Medicinal plants contain various chemical substances which can be used to treat multiple types of human diseases. The chemical compounds of the plants are reported to have antibacterial, antimicrobial, antiviral, and antifungal potentials [1]. From ancient times, the importance of medicinal plants has been recognized. Herbal medicines were used to treat diseases before the availability of synthetic drugs. Plants are employed as therapeutic agents within established systems of traditional medicine, such as Ayurveda, Siddha, and Unani, as well as in less formalized practices, including folk, tribal, and indigenous medicinal traditions. The use of herbal medicine is increasing daily, so screening medicinal plants for their active compounds and understanding innovative mechanisms of action [2, 3] is important. Exploration of Euphorbia heyneana Spreng is very important as this plant is very less studied for their medicinal values. The E. heyneana Spreng. plant is a prostrate annual herb with up to 15 cm long branches commonly known as chhotidudi and belongs to the family Euphorbiaceae. The shape of leaves is clavate or club-shaped, to 8 × 4.5 mm, base circuitously rounded or subcordate, with round apex, edge entire, or saw-like; petiole length measuring 1 mm; while stipules are typically of length 1.5 mm and often have 2–4 linear teeth. The inflorescences are cyathia solitary with cup-shaped involucres; glands four in number. The male flowers are filamentous with few small bracteoles and stamens having a length of 1 mm, and the female flower has a pedicellate type of ovary; minute styles (0.2 mm in length), spreading and bifid to halfway [4]. Fruits, vegetables, medicinal herbs, leaves, flowers, and roots all contain natural bioactive substances called phytochemicals that combine with fibers to protect against diseases [5]. Knowledge of the chemical contents of plants is crucial for discovering therapeutic agents, disclosing novel sources of economically valuable phyto-constituents for synthesizing complex chemical compounds, and finding the significance of folkloric remedies [6]. Presently, various studies have been conducted on medicinal plants that have traditional therapeutic characteristics. The present study aimed to systematically purifying, characterizing, and evaluating the phytochemicals of E. heyneana Spreng, revealing their potential antibacterial properties with molecular-level insights, while also assessing cytotoxic safety.
2. MATERIALS AND METHODS
2.1. Collection of Plant Material
Plant samples were collected from different regions of Rohtak (Longitude: 76.606613 Latitude 28.895515) (Haryana) [Figure 1]. For the collection of plant samples, several field surveys were conducted at regular intervals in different regions of Rohtak. During the visit, necessary field equipment, e.g. knife, scissors, polythene bags, etc., were used. The required information, such as the botanical name, local name, family name, English name, and habitat, was noted at collection time. The local names of the collected plant samples were recorded with the help of interviews and discussions with the local people. Further identification and authentication of the specimens were done from the Botanical Survey of India, Northern Regional Center 192, Kaulagarh Road, Dehradun 248-195 (Acc. No. 1162).
 | Figure 1: Euphorbia heyneana Spreng, grown in wild. [Click here to view] |
2.2. Preparation of Plant Extracts
The collected plants/parts were thoroughly rinsed under running tap water and then dried under shade with distilled water. The dried plants/parts were coarsely ground to powder and then filled into a paper thimble made from Whatman filter paper. Using the Soxhlet extraction method, the plant samples were extracted in different solvents (chloroform, ethanol, and petroleum ether). The extracts were stored at −4°C for further use. The chloroform extract was used for the present study [7].
2.3. Microbial Strains
Bacterial strains used in the antimicrobial study were obtained from the Microbial Type Culture Collection and Gene Bank, Chandigarh, India. Gram-positive (G+ve) strains Bacillus subtilis (MTCC 121) and Staphylococcus aureus (MTCC 96) and Gram-negative (G-ve) strains Escherichia coli (MTCC 443) and Pseudomonas aeruginosa (MTCC 424) were included in this study.
2.4. Screening of Antimicrobial Activity of Plant Extracts
The well-diffusion method described by Magaldi et al. with few modifications was used to assess the antimicrobial potency of the plant extract [8]. The nutrient agar plates were prepared under sterile conditions, and 100 µL of each microbial strain was spread separately to form a bacterial lawn. A sterile borer with a diameter of 6 mm was used to create wells in each plate. The designated wells were filled with 50 µL of extract stocks and dilutions (1:1, 1:2, 1:3, and 1:4) and incubated at 37°C overnight. The experiment was repeated three times, and the zone of inhibition was measured and recorded.
2.4. Purification of the Sample by Reverse High-performance Liquid Chromatography (HPLC)
The plant extracts with high antimicrobial activity were purified by reverse HPLC using the C18 column (Agilent 1200 infinity series). The mobile phase comprises chloroform to acetonitrile in the ratio of 60:40 V/V, and this was carried out using the isocratic mode, elution executed at a flow rate of 1 ml/min. The sample was analyzed for 12 min. and perception was done at 260 nm by an ultraviolet photodiode detector. The purified samples were subsequently used for screening in the antibacterial assay.
2.5. Cytotoxicity Studies
In Vitro assay, cytotoxicity activity was checked by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [9]. For testing, Madin-Darby canine kidney (MDCK) cells were washed with phosphate buffer saline and harvested after trypsinization and seed in 96 well plates (5000 cells/well) and then incubated for 24 h at 37°C. Then, the cells were subjected to various concentrations (1, 0.5, 0.25, 0.125, 0.062, 0.031, 0.015, 0.007 mg/mL) of plant extract. Subsequently, the cells were incubated at 37°C with 5% CO2 for another 24 h. After the incubation period, the medium was removed, and 30 µL of MTT was added to each well in the dark. After 4 h, the resulting formazan crystals were dissolved by adding 100 µL of DMSO. The plate was then kept at room temperature in a dark environment. The absorbance of the samples was measured at 570 nm using a microtiter ELISA plate reader. This entire experiment was repeated two times, and the data were analyzed to determine the cell viability and toxicity by the following formula.
The percentage growth inhibition was calculated by the formula:

Where At = absorbance value of test compound,
Ab = Absorbance value of blank and Ac = Absorbance value of the control.
2.6. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The most potent samples were further characterized by GC-MS analysis [10]. The sample was dissolved in chloroform and injected in a GC-MS-Agilent 7890B GC connected to 5977A MSD, ColumnHP_5MS 5% Phenyl Methyl Silox-60°C–325°C, 30 m × 250 µm × 0.25 µm. Injection mode: Splitless. The following were the GC-MS operating conditions for the analysis: The oven temperature was raised to 280°C and maintained isothermally for 10 min after first reaching 45°C for 2 min and then 140°C at 5°C/min. Helium was employed as the carrier gas at a 1 mL/min rate, and 2 µL of the sample was injected. At 70 eV, the constituent parts of the sample were ionized. The GC operated for 54.0 min. The compounds’ structures were subsequently compared with those in the NIST database by searching the NIST14.L library. Retention times and mass spectra were then used to identify substances that were already cataloged in the NIST library (C: \Database\NIST MS2011.L) [11].
2.7. In Silico Study of Phytochemical against Microbes
2.7.1. Molecular docking
Molecular docking was carried out using AutoDock4.2.6 (https://autodock.scripps.edu/download-autodock4/) with 100 Lamarckian genetic algorithm runs along with the default parameters. Confirmations with the lowest free binding energies, hydrogen bonds, and the most populated cluster were selected for further analysis. Interaction analyses were carried out using the BIOVIA Discovery Studio visualizer.
3. RESULTS AND DISCUSSION
3.1. Screening of Antimicrobial Activity of Plant Extracts
The present study was conducted with the objectives of carrying out the phytochemical screening and pharmacological evaluation of plant extracts of E. heyneana Spreng., extracted with chloroform, petroleum ether, and ethanol [Table 1].
Table 1: Assessment of the growth inhibitory potential of the Euphorbia heyneana Spreng. plant extracts against bacteria.
| Plants | Zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| Solvents | Pseudomonas aeruginosa | Escherichia coli | Staphylococcus aureus | Bacillus subtilis | |
| Euphorbia heyneana Spreng. | Chloroform | - | 16 | 7 | - |
| Petroleum ether | - | - | - | - | |
| Ethanol | 8 | - | - | - | |
After the primary screening of the crude plant extract, crude plant extracts with antimicrobial activity were selected for purification using reverse-phase HPLC (RP-HPLC). The fraction of the major peak was collected and further analyzed for antimicrobial activity.
3.2. Purification of E. heyneana Spreng Extract
The chloroform extract of E. heyneana Spreng was purified using RP-HPLC and the chromatogram showed three major peaks of NPE.PK1, NPE.PK2, and NPE.PK3 [Figure 2]. The first peak after the injection of the compound at 8.701 min, the peak area was 1840019, and the peak height was 124277. The second peak was after 14.181 min of injecting the sample with an area of 764775, and the peak height was 44571. The third peak was after 15.526 min of injecting the sample with area 485622, and the peak height was 42280 [Table 2]. The area covered by the NPE.PK1 was more in comparison to NPE.PK2 and NPE.PK3, and it was assumed that NPE.PK1 comprises more compounds. These major peaks were collected and further evaluated for antimicrobial activity.
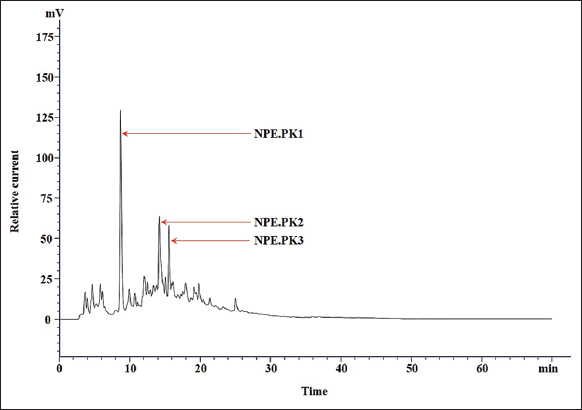 | Figure 2: Chromatogram of crude extracts from Euphorbia heyneana Spreng chloroform. The chromatogram showed the X-axis (horizontal) which represented retention time (minutes) and Y-axis (vertical) represented relative current (Mv). The time interval between the compound’s injections and the compound’s detection is described as retention time and plays a significant role in the identification of the compound. The peak area represents the amount of compounds that have passed the detector. [Click here to view] |
Table 2: Reverse phase high-performance liquid chromatography analysis of chloroform extract of Euphorbia heyneana.
| Peaks | Retention time | Area | Height |
|---|---|---|---|
| NPE.PK1 | 8.701 | 1840019 | 124277 |
| NPE.PK2 | 14.181 | 764775 | 44571 |
| NPE.PK3 | 15.526 | 485622 | 42280 |
3.3. Antimicrobial Activity of Purified Fraction of E. heyneana Spreng
The major three fractions of NPE.PK1, NPE.PK2, and NPE.PK3 were obtained after the purification of E. heyneana Spreng extract. These fractions showed varied antimicrobial activity [Table 3]. The NPE.PK2 showed more antimicrobial activity against P. aeruginosa with a zone of inhibition of 15 mm diameter. Another fraction of NPE.PK1 showed activity against P. aeruginosa only with a 10 mm zone of inhibition. The NPE.PK3 fraction showed activity against B. subtilis and P. aeruginosa with 10 mm and 5 mm zones of inhibition, respectively. However, no antimicrobial activity was observed against E. coli and S. aureus.
Table 3: Antimicrobial activity of the purified compounds from chloroform extract of Euphorbia heyneana.
| Purified compounds | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Bacillus subtilis |
|---|---|---|---|---|
| NPE.PK1 | − | ++ | − | − |
| NPE.PK2 | − | +++ | − | + |
| NPE.PK3 | − | + | − | ++ |
Each “+” sign was equal to 5 mm of the zone of inhibition.
The purified fractions showed maximum activity against the bacteria P. aeruginosa and mild activity against B. subtilis; however, no activity was observed against E. coli and S. aureus. The active fractions were further characterized using the GC-MS technique. In the subsequent sections, these fractions were abbreviated as NPE1 and NPE2.
3.4. Characterization of NPE1 Fraction of E. heyneana Spreng
The NPE1 fraction of E. heyneana Spreng was analyzed using GC-MS and a chromatogram showed that this fraction consists of 28 different compounds [Table 4]. The compound 3-chloropropionic acid, heptadecyl ester makes a high peak with the largest percent area coverage, i.e., 10.192% at a retention time of 32.041 min [Figure 3]. The second largest percent area coverage was showed by 2-isopropyl-5-methyl-1-heptanol which is 5.791% at retention time 36.072 min, although it also appeared at 34.671 min but percent area coverage was 0.437%. Five compounds with least percent area coverage were 3-Carene, Trichloroacetic acid, hexadecyl ester, 10-Methylnonadecane, Ethanol, 2-(octadecyloxy), and Dodecane, 2,6,11-trimethyl, and their percent area coverage and retention time were 0.169% (9.443 min), 0.181% (45.947%), 0.190% (25.977 min), 0.203% (43.511 min), and 0.237% (20.547 min), respectively. The compound detection in GC-MS is listed in Table 4.
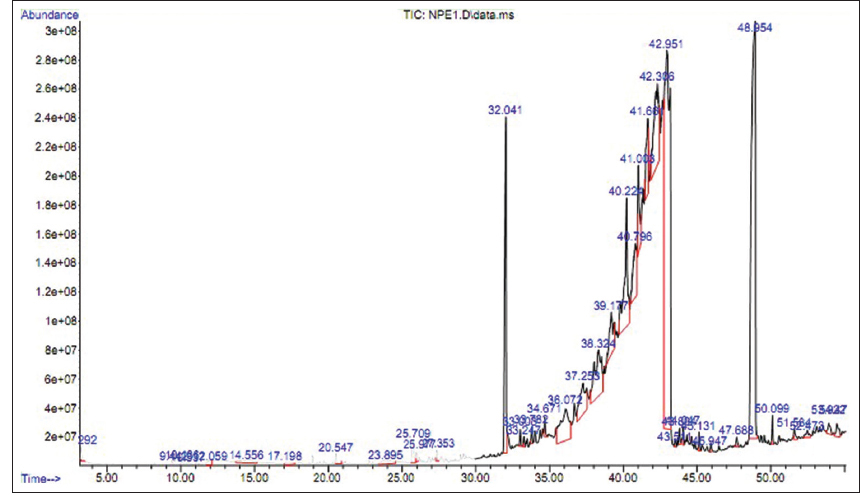 | Figure 3: Gas chromatography-mass spectrometry chromatogram for chloroform extract (NPE1 fraction) of Euphorbia heyneana Spreng. [Click here to view] |
Table 4: Spectral analysis of chloroform extract (NPE1, fraction) of Euphorbia heyneana shoot.
| Name | RT. Min | MW | SF | PA% |
|---|---|---|---|---|
| Isobutyl acetate | 3.292 | 116 | C6H12O2 | 0.358 |
| 3-Carene | 9.443 | 136 | C10H16 | 0.169 |
| Cyclohexene,1-methyl-5- (1-methylethenyl)-, (R)- | 10.136 | 136 | C10H16 | 0.325 |
| 1-Hexanol, 2-ethyl- | 10.537 | 130 | C8H18O | 0.257 |
| 1H-Imidazole | 12.059 | 68 | C3H4N2 | 0.392 |
| Glycerin | 14.556 | 92 | C3H8O3 | 0.811 |
| 2,4-Imidazolidinedione, 3-methyl | 17.198 | 114 | C4H6N2O2 | 0.396 |
| Dodecane, 2,6,11-trimethyl | 20.547 | 212 | C15H32 | 0.237 |
| Tromethamine | 23.895 | 121 | C4H11NO3 | 0.957 |
| 1-Undecanol | 25.709 | 172 | C11H24O | 0.881 |
| 10-Methylnonadecane | 25.977 | 282 | C20H42 | 0.190 |
| Hexadecane, 2,6,11,15-tetramethyl | 27.353 | 282 | C20H42 | 0.256 |
| 3-Chloropropionic acid, heptadecyl ester | 32.041 | 346 | C20H39ClO2 | 10.192 |
| 1-Dodecanol, 2-octyl- | 33.003 | 298 | C20H42O | 0.285 |
| Trichloroacetic acid, hexadecyl ester | 33.782 | 386 | C18H33Cl3O2 | 0.340 |
| 2-Isopropyl-5-methyl-1- heptanol | 34.671 | 172 | C11H24O | 0.437 |
| 36.072 | 172 | C11H24O | 5.791 | |
| 1,4-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester | 37.253 | 390 | C24H38O4 | 4.171 |
| Terephthalic acid, 4-octyl octyl ester | 39.177 | 390 | C24H38O4 | 3.565 |
| Ethanol, 2-(octadecyloxy) | 43.511 | 314 | C20H42O2 | 0.203 |
| Decanedioic acid, dibutyl ester | 43.804 | 314 | C18H34O4 | 0.378 |
| 2-Methyl-Z-4-tetradecane | 44.047 | 210 | C15H30 | 0.347 |
| Heptacosyl pentafluoropropionate | 45.131 | 542 | C30H55F5O2 | 0.362 |
| Trichloroacetic acid, hexadecyl ester | 45.947 | 386 | C18H33Cl3O2 | 0.181 |
| Hexanedioic acid, bis (2-ethylhexylester | 47.688 | 370 | C22H42O4 | 0.288 |
| Diisooctyl phthalate | 51.584 | 390 | C24H38O4 | 0.406 |
| Eicosane, 7-hexyl- | 52.473 | 366 | C26H54 | 0.454 |
| Hexanoic acid, 2-ethyl-, octadecyl ester | 53.922 | 396 | C26H52O2 | 0.605 |
The GC-MS analysis of the chloroform-extracted NPE1 fraction from E. heyneana Spreng revealed that it contains 28 compounds of which two compounds had the highest PA. The highest peak area was observed for 3-chloropropionic acid, heptadecyl ester (10.192%) followed by the peak area of 2-isopropyl-5-methyl-1-heptanol (5.791%). Gupta and Kumar [12] reported that the plant extract of Terminalia arjuna showed antimicrobial activity against different pathogenic bacteria such as E. coli, P. aeruginosa, Raoultella planticola, Enterobacter aerogenes, B. subtilis, and Agrobacterium tumefaciens. GC-MS analysis of T. arjuna extract revealed 3-chloropropionic acid, and heptadecyl ester was one of the major components with a percent area coverage of 3.89%. Another compound 2-isopropyl-5-methyl-1-heptanol was reported to have antimicrobial activity [13].
3.5. Characterization of NPE2 Fraction of E. heyneana Spreng
The NPE2 fraction of E. heyneana Spreng was analyzed using GC-MS and a chromatogram showed that this fraction consists of 11 different compounds. The compound 1,4-benzenedicarboxylic acid, bis (2-ethylhexyl) ester was detected at 8 different retention times 27.898, 30.361, 30.93, 32.03, 32.97, 33.857, 36.625, and 36.837 min. The overall percent area coverage by 1,4-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester was 51.7% [Table 5]. Terephthalic acid, 4-octyl octyl ester was detected in the NPE2 fraction three times with a cumulative percent area coverage of 42.5%. Their retention time was 10.368, 11.863, and 20.304 min [Figure 4]. Another compound ethyl acetate, n-Propyl acetate, 2,7,7-Trimethylbicyclo [3.1.1] hept-2-en-6-yl acetate, 1-Undecanol, dodecanoic acid, 10-methyl-, methyl ester, Dodecyl acrylate, 9,12-Octadecadienoic acid, methyl ester, trans-13-Octadecenoic acid, methyl ester, Methyl stearate, and their retention time with percentage area coverage were 2.617 (0.367%), 4.55 (0.054%), 11.238 (0.019%), 21.74 (0.02%), 25.209 (0.086%), 29.103 (2.388%), 37.234 (0.135%), 37.34 (0.33%), and 37.81 (0.101%), respectively.
Table 5: GC-MS spectral analysis of chloroform extract (NPE2 fraction) of Euphorbia heyneana shoots.
| Name | RT. min | MW | SF | PA% |
|---|---|---|---|---|
| Ethyl acetate | 2.617 | 88 | C4H8O2 | 0.367 |
| n-Propyl acetate | 4.550 | 102 | C5H10O2 | 0.054 |
| Bicyclo[3.1.1]hept-2-en-6-ol, 2,7,7-trimethyl-, acetate | 11.238 | 194 | C12H18O2 | 0.019 |
| 1-Undecanol | 21.740 | 172 | C11H24O | 0.020 |
| Dodecanoic acid, 10-methyl-, methyl ester | 25.209 | 228 | C14H28O2 | 0.086 |
| Dodecylacrylate | 29.103 | 240 | C15H28O2 | 2.388 |
| 1,4-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester | 27.898 | 390 | C24H38O4 | 0.228 |
| 30.361 | 390 | C24H38O4 | 0.686 | |
| 30.930 | 390 | C24H38O4 | 3.492 | |
| 32.030 | 390 | C24H38O4 | 5.707 | |
| 32.970 | 390 | C24H38O4 | 10.258 | |
| 33.857 | 390 | C24H38O4 | 10.754 | |
| 36.625 | 390 | C24H38O4 | 7.767 | |
| 36.837 | 390 | C24H38O4 | 12.796 | |
| Terephthalic acid, 4-octyl octyl ester | 34.731 | 390 | C24H38O4 | 10.368 |
| 35.401 | 390 | C24H38O4 | 11.863 | |
| 36.042 | 390 | C24H38O4 | 20.304 | |
| 9,12-Octadecadienoic acid, methyl ester | 37.234 | 294 | C19H34O2 | 0.135 |
| trans-13-Octadecenoic acid, methyl ester | 37.340 | 296 | C19H36O2 | 0.330 |
| Methyl stearate | 37.810 | 298 | C19H38O2 | 0.101 |
GC-MS: Gas chromatography-mass spectrometry.
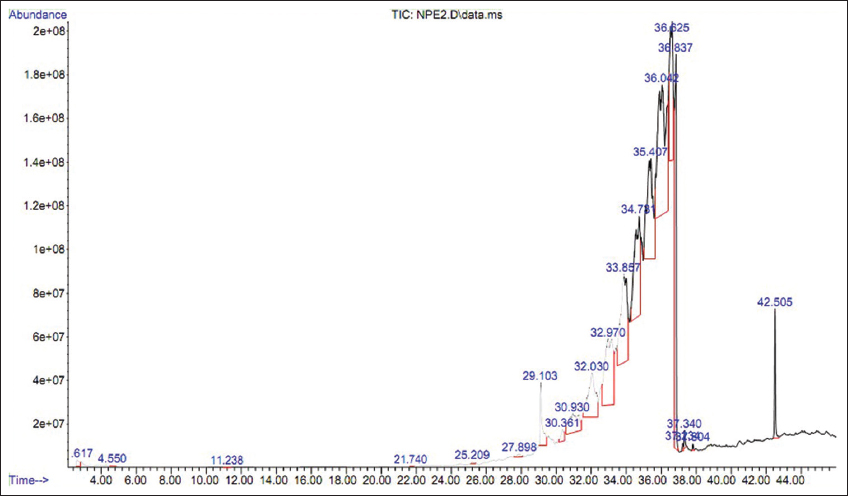 | Figure 4: Gas chromatography-mass spectrometry chromatogram for chloroform extract (NPE2 fraction) of Euphorbia heyneana Spreng. [Click here to view] |
The chloroform extracted fraction from the E. heyneana Spreng (NPE2) when analyzed with GC-MS, the compound 1,4-benzenedicarboxylic acid, bis (2-ethylhexyl) ester was detected at eight different retention times and their cumulative percent was 51.7%. Valarmathi et al. [14] reported antimicrobial activity of the plant extract from Dryopteris hirtipes (Blumze) Kuntze Linn. The GC-MS analysis of the extract demonstrated that the 1,4-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester was one of the major fraction of extract with a peak area of 7.16%. In another study by Adebiyi et al., 1,4-benzenedicarboxylic acid, bis (2-ethylhexyl) ester was the major component in Nephrolepis cordifolia (L) with PA 18.35% [15]. Terephthalic acid, 4-octyl octyl ester was detected in the NPE2 fraction three times with a cumulative percent area coverage of 42.5%. To date, no specific information about the antimicrobial activity of terephthalic acid, 4-octyl octyl ester was found.
3.6. The Minimum Inhibitory Concentration (MIC) of a Plant Extract
The MIC of a plant extract is a crucial parameter in assessing its antimicrobial properties. MIC is defined as the minimum extract concentration at which the visible growth of microorganisms, such as bacteria or fungi is inhibited. This measurement is essential in determining the effectiveness of a plant extract as a potential antimicrobial agent.
The NPE1 and NPE2 fractions of E. heyneana Spreng were subjected to evaluation for MIC against tested bacteria. The MIC of the NPE1 and NPE2 fractions were evaluated against P. aeruginosa. The minimum concentration at which bacterial growth was inhibited is exhibited in Figure 5. The MIC of both the NPE1 and NPE2 fractions against P. aeruginosa was 100 µg/mL.
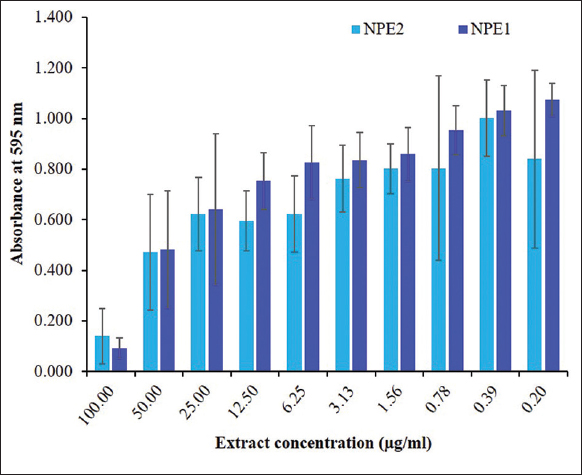 | Figure 5: Minimum inhibition concentration (µg/mL) for the chloroform extracts from Euphorbia heyneana Spreng. [Click here to view] |
In the present study, the MIC of NPE1 and NPE2 fractions of E. heyneana Spreng against P. aeruginosa was also 100 µg/mL. A similar MIC value was also reported by Perumal and Mahmud [16] that the methanol extract Euphorbia hirta L., against P. aeruginosa was 125 µg/mL and in the present study, the MIC of chloroform extract of E. heyneana Spreng against P. aeruginosa was 100 µg/mL. The higher MIC of chloroform extract of the stems of Euphorbia paralias was reported by Hlila et al. and value reported was 310 µg/mL [17]. The acetone extract of Cirsium argyracanthum showed antimicrobial activity against S. aureus and MIC reported was 30 mg/mL [18].
3.7. MTT Assay of NPE1 Extract from E. heyneana Spreng
The result of the MTT assay of E. heyneana Spreng extract NPE1on MDCK cell line exhibited that there was no severe cytotoxic potential against them. The CC50 value of NPE1 was 45.6 µg/100 µL. The dose-dependent study showed around 79.5% of cells died at 100 µg/100 µL dose whereas only 1.3% of cells died at 1.6 µg/100 µL dose [Figure 6]. Fifty percent of the cells survived at a dose of 45.6 µg/100 µL.
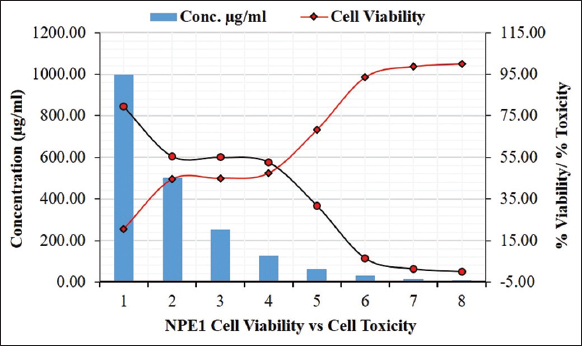 | Figure 6: Cell viability against NPE1 extract of Euphorbia heyneana Spreng. [Click here to view] |
3.8. MTT Assay of NPE2 Extract from E. heyneana Spreng
The MTT assay of NPE2 extract from E. heyneana Spreng on the MDCK cell line showed that there was no severe cytotoxic effect of the extract NPE2 on the MDCK cell line. Only 15.1% of the cells survived under 100 µg/100 µL of NPE2. However, at 50 µg/100 µL dose, concentration around 25% of cells survived and at 3.125 µg/100 µL dose, more than 75% of cells survived [Figure 7]. The CC50 value of NPE2 was 37.7 µg/100 µL.
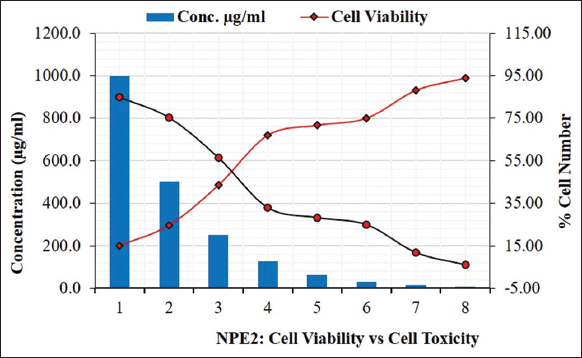 | Figure 7: Cell viability against NPE2 extract of Euphorbia heyneana Spreng. [Click here to view] |
The stability of antimicrobial metabolites from plants in terms of cytotoxicity is crucial. As part of the present study on the discovery of bioactive compounds from E. heyneana Spreng, wild plants with potential antimicrobial activity were evaluated for their cytotoxicity activity, and the chloroform extract of the E. heyneana Spreng was screened in the MTT assay. Concerning the cytotoxicity screening, it was conducted on the MDCK cell line. The determination of cytotoxicity stability of purified antimicrobial metabolites against MDCK cells involves assessing the potency and safety profiles of these compounds, highlighting their potential as effective and safe antimicrobial agents derived from natural sources.
In the present study, the cytotoxicity concentration (CC50) of NPE1 and NPE2 extract from E. heyneana Spreng was 456 µg/ml and 377 µg/ml, respectively, against the MDCK cell line. Sunmathi and Sivakumar [19] studied the effect of the ethanolic leaf extract of Alternanthera philoxeroides and Alternanthera sessilis on the growth of the human osteosarcoma cell line MG-63 [19]. They reported that the extract inhibited cell growth by 67.37% and 47.71%, respectively, at a concentration of 300 µg/ml and the value of the CC50 was found to be 249.2 and 314 µg/mL, respectively. Whereas, in the present study, the CC50 for the Alternanthera pungens against MDCK cell line was 593 µg/mL. The cytotoxic activity of methanolic extract of C. scabrum was investigated by Sahli et al. against the cell line J774 and WI38 and they reported 50% growth inhibition (CC50) at a dose of 11.53 µg/mL and 29.89#x03BC;g/mL, respectively, whereas, in the present study, the CC50 for the Cirsium arvense against MDCK cell line was 515 µg/mL [20].
3.9. In Silico Study of Phytochemical against Microbes
3.9.1. Molecular docking
The molecular docking result revealed that the dihydropteroate synthase exhibited favorable binding affinity with tromethamine. The affinity of tromethamine with dihydropteroate synthase of S. aureus was −6.09 kcal/mol, whereas with E. coli, the affinity was −6.44 kcal/mol. However, the binding affinity with 2-isopropyl-5-methyl-1-heptanol and dihydropteroate synthase of S. aureus was −6.02 kcal/mol, whereas with E. coli, the affinity was –5.15 kcal/mol. In the present study, the Muramyl ligase E (MurE) protein of E. coli had a strong binding affinity with only Terephthalic acid, 4-octyl octyl ester (−7.42 kcal/mol). No significant affinity was shown by any ligands other than Terephthalic acid, 4-octyl octyl ester with MurE protein of Gram-negative and positive bacteria. According to the present study, S. aureus and E. coli protein DNA gyrase B showed a strong binding affinity with the compounds Tromethamine and Terephthalic acid, 4-octyl octyl ester. The binding energies between DNA gyrase B of S. aureus and E. coli with tromethamine were −6.23 and −6.80 kcal/mol, respectively, whereas, it was −7.36 and −7.96 kcal/mol, respectively, for DNA gyrase B. In the current study, terephthalic acid, 4-octyl octyl ester had the highest binding affinity with S. aureus (−7.24 kcal/mol) and E. coli protein transpeptidase (−8.36 kcal/mol). The binding affinity of cyclohexene, 1-methyl-5-(1-methyl ethenyl)-(R) with S. aureus transpeptidase was 7.24 kcal/mol while 9,12-octadecadienoic acid had a binding affinity of 6.27 and 6.67 kcal/mol, respectively, with the transpeptidase protein of S. aureus and E. coli.
Antibacterial activity of the fosfomycin tromethamine in urinary tract infections has been reported by several studies in the urinary tract infection [21, 22]. In a study, the therapeutic benefit of tromethamine was also reported in cystic fibrosis airway disease as it kills the bacteria [23]. The antibacterial activity of 2-isopropyl-5-methyl-1-heptanol was reported by many groups of researchers [24,25]. The compounds isolated from the Piper nigrum contain cyclohexene, 1-methyl-5-(1-methyl ethenyl)-(R) and are reported to be antimicrobial agents [26].
4. CONCLUSION
The extracts derived from the plant E. heyneana Spreng demonstrated significant antimicrobial properties against several common pathogenic bacteria. Notably, the NPE1 and NPE2 fractions of E. heyneana Spreng effectively inhibited the growth of P. aeruginosa, a bacterium commonly associated with hospital-acquired infections and known for its multi-drug resistance. Given the alarming rise of antimicrobial resistance, the findings suggest that E. heyneana Spreng holds potential as a valuable resource for medicinal applications, particularly in the development of new antimicrobial agents. However, it is essential to conduct further research to thoroughly characterize and identify the specific compounds responsible for this antimicrobial activity, paving the way for potential therapeutic uses.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
The author AL thankfully acknowledge the UGC, New Delhi for providing SRF.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
The data supporting the findings of this study are available within the article.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Osuntokun OT, Cristina GM. Bio isolation, chemical purification, identification, antimicrobial and synergistic efficacy of extracted essential oils from stem bark extract of Spondias mombin (Linn). Int J Mol Biol. 2019;4(4):135-43. [CrossRef]
2. Ezhilarasi R, Senthikumar B, Devi K. GC-MS analysis of bio-active compounds in aqueous extract of Boerhaavia diffusa, Euphorbia hirta and Amaranthus polygonoides. Int J Trend Sci Res Dev. 2019;4(1):65-9.
3. Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice:Importance, challenges and future. J Tradit Complement Med. 2016;7(2):234-44. [CrossRef]
4. Smith AR, Carter S, Smith AR. Flora of Tropical East Africa- Euphorbiaceae. Vol. 2. Rotterdam:Balkema;1988.
5. Krishnaiah D, Devi T, Bono A, Sarbatly R. Studies on phytochemical constituents of six Malaysian medicinal plants. J Med Plants Res. 2009;3:67-72.
6. Milne A, Beamish T. Inhalational and local anesthetics reduce tactile and thermal responses in Mimosa pudica. Can J Anaesth. 1999;46(3):287-9. [CrossRef]
7. Gopalasatheeskumar K. Significant role of Soxhlet extraction process in phytochemical research. Mintage J Pharm Med Sci. 2018;7(1):43-7.
8. Magaldi S, Mata-Essayag S, De Capriles CH, Perez C, Colella MT, Olaizola C, et al. Well diffusion for antifungal susceptibility testing. Int J Infect Dis. 2004;8(1):39-45. [CrossRef]
9. Nemati F, Dehpouri AA, Eslami B, Mahdavi V, Mirzanejad S. Cytotoxic properties of some medicinal plant extracts from Mazandaran, Iran. Iran Red Crescent Med J.2013;15(11):e8871. [CrossRef]
10. Yingyuen P, Sukrong S, Phisalaphong M. Isolation, separation and purification of rutin from Banana leaves (Musa balbisiana). Ind Crops Prod. 2020;149:112307. [CrossRef]
11. Carlin S, Lotti C, Correggi L, Mattivi F, Arapitsas P, Vrhovšek U. Measurement of the effect of accelerated aging on the aromatic compounds of gewürztraminer and Teroldego wines, using a SPE-GC-MS/MS protocol. Metabolites. 2022;12(2):180. [CrossRef]
12. Gupta D, Kumar M. Evaluation of in vitro antimicrobial potential and GC-MS analysis of Camellia sinensis and Terminalia arjuna. Biotechnol Rep. 2017;13:19-25. [CrossRef]
13. Kala SM, Tresina Soris P, Mohan VR. GC-MS determination of bioactive components of Eugenia flocossa Bedd. (Myrtaceae). Int J Pharma Bio Sci. 2012;3(1):277-82.
14. Valarmathi R, Natarajan D, Suryadevara N, Nazmul M, Maziz H, Ragavan ND, et al. GC-MS analysis and antibacterial activity of Dryopteris hirtipes (Blumze) Kuntze Linn. J Surv Fish Sci. 2023;10(1S):3718-26.
15. Adebiyi AO, Oyeyemi SD, Tedela PO, Ojo VI. GC-MS analysis of bioactive compounds from n-hexane leaf extract of a tropical fern, Nephrolepis cordifolia (L) C. Presl. East Afr Sch J Biotechnol Genet. 2019;1(5):118-23.
16. Perumal S, Mahmud R. Chemical analysis, inhibition of biofilm formation and biofilm eradication potential of Euphorbia hirta L. against clinical isolates and standard strains. BMC Complement Altern Med. 2013;13:346. [CrossRef]
17. Hlila MB, Majouli K, Ben Jannet H, Aouni M, Mastouri M, Selmi B. Antimicrobial activity of Tunisian Euphorbia paralias L. Asian Pac J Trop Biomed. 2017;7(7):629-32. [CrossRef]
18. Baral R, Karki A, Karki S, Neupane B, Koirala P, Baral S, et al. Phytochemical screening, free radical scavenging, and in-vitro anti-bacterial activity study of chloroform, acetone and methanol extracts of selected medicinal plants of Nepal. Curr Perspect Med Aromatic Plants. 2021;4:22-35. [CrossRef]
19. Sunmathi D, Sivakumar R. In vitro cytotoxicity of ethanolic leaf extract of Alternanthera sessilis (L.) R. Br. Ex. DC and Alternanthera philoxeroides (mart.) griseb. Against human osteosarcoma cell line MG-63. Eur J Biomed Pharm Sci. 2016;3:416-20.
20. Sahli R, Rivière C, Dufloer C, Beaufay C, Neut C, Bero J, et al. Antiproliferative and antibacterial activities of Cirsium scabrum from Tunisia. Evid Based Complement Alternat Med. 2017;2017:7247016. [CrossRef]
21. Song F, Liu C, Zhang J, Lei Y, Hu Z. Antibacterial effect of fosfomycin tromethamine on the bacteria inside urinary infection stones. Int Urol Nephrol. 2020;52(4):645-54. [CrossRef]
22. Wang T, Wu G, Wang J, Cui Y, Ma J, Zhu Z, et al. Comparison of single-dose fosfomycin tromethamine and other antibiotics for lower uncomplicated urinary tract infection in women and asymptomatic bacteriuria in pregnant women:A systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(1):106018. [CrossRef]
23. Abou Alaiwa MH, Launspach JL, Sheets KA, Rivera JA, Gansemer ND, Taft PJ, et al. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight. 2016;1(8):e8735. [CrossRef]
24. Bora P, Devi NN. Exploration of the chemical constituents and its antioxidant, antibacterial activities of endophytic fungi isolated from the medicinal plant Dillenia indica. Arch Microbiol. 2023;205(2):67. [CrossRef]
25. Uyan A, Turan C, Erdogan-Eliuz EA, Sangun MK. Antimicrobial properties of bioactive compounds isolated from epidermal mucus in two ray species (Dasyatis marmorata and Gymnura altavela). Trop J Pharm Res. 2020;19(10):2115-21. [CrossRef]
26. Mohammed GJ, Omran AM, Hussein HM. Antibacterial and phytochemical analysis of Piper nigrum using gas chromatography-mass spectrum and Fourier-transform infrared spectroscopy. Int J Pharmacogn Phytochem Res. 2016;8(6):977-96.