1. INTRODUCTION
Medicinal properties in plants have attracted the attention of many scientists all over the world over the period of time. Plants are the source for the biosynthesis of an array of phyto-constituents which form the basis for various commercial pharmaceutical drugs and herbal remedies [1]. The extensive study on the use of the medicinal plants to treat cancer in traditional as well as scientific parlance is being carried out to design effective anticancer drugs. Many studies have revealed that secondary metabolites, like alkaloids, polyphenols, terpenoids, and phenyl propanoids, are being considered for the drug development as they are rich in anticancer and antimutagenic properties [2]. Other benefits of using medicinal plants over allopathic drugs to treat cancer include their availability, cheap price, and toxic-free nature. A major emphasis is given to the research in use of phytoconstituents in anticancer drugs that may provide prevention and treatment of cancer, without any side-effects, which are seen with chemo and radio therapies [3,4].
Solanaceae family constitutes around 80 genera consisting of 3,000 species, from which 1,500 species comes under the genus Solanum [5]. Solanum, known for the abundant presence of steroidal glycoalkaloids and flavonoids, is the important traditional medicinal plants with pharmacological properties, like antioxidant, anti-ulcerogenic, hepatoprotective and neuroprotective effects, and anticancer activities. It is widely used in various Ayurveda formulations in the form of capsules and syrup [6,7]. Solanum species contain a number of free and glycosylated alkaloids those gradually get converted into the precursor for biosynthesis of steroids and used in the formulation of steroidal drugs [8]. Conventional secondary metabolites, especially steroidal alkaloids and steroidal glycosides constitute an essential compound in plants and exhibits anti-inflammatory, anti-bacterial, anti-parasitic, anti-fungal, and anti-cancer activities [9,10].
Solasodine is the principal constituent of Solanum spp., which is a steroidal glycoalkaloid, known for the wide range of pharmacological activities, like hepatoprotective, antifungal, antiandrogenic, anticancer, antipyretic, diuretic, immunomodulatory, cardiotonic, and anticancer activity [11–13]. Solasodine exhibited higher efficacy of anticancer activities on a varied range of cancer cell lines and may come up as a new chemotherapeutic agent for the treatment of cancer [14,15].
Although solasodine exhibits such important therapeutic properties, its concentration in in vivo plant is low which may not be sufficient to fulfill the increasing demand of the pharmaceutical industry. The present investigation is carried out to check the cytotoxic activity of solasodine extracted from four different species of Solanum, including S. nigrum, S. surattense, S. villosum, and S. incanum against cancer cells lines HT-29 (Human Colorectal Adenocarcinoma Cell Line), MG-63 (Human Osteosarcoma Cells Line), and normal cell line L-929 and evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays.
2. MATERIALS AND METHODS
2.1. Chemicals
Antibiotic-antimycotic 100× solution was purchased from Thermo fisher Scientific. 200 mM L-glutamine, 0.25% trypsin/0.038% Ethylenediamine tetraacetic acid (EDTA), Dulbecco’s Modified Eagle Medium (DMEM), MTT dye were procured from HiMedia. Heat inactivated fetal bovine serum (FBS) was obtained from Gibco, Invitrogen. Purified Solasodine procured from Sigma (SML 1141) was used as the standard.
2.2. Extraction of Solasodine
Elicitation using cell suspension culture to enhance the solasodine concentration was carried out in four different varieties of Solanum, including S. nigrum, S. surattense, S. villosum, and S. incanum at our laboratory. Solasodine extracted from the said studies, i.e., from four different Solanum species were used to check the cytotoxic activity.
Solasodine was extracted by using the method described by Desai et al. [16] with some modifications. Cell suspensions were filtered through a sterile nylon mesh (100 mm) after the incubation and the filtrate having solasodine were collected into a sterile flask, and the cells biomass was separated. Filtrate was concentrated by heating at 37°C and obtained in the powdered form. One gram powder from suspension cultures of each variety was dried at 60°C and extracted with 25 ml of methanol separately and hydrolysed with 1 M HCl under reflux for 3 hours so as to remove the sugar residues. The hydrolysed material was cooled and the residue was dissolved in 10 ml ethanol and filtered through 0.2 μM membrane filter.
2.3. Cell Cultures
Human Colorectal Adenocarcinoma cell line (HT-29), Osteosarcomacell line (MG-63), and normal fibroblast cell line (L-929) were obtained from National Center for Cell Sciences, Pune and were maintained with DMEM medium supplemented with 10% heat inactivated FBS and 1% antibiotic-antimycotic 100× solution.
2.4. Cytotoxicity Assay
MTT assay is a quantitative colorimetric assay which indicates the proliferation of cells. The cells were maintained in DMEM with 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and supplemented with 10% FBS procured from Gibco Life Technologies, Bangalore-India.
The cells were seeded with a density of 5 × 103 cells/well in a 96-well micro titre plate and final volume made up to 150 μl with DMEM media. It was maintained in the presence of 5% CO2 at 37°C into CO2 incubator overnight. Cell lines were treated with solasodine extracts in different concentrations, namely, 31.25, 62.5, 125, 250, 500, and 1,000 μg/ml and incubated for 24 hours. Standard solasodine was used as a control. 20 μl of the freshly prepared MTT reagent (5 mg/ml in PBS) procured from Hi Media laboratories was added to each of the well containing cells followed by 4 hours incubation. After incubation, 100 μl of dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The absorbance was recorded at 570 nm using Enzyme-linked immunoassay (ELISA) plate reader (Lisa plus, India) [17]. The assay was performed in triplicates and percent cell viability was calculated. The optical density of absorbance is directly proportional to the number of live cells.
2.5. Statistical Analysis
All data reported are the arithmetic mean from three independent experiments performed in triplicate and ± standard deviation unless stated otherwise. For calculation of IC50 value, % cell inhibition was determined by using following formula
% Cell inhibition = 100−{Mean Optical density (OD) of test compound/Mean Optical density (OD) of Negative control × 100}
The graph Pad Prism Version 5.1 was used for the same.
3. RESULTS AND DISCUSSION
3.1. Cytotoxic Effect of Standard Solasodine on Cancer Cells (HT-29 and MG-63) and on Normal Fibroblast Cells (L-929)
Standard solasodine (Sigma—SML 1141) was tested at different concentrations, namely, 2.5, 5, 10, 20, 40, and 80 μg/ml in order to observe the growth inhibition of cancer cell lines HT-29 and MG-63 and normal L-929 cell lines. As illustrated in Figure 1, percent cell viability showed inversely proportional trend with increasing solasodine concentration in all cell lines. The percentage of cell growth inhibition in normal fibroblast L-929 cell line was the lowest, namely, 2.7% at 2.5 μg/ml concentrations and even at a higher concentration of 80 μg/ml, this cell line demonstrated percent cell growth inhibition as low as 15.8%. This reflects a potent feature of solasodine being less toxic to the normal cells [18]. Human Colorectal Adenocarcinoma cell line (HT-29) and Osteosarcoma cell line (MG-63) followed suit producing same results as normal cell line (L-929) with a higher proportion of percent cell growth inhibition. Alkaloids block various chemical activities that cause cancer [19], making solasodine an effective anti-cancer drug.
HT-29 cancer cell line was the most responsive to standard solasodine in all concentrations studied. These cell lines exhibited 43.8% cell growth inhibition at the lowest concentration of 2.5 μg/ml solasodine, increased with concentrations, and to 76.9% cell growth inhibition at 80 μg/ml. This was in accordance with the recent study conducted by Zhuang et al.[20], where in solasodine had a greater suppressive effect against few colorectal cell lines, such as HCT116, HT-29, and SW480, whereas MG-63 cell line indicated 20.9% cell growth inhibition at 2.5 μg/ml and highest cell growth inhibition of 63.9% at 80 μg/ml concentration, which is 13% lower than that of HT-29. MG-63 cell line has consistently demonstrated lower percent cell viability, like 20.9%, 22.2%, 31.3%, 35.8%, 49.1%, and 63.9% at 2.5, 5, 10, 20, 40, and 80 μg/ml standard solasodine concentrations, respectively, as opposed to 43.8%, 54.7%, 63.4%, 68.7%, 72.4%, and 76.9% cell growth inhibition of HT-29 at the same concentration levels (Fig. 1).
Another peculiar trend of HT-29 is the decreasing difference between percent cell inhibitions as the concentration increases. Cell growth inhibition of HT-29 was 43.8% and 54.7% at 2.5 and 5 μg/ml concentrations, respectively, which showcases the huge difference of 10.9% cell growth inhibition with just 2.5 μg/ml hikes in concentration. On the contrary, it manifests meager 4.5% difference in cell growth inhibition even after doubling the concentration from 40 to 80 μg/ml, which is significantly higher than 2.5 μg/ml concentration. This difference is even more pronounced in MG-63 except it is exactly the opposite—the gap of percent cell growth inhibition widens from low to high concentration. As the concentration increases from 2.5 to 5 μg/ml, the percent cell growth inhibition increases by a mere 1.3%, that is, from 20.9% to 22.2%. This variation, however, broadens at 40 and 80 μg/ml concentrations to 14.8% cell inhibition, from 49.1% to 63.9% cell growth inhibition, respectively. An objective analysis of these two stark observations indicates that the MG-63 cancer cell line is likely to have higher percent cell growth inhibition if treated at concentrations higher than 80 μg/ml standard solasodine [21]. Conversely, HT-29 seems to have slowed down in its response to anticancer activity that percent cell growth inhibition would not raise significantly at higher concentrations after 80 μg/ml.
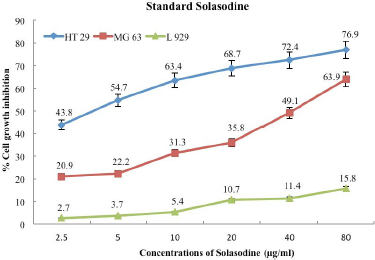 | Figure 1: Effect of standard solasodine on cell growth inhibition of cancer cell line- HT-29 and MG-63 and normal fibroblast L- 929 cell lines at various concentrations. [Click here to view] |
3.2. Cytotoxic Effects of Solasodine Extracted from In Vitro Cultured Cells of S. surattense, S. villosum, S. nigrum, and S. incanum on Cancer Cell Lines (HT-29 and MG-63) and on Normal Fibroblast Cell Line L-929
The MTT assay was carried out with the solasodine extracted from cultured cells of S. surattense, S. villosum, S. incanum, and S. nigrum at different concentrations, namely, 31.25, 62.5, 125, 250, 500, and 1,000 μg/ml with the motive of examining the effect of extracted solasodine on HT-29 and MG-63 cancer cell lines. Solasodine indicated high rate of inhibition in the proliferative capability of HT-29 and MG-63 cell lines as compared to the normal cell line (Fig. 2).
 | Figure 2: IC50 values of solasodine extracted from cell culture of S. surattense, S. villosum, S. incanum, and S. nigrum on (a) L-929 (b) HT-29 (c) MG-63 cell lines. [Click here to view] |
According to the results, normal fibroblast cells L-929 displayed 10.2% cell growth inhibition at 31.25 μg/ml concentration of S. nigrum extract, highest among the lower concentration of solasodine extracts from four species of Solanum, i.e., S. surattense, S. villosum, S. incanum, and S. nigrum (Fig. 3). The lowest percent cell growth inhibition was observed in S. villosum extract, which was recorded as 0.6% at 31.25 μg/ml concentration.
 | Figure 3: Effect of solasodine extracted from a) S. surattense b) S. villosum c) S. incanum and d) S. nigrum on cell growth inhibition on L-929 normal cell line at various concentrations. [Click here to view] |
Interestingly, in the case of HT-29 cancer cells, S. surattense extract at 31.25 μg/ml concentration started from 2.7% cell growth inhibition which is the lowest percentage of cell growth inhibition among all four extracted solasodine samples (Fig. 4). The modest escalations of S. incanum extract and S. villosum extract in cell growth inhibition percentage indicate the likelihood of flaccid response at concentrations higher than 1,000 μg/ml. On the other hand, S. surattense extract followed by S. nigrum extract point toward more positive results at higher concentrations (1,000 μg/ml) than the ones used in this study, based on the rapid increase in cell growth inhibition percentage.
While, S. nigrum extract has consistently displayed higher % cell growth inhibition, namely, 21.9, 38.2, 40.6, 53.1, 57.9, and 59.2 at 31.25, 62.5, 125, 250, 500, and 1,000 μg/ml concentrations, respectively, with respect to Osteosarcoma cell line MG-63 (Fig. 5). Contrarily, S. incanum has underperformed at all concentrations with respect to other extracted solasodine samples. Solanum incanum manifested 11.4% cell growth inhibition at 31.25 μg/ml concentrations and rested at 38.2% cell growth inhibition after being treated at 1,000 μg/ml concentration. The present result indicates that solasodine extracts from cell cultures of S. surattense and S. nigrum response better in terms of cell growth inhibition of cancer cell lines HT-29 and MG-63 compare to the other species studied. Many studies have outlined that the MTT is a rapid, consistent, and less variation method to ascertain the sensitivity of anticancer drugs [22]. The cytotoxic effect of solasodine has also been reported in other cancer studies, namely, human hepatocellular [23], ovarian [24], pancreatic [25], and lung [26]. In the present study, the solasodine extracts had anti-proliferative effects against HT-29 and MG-63 cancerous cells and exerted fewer effects on normal cells. Thus, the cytotoxicity effects were dose-dependent; hence, the maximum cytotoxicity was sighted for HT-29 cancer cells.
 | Figure 4: Effect of solasodine extracted from a) S. surattense, b) S. villosum, c) S. incanum, and d) S. nigrum on cell growth inhibition on HT-29 cancer cell line at various concentrations. [Click here to view] |
 | Figure 5: Effect of solasodine extracted from a) S. surattense, b) S. villosum, c) S. incanum, and d) S. nigrum on cell growth inhibition on MG-63 cancer cell line at various concentrations. [Click here to view] |
4. CONCLUSION
Medicines derived from natural sources can rescue the world from the vicious cycle of high cost-high adverse effects caused by the synthetic drugs. It is the need of the hour that the scientists combine modern science and natural products to effectively address the shortfall of anticancer drugs with the least hazardous effects. According to the study, herbal medicines are gaining popularity even in the developed countries because of its safe nature [27]. This study highlights the positive effects of extracted solasodine from S. surattense, S. villosum, S. incanum, and S. nigrum on HT-29 and MG-63 cancer cell lines and underlines the fact that solasodine is an extremely efficient anti-cancer agent which helps in high cell growth inhibition and low cell viability. The degree of effectiveness varies with different levels of concentration and is mostly dependent on the cancer line, it is being used upon. The results obtained in this review provide reliable information for researchers who are working in the area of anticancer activity of secondary metabolites in general and solasodine in particular.
5. ACKNOWLEDGMENTS
The authors are grateful to the Director, Dr. D.Y. Patil Biotechnology and Bioinformatics Institute, Pune for providing the research facilities needed in carrying out this research work. The authors are also thankful to Secretary, Mahatma Gandhi Mission—Aurangabad, Principal, MGM College of Agricultural Biotechnology, Aurangabad and KAHER’s BSRC for providing the laboratory/cell culture facility.
6. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
11. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem 2020;148:80–9; http://doi.org/10.1016/j.plaphy.2020.01.006. CrossRef
2. Sanchita and Sharma A. Gene expression analysis in medicinal plants under abiotic stress conditions. Plant metabolites and regulation under environmental stress, Academic press, pp 407–14, 2018. CrossRef
3. Deshpande J, Bodas M, Wani M, Khetmalas M. Plumbagin: a potential drug candidate for treatment of oral cancer. Trends Life Sci 2015;4(4), 2319–5037(e).
4. Ahmad R. Steroidal glycoalkaloids from S. nigrum target cytoskeletal proteins: an in silico analysis. Peer J 2019;7:e6012; http://doi.org/10.7717/peerj.6012
5. Gebhardt C. The historical role of species from the Solanaceae plant family in genetic research. Theor Appl Genet 2016;129:2281–94; http://doi.org/10.1007/s00122-016-2804-1
6. Abdel-Rahim EA, Abdel-Mobdy YE, Ali RF, Mahmoud HA. Hepatoprotective effects of S. nigrum Linn fruits against cadmium chloride toxicity in albino rats. Biol Trace Elem Res 2014;160(3):400–8. CrossRef
7. Chester K, Paliwal S, Khan W, Ahmad S. UPLC-ESI-MS/MS and HPTLC method for quantitative estimation of cytotoxic glycosides and aglycone in bioactivity guided fractions of S. nigrum L. Front Pharmacol 2017;8:434. CrossRef
8. Solouki M, Hoshyar H, Ramroudi M, Tavassoli A. Comparison and evaluation of steroid alkaloid solasodine on in vivo and in vitro cultures of S. surattense Burm L. Afr J Microbiol Res 2011;5(22):3810–4. CrossRef
9. Agostini C, Vieira RF, Bizzo HR. Secondary metabolites. In: Dhanarasu S (ed.). Chromatography and its applications, InTech, Rijeka, Croatia, 2012.
10. Sparg SG, Light ME, Van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol 2004;94(2–3):219–43; http://doi.org/10.1016/j.jep.2004.05.016
11. Desai AB, Kagathara VG, Joshi H, Rangani AT, Mungra H. Evaluation of antiamnesic effect of solasodine in mice. Int J Pharm Tech Res 2011;3(2):732–40.
12. Thomas S, Gunasagaran G, Arumugam VA, Muthukrishnan S. Synthesis and characterization of zinc oxide nanoparticles of S. nigrum and its anticancer activity via the induction of apoptosis in cervical cancer. Biol Trace Elem Res 2021; http://doi.org/10.1007/s12011-021-02898-6
13. Montagner GFFDS, Barbisan F, Ledur PC. In vitro biological properties of S. sessiliflorum (dunal), an amazonian fruit. J Med Food 2020;23(9):978–87; http://doi.org/10.1089/jmf.2019.0193
14. Munari CC, de Oliveira PF, Campos JC. Antiproliferative activity of S. lycocarpum alkaloidic extract and their constituents, solamargine and solasonine, in tumor cell lines. J Nat Med 2014;68(1):236–41; http://doi.org/10.1007/s11418-013-0757-0
15. Cham BE. Cancer intralesion chemotherapy with solasodine rhamnosyl glycosides. Res J Biolo Sci 2008;(3)1008–17.
16. Desai S, Tatke P, Gabhe S. A new HPLC-DAD method for estimation of solasodine from Solanum nigrum Linn. extracts and formulations. Nat Prod J 2014;4(3):1–5. CrossRef
17. Cota DL, Mishra S, Shengule SA, Patil D. Assessment of in vitro biological activities of Terminalia arjuna Roxb. bark extract and Arjunarishta in inflammatory bowel disease and colorectal cancer. Indian J Exp Biol 2020;58:306–13.
18. Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol 2020;10:1614. CrossRef
19. Prajapati ND, Purohit SS, Sharma AK, Kumar T. A hand book of medicinal plants. 1st edition, Agrobios Publisher, Jodhpur, India, 2003.
20. Zhuang YW, Wu CE, Zhou JY. Solasodine inhibits human colorectal cancer cells through suppression of the AKT/glycogen synthase kinase-3β/β-catenin pathway. Cancer Sci 2017;108 (11):2248–64; http://doi.org/10.1111/cas.13354
21. Ding L, Bailey MH, Porta-Pardo E, Thorsson V, Colaprico A, Bertrand D, et al. Cancer genome atlas research network. Perspective on oncogenic processes at the end of the beginning of cancer genomics. Cell 2018;173(2):305–20.e10; http://doi.org/10.1016/j.cell.2018.03.033
22. Papadimitriou M, Hatzidaki E, Papasotiriou I. Linearity comparison of three colorimetric cytotoxicity assays. J Cancer Ther 2019;10(07):580–90. CrossRef
23. Fekry MI, Ezzat SM, Salama MM, Alshehri OY, Al-Abd AM. Bioactive glycoalkaloids isolated from S. melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci Rep 2019;9(1):1746; http://doi.org/10.1038/s41598-018-36089-6
24. Xu XH, Zhang LL, Wu GS. Solasodine induces apoptosis, affects autophagy, and attenuates metastasis in ovarian cancer cells. Planta Med 2017;83(3–04):254–60; http://doi.org/10.1055/s-0042-113000
25. Zhuang YW, Wu CE, Zhou JY, Zhao ZM, Liu CL, Shen JY, et al. Solasodine reverses stemness and epithelial-mesenchymal transition in human colorectal cancer. Biochem Biophys Res Commun 2018;505(2):485–91; http://doi.org/10.1016/j.bbrc.2018.09.094. CrossRef
26. Shen KH, Hung JH, Chang CW, Weng YT, Wu MJ, Chen PS. Solasodine inhibits invasion of human lung cancer cell through down regulation of miR-21 and MMPs expression. Chem Biol Interact 2017;268:129–35; http://doi.org/10.1016/j.cbi.2017.03.005
27. Ponsoni K, Raddi MSG, de Almeida DV, Almeida AE, Alecio AC. Effects of liver S9 enzymes on somalargine and solasodine cytotoxicity and mass spectrometric fragmentation. Eur Food Res Technol 2013;237; http://doi.org/10.1007/s00217-013-1977-y