ning S, Yuhe W, Li L. The anti-tumor activity of Mikania micrantha aqueous extract in vitro and in vivo. Cytotechnology 2013;66:107-17. [CrossRef]
46. Rufatto LC, Finimundy TC, Roesch-Ely M, Moura S. Mikania laevigata:Chemical characterization and selective cytotoxic activity of extracts on tumor cell lines. Phytomedicine 2013;20:883-9. [CrossRef]
47. Sekender Ali M, Islam MS, Rahman MM, Islam MR, Sayeed MA, Islam MR. Antibacterial and cytotoxic activity of ethanol extract of Mikania cordata (Burm.f.) B.L. Robinson leaves. J Basic Clin Pharmacol Toxicol 2015;2:103-7.
48. Nicollier G, Thompson AC. Essential oil and terpenoids of Mikania micrantha. Phytochemistry 1981;20:2587-8. [CrossRef]
49. Saikia S, Tamuli KJ, Narzary B, Banik D, Bordoloi M. Chemical characterization, antimicrobial activity, and cytotoxic activity of Mikania micrantha Kunth fower essential oil from North East India. Chem Papers 2020;74:2515-28. [CrossRef]
50. Sukjamnong S, Santiyanont R. Antioxidant activity of Fimbristylis ovata and its effect on RAGE gene expression in human lung adenocarcinoma epithelial cell line. J Chem Pharm Res 2012;4:2483-9.
51. Kashyap CP, Tikka B, Sharma S, Kumari S, Verma P, Sharma S, Arya V. Human cancer cell lines a brief communication. J Chem Pharm Res 2011;3:514-20.