Pulicaria jaubertii Gamal Eldin, also known as Pulicaria orientalis Jaub., which is a member of the family Asteraceae, is one of the Pulicaria species endogenous to the Arabian Peninsula, particularly in Yemen. It is a perennial aromatic plant with erect branches that may reach 50 cm in height. In Arabic, it is officially called “Eter Elraee” [1,2]. However, it is known locally in Yemen as “Anssif” or “Alkhaoah” and is used as a spicing herb for making some food, such as laban (fermented milk) and soup. Traditionally, it is used as an insecticide, antipyretic, diuretic, and for treating urogenital system disorders, colds, malaria, inflammation, and microbial infections [3-6].
P. jaubertii is a perennial aromatic herb with erect, tomentose, grey-green branches reaching 30–50 cm in height. The plant is strongly fragrant and partially woody at the base. Leaves are sessile, oblong to oblanceolate, measuring 1.5–4.5 cm in length and 0.4–2 cm in width, with toothed margins and an obtuse apex. The inflorescence consists of a few capitula (flower heads), each 1–2 cm in diameter, with yellow florets. Involucral bracts are oblanceolate and densely villous. The ray florets are 3–5 mm long. The fruit is an achene, setulose, and obscurely ribbed, containing small seeds adapted for wind dispersal [2,7].
Several studies revealed that Pulicaria species, including P. jaubertii, have been found to have various bioactive characteristics such as cytotoxic and anticancer [2,8-11], antioxidant [3,5,12-14], antibacterial [2,13,15], antifungal and immuno-regulatory [16], antiinflammatory and antihistaminic [9,10,17], antidiabetic [9], antihypertensive [8,18], and antispasmodic [19] activities.
Detailed agricultural statistics on this species are rare due to its large-scale harvesting rather than cultivation. Ethnobotanical surveys and herbarium records in Yemen indicate that P. jaubertii is seasonally collected because of its high demand for culinary and medicinal uses in rural areas [20,21]. In addition, it is highly sold in local markets; thus, formal data on annual harvest volumes are also lacking. Therefore, the emergence and worsening of habitats have increased concern for the stability of wild populations, which outlines the need for protective measures and further research [22].
P. jaubertii is not yet recorded on the International Union for Conservation of Nature Red List of endangered species. However, many factors like drought, overexploitation, uncontrolled overcutting, and overgrazing in Yemen and Saudi Arabia may put many native species, including P. jaubertii, at high risk and worsen this decline [7]. Therefore, conservation efforts should consist of protecting their natural habitats and preserving them in ex-situ environments through seed banks and botanical gardens [16]. However, some recent techniques, including the micropropagation technique, could be used to improve their production. This technique is an important in vitro tissue culture method that may be used to conserve a great number of threatened and rare crops, medicinal, and economically important plants [23-27]. It could be used to provide several benefits for the plant, such as producing a large amount of pathogen-free and healthy plants, as well as genetically improved crops within a relatively short time and small space [28-32]. Interestingly, the rate of proliferation and other variables could be affected by the use of diverse types and concentrations of plant growth regulators (PGRs), chiefly auxins and cytokinins [33,34]. These types of PGRs are commonly used in combination with plant tissue culture [35,36], and the research area for their action is still broad. Phytochemical composition in plants varies due to genetic, environmental, and methodological factors, which could influence their biological properties and therapeutic potential [37].
P. jaubertii has been widely investigated for its essential oils and secondary metabolites, which vary significantly with geographic origin, extraction method, and plant part used [2,5,13,38]. However, in vitro techniques such as callus culture remain unexplored for this species. Callus cultures offer a controlled environment for enhancing the production of bioactive compounds [16]; and understanding the biochemical and genetic factors influencing such variation may facilitate the synthesis of pharmacologically and industrially valuable phytochemicals [39]. To date, no study has reported a micropropagation protocol or gas chromatography-mass spectrometry (GC-MS)-based phytochemical profiling for P. jaubertii. This study, therefore, presents the first successful micropropagation strategy for the species, employing seed-derived callus as the primary explant source. Although the approach follows standard protocols used in related Asteraceae species such as Pulicaria incisa and Achillea spp., its application to P. jaubertii addresses a critical gap. The developed protocol not only supports conservation but also establishes a reproducible platform for secondary metabolite production under controlled conditions, which aims to assess growth responses in Murashige and Skoog (MS) media supplemented with different types and concentrations of PGRs, specifically cytokinins and auxins.
2. MATERIALS AND METHODS
2.1. Chemicals
Cytokinins (kinetin [Kin] and 6-benzylaminopurine [BAP]) and Auxins (1-naphthaleneacetic acid [NAA] and 2,4-dichlorophenoxyacetic acid [2,4-D]), plant hormones, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethanol, n-hexane, and other chemicals used in the study were of the highest quality available.
2.2. Plant Material and Study Design
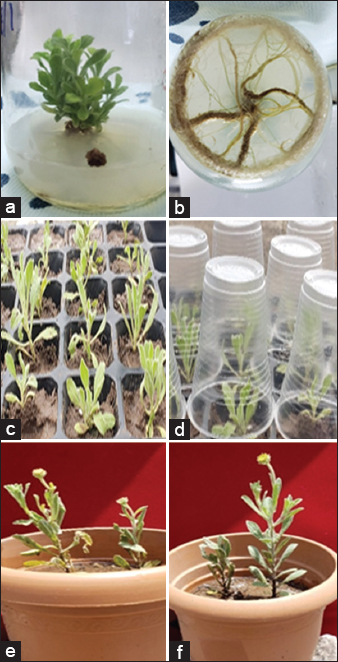
P. jaubertii [Figure 1a] was collected from Ibb City, Yemen, from March to April 2021 at GPS coordinates of 13º 57’ 46.44” N and 44º 10’ 23.88” E. The taxonomist, Dr. Esam Aqlan, identified the plant at the Biology Department, Faculty of Science, Ibb University. The experimental study was conducted in the Tissue Culture Laboratory, Department of Biology, Faculty of Sciences, Ibb University, in 2021.
 | Figure 1: (a) Morphology of Pulicaria jaubertii; (b) Seeds; (c) Callus used as explants; (d) Harvested callus for extraction. [Click here to view] |
2.3. Medium and Cultural Conditions
Several types of plant parts (leaves, nodes, and seeds) were surface-sterilised and inoculated in a full Murashige and Skoog [40] medium as performed by Salam et al. [41] [Figure 1b]. Preliminary trials also tested leaf and stem explants under various hormonal regimes, including high cytokinin and low auxin combinations; however, these explants failed to produce callus or shoots and often exhibited necrosis. Seeds, used with their coats intact, germinated successfully and induced callus without the need for decoating. The MS medium contains specific concentrations of nutrients necessary for most plant growth, but in hormone-free conditions, only seeds produce roots and shoots.
For micropropagation, seeds were supplemented with 0.5 mg/L Kin and 0.1 mg/L 1-naphthalene acetic acid (NAA) in MS medium. Callus was formed within 1–2 weeks [Figure 1c and d], followed by shoot formation and then root production after 3–4 weeks. Explants obtained from these calli were then subcultured with various types and concentrations of PGRs.
2.4. Hormonal Effects on Shoot, Root and Callus Regeneration
2.4.1. Shoot induction and multiplication
The explants’ response to growth was analysed in full MS media with different concentrations of the cytokinin Kin (0, 0.25, 0.5, and 1 mg/L) and a fixed concentration of the auxin NAA (0.1 mg/L).
2.4.2. Root induction
The explants’ response to growth was investigated in MS media supplemented with various concentrations (0, 0.25, 0.5, and 1 mg/L) of the auxin indole-3-acetic acid (IAA) and a fixed concentration of the cytokinin 6-benzylamino purine (BAP) (0.1 mg/L).
2.4.3. Callus induction
Likewise, explants’ response was tested with different concentrations of growth regulators 2,4-dichlorophenoxyacetic acid (2,4-D) (0, 0.25, 0.5, and 1 mg/L) and a fixed concentration of Kin (0.1 mg/L).
The response included the regeneration of shoots, roots, and callus, as well as their related growth parameters. Cultures were kept at 25 ± 2°C in an air-conditioned environment under a 16-h light/8-h dark photoperiod. Since no previous micropropagation study has focused on P. jaubertii, a stepwise method was used to understand the basic hormonal needs for shoot and callus development. Each experiment changed one growth regulator (cytokinin or auxin) while keeping the concentration of the other constant to find effective baseline conditions.
2.4.4. Acclimatisation of in vitro derived plantlets
Acclimatisation was carried out on 50 rooted shoots from 35-day-old in vitro plantlets. These plantlets were moved to plastic pots filled with a soil-sand mixture (70:30, v/v) and kept under laboratory conditions (25 ± 2°C, 16-h photoperiod) for 4 weeks. The survival rate was noted, and 45 plantlets (90%) successfully acclimatised. Among these, 20 plantlets were later transplanted into larger pots and grown under outdoor garden conditions. All 20 plantlets survived and showed healthy growth (100%) after another 4 weeks.
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
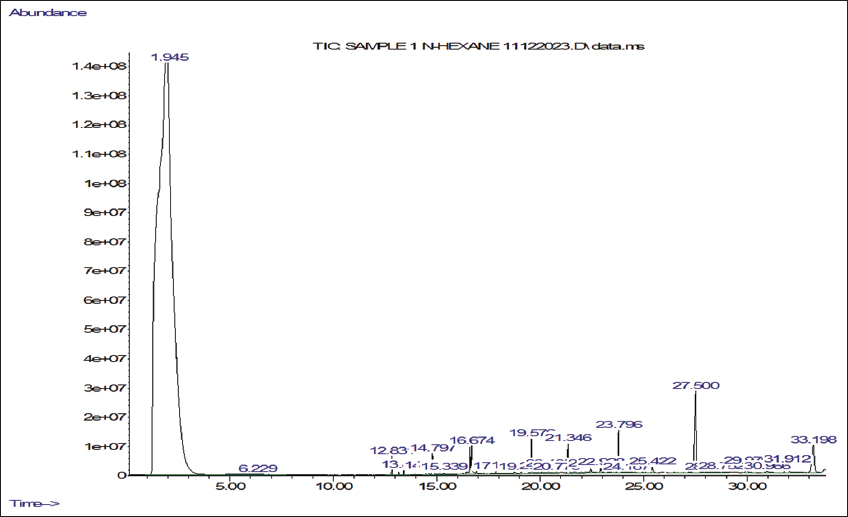
Ethanol and n-hexane extracts were made from shade-dried powder obtained from the mother and callus derived from micropropagated explants of P. jaubertii. The four extracts were prepared using the cold maceration method and then concentrated at 45°C using a rotary evaporator (Büchi, Switzerland). We determined the volatile phytochemicals following the procedure by Yisak et al. [42] with some modifications. GC-MS analysis was done using an Agilent 7890A gas chromatograph coupled with a 5975C mass spectrometer (Agilent, USA) equipped with electron ionisation (EI) for phytochemical analysis. Chromatographic separations were carried out using an HP-5MS (19091S-433) capillary column that is 30 m long, has a 0.25 mm internal diameter, and a 0.25-µm column phase film thickness. The injection mode was split-less, Helium served as the carrier gas, and we injected 1 µL of the sample at a consistent flow rate of 1 mL/min into an inlet heated to 275°C. The initial oven temperature was 60°C with a 2-minute hold time, then increased to 200°C with a ramp of 10°C/min and 3°C/min to 240°C. The ion source temperature was set to 230°C for the mass spectrometer settings, and the quadrupole temperature was 150°C. The system ran in positive electron impact mode at 70 eV, scanning from 40 to 650 m/z. The total run time was 45 minutes. Phytochemical components were qualitatively identified by comparing their retention times and mass spectral data with those in the NIST98 library.
2.6. Statistical Analysis
Data were summarized in tables as frequencies and proportions. Crosstabulation and Fisher’s exact test were used to study the effect of different concentrations of the hormones on the non-parametric markers of plant growth. One-way analysis of variance, followed by Duncan’s Multiple Range test, was used to compare the means of the parametric values produced at various concentrations of the hormones. Data analysis was performed using the statistical software Statistical Package for the Social Sciences version 20.0, and P ≤ 0.05 was taken as significant.