1. INTRODUCTION
Thirty-five percent of rice fields in Northeast Thailand are unfavorable for the development of soil invertebrates [1]. This is due to three typical abiotic stresses in Thailand: flooding, drought, and heat, which result in anoxic soil conditions that limit the activities of soil invertebrates. Acid sandy soils are susceptible to erosion and compaction, low clay and nutrient contents, low water infiltration, and holding capacities. These factors decreased the yield potential of KDML105 and RD6 to about 4 t/ha [2]. Organic nutrients are suitable in soils with clay contents below 5%, whereas soil invertebrates such as termites and earthworms improve soil’s chemical and physical properties. They create biological structures, such as nests and organic matter (OM), which influence biological and chemical soil cycling in the ecosystem and ultimately affect the growth of crops such as rice [3]. Paddy field ecosystems consist of small soil plots separated by a levee height of 40 cm and various types of grasses. Termites often build mounds under trees and are beneficial for preserving soil biodiversity, protecting soil macrofauna, and providing adequate shade and humidity during the dry season [4]. Termites usually improve soil properties by producing soil organo-mineral aggregates such as feces and mounds. They also create macropores in the form of galleries and chambers, which have unique physical, chemical, and biological properties for biogenic structures [5]. Termites have microorganisms in their stomachs, especially bacteria that can decompose and accumulate in the soil in termite mounds [4]. To increase the surface area accessible to soil microorganisms and own symbionts intestinal and speed up net decay, termites will chop up plant material with their mandibles and grind it with their gizzard. OM and mineral nutrients return to the soil through feces, salivary secretions, corpses, and predators, especially ant predation and mound erosion, which are important contributors to the ecosystem. Higher levels of total N, cationic exchange capacity, and mineral nutrients (Ca2+, Mg2+, K+, and P) were found in termite mound soils compared to surrounding soils [6-10]. In addition, earthworms are also important as they can penetrate the soil and create flakes to enhance soil fertility in the ecosystem [3]. Earthworm caves affect N and P cycling increase [11,12], the soil particle size composition [13], and the quantity, composition, and function of soil microorganisms, which indirectly affect the distribution of organic C [14,15] through their feeding, defecation, and soil mixing activities. Growing wheat with different earthworms increases bacterial and fungal contents in the soil [16].
Termite nest structures created to keep the humidity and temperature constant throughout all seasons make termites active in harsh environments. There are decomposition processes [17,18] and essential ecosystem services [19] during the dry season, where other soil macroinvertebrates are diminished. Termite mound soils maintain several microorganisms, especially bacteria [20], and nutrient turnover in the ecosystem [21-23]. Some bacteria [24-27] isolated from termite mound soil could be utilized as a potential material for antimicrobial production, material for antimicrobial production [28,29], biofertilizers, and biocontrol [30], thereby environmental sustainability [31,32]. Acidic sandy soil in Northeast Thailand has low OM, water-holding capacity, and soil fertility, especially low levels of available P. To compensate for this, chemical fertilizers have been increasingly used in crop production and adversely impact phosphate solubilizing bacteria (PSB) diversity. To sustainable soil utilization, 10% of phosphate-dissolving microorganisms are associated with plant nutrition management [33,34]. PSBs are usually used to enhance P availability, cytokinin, and auxin to promote plant growth by acidification, chelation, and exchange reactions [35-37]. Inorganic fertilizer with PSB changes the P acquirement and dispensation to improve crop yield [38]. Meanwhile, it also enhances the availability of definite micronutrients and mitigates and inhibits pathogenic microorganisms, consequently the nutritional status and crop health at a low cost [39,40]. The change of insoluble P by PSB is the main core for biofertilizers. The highest rice dry matter (1,101.11 g) was accumulated with NPK + PSB, which was higher than NP0K, NP0K + PSB, and NPK at 7.9%, 8.6%, and 13.27%, respectively. NP0K+PSB gave the highest (442.22) number of panicle m-2, which was significantly 40.70% and 37.68% higher over N0P0K0 + PSB and N0P0K0, respectively [41]. The application of di-ammonium phosphate (DAP) + rock phosphate + PSB had 20.7, 24.3,19.6, 21.0, 28.1, 33, 15.2, 14.4 and 29.4% higher rice grain and straw yields, grain and straw P uptake, available and saloid P, dehydrogenase, acid phosphatase, and alkaline phosphatase activities, respectively, as compared to DAP treatment. Thus, the direct application of phosphorus (P) hardly exceeds 15–20% [42]. Enterobacter asburiae 30FPSSB1, E. mori NTTC11, Priestia aryabhattai KNB6, P. aryabhattai 49KNA2, P. megaterium 65KNA2, and Bacillus spp. 38DFWA exhibited rice growth-promoting traits through phosphate solubilization and indole acetic acid (IAA) production, respectively, ranging within 32–42 mg/L and 5.3–340 mg/L. Some of these PSBs presented a broad range of antifungal activities, reducing the growth of phytopathogens by 10–35%. Bacillus spp. 38DFWA, P. aryabhattai KNB6, and P. megaterium 65KNA2 had increased biomasses of shoots by 33, 26 and 23%, respectively, and roots by 34, 48 and 48%, respectively, which facilitated P availability and increased nutrient uptake in the plant tissues by 89, 96 and 143%, respectively. P. aryabhattai KNB6 and E. mori NTTC11 greatly increased rice plant biomass and gave the highest P accumulation in plant tissues. Interestingly, P. aryabhattai KNB6 strongly increased P uptake in plant tissue by 121% and promoted rice growth by 22% in P-deficient soil [43].
However, phosphorus-solubilizing microorganisms inoculants, when introduced into the soil, may encounter furious competition from the indigenous soil microflora. Therefore, how quickly and efficiently do such microbes overcome stressful environmental variables [44]. Nitrogen (N) functionality of soil-inhabiting PSM and P solubilization activity [45]. It has been reported that with the decrease in pH, there was significantly more P solubilized in the presence of NH4Cl (129.65 mg/L) than in the presence of NH4NO3 (109.25 mg/L) [45]. Furthermore, ammonium is a better N source than nitrate [46]. Calcium added as CaCl2, CaCO3, and Ca(OH)2 reduces the P solubilization by Rhizobium and Bradyrhizobium from rock phosphate. The authority of CaCO3 enhances the pH of the medium toward alkalinity, which inhibits the growth of bacteria and results in little solubilization [47]. In addition, heterogeneously distributed microbial communities exhibit varying capacities to solubilize P [48]. The genera Bacillus (B. polymyxa) and Pseudomonas (P. striata) have shown maximum P-solubilizing activity [49], followed by Penicillium and Aspergillus [50], whereas Streptomyces is the least effective one. At the center of Laos, termite mounds are used to grow vegetables and make charcoal kilns in a rice-growing village. Adebajo et al. [51] explored termite mounds as biofertilizers for solubilizing phosphate and potassium, producing IAA, and suppressing plant soil pathogens. Muon et al. [52] claim that using termite mounds to amend the soil increases the rice plants’ ability to withstand drought and pathogens. Miyagawa et al. [53] suggest that the traditional wisdom of termite mound utilization, including termite mushrooms, will be lost if the termite mounds have been completely used or destroyed. However, termites can be fed over 1–10 m where the humidity and temperature remain constant throughout all seasons. This makes them not far away from nest structures. Termites find their food in areas not far from their nests, and their waste is often deposited in the soil [4]. There is little research involving the diversity and function of bacteria in termite gut and the surrounding soil. Therefore, it is important to explore the effects of selected PSB on phosphate solubility, population size, and IAA hormone production capacity. This study aims to assess the role of termite mounds in increasing PSB diversity and bioavailability in paddy fields. The soil at a distance of 2 and 5 m from termite mounds was compared with soil in paddy fields without termite mounds. Understanding the potential of PSB in the rice paddy ecosystem can help manage rice cultivation efficiently and encourage farmers to use termite mound soil as a biofertilizer for various crops.
2. MATERIALS AND METHODS
2.1. Soil Sampling
The experimental site was conducted in rice fields with and without termite mounds in Wang Saeng sub-district, Chonnabot district, Khon Kaen province, Northeast Thailand, from 2021 to 2022. Soil samples in rice fields were collected at 0–10 cm depth from 2 and 5 m away from termite mounds soil and without termite mounds. The samples were collected at 10 points and mixed before keeping in ziplock plastic bags at 4°C for chemical and physical analysis and PSB isolation.
2.2. Soil Chemical and Physical Measurement
The soil samples were air-dried, ground, and passed through a 2 mm sieve before being sent for analysis. Soil chemical and physical properties, including total N, available P, exchangeable K, Ca, and Mg, OM, soil pH, electrical conductivity (EC), cation exchange capacity (CEC), soil texture, percentage of sand, silt, and clay were measured at the Northeast Agriculture Research and Development Centre in the Faculty of Agriculture at Khon Kaen University, Thailand.
2.3. Isolation of Phosphate-Solubilizing (PS) Bacteria
The soil sample of 5 g was used to isolate PSB in the liquid selective medium (NBRIP). Sugar was prepared with 10 g of glucose per liter. The stock of NBRIP was prepared as follows: 5 g MgCl2·6H2O, 0.25 g MgSO4·7H2O, 0.2 g KCl, and 0.1 g (NH4)2SO4 per liter. The insoluble P source of AlPO4, FePO4, and Ca3(PO4)2 were used to modify the NBRIP medium for initial PSB screening. Insoluble P, sugar, and stock were autoclaved separately at 121°C for 20 min and mixed inside the laminar air flow of BIO-CLEAN BENCH, model MCV-13BSS. The inoculants containing 50 mL of the medium in Erlenmeyer flasks were incubated at 30°C and shaker speed (150 rpm/min) for 1 week. The next week, 5 mL of this incubated medium with inoculants was transferred into 50 mL again with the new liquid medium for 7 more days at 30°C and shaker speed (150 rpm/min). By the end of each week, 0.1 mL of the inoculant sample was diluted to 0.9 mL of sterile H2O and mixed 3 times. The aliquots of each sample were spread on the solid NBRIP medium and incubated at 30°C for 14 days. The selective bacteria with a clear halo were selected for further purification and counted for the population size from rice field soil [54].
2.4. Determination of Mineral Phosphate Solubilization
The selective bacterial was tested by determining PS activity with the molybdenum-blue method for four replicates [55]. The liquid NBRIP media containing different insoluble forms of phosphate (AlPO4, Ca3(PO4)2, and FePO4) were used to grow selective bacteria at 30°C in an incubator shaker with a speed of 150 revolutions min−1 for 3 days. Color development occurs after the mixed reagent is incubated at room temperature for 30 min. Ascorbic acid was used to measure phosphorus compounds for the efficiencies of solubilizing activity when the reaction of ammonium phosphomolybdate is reduced to molybdenum blue. The concentration of phosphate solubilization was measured with the absorption of light wavelength 880 nm by Shimadzu UV-120-01 spectrophotometer and compared to the concentrations of KH2PO4 ranging from 0 to 0.9 mL/L.
2.5. IAA Production
Luria–Bertani medium (LB) 1 l contained 10 g of tryptone, 5 g of NaCl, and 5 g of yeast extract. The selective bacteria isolates were cultured in 50 mL of LB at 30 °C in an incubator shaker with a speed of 150 rpm/min for 3 days [56]. The aliquots sample was centrifuged at 14,000 rpm for 5 min, and the suspended supernatant reacted with 0.01 of M FeCl3 in 35% HClO4 at 25°C in dark conditions for 30 min. IAA concentration was determined with the absorption of light wavelength 530 nm of Shimadzu UV-120-01 spectrophotometer and compared with a standard curve ranging from 0 to 150 μg/mL [57].
2.6. Identification of PS Bacteria
The extraction of genomic DNA was done using the Genomic DNA mini kit (Blood/culture cell) (Geneaid Biotech Ltd., Taiwan). The genomic DNA extracted was utilized as a template to amplify 16S rDNA. The amplified product was then evaluated using agarose gel electrophoresis with a 0.8% concentration. The polymerase chain reaction (PCR) product was purified using the GenepHlowTM Gel/PCR Kit (Geneaid Biotech Ltd., Taiwan). The concentration of DNA in the purified product was evaluated to be within the suggested range of 20–50 ng/μL, as advised by the sequencing service provider. The sequencing was performed by a professional sequencing service provider using an ABI Prism® 3730XL DNA Sequence instrument. The PCR amplification sequencing of 16S rDNA regions was carried out using Taq polymerase by Kawasaki et al. [58], Yamada et al. [59], and Katsura et al. [60]. Two primers of 20F (5’-GAG TTT GAT CCT GGC TCA G-3’, positions 9–27 on 16S rDNA by the Escherichia coli numbering system) and 1500R (5’-GTT ACC TTG TTA CGA CTT-3’, position 1509–1492 on 16S rDNA by the E. coli numbering system [61]), were used to prepare a PCR product.
The PCR products, purified and single-banded (approximately 1500 bases), were directly sequenced. The nucleotide sequences were compared using the BLASTN program [62] against the 16S rDNA sequence database of validly published prokaryotes. The highest scores sequences were calculated pairwise sequence similarity using the global alignment algorithm [63]. The sequences of PSB isolates were submitted to NCBI. The neighbor-joining method uses the distance matrix of the alignment for a phylogenetic tree based on 16S rDNA gene sequences of PSB strains and their phylogenetically related closest relatives. Bootstrap values (>50%) are shown at branch nodes. Nguyenibacter vanlangensis (NR 125459) was used as the outgroup. Phylogenetic analysis and molecular evolution were performed using the MEGA11.
2.7. Measurement of Rice Yield
RD. 6 was planted in early July of 2021–2022 after the field was plowed once and fertilized with chicken manure of 1,250 kg/ha and inorganic fertilizer 15–15–15 of 156.25 kg/ha. RD. 6 seedlings at 14 days were transplanted at a spacing of 20 × 20 cm, with one plant per hole. The plants reached the physiological maturity stage around December 2021–2022, and yield data were collected. To collect the yield data, six plants per plot were randomly selected at three different distances from the termite mound base – 1 m, 2 m, and 5 m. At each distance, 20 clumps were randomly collected, and three replicates were used. The growth of the plants was analyzed by measuring plant height, and rice yield weighed at 14% moisture content. The data collected was used to calculate the yield of rice for each treatment.
2.8. Statistical Analysis
The average data were analyzed using an analysis of variance with an F-test. If the results were significant, the least significant difference test was conducted at a 0.05 and 0.01 level. The Statistix 10 software was used to analyze each treatment.
3. RESULTS
3.1. Soil Fertility
When total N was measured by the Kjeldahl method [64], available P by Bray II and molybdenum-blue method [65], exchangeable K, Ca, and Mg by 1 N NH4OAc, pH 7 and flame photometry method, OM by Walkley and Black method [66], soil pH by pH meter [67], EC by EC meter [68], CEC by ammonium saturation, distillation for NH4+ and physical properties such as soil texture by pipette method. The results of soil chemical and physical are shown in Table 1. Significant differences P < 0.05 were observed in the soil pH, OM, EC or salinity (EC), total N, exchangeable Ca and Mg, CEC, and sand and clay content. However, no significant differences P < 0.05 were found in available P, exchangeable K, and soil content. The pH level of the rice soil fell within the neutral range, which was suitable for the growth of most plants. The exchangeable K and Ca levels in the soil are sufficient to meet the plants’ requirements. The EC or salinity was found to be poor in the rice soil fields. Termite mound soil tends to have higher levels of soil pH, OM, total N, available P, exchangeable K, Ca and Mg, CEC, and clay content.
Table 1: Chemical and physical properties of the termite mound soil and the RD.6 field with and without termite mounds in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province.
| Soil properties | Termite mound soil | 2 m from termite mounds | 5 m from termite mounds | Without termite mounds (Control) |
|---|---|---|---|---|
| pH (1:1 H2O) | 7.07±0.20a | 5.83±0.56b | 5.94±0.44b | 5.45±0.26b |
| Organic matter (%) | 2.11±0.35a | 1.18±0.13b | 0.86±0.15b | 0.79±0.06b |
| EC (dS/m) | 0.41±0.28a | 0.04±0.00b | 0.05±0.03b | 0.04±0.00b |
| Total N (mg/g) | 1.00±0.02a | 0.50±0.01b | 0.40±0.01b | 0.40±0.01b |
| Available P (mg/kg) | 49.58±35.01a | 19.17±1.44a | 9.50±4.77a | 5.67±0.52a |
| Exchangeable K (mg/kg) | 230.29±145.18a | 85.78±11.76a | 82.18±59.01a | 97.86±81.96a |
| Exchangeable Ca (g/kg) | 4.42±0.33a | 1.03±0.85b | 0.41±0.14b | 0.28±0.34b |
| Exchangeable Mg (mg/kg) | 253.28±51.53a | 45.88±6.01b | 35.16±6.15b | 27.32±2.13b |
| Cation exchange capacity (c mol+/kg) | 14.36±1.04a | 7.40±0.28b | 5.59±0.93b | 6.13±0.77b |
| Soil texture | Loamy and sticky | Sandy loam | Sandy loam | Sandy loam |
| Sand (%) | 53.80±6.63b | 68.17±4.25a | 68.42±5.39a | 70.09±1.65a |
| Silt (%) | 27.39±5.55a | 23.99±4.42a | 24.88±4.91a | 22.88±1.09a |
| Clay (%) | 18.82±2.57a | 7.84±0.37b | 6.70±0.97b | 7.03±0.56b |
Different letters within the same row indicate significant differences based on the least significant difference test at P<0.05
3.2. PS Bacteria Isolated
An experiment was conducted to isolate PSB from soil samples taken from rice fields with and without termite mounds in the rice field ecosystem RD.6, Wang Saeng subdistrict, Chonnabot district, Khon Kaen province. There were four isolates of phosphate-dissolving bacteria, out of which three were found in rice field soil at a distance of 5 m from termite mounds, and one isolate was found in rice field without termite mounds. The bacteria were not found in termite mound soil. The population sizes of bacteria in rice fields at 5 m from termite mounds and rice fields without termite mounds are shown in Table 2. The PSB isolate R111 showed the highest significant differences at P < 0.01 of 75.67 × 106 CFU compared to the PSB isolate R180.
Table 2: The density of phosphate-solubilizing bacteria from RD.6 field with and without termite mounds in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province.
| PSB isolates | Solubilized phosphate bacteria (CFU/L) |
|---|---|
| R111 (5 m from termite mounds) | 75.67×106a |
| R150 (5 m from termite mounds) | 33.67×106ab |
| R180 (5 m from termite mounds) | 4.33×106b |
| R200 (without termite mounds) | 52.33×106ab |
Different letters within the same column indicate significant differences based on the least significant difference test at P<0.01
3.3. Phosphate-Solubilization Efficiency and IAA Production
Our study found that bacteria isolated from rice fields at a distance of 5 m from termite mounds can dissolve phosphate in the forms of FePO4, Ca3(PO4)2, and AlPO4. AlPO4 and FePO4 are the main forms that cause phosphorus uptake issues in sandy soils with low pH [69]. The highest solubility of phosphate in the form of FePO4 was 523.99 mg/L at P < 0.01, followed by Ca3(PO4)2 at 443.69 mg/L and AlPO4 at 103.42–145.46 mg/L [Table 3]. Moreover, one PSB, isolated from a rice field without termite mounds, can dissolve phosphate in the form of Ca3(PO4)2 as opposed to AlPO4 and FePO4. Ca3(PO4)2 is the main form that causes phosphorus uptake in alkaline soil and affects soil organic carbon stocks [70].
Table 3: Phosphate-solubilizing effectiveness of tested bacteria from the RD.6 field with and without termite mounds in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province.
| PSB isolates | Solubilized phosphate (mg/L) Form | ||
|---|---|---|---|
| AlPO4 | Ca3(PO4)2 | FePO4 | |
| R111 (5 m from termite mounds) | 103.42ab | 11.23d | 238.36b |
| R150 (5 m from termite mounds) | 109.43ab | 229.61c | 234.93b |
| R180 (5 m from termite mounds) | 145.46a | 443.69a | 523.99a |
| R200 (without termite mounds) | 69.00b | 253.68b | 0.24c |
Different letters within the same column indicate significant differences based on the least significant difference test at P<0.01
The bacterial samples collected from rice fields were analyzed for their potential to produce IAA hormones. The bacteria isolated from rice fields without termite mounds revealed the highest potential for IAA production at P < 0.01, accounting for 2,131.80 mg/L at 2 days of incubation [Table 4]. In contrast, the bacteria isolated from rice fields located 5 m away from termite mounds were not effective in dissolving different types of phosphates. It indicates that PSB isolated under varying environmental conditions may have different abilities to dissolve phosphate or produce hormones (IAA) at different levels, which could be contributed by the interactions with other microorganisms in the soil, as well as the media formula used for cultivation and the incubation time [71]. After collecting and isolating PSB from rice fields with and without termite mounds, the sequences of PSB isolates were submitted to NCBI (GeneBank) as listed with the bar representing 0.05 substitutions per nucleotide position. The results showed that R111 and R150 matched with Burkholderia gladioli accession numbers SUB13925899A11SOR726098 and SUB13926221F15-2OR726100, respectively [Table 5]. R180 matched with Priestia spp. accession number SUB13926226C18-2OR726101, and R200 matched with Burkholderia spp. accession number SUB13926232A20OR726104 [Figure 1]. PSBs that are effective in dissolving phosphate in various forms and producing IAA were B. gladioli and Priestia spp.
Table 4: Indole acetic acid production of PSB isolates from RD.6 field with and without termite mounds in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province.
| PSB isolates | IAA Production (mg/L) |
|---|---|
| R111 (5 m from termite mounds) | 382.01b |
| R150 (5 m from termite mounds) | 586.58ab |
| R180 (5 m from termite mounds) | 487.95ab |
| R200 (without termite mounds) | 2,131.80a |
Different letters within the same column indicate significant differences based on the least significant difference test at P<0.01
Table 5: Identification of PSB isolates from RD.6 field with and without termite mounds in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province by 16S rDNA sequencing after inoculation.
| Isolate code | Name | Strain | Accession No. | Gene Identity (%) |
|---|---|---|---|---|
| R111 (5 m) | Burkholderia gladioli | NBRC13700 | SUB13925899 A11S OR726098 | 99.50 |
| R150 (5 m) | Burkholderia glumae | LMG 2196 | SUB13926221 F15-2 OR726100 | 99.01 |
| R180 (5 m) | Priestia spp. | - | SUB13926226 C18-2 OR726101 | had the highest similarity with P. aryabhattai at 99.86% |
| R200 (without termite mounds) | Burkholderia spp. | - | SUB13926232 A20 OR726104 | and a 98.3% similarity with B. humptydooensis |
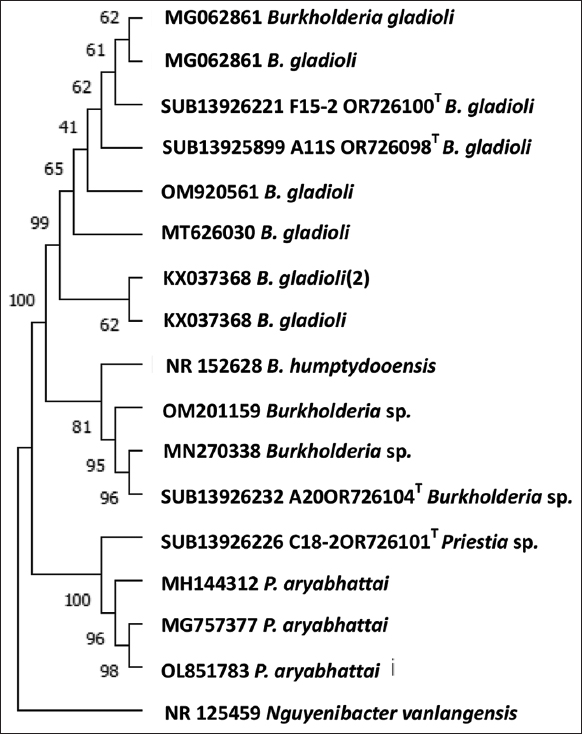 | Figure 1: Phylogenetic tree of PSB isolates from RD.6 field with and without termite mounds in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province by 16S rDNA sequencing after inoculation and the deposited sequence in NCBI. [Click here to view] |
3.4. Response of Rice Plant Height and Yield to the Termite Mound Soil
Rice plants growing near termite mounds had an average height of 84.30 cm [Table 6]. In contrast, the height of rice plants that were far from the termite mound decreased, with plants being 132.00 cm, 125.26 cm, and 118.93 cm in height at P < 0.05, respectively [Table 6]. The results showed that plots with termite mounds produced a substantially higher rice yield than plots without termite mounds. The average yield of rice in plots without termite mounds was 667.38 kg/ha, which was significantly less than plots with termite mounds at a distance of 5 m from the termite mounds, representing 1,640.88 kg/ha at P < 0.05. There was no statistical difference in rice yield between plots with termite mounds at 1 and 2 m distance from the mounds, with the average rice yields being 1,157.38 and 1,260.69 kg/ha at P < 0.05, respectively [Table 6].
Table 6: Plant height and yield of RD.6 growing in the field without termite mounds and with termite mounds at distances of 1, 2, and 5 m in Wang Saeng subdistrict, Chonnabot district, Khon Kaen province.
| Treatments | Plant Height (cm) | Yield (kg/ha) |
|---|---|---|
| RD.6 without termite mounds | 84.30b | 667.38b |
| RD.6 with termite mounds at 1 m | 132.00a | 1,157.38ab |
| RD.6 with termite mounds at 2 m | 125.26a | 1,260.69ab |
| RD.6 with termite mounds at 5 m | 118.93a | 1,640.88a |
Different letters within the same column indicate significant differences based on the least significant difference test at P<0.05
4. DISCUSSION
Termite mound soil showed higher levels of total N, available P, exchangeable K, Ca, and Mg, OM, CEC, and EC. These results were consistent with the research work of Tuma et al. [72]. As a result, rice plants tend to grow and yield better in rice fields that have termite mounds than those without termite mounds [73]. Rice height was significantly lower (84.30 cm) in the rice fields without termite mounds as compared to those with termite mounds at 1, 2, and 5 m distances. The rice height showed the opposite response to the distance from the termite mounds increased (132.00 cm, 125.26 cm, and 118.93 cm, respectively). This may be due to the fact that most termite mounds are covered with perennials and shrubs, resulting in taller rice plants near the mounds. It is primarily due to shading, water retention, and nutrient availability from the mounds. The average rice yield at 5 m away from a termite mound was high (1,640.88 kg/ha) and not significantly different from the rice yield at 1 and 2 m from the termite mounds (1,157.38 kg/ha and 1,260.69 kg/ha, respectively), but significantly different from the rice yield in the field without termite mounds. The average lower yield was 667.38 kg/ha, which may be linked to the low availability of P in the rice fields without termite mounds compared to those with termite mounds. However, in the plain areas of Laos, the results of the rice yield survey suggested that higher yields near trees than in open areas were due to the presence and development of termite mounds around trees in the paddy fields [74]. B. gladioli, Burkholderia glumae, and Priestia spp. are PS bacteria in paddy fields that can solubilize a wide range of AlPO4 and FePO4, which are the main inorganic phosphate compounds in acidic soil. The availability of P in the soil increases with the increased activity of phosphatase and dehydrogenase enzymes. Despite the contributions of termite mound bacteria in improving soil fertility, there is little research involving the assessment of the bacterial richness and functional diversity in termite mound soil when compared to the diversity of bacteria in termite gut and the surrounding soil [75]. Termite mound soils can absorb water and plant nutrients for crop growth [76]. Termite nests are created with OM derived from the digestion of plant tissues combined with soil particles. Enagbonma and Babalola [30] assessed the potential of bacteria in termite mound soil in South Africa to serve as biofertilizers and biocontrol as a promising tool for sustainable agriculture. This review mentions the role of bacteria in termite mound soil to improve the fertility of the soil and suppress soil-borne plant pathogens through the production of antibiotics, and nutrient fixation. These bacteria could reduce the reliance on the usage of chemical fertilizers and pesticides in farming, thereby increasing crop yield. This process increases the levels of nitrogen, phosphorus, potassium, iron, and calcium [77]. However, soil fertility is reduced after erosion of the termite mound soil down to the surface of the paddy field far from the termite mound. In addition, villagers used the termites for food and as feed for breeding fish, and they used their mounds for vegetable seedling beds and charcoal kilns, among other uses. Many reports have mentioned the possibility of using termite mounds for fertilizer and have described examples of its use in crop fields in Africa [53] and for construction materials in Northwestern Namibia [78]. Further studies need to be conducted in both pot and field experiments to address the soil fertility of different crop growth.
The soil pH in all sites was above 5.4, ideal for the growth of useful bacteria. Microorganisms in soil play relevant roles in soil morphology, air circulation, water penetrability, and nutritious availability. Effect of soil pH on the availableness of N, P, and K minerals. This initial observation suggests that different bacterial isolates exhibit varying degrees of phosphate solubilization efficiency in the collected soil samples [79]. The PSB isolated from rice field soil, at 5 m from termite mounds, solubilized significantly more FePO4, Ca3(PO4)2, and AlPO4, respectively. Phosphate-dissolving bacteria isolated from the soil around rice roots were Enterobacter spp., Micrococcus spp., Pseudomonas spp., Bacillus spp., Klebsiella spp., Serratia spp., Burkholderia multivorans, and Pantoea dispersa [80]. In contrast, bacteria from the rhizosphere of potato roots solubilized significantly more Ca3(PO4)2 than those from tomato roots. This difference may be due to the variations in rhizospheric microbial communities among various plant species [81]. Plant growth-promoting bacteria promote the hydroxylation of organophosphates into inorganic phosphate anions through phosphatases [82]. In acidic environments, insoluble complexes form as aluminum (Al3+) and iron (Fe3+) fixed with the phosphate ion (PO43−) [83]. Therefore, PSB isolates from this investigation were efficient in solubilizing FePO4 and were capable of solubilizing the phosphate complexed with aluminum. The microorganisms, soil degrees, moisture, soil pH, the correlated bacterial neighborhood [84], and carbon and nitrogen sources [85] are related to the efficiency of phosphatase. Our results found that the Priestia spp. strain isolated from rice field soil at 5 m from termite mounds appeared to be more capable of solubilizing FePO4 (523.99 mg/L) and AlPO4 (145.46 mg/L), which are the most irresolvable forms in sandy ground soil. The solubilization potential rate of FePO4 significantly exceeded the several research microfauna, especially Pantoea spp. (34–60 mg/L) and Enterobacter spp. (33–41 mg/L). Pantoea spp. and Enterobacter spp. which were unable to solubilize the phosphate complexed with AlPO4 and FePO4 [81], whereas AlPO4 solubilization was significantly lower in Paenibacillus elgii (16 mg/L) [86]. The bacteria isolates BTPK 5-3, BGNACMC 4-3, and BTNA 5-1 from termite nests and guts in the center of Thailand were found to be closely related to Bacillus cereus, whereas the bacterial isolate BTNASP 5-2 was closely related to Bacillus subtilis. B. cereus exhibited a variety of biological activities, denoting the highest cellulase, PS, and antifungal activities, whereas B. subtilis produced only a siderophore [87]. Bacillus spp. was isolated from nine different mounds of soil from households, farms, and water in Nigeria. The phosphate and potassium solubilization test and IAA production were positive for these bacteria. Furthermore, these bacteria had antagonistic activities against Fusarium oxysporum and Ralstonia solanacearum [51]. The PSB with phosphorus fertilizers enhanced the sugarcane yield from 17.03 to 38.42% and commercial cane sugar percent from 4.8 to 19.96% compared to no fertilizer application. B. subtilis with DAP showed effective results in soil phosphorus content and sugarcane yield. Sugarcane yield was highest in B. subtilis with DAP or rock phosphate [88].
5. CONCLUSION
Under good agricultural practice standards, the health of producers and consumers and the decrease of chemical residues in the environment are evaluated for crop production using biological agents to promote plant growth. Therefore, termite mound soil is a suitable source for extracting useful agricultural microorganisms. B. gladioli, B. glumae, and Priestia spp. are types of PS bacteria found in paddy fields, particularly in soil located 5 m away from termite mounds. These bacteria can solubilize a wide range of inorganic phosphate compounds, such as AlPO4 and FePO4, which are commonly found in acidic soil. These bacteria also have the potential to produce IAA, making them promising candidates for promoting the growth of rice and other crops. The average rice yield at 5 m from a termite mound (1,640.88 kg/ha) was significantly higher than in the field without termite mounds. The termite’s activity in facilitating bacteria growth and maintaining fertile land is important in rice fields. PSB connected with termite mounds enhanced phosphorus availability, cytokinin and IAA, nitrogen cycling, carbon dioxide, and cellulose breakdown to improve agricultural yield. These bacteria could reduce dependency on inorganic fertilizers and herbicides. Further research should find out whether these PSBs come from the termite stomach or the rice root rhizosphere. It is important to consider the acid type of these bacteria, environmental factors such as interactions with other microorganisms, plant development, climate, and soil category. Moist soil is more conducive to PSB growth and efficiency in dissolving phosphate than dry soil. Moreover, the long-term stability of bacterial activity in soil-field trials over multiple seasons should be carried out.
6. ACKNOWLEDGMENTS
The “Potential of phosphate solubilizing bacteria from paddy fields rhizosphere with termite mounds ecosystems” has received funding from the Fundamental Fund of Khon Kaen University from the National Science, Research and Innovation Fund or NSRF, Thailand.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. FUNDING
The Fundamental Fund of Khon Kaen University 2022 from the National Science, Research, and Innovation Fund or NSRF, Thailand, supported the research work.
9. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
12. Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
13. Use of artificial intelligence (AI)-assisted technology
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Tomita S, Nawata E, Kono Y, Inamura T, Nagata Y, Noichana C, et al. Impact of direct dry seeding on rainfed paddy vegetation in North-East Thailand. Weed Biol Manag 2003;3:68-76. [CrossRef]
2. Haefele SM, Naklang K, Harnpichitvitaya D, Jearakongman S, Skulkhu E, Romyen P, et al. Factors affecting rice yield and fertilizer response in rainfed lowlands of northeast Thailand. Field Crops Res 2006;98:39-51. [CrossRef]
3. Jouquet P, Chaudhary E, Kumar AR. Sustainable use of termite activity in agro-ecosystems with reference to earthworms. A review. Agron Sustain Dev 2018;38:3. [CrossRef]
4. Jouquet P, TraoréS, Choosai C, Hartmann C, Bignell D. Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur J Soil Biol 2011;47:215-22. [CrossRef]
5. Jouquet P, Dauber J, Lagerlof J, Lavelle P, Lepage M. Soil invertebrates as ecosystem engineers:Intended and accidental effects on soil and feedback loops. Appl Soil Ecol 2006;32:153-64. [CrossRef]
6. Holt JA, Lepage M. Termites and soil properties. In:Abe T, Bignell DE, Higashi M, editors. Termites:Evolution, Sociality, Symbioses, Ecology. Dordrecht:Kluwer Academic Publishers;2000. 389-407. [CrossRef]
7. Mujinya BB, Van Ranst E, Verdoodt A, Baert G, Ngongo LM. Termite bioturbation effects on electro-chemical properties of Ferralsols in the Upper Katanga (D.R. Congo). Geoderma 2010;158:233-41. [CrossRef]
8. Lopez-Hernandez D, Brossard M, Fardeau JC, Lepage M. Effect of different termite feeding groups on P sorption and P availability in African and South American savannas. Biol Fert Soils 2006;42:207-14. [CrossRef]
9. Mamo M, Wortmann C. Phosphorus sorption as affected by soil properties and termite activity in eastern and southern Africa. Soil Sci Soc Am J 2009;73:2170-6. [CrossRef]
10. Rückamp D, Amelung W, Theisz N, Bandeira AG, Martius C. Phosphorus forms in Brazilian termite nests and soils:Relevance of feeding guild and ecosystems. Geoderma 2010;155:269-79. [CrossRef]
11. Blouin M, Hodson ME, Delgado EA, Baker G, Brussaard L, Butt KR, et al. A review of earthworm impact on soil function and ecosystem services. Eur J Soil Sci 2013;64:161-82. [CrossRef]
12. Suarez ER, Pelletier DM, Fahey TJ, Groffman PM, Bohlen PJ, Fisk MC. Effects of exotic earthworms on soil phosphorus cycling in two broadleaf temperate forests. Ecosystems 2004;7:28-44. [CrossRef]
13. Ma L, Shao MA, Li TC. Characteristics of soil moisture and evaporation under the activities of earthworms in typical Anthrosols in china. Sustainability 2020;12:6603. [CrossRef]
14. Lin H, Xia M, Lin X, Xu C, Wu L, Jie W, et al. Earthworm gut bacteria increase silicon bioavailability and acquisition by maize. Soil Biol Biochem 2018;125:215-21. [CrossRef]
15. Wilpiszeski RL, Aufrecht JA, Retterer ST, Sullivan MB, Graham DE, Pierce EM, et al. Soil aggregate microbial communities:Towards understanding microbiome interactions at biologically relevant scales. Appl Environ Microbiol 2019;85:324. [CrossRef]
16. Asshoff R, Scheu S, Eisenhauer N. Different earthworm ecological groups interactively impact seedling establishment. Eur J Soil Biol 2010;46:330-4. [CrossRef]
17. Collins NM. The utilization of nitrogen resources by termites (Isoptera). In:Lee JA, McNeill S, Rorison IH, editors. Nitrogen as an Ecological Factor. Oxford:Blackwell Scientific Publications;1983. 381-412.
18. Wood TG, Sands WA. The role of termites in ecosystems. In:Brian MV, editor. Production Ecology of Ants and Termites. Cambridge:Cambridge University Press;1978. 245-92.
19. Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F, et al. Soil invertebrates and ecosystem services. Eur J Soil Biol 2006;42:S3-15. [CrossRef]
20. Kumari R, Sachdev M, Prasad R, Garg AP, Sharma S, Giang PH, et al. Microbiology of termite hill (mound) and soil. In:Intestinal Microorganisms of Termites and Other Invertebrates. Berlin, Germany:Springer;2006. 351-72. [CrossRef]
21. Dhembare A. Physico-chemical properties of termite mound soil. Arch Appl Sci Res 2013;5:123-6.
22. Jouquet P, Guilleux N, Shanbhag RR, Subramanian S. Influence of soil type on the properties of termite mound nests in Southern India. Appl Soil Ecol 2015;96:282-7. [CrossRef]
23. Menichetti L, Landi L, Nannipieri P, Katterer T, Kirchmann H, Renella G. Chemical properties and biochemical activity of colonized and abandoned litter-feeding termite (Macrotermes spp.) in chromic Cambisol Area on the Borana Plateau, Ethiopia. Pedosphere 2014;24:399-407. [CrossRef]
24. Kumar P, Tilak M, Sivakumar K, Saranya K. Studies on the assessment of major nutrients and microbial population of termite mound soil. Int J For Crop Improv 2018;9:13-7. [CrossRef]
25. Devi R, Thakur R. Screening and identification of bacteria for plant growth promoting traits from termite mound soil. J Pharm Phytochem 2018;7:1681-6. [CrossRef]
26. Poulsen M, Hu H, Li C, Chen Z, Xu L, Otani S, et al. Complementary symbiont contributions to plant decomposition in a fungus-farming termite. Proc Natl Acad Sci USA 2014;111:14500-5. [CrossRef]
27. Benndorf R, Guo H, Sommerwerk E, Weigel C, Garcia-Altares M, Martin K, et al. Natural products from actinobacteria associated with fungus-growing termites. Antibiotics 2018;7:83. [CrossRef]
28. Sujada N, Sungthong R, Lumyong S. Termite nests as an abundant source of cultivable actinobacteria for biotechnological purposes. Microbes Environ 2014;29:211-9. [CrossRef]
29. Krishanti NP, Zulfina D, Wikantyoso B, Zulfitri A, Yusuf S. Antimicrobial production by an actinomycetes isolated from the termite nest. J Trop Life Sci 2018;8:279-88. [CrossRef]
30. Enagbonma BJ, Babalola OO. Potentials of termite mound soil bacteria in ecosystem engineering for sustainable agriculture. Ann Microbiol 2019;69:211-9. [CrossRef]
31. Yêyinou Loko LE, Orobiyi A, Agre P, Dansi A, TamòM, Roisin Y. Farmers'perception of termites in agriculture production and their indigenous utilization in Northwest Benin. J Ethnobiol Ethnomed 2017;13:64. [CrossRef]
32. Bama PS, Ravindran AD. Dynamics of P sorption and solubilising activity in termite nest material. Asian J Res Soc Sci Hum 2012;2:231-7.
33. Fujita Y, Venterink HO, Van Bodegom PM, Douma JC, Heil GW, Hölzel N, et al. Low investment in sexual reproduction threatens plants adapted to phosphorus limitation. Nature 2013;505:82-6. [CrossRef]
34. Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002;245:83-93. [CrossRef]
35. Wang C, Liu Y, Li SS, Han GZ. Origin of plant auxin biosynthesis in charophyte algae. Trends Plant Sci 2014;19:741-3. [CrossRef]
36. Rodriguez H, Gonzalez T, Goire I, Bashan Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004;91:552-5. [CrossRef]
37. Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, et al. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 2005;37:1970-4. [CrossRef]
38. Raymond NS, Gómez-Muñoz B, van der Bom FJ, Nybroe O, Jensen LS, Müller-Stöver DS, et al. Phosphate-solubilising microorganisms for improved crop productivity:A critical assessment. New Phytol 2021;229:1268-77. [CrossRef]
39. Mosimann C, Oberhänsli T, Ziegler D, Nassal D, Kandeler E, Boller T, et al. Tracing of two Pseudomonas strains in the root and rhizoplane of maize, as related to their plant growth-promoting effect in contrasting soils. Front Microbiol 2017;7:2150. [CrossRef]
40. Owen D, Williams AP, Griffith GW, Withers PJ. Use of commercial bioinoculants to increase agricultural production through improved phosphorus acquisition. Appl Soil Ecol 2015;86:41-54. [CrossRef]
41. Biswakarma B, Verma H, Sarkar NC. Effect of phosphate solubilizing bacteria on yield of transplanted rice under lateritic belt of West Bengal, India. Int J Curr Microbiol App Sci 2018;7:3192-204. [CrossRef]
42. Biswas SS, Biswas DR, Purakayastha TJ, Sarkar A, Kumar R, DasTK, et al. Residual effect of rock-phosphate and PSB on rice yield and soil properties. Indian J Agric Sci 2021;91:440-4. [CrossRef]
43. Phringpaen W, Aiedhet W, Thitithanakul S, Kanjanasopa D. Ability of phosphate-solubilizing bacteria to enhance the growth of rice in phosphorus deficient soils. Trends Sci 2023;20:12. [CrossRef]
44. Musarrat J, Saghir Khan MD. Factors affecting phosphate-solubilizing activity of microbes:Current status. In:Khan MS, editor. Phosphate Solubilizing Microorganisms. Switzerland:Springer International Publishing;2014. [CrossRef]
45. Habte M, Osorio NW. Effect of nitrogen form on the effectiveness of a phosphate-solubilizing fungus to dissolve rock phosphate. J Biofertil Biopestici 2012;3:127.
46. Wenzel CL, Ashford AE, Summerell BA. Phosphate-solubilizing bacteria associated with proteoid roots of seedlings of waratah [Telopea speciosissima (Sm.) R.Br.]. New Phytol 1994;128:487-96. [CrossRef]
47. Halder AK, Mishra AK, Bhattacharyya P, Chakrabartty PK. Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. J Gen Appl Microbiol 1990;36:81-92. [CrossRef]
48. Khan MS, Zaidi A, Wani PA. Role of phosphate-solubilizing microorganisms in sustainable agriculture-a review. Agron Sustain Dev 2007;27:29-43. [CrossRef]
49. Khan MS, Zaidi A, Wani PA, Ahemad M, Oves M. Functional diversity among plant growth-promoting rhizobacteria. In:Khan MS, Zaidi A, Musarrat J, editors. Microbial Strategies for Crop Improvement. Berlin:Springer;2009. 105-32. [CrossRef]
50. Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA. Plant growth promotion by phosphate solubilizing fungi-current perspective. Arch Agron Soil Sci 2010;56:73-98. [CrossRef]
51. Adebajo SO, Akintokun PO, Ezaka E, Ojo AE, Olannye DU, Ayodeji OD. Use of termitarium soil as a viable source for biofertilizer and biocontrol. Bull Natl Res Cent 2021;45:100. [CrossRef]
52. Muon R, Lai CD, Herve V, Rainer Z, Chassagne F, Bureau E, et al. Abundance, perceptions and utilizations of termite mounds in Cambodia. Soil Use Manag 2023;39:1172-84. [CrossRef]
53. Miyagawa S, Koyama Y, Kokubo M, Matsushita Y, Adachi Y, Sivilay S, et al. Indigenous utilization of termite mounds and their sustainability in a rice growing village of the central plain of Laos. J Ethnobiol Ethnomed 2011;7:24. [CrossRef]
54. Nautiyala CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 1999;170:265-70. [CrossRef]
55. Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 1962;27:31-6. [CrossRef]
56. Nuntagij A, Abe M, Uchimi T, Seki Y, Boonkerd N, Higashi S. Characterization of Bradyrhizobium strains isolated from soybean cultivation in Thailand. J Gen Appl Microbiol 1997;43:183-7. [CrossRef]
57. Sarwar M, Arshad MG, Frankenberger WT. Tryptophan-dependent biosynthesis of auxins in soil. Plant Soil 1992;147:207-15. [CrossRef]
58. Kawasaki H, Hoshino Y, Hirata A, Yamasato K. Is intracytoplasmic membrane structure a generic criterion?It does not coincide with phylogenetic interrelationships among photosynthetic purple non-sulfur bacteria. Arch Microbiol 1993;160:358-62. [CrossRef]
59. Yamada Y, Katsura K, Kawasaki H, Widyastuti Y, Saono S, Seki T, et al. Asaia bogorensis gen. ., sp. nov., an unusual acetic acid bacterium in the alpha-Proteobacteria. Int J Syst Evol Microbiol 2000;50:823-9. [CrossRef]
60. Katsura K, Kawasaki H, Potacharoen W, Saono S, Seki T, Yamada Y, et al. Asaia siamensis sp. ., an acetic acid bacterium in the alpha-Proteobacteria. Int J Syst Evol Microbiol 2001;51:559-63. [CrossRef]
61. Brosius J, Dull TJ, Sleeter DD, Noller HF. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol 1981;148:107-27. [CrossRef]
62. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST:A new generation of protein database search programs. Nucleic Acids Res 1997;25:3389-402. [CrossRef]
63. Myers EW, Miller W. Optimal alignments in linear space. Comput Appl Biosci 1988;4:11-7. [CrossRef]
64. Bremner JM. Nitrogen. In:Black CA, editor. Methods of Soil Analysis Part 2:Chemical and Microbiological Properties. Madison, Wisconsin, USA:American Society of Agronomy Inc.;1965. 699-799.
65. Bray RH, Kurtz LT. Determination of total organic and available forms of phosphorus in soils. Soil Sci 1945;59:39-45. [CrossRef]
66. Walkley A, Black IA. Chromic and titration method for determination of soil organic matter. Soil Sci Am 1947;63:257. [CrossRef]
67. Peech M. Soil pH by glass electrode pH meter. Methods of soil analysis. Am Soc Agron 1965;60:914-25.
68. Bower CA, Wilcox LV. Soluble salts. In:Black CA, editor. Methods of Soil Analysis. Madison:American Society of Agronomy;1965. 933-40. [CrossRef]
69. Sungthongwises K. Phosphate-solubilizing bacteria:An alternative to increase availability of phosphorus for crop production. J Acad Serv 2012;3-4:15-24.
70. Cortijos-López M, Sánchez-Navarrete P, Lasanta T, Nadal-Romero E. How do acid or alkaline soil environments affect soil organic carbon stocks in a post-abandonment secondary succession process in Mediterranean mountain areas. Catena 2015;232:107384. [CrossRef]
71. Van Waasbergen LG. What makes a bacterial species? When molecular sequence data are used, is rRNA enough? In:Miller RV, Day MJ, editors. Microbial Evolution:Gene Establishment, Survival and Exchange. Ch. 21. Washington, DC, USA:ASM Press;2004. 339-56. [CrossRef]
72. Tuma J, Frouz J, Vesela H, Krivohlavy F, Fayle TM. The impacts of tropical mound-building social insects on soil properties vary between taxa and with anthropogenic habitat change. Appl Soil Ecol 2022;179:104576. [CrossRef]
73. Choosai C, Mathieu J, Hanboonsong Y, Jouquet P. Termite mounds and dykes are biodiversity refuges in paddy fields in North-Eastern Thailand. Environ Conserv 2009;36:71-9. [CrossRef]
74. Miyagawa S, Kokubo M, Harada M, Koyama Y, Adachi Y, Kawakubo N, et al. The possibility of small animals living on trees as factor of rice growth, and natural resources in rain-fed paddy fields of Laos. Trop Agric 2010;3:125-6.
75. Fall S, Hamelin J, Ndiaye F. Differences between bacterial communities in the gut of a soil-feeding termite (Cubitermes niokoloensis) and its mounds. Appl Environ Microbiol 2007;73:5199-208. [CrossRef]
76. Aenod N, Daoh M, Chanlert P, Setapong N, Tamad A. Analysis of elements inanthill soil samples in some areas of the Songkhla province by wavelength dispersive X-ray fluorescence techniques. J Phys Gen Sci 2020;4:??42-49.
77. Apori SO, Murongo M, Hanyabui E, Atiah K, Byalebeka J. Potential of termite mounds and its surrounding soils as soil amendments in smallholder farms in central Uganda. BMC Res Notes 2020;13:397. [CrossRef]
78. Yamashita C. Interactions between termite mounds, trees, and the Zemba people in the Mopane savanna in Northwestern Namibia. Afr Study Monogr 2010;40:115-28.
79. Kalayu G. Phosphate solubilizing microorganisms:Promising approach as biofertilizers. Int J Agron 2019;7:4917256. [CrossRef]
80. Somtrakoon K, Chouychai W. Phosphorus deficiency in plant and roles of phosphate-solubilizing bacteria. J Agric Res Ext 2020;38:39-49.
81. Sharon JA, Hathwaik LT, Glenn GM, Imam SH, Lee CC. Isolation of efficient phosphate solubilizing bacteria capable of enhancing tomato plant growth. J Soil Sci Plant Nutr 2016;16:525-36. [CrossRef]
82. Seangsanga T. Biosynthesis of Indole-3-Acetic Acid (IAA) of Nitrogen Fixing Bacteria Isolated from Rubber Tree Hevea Brasiliensis Mull-Arg. In:Proceedings of the 7th National Science Research Conference. Naresuan University;2015.
83. Rawat P, Das S, Shankhdhar D, Shankhdhar SC. Phosphate-solubilizing microorganisms:Mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nutr 2020;21:49-68. [CrossRef]
84. Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 1999;63:968-89. [CrossRef]
85. Mujahid TS, Subhan SA, Wahab A, Masnoon J, Ahmed N, Abbas T. Effects of different physical and chemical parameters on phosphate solubilizing activity of plant growth promoting bacteria isolate, indigenous soil. J Pharm Nutr Sci 2015;5:64-70. [CrossRef]
86. Oliveira CA, Alves VM, Marriel IE, Gomes EA, Scotti MR, Carneiro NP. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem 2009;41:1782-7. [CrossRef]
87. Kunhachan P, Sirithana W, Komutiban O, Limkhuansuwan V, Menchai P, Trakunjae C, et al. Selection of potential bacteria in termite nest and gut for sustainable agriculture. Trends Sci 2024;21:7794. [CrossRef]
88. Aye PP, Pinjai P, Tawornpruek S. Effect of phosphorus solubilizing bacteria on soil available phosphorus and growth and yield of sugarcane. Appl Sci 2021;18:10754. [CrossRef]