Abiotic stressors, or environmental stressors, are events that put plants under stress. They are the element of the ecosystem that is not biological but yet affects every living part of the system. Natural systems depend on a delicate balance, and when either of these factors is out of the ordinary, the ecosystem is stressed, endangering the health of all living creatures. Some of the main abiotic elements of the agricultural ecosystem that affect plants are water, nutrients, salts, temperature, and pH. These abiotic stresses affect both plants and microbes. Even though bacteria compete fiercely with one another, especially in the rhizosphere, some microbes possess the cellulase enzyme that allows them to dissolve the cellulose cell wall of plants and allow roots to enter the apoplast, or inside the cell wall, as well as the vascular bundle-xylem, where they live and perform normal metabolic functions [1].
Abiotic stressors are adverse environmental elements that limit the physicochemical qualities of soil and the functional diversity of micro-organisms, leading to significant yield loss [2]. The intricate effects of climate change on the outcomes of abiotic stress pose an imminent threat to the viability and productivity of agricultural systems. The decrease in agricultural output is primarily caused by a few abiotic factors which include drought, salinity, heavy metals, flooding, and extremes of temperature [3]. The hydrological cycle is severely disrupted by high salinity drought, which is also extremely stressful and has negative effects on the environment, society, and economy [2,4]. Drought or salinity causes a variety of physiological, metabolic, and biochemical alterations in a wide range of plant species [5,6]. Decreases in respiration, photosynthesis, and protein synthesis caused by salt and osmotic stressors have a detrimental effect on the growth and development of sensitive species [7].
Plant-associated microbes, also referred to as plant growth-promoting rhizobacteria (PGPR), are thought to stimulate plant defenses and have positive effects on plants, such as increased growth and decreased susceptibility to pathogen-caused diseases [8]. In this context, we have focused on the various types of abiotic stress that plants endure. Rhizobacteria are those essential micro-organisms that are able to help plants both directly and indirectly. The exopolysaccharide (EPS), volatile compounds, and aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing plant PGPR, which is found in the rhizosphere and endorhizosphere, stimulates the production of osmolytes and antioxidants, controls the gene expression that responds to stress and modifies the morphology of the roots, resulting in plants resistance to drought [Figure 1]. For plants to thrive under drought stress, PGPR needs to promote the genes that respond to osmotic pressure and stress. In addition to enduring plants’ resistance and adaptation to drought challenges, PGPR is crucial in identifying potential issues with future food security. Beyond contributing to the osmotic and metabolic changes that the PGPR treatment causes in plants, it also alters the properties of the soil to encourage plant development [9]. The phrase “induced systemic resistance,” or ISR, refers to how some PGPR results in chemical or physical alterations that are considered crucial to plant defense. Although it is widely recognized that ISR produced by PGPR confers resistance against plant diseases caused by pathogens, a few published findings imply that PGPR may also function as an elicitor of abiotic stressors in plants [8]. PGPR has the capability to help plants adapt to many forms of stress and in addition PGPR has minimized the need for fertilizer and has reduced environmental pollution.
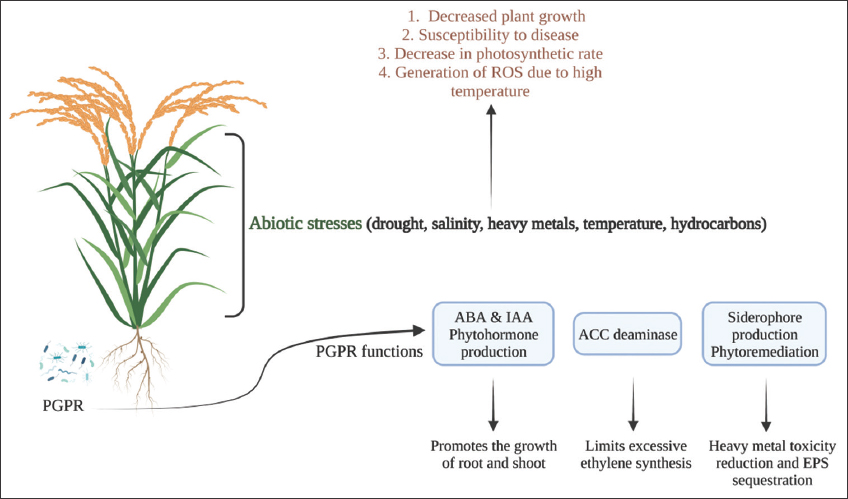 | Figure 1: Plant abiotic stress tolerance and the role of plant growth-promoting rhizobacteria in plants. [Click here to view] |
2. IMPACT OF VARIOUS ABIOTIC FACTORS ON PLANTS
Abiotic variables that are crucial include temperature, salt, drought, and heavy metals. Abiotic stressors have the potential to restrict crop yield pose challenges for crop cultivation in specific regions, and generally make conditions unfavorable. The degree of biotic stress is significantly influenced by abiotic stress. All of these stressors together have a major effect on the physiology, metabolic makeup, and gene expression of plants [10]. The disappearance of the soil microbiota might also result from abiotic stress. A sustainable approach to overcoming environmental instability and achieving agricultural sustainability might be to replenish rhizobacteria that promote plant growth (PGPR) in an environment under abiotic stress.
2.1. Drought Stress on Rice (Oryza sativa)
Drought stress limits rice productivity and generally inhibits it from growing by negatively influencing water absorption and nutrient uptake. The rice plant’s physiological, molecular, biochemical, and morphological responses are altered by drought stress. Water stress is a significant factor in the agriculture sector as it is a necessary component of cropland. Just over 60% of the 40 million ha of rice farms are irrigated. Rest is somewhat dependent on rainfall because droughts are prone to them. Drought drastically restricts rice production since crop failure is the result [11], which lowers the world’s food supply. Plants with low water content may experience physiological, biochemical, and morphological damage that interferes with critical cellular processes [12]. According to Chaves et al., 2009 [13] and Zlatev and Lidon 2012 [14], the main adverse effects of drought are oxidative damage to proteins, membrane lipids, and other cell components as well as damage to the chloroplast’s photosynthetic machinery. Crops may also develop more slowly, mature later, and yield grains of lesser quality in situations where there is a water shortage [12].
According to Fang and Xiong 2015 [15], drought avoidance, drought tolerance, and drought recovery are some of the many mechanisms that make up the complex quality known as drought resistance. Of note, resistance to drought is also significantly influenced by interactions with the environment [16]. As a result, the molecular basis for rice’s drought resilience and the course of its evolutionary development are still unknown. Elite cultivars of irrigated rice cultivated for excellent quality and production are particularly vulnerable to drought on an individual level [17]. These comments outline a possible trade-off between production and drought resilience. If a trade-off occurs, it must be addressed when breeding for drought-resistant cultivars since it will significantly influence how rice adaptively evolves its resistance to drought. Upland rice and lowland rice are two rice ecotypes that may be distinguished based on their tolerance to drought in agroecosystems with differing soil-water conditions. Upland rice has accrued genetic diversity via adaptation to drought-prone upland locations and is grown in rain-fed, unmanaged fields, increasing its drought tolerance [18] and has much stronger resistance to drought than lowland rice, as evidenced by field performance measurements conducted during drought conditions.
2.2. Effect of Soil Salinity on Plants
One of the main abiotic stresses that inhibit plant growth and yield worldwide is salinity stress. Saline soils affect about 7% of the surface area on earth [19]. Cultivable land can have its secondary salinity increased by up to 20% by inappropriate irrigation [20]. These salts can accumulate in the soil over time either through human activities or naturally such as irrigation practices and as a result, this leads to soil salinity [21]. The salinity of the soil affects almost every aspect of a plant’s life, including germination of seeds, growth, uptake of nutrients and water, transpiration rate, physiological development, production, ecological balance, etc. [22]. According to Shrivastava and Kumar 2015 [21], there are several ways in which soil salinity influences plant growth. For example, saline soils have a higher osmotic potential than roots, which means that if soil salt concentrations increase drastically, plants will have less access to water. As a result, plant experiences water stress that exhibits symptoms like wilting. Saline soils also lead to nutrient imbalance, ion toxicity, disruption of soil structure, and also osmotic stress. Ions such as salt, chlorine, and boron have a deleterious effect on plants. Plant cell death due to osmotic stress may be caused by an increase in sodium ions in the cell walls [23]. Crop development processes such as microsporogenesis, stamen elongation, ovule development, and embryogenesis can be impacted by the soil’s salinity, which also promotes programmed cell death [24]. Plant species vary in their tolerance to salinity, with some being tolerant and some being non-tolerant. PGPR, or beneficial bacteria that colonize the rhizosphere and improve plant growth and health, helps to lessen the detrimental effects of soil salinity on plant growth.
2.3. Effects of Heavy Metals on Rice Plant Growth
As a result of human activities such as urbanization and industrialization, heavy metals find their way into the soil. Among the dangerous and non-degradable, heavy metals that can be detrimental to plant and human health are Al, Cr, As, Cu, Hg, Pb, Se, Hg, Zn, and Mn. When humans ate rice and other grains grown on contaminated land, heavy metals seeped into the body and caused health problems. Heavy metals have detrimental effects on plant physiology, metabolism, senescence, water and nutrient absorption, growth, and physiology. It hinders the generation of plant growth hormones and damages the integrity of cell membranes, which both have an impact on plant development [25]. This results in stunted development, poor biomass, chlorosis, browning of the roots, and finally death in plants grown in heavy metal-contaminated areas. Plant absorption of heavy metals leads to a higher production of reactive oxygen species (ROS), which ultimately causes cell death. To maintain environmental sustainability, protect the ecosystem, and increase agricultural productivity, heavy metals need to be eliminated in a way that is environmentally friendly. In this sense, PGPR can promote plant growth and mitigate the detrimental effects of heavy metals on plants [26].
2.4. Impact of Temperature Stress on Rice Plant (Phytoretardation)
Among the primary abiotic stress factors influencing plant development and output is temperature, which may be either excessive or low. Nearly all of the physiological functions of the plant are impacted, reduced photosynthesis, cell division, increased transpiration, protein denaturation, and total plant development. Extreme heat increases the fluidity of the cell membrane whereas extreme cold increases its rigidity. A higher temperature increases the generation of ROS, which may cause oxidative cell damage [27]. At low temperatures, plants exhibit obvious deterioration including wilting, necrosis, and chlorosis [28]. The content of calcium inside plant cells may fluctuate in response to temperature variations [29]. According to Ruelland and Zachowski 2010 [28], it may also phosphorylate both mitochondrial and thylakoid proteins and alter the structure of plastids and the thylakoid membrane. In all of these major problems, PGPR can assist plants in coping with heat stress and subsequently enhance plant growth development.
3. DROUGHT RESISTANCE MEDIATED BY PGPR
Rhizospheric bacteria, which naturally adapt to and live in arid environments, can aid in the growth of plants. These PGPR strains increase drought resistance in plants through a number of different processes, including ACC deaminase biosynthesis, EPS synthesis, phytohormone synthesis, and ISR [30]. When rice lacks water, phytohormones such as abscisic acid (ABA) and IAA are released by PGPR stimulating the growth of the shoots and roots. Drought tolerance genes are activated and hydraulic conductivity is increased by phytohormone ABA, which reduces the stress brought on by drought. Belimov et al., 2001 [31] state that the enzyme ACC deaminase, which generates PGPR, can promote plant growth in rice plants exposed to unfavorable climatic conditions by limiting the synthesis of excessive ethylene by converting ACC, an ethylene precursor, into ammonia and F-ketobutyrate. Further, the synthesis of sugar, amino acids, water, and proline is increased by PGPR inoculation, which also increases the plant’s biomass and water potential [32]. Plants under Pseudomonas putida treatment experience less stress from dry circumstances because their levels of biomass and chlorophyll are higher [33]. The combined application of mycorrhizae and PGPR may help to mitigate the harmful impacts of drought. A brief description of the PGPR strains that have been used in O. sativa and have demonstrated tolerance to abiotic stress is listed in Table 1.
Table 1: List of few researches that explored Oryza sativa’s ability to withstand abiotic stress using the PGPR strains.
| Crop | Abiotic stress | PGPR | References |
|---|---|---|---|
| Oryza sativa | Salinity | Alcaligenes faecalis | [68,69] |
| Drought | Osmotolerant bacteria | [70-72] | |
| Heavy metal | Bacillus subtilis | [73] |
4. PGPR’S SALINITY-REDUCTION MECHANISM
The stress of salt on plants can be reduced using rhizobacteria that promote plant development PGPR. It reduces stress and promotes plant development both indirectly and directly. Nutrient mobilization, siderophore synthesis, phytohormone synthesis, and nitrogen fixation are a few of the direct mechanisms [34]. While the indirect method entails keeping a variety of plant pathogens from infecting plants. PGPR not only helps convert ACC into α-ketobutyrate and ammonia but also produces the enzyme ACC deaminase, which prevents plants from producing excessive ethylene. By controlling the production of rhizobitoxine enzyme in salt-challenged conditions, it also promotes the development of root nodules in plants [35]. According to Kasim et al., 2016 [4], the EPS generated by the PGPR functions as an inhibitor that binds with the cations and aids in the plant’s reduction of salt stress.
Many rhizobacteria have PGPR characteristics that enable them to resist salt stress. According to Bano and Fatima, 2009 [36], Pseudomonas and Rhizobium bacteria are helpful for fostering plant growth in salt. It has been observed that inoculating rice plants with Pseudomonas pseudoalcaligenes and Bacillus pumilus inhibits the activities of superoxide dismutase and lipid peroxidation under salt stress [37]. In addition, they increased the rice plant’s ability to produce glycine betaine and made it more resistant to salt [38]. Bacillus subtilis and B. pumilus promote the growth of plants in saline soil by solubilizing phosphate and producing HCN, IAA, and ammonia [39]. Rice plants become salt-resistant due to the production of phytohormones such as auxins and ABA by Bacillus amyloliquefaciens, as reported by Shahzad et al., 2017 [40]. According to Nautiyal et al., 2013 [41], the B. amyloliquefaciens bacterium is necessary for the regulation and repression of 14 different rice plant genes to mitigate the impact that saline stress has on rice plants.
5. HEAVY METAL SEQUESTRATION VIA PGPR MECHANISM
When plants are under significant metal stress, PGPR might accelerate plant growth. PGPR reduces the toxicity of heavy metals by several methods, such as detoxification, volatilization, heavy metal efflux, limiting metal entrance into cells, and complex formation. Furthermore, PGPR reduces the synthesis of ethylene while stimulating the synthesis of other plant growth regulators, such as ACC deaminase and IAA [42]. PGPR facilitates plant development by mobilizing essential nutrients, solubilizing phosphorus, fixing atmospheric nitrogen, and producing siderophore [43]. According to Zhang et al., 2020 [44], phylum Actinobacteria and Proteobacteria are used for the removal of As, Mn, and Pb from polluted soil. Cu phytotoxicity decreased when PGPR was applied to the agricultural plants [45]. Cadmium is mobilized from contaminated soil by Klebsiella sp. and Micrococcus sp. and encourages plant growth by lengthening their shoots and roots [46]. Arsenic is incorporated into biomass by certain bacteria such as Azospirillum brasilense and Bradyrhizobium japonicum, which also promotes plant growth and development when exposed to heavy metal stress [47].
A novel approach called “phytoremediation” refers to using plants to extract heavy metals from soil. Micro-organisms are utilized in phytoremediation to boost its effectiveness and create a biomarker for heavy metal pollution [48]. According to Babu et al., [49], certain bacteria can eliminate metal contaminants from the environment, while other bacteria can change the highly poisonous form of the metal ion into a less hazardous one. Heavy metals are detoxified by Pseudomonas spp. in the biotransformation of cadmium citrate and the Fe (III)-Zinc complex [50]. According to Saha et al., [51], bacteria-produced siderophore chelate additional metals in addition to ferric ions and aid in phytoremediation. As a result, PGPR promotes plant growth by alleviating the impacts of heavy metal stress.
6. INFLUENCE OF PGPR ON THE MECHANISMS UNDERLYING THERMOGENIC STRESS AND ADAPTATION
Thermogenic stress refers to the adverse effects of high temperatures on plants, which might eventually lead to biochemical or physiological disruptions. By utilizing micro-organisms that encourage plant development, plants can be protected from the harmful effects of thermic stress. As they are ubiquitous, microbes can be used as a tool to promote the growth of plants in a variety of temperature circumstances. Under extremely cold conditions, psychrophilic bacteria such as Arthrobacter nicotianae, Brevundimonas terrae, and Pseudomonas cedrina showed the ability to promote plant growth and development [52]. The effectiveness of psychrophilic bacteria in boosting plant development and their antibacterial activity has been the subject of several publications [53,54]. Similarly, research on thermotolerant bacteria that can produce organic acids to convert phosphate from an insoluble form to a soluble state has been conducted for a very long time [55]. Plants develop their own osmolytes, polyphenols, and other secondary metabolites that help them cope with temperature fluctuations and prevent oxidative damage to plant cell membranes at high temperatures [56].
Plants can also produce antioxidants like glutathione to protect cells from the damaging effects of ROS. Under circumstances of high temperature, the bacteria Paecilomyces formosus demonstrated features that promoted plant development. Comparable to bacteria, endophytic fungi and other symbiotic fungi, such as Curvularia protuberata, enhanced plants’ ability to tolerate heat and had an impact on their development [57,58]. Furthermore, it is critical to note that the capability of the bacterial strain, the surrounding environment, and the type of plant species all affect PGPR in thermogenic stress adaptation. Collectively use of PGPR reduces the detrimental effect of high temperatures on plant growth and promotes adaptability so that plants may survive in thermogenic stress environments [26].
7. CHALLENGES AND LIMITATIONS OF PGPR IN RICE PLANT ABIOTIC STRESS TOLERANCE
Rhizobacteria that encourage plant growth have been extensively researched for their potential to improve abiotic stress tolerance in plants. However, the usage of PGPR is not without its difficulties and restrictions. Below is a list of the majority of them.
7.1. Variability and Strain Specificity
Different PGPR strains have variable impacts on the capacity of rice plants to withstand stress, and environmental conditions can affect how well they work. It can be difficult to identify and choose the best strains for certain abiotic stressors and then optimize their use [59]. All PGPR strains will not be equally effective in promoting stress tolerance to rice plants.
7.2. Interaction with other Micro-organisms
The performance of PGPR may be impacted by the presence of other microbes in the rhizosphere. The colonization and advantageous effects of PGPR in rice plants may be constrained by competition or antagonistic interactions with other micro-organisms [60].
7.3. Inconsistent Performance
The efficacy to which PGPR improves rice’s abiotic stress tolerance varies under different field conditions, making it challenging to achieve consistent results. Factors such as soil type, climatic conditions, pH, nutrient availability, and agronomic practices can influence the performance of PGPR [61].
7.4. Lack of Standardized Protocol and Limited Understanding of the Mechanisms
There is a lack of standardized protocols for the isolation, characterization, and application of PGPR strains for abiotic stress tolerance in rice. This hinders the comparability of results across different studies and makes it difficult to identify the most effective strains and application methods [61].
7.5. Field-scale Application and Cost-effectiveness
Scaling up the application of PGPR in rice fields can be challenging due to the cost of production, formulation, and application methods. Developing cost-effective and practical strategies for large-scale applications is essential for their widespread adoption [62].
7.6. Compatibility with Other Inputs
The compatibility of PGPR with other agricultural inputs, such as fertilizers, pesticides, and fungicides, needs to be considered, as some of these inputs may interfere with PGPR colonization and growth or affect their efficacy in improving abiotic stress tolerance [8].
8. FUTURE PERSPECTIVES
Several studies indicate the potential of PGPR to enhance the growth of rice plants and stress tolerance. For example, B. pumilus, a commonly studied PGPR, was found to promote tolerance to rice plants against salt stress by lowering Na+ accumulation in plant tissues and enhancing antioxidant enzyme activity [63]. Similarly, it has been found that B. subtilis increases rice tolerance to drought stress through enhanced osmotic adjustment and water usage efficiency [64]. In addition, it has been found that PGPR increases the uptake of nutrients in rice plants, which can further improve their stress tolerance. For instance, a well-known PGPR, A. brasilense was found to enhance nitrogen uptake and promote root growth, which improves rice growth and drought tolerance [65]. Overall, the application of PGPR in the cultivation of rice shows great promise for improving plant development and stress tolerance, which would ultimately result in higher yields and a more secure food supply. More research is required to fully comprehend the mechanisms through which PGPR promotes plant development and stress tolerance to maximize its use in rice cultivation. Further, the rice plant’s resistance to drought stress was enhanced with the application of PGPR spraying. The researchers discovered that plants treated with PGPR strains had more antioxidant activity and suffered less oxidative damage in areas that experienced drought [66].
Similar results were found in another study by Khan et al., 2020 [62] which showed that PGPR inoculation increased rice plants’ ability to tolerate salt. Under salt stress, the researchers found that plants treated with PGPR developed more roots, had more chlorophyll, and were less harmful to ions. Further studies were performed to look at the potential of PGPR to minimize the negative impacts of heavy metal stress on rice plants, where many scientists discovered that PGPR inoculation enhanced the plants’ antioxidant defense mechanisms and reduced the accumulation of heavy metals in plant tissues [67]. The results of these studies suggest that the application of PGPR may improve rice plants’ resistance to abiotic stress. Further, to fully understand the mechanisms behind the beneficial effects of PGPR on plant stress responses, more research is needed to maximize their application in agricultural environments. In conclusion, PGPR holds great promise for sustainable agriculture, crop yield improvement, and environmental conservation at the same time.
9. CONCLUSIONS
The comprehensive and current research information on the effects of PGPR on rice plant resistance to abiotic stress is presented in this review. The study results mentioned throughout the paper offer strong support for the idea that PGPR can significantly enhance rice plants’ resilience to and ability to adapt to a variety of abiotic stimuli. The significance of abiotic stresses in influencing rice plant growth and productivity was underlined in the article. Drought, salinity, and temperature extremes are some of the challenges that PGPR encountered. Given that these pressures pose significant challenges to the cultivation of rice, finding workable solutions to decrease their negative impacts is crucial for sustainable agriculture. Future PGPR research has a wide range of potential applications, including combining it with other bio-fertilizers or bio-pesticides to increase their overall potency as well as commercial development of effective PGPR products. Therefore, more extensive research is required to find and characterize the noble PGPR strains with enhanced features as well as to comprehend the full mechanisms by which PGPR interacts with plants and encourages vital growth.
10. ACKNOWLEDGMENTS
The authors would like to express thanks to Chandigarh University.
11. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
12. FUNDING
There is no funding to report.
13. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
14. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
15. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
16. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Enebe MC, Babalola OO. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress:A survival strategy. Appl Microbiol Biotechnol 2018;102:7821-35. [CrossRef]
2. Goswami M, Deka S. Plant growth-promoting rhizobacteria alleviators of abiotic stresses in soil:A review. Pedosphere 2020;30:40-61. [CrossRef]
3. Kumar A, Patel JS, Meena VS, Srivastava R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal Agric Biotechnol 2019;20:101271. [CrossRef]
4. Kasim WA, Gaafar RM, Abou-Ali RM, Omar MN, Hewait HM. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann Agric Sci 2016;61:217-27. [CrossRef]
5. Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses:Consequences for changing environment. Environ Sci Pollut Res Int 2015;22:4907-21. [CrossRef]
6. Ma Y, Dias MC, Freitas H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci 2020;11:591911. [CrossRef]
7. Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol 2008;59:651-81. [CrossRef]
8. Gururani MA, Upadhyaya CP, Baskar V, Venkatesh J, Nookaraju A, Park SW. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ros-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul 2013;32:245-58. [CrossRef]
9. Subiramani S, Ramalingam S, Muthu T, Nile SH, Venkidasamy B. Development of abiotic stress tolerance in crops by plant growth-promoting rhizobacteria (PGPR). In:Kumar M, Kumar V, Prasad R, editors. Phyto-microbiome Stress Regulation. Environmental and Microbial Biotechnology. Singapore:Springer;2020. 125-45.
10. Chodak M, Golebiewski M, Morawska-Ploskonka J, Kuduk K, Niklinska M. Soil chemical properties affect the reaction of forest soil bacteria to drought and rewetting stress. Ann Microbiol 2015;65:1627-37. [CrossRef]
11. Mahajan S, Tuteja N. Cold, salinity and drought stresses:An overview. Arch Biochem Biophys 2005;444:139-58. [CrossRef]
12. Farooq M, Wahid A, Lee DJ, Ito O, Siddique KH. Advances in drought resistance of rice. Crit Rev Plant Sci 2009;28:199-217. [CrossRef]
13. Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress:Regulation mechanisms from whole plant to cell. Ann Bot 2009;103:551-60. [CrossRef]
14. Zlatev Z, Lidon FC. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir J Food Agric 2012;24:57-72. [CrossRef]
15. Fang Y, Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 2015;72:673-89. [CrossRef]
16. Hu H, Xiong L. Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol 2014;65:715-41. [CrossRef]
17. Luo LJ. Breeding for water-saving and drought-resistance rice (WDR) in China. J Exp Bot 2010;61:3509-17. [CrossRef]
18. Bernier J, Atlin GN, Serraj R, Kumar A, Spaner D. Breeding upland rice for drought resistance. J Sci Food Agric 2008;88:927-39. [CrossRef]
19. Szabolcs I. Salt affected soils as the ecosystem for halophytes. In:Squires VR, Ayoub AT, editors. Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands. Dordrecht:Springer;1994. 19-24.
20. Al-Maskri A, Al-Kharusi L, Al-Miqbali H, Khan MM. Effects of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Int J Agric Biol 2010;12:377-80.
21. Shrivastava P, Kumar R. Soil salinity:A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 2015;22:123-31. [CrossRef]
22. Netondo GW, Onyango JC, Beck E. Sorghum and salinity. Crop Sci 2004;44:797-805. [CrossRef] [CrossRef] [CrossRef] [CrossRef]
23. Munns R. Comparative physiology of salt and water stress. Plant Cell Environ 2002;25:239-50. [CrossRef]
24. Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora Morphol Distrib Funct Ecol Plan 2004;199:361-76. [CrossRef]
25. Chibuike GU, Obiora SC. Heavy metal polluted soils:Effect on plants and bioremediation methods. Appl Environ Soil Sci 2014;2014:752708.
26. Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M. Abiotic Stress and reactive oxygen species:Generation, signaling, and defense mechanisms. Antioxidants (Basel) 2021;10:277. [CrossRef]
27. Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction:Uncovering the weakest links. J Exp Bot 2010;61:1959-68. [CrossRef]
28. Ruelland E, Zachowski A. How plants sense temperature. Environ Exp Bot 2010;69:225-32. [CrossRef]
29. Knight H, Brandt S, Knight MR. A history of stress alters drought calcium signaling pathways in Arabidopsis. Plant J 1998;16:681-7. [CrossRef]
30. Goswami A, Banerjee R, Raha S. Drought resistance in rice seedlings conferred by seed priming:Role of the anti-oxidant defense mechanisms. Protoplasma 2013;250:1115-29. [CrossRef]
31. Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE, et al. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 2001;47:642-52. [CrossRef]
32. Naseem H, Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact 2014;9:689-701. [CrossRef]
33. Kang SM, Radhakrishnan R, Khan AL, Kim MJ, Park JM, Kim BR, et al. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol Biochem 2014;84:115-24. [CrossRef]
34. Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion:A review. Ann Microbiol 2010;60:579-98. [CrossRef]
35. Vijayan R, Palaniappan P, Tongmin SA, Elavarasi P, Manoharan N. Rhizobitoxine enhances nodulation by inhibiting ethylene synthesis of Bradyrhizobium elkanii from Lespedeza species:Validation by homology modeling and molecular docking study. World J Pharm Pharm Sci 2013;2:4079-94.
36. Bano A, Fatima M. Salt tolerance in Zea mays (L). Following inoculation with Rhizobium and Pseudomonas. Biol Fertil Soils 2009;45:405-13. [CrossRef]
37. Jha Y, Subramanian RB. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol Mol Biol Plants 2014;20:201-7. [CrossRef]
38. Jha Y, Subramanian RB, Patel S. The combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 2011;33:797-802. [CrossRef]
39. Damodaran T, Sah V, Rai RB, Sharma DK, Mishra VK, Jha SK, et al. Isolation of salt tolerant endophytic and rhizospheric bacteria by natural selection and screening for promising plant growth-promoting rhizobacteria (PGPR) and growth vigour in tomato under sodic environment. Afr J Microbiol Res 2013;7:5082-9.
40. Shahzad R, Khan AL, Bilal S, Waqas M, Kang SM, Lee IJ. Inoculation of abscisic acid producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ Exp Bot 2017;136:68-77. [CrossRef]
41. Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopory SK. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem 2013;66:1-9. [CrossRef]
42. Glick BR. Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 2010;28:367-74. [CrossRef]
43. Verma JP, Yadav J, Tiwari K, Kumar A. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol Eng 2013;51:282-6. [CrossRef]
44. Zhang Y, Wang X, Ji H. Co-remediation of Pb contaminated soils by heat modified sawdust and Festuca arundinacea. Sci Rep 2020;10:4663. [CrossRef]
45. Fatnassi IC, Chiboub M, Saadani O, Jebara M, Jebara SH. Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. C R Biol 2015;338:241-54. [CrossRef]
46. Prapagdee B, Chanprasert M, Mongkolsuk S. Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 2013;92:659-66. [CrossRef]
47. Armendariz AL, Talano MA, Wevar Oller AL, Medina MI, Agostini E. Effect of arsenic on tolerance mechanisms of two plant growth-promoting bacteria used as biological inoculants. J Environ Sci (China) 2015;33:203-10. [CrossRef]
48. Chen L, Luo S, Li X, Wan Y, Chen J, Liu C. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol Biochem 2014;68:300-8. [CrossRef]
49. Babu AG, Kim JD, Oh BT. Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J Hazard Mater 2013;250-1:477-83.
50. Qian J, Li D, Zhan G, Zhang L, Su W, Gao P. Simultaneous biodegradation of Ni-citrate complexes and removal of nickel from solutions by Pseudomonas alcaliphila. Bioresour Technol 2012;116:66-73. [CrossRef]
51. Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P. Microbial siderophores and their potential applications:A review. Environ Sci Pollut Res Int 2016;23:3984-99. [CrossRef]
52. Lavakusha, Yadav J, Verma JP, Jaiswal DK, Kumar A. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol Eng 2014;62:123-8. [CrossRef]
53. Meena RK, Ramesh KS, Norang PS, Sunita KM, Vijay SM. Isolation of low temperature surviving plant growth-promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal Agric Biotechnol 2015;4:806-11.
54. Javani S, Marín I, Amils R, Abad JP. Four psychrophilic bacteria from Antarctica extracellularly biosynthesize at low temperature highly stable silver nanoparticles with outstanding antimicrobial activity. Colloids Surf A Physico Chem Eng Asp 2015;483:60-9. [CrossRef]
55. Chang CH, Yang SS. Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour Technol 2009;100:1648-58. [CrossRef]
56. Cheruiyot EK, Mumera LM, Ng'etich WK, Hassanali A, Wachira F. Polyphenols as potential indicators for drought tolerance in tea (Camellia sinensis L.). Biosci Biotechnol Biochem 2007;71:2190-7.
57. Hubbard M, Germida JJ, Vujanovic V. Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J Appl Microbiol 2014;116:109-22. [CrossRef]
58. Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM. Thermotolerance generated by plant/fungal symbiosis. Science 2002;298:1581. [CrossRef]
59. Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 2014;32:429-48. [CrossRef]
60. Beneduzi A, Ambrosini A, Passaglia LM. Plant growth-promoting rhizobacteria (PGPR):Their potential as antagonists and biocontrol agents. Genet Mol Biol 2012;35:1044-51. [CrossRef]
61. Shah A, Nazari M, Antar M, Msimbira LA, Naamala J, Lyu D, et al. PGPR in agriculture:A sustainable approach to increasing climate change resilience. Front Sustain Food Syst 2021;5:667546. [CrossRef]
62. Khan N, Bano A, Ali S, Babar MA. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul 2020;90:189-203. [CrossRef]
63. Khan A, Sirajuddin, Zhao XQ, Javed MT, Khan KS, Bano A, et al. Bacillus pumilus enhances tolerance in rice (Oryza sativa L.) to combined stresses of NaCl and high boron due to limited uptake of Na+. Environ Exp Bot 2016;124:120-9. [CrossRef]
64. Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 2009;14:1-4. [CrossRef]
65. García de Salamone IE, Funes JM, Di Salvo LP, Escobar-Ortega JS, D'Auria F, Ferrando L, et al. Inoculation of paddy rice with Azospirillum brasilense and Pseudomonas fluorescens:Impact of plant genotypes on rhizosphere microbial communities and field crop production. Appl Soil Ecol 2012;61:196-204. [CrossRef]
66. Ahluwalia O, Singh PC, Bhatia R. A review on drought stress in plants:Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour Environ Sustain 2021;5:100032.
67. Khan MS, Zaidi A, Wani PA, Oves W. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 2009;7:1-19. [CrossRef]
68. Bal HB, Nayak L, Das S, Adhya TK. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013;366:93-105. [CrossRef]
69. Jha M, Chourasia S, Sinha S. Microbial consortium for sustainable rice production. Agroecol Sustain Food Syst 2013;37:340-62. [CrossRef]
70. Yuwono T, Handayani D, Soedarsono J. The role of osmotolerant rhizobacteria in rice growth under different drought conditions. Aust J Agric Res 2005;56:715-21. [CrossRef]
71. Gusain YS, Singh US, Sharma AK. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr J Biotechnol 2015;14:764-73.
72. Cassan F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O. Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 2009;45:12-9. [CrossRef]
73. Liu A, Wang W, Zheng X, Chen X, Fu W, Wang G, et al. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 2022;302:134900. [CrossRef]