1. INTRODUCTION
The present global human population of 7.7 billion is expected to cross over 9 billion by 2050. Feeding this burgeoning population will require 70%–100% increase in food grain production globally in future [1]. Amongst several biotic and abiotic constraints to food crop production, weeds are one of the most important biotic factors causing nearly 13% reduced food crop yields globally [2]. More than 11 billion dollars of Indian agricultural produce was lost due to weeds [3]. Despite many limitations, the uses of herbicides to eradicate weeds in agri-ecosystems allow to grow crop plants at a competitive advantage is one of the important strategies [4]. However, the use of herbicides on large scale in crops affects non-target beneficial microbial flora including cyanobacteria for which use is not intended [5–7]. Cyanobacteria are most primitive Gram negative photoautotrophic unicellular to multicellular organisms exhibiting a great structural and distributional diversity [8,9]. The interdependence of carbon and nitrogen assimilation in cyanobacteria makes these organisms to grow and tolerate different adverse environmental conditions. This feature of cyanobacteria makes this microbial group a ubiquitous in distribution and makes considerable contribution in productivity of agri-ecosystem [4,10]. Cyanobacteria are considered to be a well-known natural biofertilizer, since these organisms build up soil fertility by enhancing soil nitrogen and liberating growth promoting substances such as amino acids, vitamins, and hormones, increases phosphate solubilization, water holding capacity, and also decreases soil salinity [11–13].
Many studies analyzed the harmful effects of herbicides in single or mixed form on cyanobacteria [11,14–16]. Among various herbicides, the inhibitory effect of herbicide 2, 4-D on the growth, development, and physiology of cyanobacteria has been widely investigated [10,14,17,18]. The impact of other herbicides such as bromacil and thiobendazole on cyanobacterial mat [19], glyphosate on Microcystis aeruginosa, Anabaena variabilis, Chroococcus minutus, Fischerella sp., and Nostoc muscorum [20,21], 2,4 D on Scytonema geitleri, A. variabilis, and Hapalosiphon sp. [22,23], anilofos on Synechocystis sp. PUPCCC 64 and Anabaena torulosa [24,25], pretilachlor on Anabaena sp., N. muscorum, and Desmonostoc muscorum [15,26,27] has been reported.
Pendimethalin (N-(1-ethylpropyl)-2,6-dinitro-3,4-xylidine, an orange yellow color chemical compound belong to class dinitroaniline, is the third after paraquat and glyphosate, most often used herbicide worldwide. The estimated annual consumption of pendimethalin in 2014 was about 10 million pounds [18]. This pre- and post-emergent herbicide commonly used for the eradication of broadleaf weeds of cotton, soybeans, wheat, rice, maize, and annual grasses [28,29]. Pendimethalin kills weeds through inhibition of cell elongation and division. Compared to other dinitroaniline herbicides, pendimethalin has low volatility which makes it persistent in soil for a longer period of 10–92 days and its residual amount has been frequently detected in soil, ground, and surface water [30,31]. Commercially, pendimethalin is available as both granular and emulsifiable concentrate, under the trade names of ProwlTM, StompTM, HerbadoxTM, and Pay-offTM. As a matter of fact, there is no substantial amount of literature available on the toxic effect of pendimethalin on the growth and development of cyanobacteria. The assimilation of carbon and nitrogen is an important interdependent growth and development regulatory metabolic pathway of cyanobacteria. These two pathways in cyanobacteria are the sources of all cellular components such as photo-pigments, different proteins, genetic materials, and energy rich compounds such as adinosine triphosphate (ATP) and nicotine adenine dinucleotide hydride (NADH)/nicotine adenine dinucleotide hydrogen phosphate (NADPH). Nitrogen assimilation in cyanobacteria depends upon the carbon skeleton for biosynthesis of compounds thus the limitation or oversupply of one of these elements strongly affects the metabolism of the other [32]. Thus, this present study was undertaken to understand the mechanism of pendimethalin interaction with carbon and nitrogen assimilation by the abundantly growing paddy field cyanobacterium D. muscorum PUPCCC 405.10.
2. MATERIALS AND METHODS
2.1. Microorganism and Culture Conditions
The microalga D. muscorum PUPCCC 405.10 employed in the present study was isolated from rice fields of Punjab, India by our laboratory [26] and grown photoautotrophically in slight modified Chu-10 medium [33] supplemented with micronutrients of Allen and Arnon [34]. The stock and experimental cultures were maintained in algal culture room at 28°C ± 2°C illuminated with LED tubes giving a light intensity of 44.5 µmol photons flux/m2/s (µE) on the surface of culture flasks for 14 hours every day. The cultures were maintained in homogenous state by shaking manually 4–6 times daily and kept in actively dividing stage by transferring into fresh medium after every 6–8 days. Six-day-old exponentially growing cultures were used throughout the study and each experiment was repeated three times.
2.2. Tolerance Limit Determination
The tolerance limit of the D. muscorum towards pendimethalin was determined by measuring its growth in terms of increase in absorbance with time in Chu-10 medium containing 2.0 to 20 mg/l pendimethalin as per the protocol described by Swatch et al. [15]. The experiment was conducted in 250 ml Erlenmeyer flasks. The log phase stock cultures condensed by centrifugation (5,000 g, 10 minutes) were washed thrice with sterilized double distilled water and were inoculated in flasks containing 150 ml Chu-10 medium supplemented with different doses of pendimethalin to get an initial absorbance of 0.1 at 720 nm. Ten ml of the cultures were withdrawn at intervals of 2 days, up to 12 days and the absorbance of cultures was noted at a wavelength of 720 nm using UV-Visible spectrophotometer (Shimadzu 1280, Kyoto, Japan). Percent inhibition in growth was calculated using growth data of 2–8 days. The generation time was calculated according to Singh et al. [35]. Folin-phenol reagent was used to determine protein content of experimental cultures [36].
2.3. Photosynthetic Pigments Estimation
Aliquots of 10 ml pendimethalin treated and untreated experimental cultures were centrifuged at 5,000 g for 5 minutes. The obtained pellet, washed thrice with double distilled water was resuspended in 5 ml of 80% acetone. After shaking vigorously, the mixture was kept at 4°C overnight to release the pigments from cells and centrifuged. The absorbance of the supernatant was noted at 660, 645, and 450 nm and the amount of chlorophyll a and carotenoids was quantified according to Holm [37] and Myers and Kratz [38], respectively. Phycobiliproteins such as phycocyanin, allophycocyanin, and phycoerythrin were extracted in water from the biomass using freeze-thaw method [39]. A known volume of herbicide treated and untreated culture was centrifuged at 5,000 g for 10 minutes and the pellet obtained was resuspended in double distilled water and subjected to freeze-thaw cycles till all the pigments from the cells were released in water. The solution was then centrifuged at 5,000 g for 10 minutes and absorbance of the supernatant was noted at 562,615 and 652 nm using UV visible spectrophotometer. The phycobiliproteins were calculated using the equation of Bennett and Bogorad [40].
2.4. Photosynthesis, Photochemical Activities, and Respiration
The rate of photosynthesis, photochemical and respiratory activities were determined according to Chen et al. [5]. Ten ml of each 12 hours pendimethalin treated and untreated control cultures were centrifuged at 5,000 g for 10 minutes and made a thick biomass suspension equivalent to 10 µg chlorophyll a/ml in 3 ml of 25 mM bis tris propane buffer (BTP) of pH 7.8. This mixture was kept in a reaction chamber fitted with an oxygen electrode of dissolved oxygen analyzer (Model 5300A, YSI Bioanalytical Products, Yellow Springs, OH) and stirred with magnetic stirrer. The surface of reaction chamber was illuminated with 225 µE light intensity and the rate of increase in dissolved oxygen was followed for 10 minutes. The rate of photosynthesis was expressed as nmol O2 evolved/µg chlorophyll a.minute. The rate of respiration was measured in terms of uptake of dissolved oxygen with time in dark and expressed as nmol O2 consumed/µg chlorophyll a.minute.
The activity of photosystem (PS)-Ι was measured in terms of O2 uptake in presence of light by thick cell suspension of pendimethalin treated and untreated control culture prepared as described above in 25 mM BTP buffer supplemented with electron donor (0.1 mM 2, 6-dichlorophenol indophenols; DCPIP), 5 mM ascorbic acid for reducing DCPIP to DCPIPH2, electron acceptor (0.1 mM methyl viologen; MV), inhibitor of respiration (1 mM NaN3), and PS-Π system inhibitor [10 µM 3-(3,4- dichlorophenyl)-1,1- dimethylurea (DCMU)]. The activity of PS-II was measured in terms of O2 evolved in 5 mM BTP buffer (pH 7.8) containing 1 mM p-benzoquinone (electron acceptor) using water as the source of electron donor. The whole chain photosynthetic electron transport (PET) activity was measured as uptake of dissolved oxygen in presence of light using water as the electron donor in BTP (25 mM, pH 7.8) buffer containing 0.1 mM MV as electron acceptor and 1 mM NaN3 as respiratory inhibitor.
2.5. Nitrogen Source Uptake and Enzyme Assay
Potassium nitrate, sodium nitrite, and ammonium chloride were employed as nitrate, nitrite, and ammonium source, respectively for uptake studies. Nitrogen uptake by the test microorganism was measured in terms of its depletion with time from the medium. A known volume of untreated and pre-treated with pendimethalin for 12 hours was harvested, washed with double distilled water, and inoculated in separate 100 ml Erlenmeyer flasks containing 50 ml Chu-10 medium supplemented with 100 µM nitrate, nitrite, or ammonium. The flasks were kept at 28oC ± 2oC in the culture room and illuminated with LED lights providing a light intensity of 44.5 µE on the surface of vessels. At desired time, the biomass was separated by centrifugation (5,000 g, 10 minutes) and the supernatant was used for the estimation of residual amount of nitrate, nitrite, or ammonium. The protocol developed by Robinson et al. [41] and Nicholas and Nason [42] was used for the estimation of nitrate and nitrite, respectively. The method of Solarzano [43] was used for the estimation of ammonium. The kinetics of nitrate, nitrite, and ammonium uptake was studied by measuring the respective nitrogen uptake by the pendimethalin treated and untreated cell suspension kept in different concentrations of individual nitrogen source (10–100 µM). The Km and Vmax values for each substrate were calculated by drawing Lineweaver–Burk double reciprocal plots.
The biomass pellet obtained above was used for enzyme assay. The protocol of Herrero et al. [44] was used to measure the activity of nitrate reductase (NR) and nitrite reductase (NiR) as described earlier in detail [26]. The enzyme activity in term of enzyme units (U) is expressed as µmol nitrite formed or reduced/mg protein.minute under standard assay conditions. The activity of whole cell glutamine synthetase (GS) was measured according to the method of Shapiro and Stadtman [45]. One unit of GS enzyme is expressed as µmol of L-glutamic acid ɤ-monohydroxamate formed/mg protein.minute under standard assay conditions.
2.6. Chemicals
The chemicals used in the present study for the preparation of nutrient medium and assay of enzymes were procured from SD Fine Chemical Limited, India, Merck, India and Sigma Aldrich, USA. Commercial grade pendimethalin (Dhanutop 30%) manufactured by Dhanuka Agritech Limited, Gurgaon, Haryana, India was purchased from the local market.
2.7. Statistical Analysis
The average data of three independently performed experiments ± standard deviation (SD was used throughout the current study. The whole data were analyzed statistically by employing one-way analysis of variance and Tukey’s post hoc significance difference test.
All statistical analyses were tested at 5% level of significance against probability value at 95% confidence level (p < 0.05) using software Graph Pad Prism 6.0 version 6.0 (http://www.graphpad.com).
3. RESULTS
3.1. Tolerance Limit
Percent inhibition in growth of D. muscorum in terms of absorbance at 720 nm is given in Figure 1. The test organism showed a reduction in growth by 16%, 25%, 36%, 50%, 66%, 75%, 82%, and 93% in presence of 2, 4, 6, 8, 10, 12, 14, 16, and 18 mg pendimethalin/l, respectively. The test organism failed to grow in 20 mg pendimethalin/l. The cultures grown in different concentrations of herbicide were also examined microscopically. The cultures in 4 mg pendimethalin/l showed slight change in color from bluish green to yellowish. Fragmentation of filaments started after 6 mg/l pendimethalin containing culture and severe fragmentation occurred in 12 mg/l herbicide supplemented cultures. Microscopic examination of culture containing 20 mg/l herbicide revealed that above 97% of cells were lysed to release of pigments in the medium (Fig. 2). The growth data was further analyzed by calculating the generation time. The results revealed that generation time increased to 32–85 hours in the presence of 4–12 mg pendimethalin/l compared to 25.2 hours doubling time of control culture (Fig. 1 inset). Based on percent inhibition in growth, three concentrations of pendimethalin, viz. 4, 8, and 12 mg/l equivalent to inhibitory concentration (IC) IC25, IC50, and IC75 were selected for further studies.
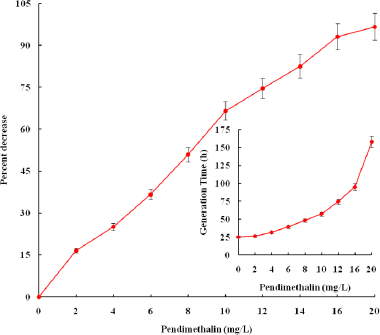 | Figure 1: Percent inhibition in growth (A720) of D. muscorum in the presence of pendimethalin. Inset figure: Generation time. The growth data of day 2–8 was taken to calculate the generation time. All data in the figure are different from each other at 5% level of significance (p < 0.05). [Click here to view] |
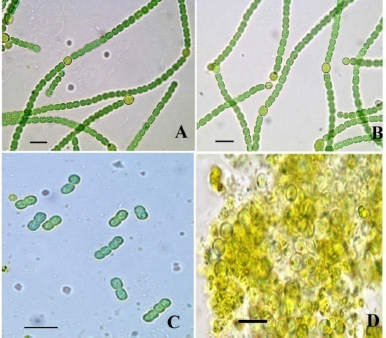 | Figure 2: Microphotographs of D. muscorum in different concentrations of pendimethalin on day 4 (Scale bar 10 µm). (A) Control (0 mg/l), (B) 04 mg/l, (C) 12 mg/l, and (D) 20 mg/l. [Click here to view] |
3.2. Effect of Pendimethalin on Growth Parameters
The results related to the growth parameters obtained in this study exhibited a dose-dependent effect of pendimethalin on dry biomass and protein content of D. muscorum. On day 6, the test organism registered a decrease of 24%, 41%, and 58% in dry biomass in presence of 4, 8, and 12 mg pendimethalin/l, respectively. The protein content of test organism recorded a decrease of 23%, 42%, and 59% in presence of respective selected doses of pendimethalin (Fig. 3).
3.3. Effect of Pendimethalin on Photosynthetic Pigments, Photosynthesis, Photosynthetic and Respiratory Activities
The inhibitory effect of pendimethalin on chlorophyll a, carotenoids, phycocyanin, allophycocyanin, and phycoerythrin of the test microorganism was also concentration dependent (Figures 4 and 5). Chlorophyll a and carotenoid contents were decreased in the range of 13%–86% and 8%–45%, respectively (Fig. 4), while phycocyanin, allophycocyanin, and phycoerythrin contents were decreased by 25%–84%, 21%–48%, and 24%–60%, respectively (Fig. 5). Pendimethalin deleteriously affected the performance of photosynthetic as well as respiratory activity of the test organism (Table 1). Treatment with 4–12 mg pendimethalin/l caused 29%–58% and 25%–61% reduction in rate of photosynthesis and respiration, respectively. The results of photochemical activity of test organism revealed that pendimethalin decreased the activity of PS-I and PS-II by 33%–64% and 24%–62%, respectively. The whole PET activity exhibited a decrease of 16%–41% in presence 4–12 mg pendimethalin/l (Table 1).
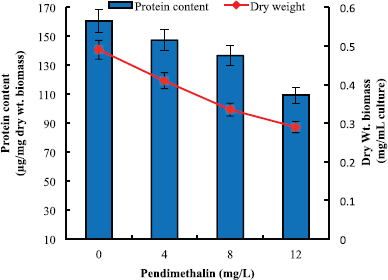 | Figure 3: Effect of pendimethalin (mg/l) on dry biomass and total protein content of D. muscorum on 6 days. Growth data of each parameter in different concentrations of pendimethalin as well as control are different from each other at 5% level of significance (p < 0.05). [Click here to view] |
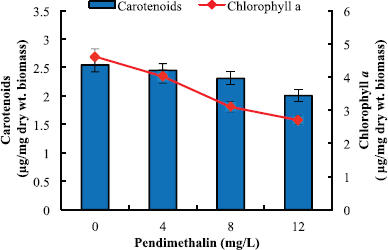 | Figure 4: Effect of pendimethalin (mg/l) on Chlorophyll a and carotenoid content of D. muscorum on 6 days. Data presented in figure of each pigment in presence of different concentrations of pendimethalin as well as of control are different from each other at 5% level of significance (p < 0.05). [Click here to view] |
3.4. Effect of Pendimethalin on Nitrate and Nitrite Uptake and Its Assimilation
The incubation of untreated control cultures of D. muscorum in 100 µM nitrate and 50 µM nitrite for 6 hours took up 0.84 and 1.2 µmol of nitrate and nitrite/mg protein. The culture treated with pendimethalin (4–12 mg/l) showed an uptake of nitrate and nitrite in the range of 0.29–0.51 and 0.20–0.75 µmol/mg protein, respectively (Table 2). A Lineweaver–Burk double reciprocal plot for kinetics of nitrate uptake showed a Vmax value of 26.6 and 47.6 µmol nitrate taken/mg protein.hour and same Km values of 0.59 mmol/l for pendimethalin treated and untreated control cultures, respectively. The trend of effect of herbicide on kinetics of nitrite uptake was similar to nitrate uptake. The kinetics of nitrite uptake showed a Vmax value of 1.17 and 2 µmol nitrite taken/mg protein.hour and same Km values of 50 µmol/l for pendimethalin treated and untreated control cultures, respectively (Table 3). The effect of pendimethalin on NR and NiR activities was also dose-dependent. The test organism showed a 32.6–39.2 U of NR and 52–62 U of NiR with treatment of 4–12 mg pendimethalin/l (Table 2). The kinetics of NR activity showed a Vmax value of 25 and 33 nmol nitrite formed/mg protein.minute and same Km value of 3.3 mmol/l for pendimethalin treated and untreated control cultures (Table 3). The Vmax value for NiR activity was 166 and 125 nmol nitrite decreased/mg protein.minute and the Km value of 40 mmol/l for both control and pendimethalin treated cultures remained same (Table 3).
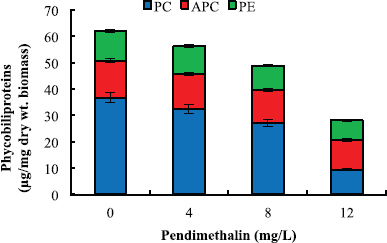 | Figure 5: Effect of pendimethalin (mg/l) on phycobiliprotein content of D. muscorum on 6 days. Data presented in figure of each pigment in presence of different concentrations of pendimethalin as well as of control are different from each other at 5% level of significance (p < 0.05). [Click here to view] |
3.4.2. Effect of Pendimethalin on Ammonium Uptake and Its Assimilation
Pendimethalin (4–12 mg/l) treated cells incubated in 200 µM ammonium for 6 hours decreased the uptake of ammonium to 0.65–0.92 µmol/mg protein compared to 1.02 µmol uptake by control culture (Table 2). A Lineweaver–Burk double reciprocal plot for kinetics of ammonium uptake showed a Vmax value of 0.33 and 0.8 µmol ammonium taken up mg/protein.minute and Km value of 11.1 and 33.3 mmol ammonium/l for pendimethalin treated and untreated control cultures, respectively (Table 3). Pendimethalin decreased the GS activity of test organism to 7–10 U (Table 3). The kinetic studies of GS showed Vmax of 9.09 and 16.6 µmol ɤ glutamate hydroxamate formed/mg protein.minute and same Km value of 50 mmol/l for pendimethalin treated and untreated control cultures, respectively (Table 3).
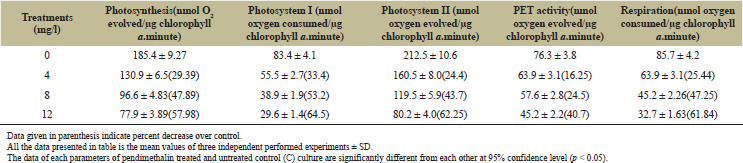 | Table 1: Effect of pendimethalin on rate of photosynthesis, photochemical activities, and respiration of D. muscorum after 12 hours treatment. [Click here to view] |
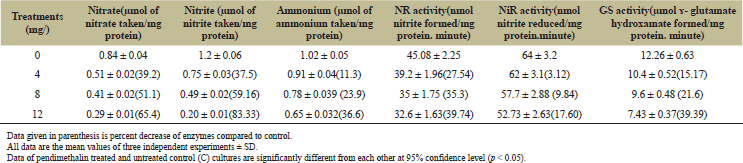 | Table 2: Pendimethalin effect on nitrogen source uptake and assimilation enzymes of D. muscorum after 12 hours treatment. [Click here to view] |
4. DISCUSSION
Diazotrophic cyanobacteria are well-known natural nitrogen fixing microbes enormously contributing to the fertility of the soil ecosystem. This necessitates to examine the effect of agricultural pollutants such as pesticides including herbicides on their growth, development, and nitrogen assimilation [4,11]. The cyanobacterium D. muscorum employed during the present study is commonly occurring in the paddy fields of Punjab, India [26]. Pendimethalin selected for this study is employed by the farmers on a large scale in agri-ecosystems such as rice, cotton, soya bean, and tobacco to kill annual grasses and many broadleaf weeds [46–48]. A decrease in growth of the organism was observed with increase in the herbicide as evidenced from percent inhibition in growth and increased generation time (Fig. 1). The test microorganism failed to grow in 20 mg pendimethalin/l and the culture showed above 97% cell lyses as reported in other studies [25,27,49].
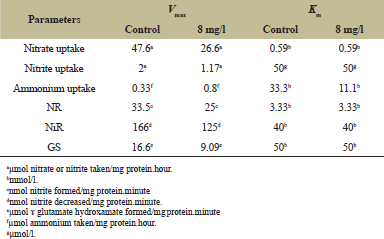 | Table 3: Pendimethalin effect on nitrogen source uptake kinetics. [Click here to view] |
The tolerance level and growth performance of any microorganism are the manifestation of prevailing environmental conditions. The inhibitory effect of herbicide on growth of cyanobacteria rely on the nature of microorganism and herbicide used [14–16,27]. Pendimethalin exhibited a dose-dependent effect on cellular activities which ultimately led to decrease in dry biomass and protein content. Treatment with pendimethalin (4–12 mg/l) decreased dry biomass and protein content by 23%–59% (Fig. 3). Similar types of reports on other cyanobacteria with other herbicides are available in the literature [21,22,27]. The inhibition in growth of the cyanobacterium in presence of different concentrations of pendimethalin may be due to its interaction with cells structural and functional membranous proteins or other component of photosynthetic machinery such as photo pigments, PSs or interaction with nitrogen assimilation [15,50–52].
Since most of the earlier studies were targeted either towards carbon or nitrogen assimilation, the exact mechanism by which pendimethalin exhibits its toxic effect on cyanobacteria is lacking. Thus, the effect of pendimethalin on individual components of carbon and nitrogen parameters was targeted in this study. Pendimethalin caused a dose-dependent decrease in chlorophyll a, carotenoid, phycocyanin, allophycocyanin, and phycoerythrin content of the test cyanobacterium. Chlorophyll a and carotenoid contents were decreased by 8%–86% (Fig. 4). The contents of phycocyanin, allophycocyanin, and phycoerythrin were decreased in range 25%–84%, 21%–48%, and 24%–60%, respectively (Fig. 5). The reduction in phycobiliproteins with pendimethalin treatment might be due to their direct exposure to herbicide because of their extrinsic localization on the thylakoid membrane [27]. The decrease in carotenoid content in the presence of pendimethalin could be attributed to its protective role against stress caused by the herbicide [53]. The reduction in growth of D. muscorum in presence of pendimethalin may also be as a result of degradation of photosynthetic pigments by pendimethalin or due to its interference in synthesis of these pigments.
The role of photosynthesis and respiration is extremely crucial in microbes and plants due to generation of reducing power NADPH/NADH and ATP. The rates of photosynthesis and respiration in this cyanobacterium were clearly compromised upon exposure to pendimethalin (Table 1). Treatment of the organism with 4–12 mg pendimethalin/l caused dose dependent reduction in rate of photosynthesis by 29%–58%. Pendimethalin may block energy transfer from light harvesting phycobiliproteins to PS II due to the blockage of PET, thereby reducing photophosphorylation and hence the photosynthetic oxygen evolution as studied in other cyanobacteria with other herbicides [15,54]. To substantiate this, the interaction of pendimethalin with PSs and whole PET activity of test microorganism was studied. The results of photochemical activity of test organism revealed that pendimethalin decreased the activity of PS-I, PS-II, and whole PET in the range 33%–64%, 24%–62%, and 16%–41%, respectively (Table 1). These results confirmed that pendimethalin inhibits the energy transfer from phycobiliproteins to PS-II by blocking of PET at or near PS-I resulting in a low rate of photosynthesis in test organism. Similar to our observations, herbicides such as ioxynil, DCMU, and terbutryn have also been shown to affect PS-II in other photoautotrophic cyanobacteria [55–57]. Herbicides block electrons from sites QA to QB by binding with the D1 protein subunit of QB of PS-II subunits of cyanobacteria [56,57].
The inhibitory effect of pendimethalin on respiration of the test organism was also observed to be dose-dependent. The decrease in the rate of respiration of the test organism ranged between 25% and 62% with treatment of 4–12 mg pendimethalin/l (Table 1). These observations revealed that together with photosynthesis, respiration of the organism was also affected. Thus, additional studies are required to know the effect of herbicide on respiratory electron transport chain of cyanobacteria. Similar observations have been reported for other cyanobacteria with different herbicides [14]. Bensulfuron methyl, butachlor, and dimethoate have also been reported to decrease the rate of respiration in Nostoc sp. [5].
Cyanobacteria use nitrate, nitrite, and ammonium for their growth and development [58]. This necessitates to study the effect of pendimethalin on nitrogen uptake and its assimilating enzymes. Pendimethalin (4–12 mg/l) treated cultures exhibited a decrease in nitrate and nitrite uptake by 39%–65% and 37%–83%, respectively (Table 2). A Lineweaver–Burk double reciprocal plot for kinetics of nitrate and nitrite uptake showed different Vmax and similar Km values for pendimethalin treated and untreated control cultures (Table 3). The unchanged value of Km for nitrate and nitrite uptake by pendimethalin treated and untreated control cultures of test microorganism gives an indication that herbicide did not have any inhibitory effect on the affinity of nitrate and nitrite uptake system. It is well known that nitrogen (nitrate and nitrite) uptake in photosynthetic cyanobacteria is an energetically light dependent process controlled by a set of nitrate/nitrite transporters [59,60]. The decrease in Vmax for pendimethalin treated compared to control cultures points towards the interference of herbicide with photosynthetically generated energy. The results of this study corroborate to our earlier report on this organism with another herbicide, pretilachlor [26].
Pendimethalin exhibited dose-dependent decrease in NR and NiR activities by 27%–40% and 3%–18%, respectively (Table 2). The results of kinetics of NR activity showed similar Km value of 3.33 mmol/l and different Vmax value of 25 and 33 nmol nitrite formed/mg protein.minute for pendimethalin treated and untreated control cultures (Table 3). The same is true for NiR activity as well, where a value of 166 and 125 nmol nitrite decreased/mg protein.minute as Vmax was observed for control and pendimethalin treated cultures, respectively. The Km value of 40 mmol nitrate/l for both control and pendimethalin treated cultures remained same (Table 3). These results showed that pendimethalin interacted in non-competitive manner with both these enzymes in this microorganism. It is thus clear that pendimethalin caused a reduction in uptake of nitrate and nitrite as well as the activities of NR and NiR. This is because of less available energy as result of pendimethalin inhibition in photosynthetically generated reduced ferredoxin that donates electrons for nitrogen assimilation in cyanobacteria [60,61]. This was confirmed from the low rate of photosynthesis, photochemical activities, and even low respiration of the test microorganism in presence of pendimethalin (Table 1). Many reports related to progressive decrease in activity of NR and NiR in cyanobacteria Anabaena sp, Aulosira fertilissima, Fischerella muscicola, N. muscorum, and Westiellopsis prolifica by pesticides carbaryl, endosulfan, malathion, pretilachlor, trichlorfon, and tebuconazole have also supported our observation [26,52,62].
The effect of pendimethalin on ammonium uptake revealed the same pattern as of nitrate and nitrite uptake. Pendimethalin (4–12 mg/l) treatment decreased the uptake of ammonium by 11%–37.6%. The results of ammonium uptake kinetic studies revealed different value of Vmax (0.33 and 0.8 µmol ammonium taken up mg/protein.minute) as well as Km (11.1 and 33.3 mmol ammonium/l) for pendimethalin treated and control culture, respectively (Table 2 and 3). These results point towards the interaction of pendimethalin with the affinity of ammonium uptake transporter system of the cyanobacterium. It has been reported that PII signalling regulates ammonium uptake in Synechocystis sp. strain PCC 6803 through Amt1 ammonium permease similar to the known in bacterium Escherichia coli [58]. Thus, it appears that pendimethalin interacted with ammonium uptake regulator permease in this organism which needs further investigation. Ammonium produced in cyanobacteria through the activity of NR and NiR or taken up directly from the environment is further incorporated into the carbon skeleton in the sequential action of regulatory enzyme GS through the glutamine synthetase-glutamine-2-oxo-glutarate aminotransferase cycle [63]. Pendimethalin also decreased the GS activity of test organism by 15%–39% (Table 2). Similar to our results, earlier studies also reported effect on the GS activity in cyanobacteria by bagalol, mancozeb, thiodan, and phorate [64]. The reduction in GS activity by pendimethalin is correlated with the less reduction of nitrate and nitrite by the NR or NiR within the cells. The kinetics of GS showed different Vmax (9.09 and 16.6 µmol ɤ glutamate hydroxamate formed/mg protein.minute) and similar Km (50 mmol/l) values for pendimethalin treated and untreated cultures (Table 3). These results indicate that pendimethalin directly interacted with GS in a non-competitive manner to lower down its activity. Our results were supported by other studies where other herbicides also inhibited the GS activity in other cyanobacteria [24,26,62].
5. CONCLUSION
The overall results assert beyond doubt that pendimethalin negatively affected both carbon and nitrogen assimilation of cyanobacterium D. muscorum. The toxicity caused by iterated use of the pendimethalin can pose more serious negative impacts on the growth and development of farmer’s eco-friendly paddy field diazotrophic cyanobacteria. Thus, there is a need to be cautious during the application of such herbicide in agricultural fields to protect the benevolent microbes that are the key component in maintaining the integrity and fertility of soil for sustainable agriculture.
6. ACKNOWLEDGMENTS
The authors are grateful to Head and Coordinator, FIST of DST, DRS SAP-II of UGC, Department of Botany, Punjabi University, Patiala, for providing laboratory and other infrastructure facilities. Mr Manzoor Ahmad Bhat acknowledges UGC, New Delhi for financial assistance under MANF-SRF research scheme.
7. AUTHOR’S CONTRIBUTION
DPS and JISK: Conceived the idea and designed the experiments. MAB: Performed the experiments and wrote the manuscript. DPS, JISK and RSS: Corrected, edited, and finalized the MS. All the authors contributed equally in this MS and agree to submit it for publication.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
REFERENCES
1. Chauhan BS. Grand challenges in weed management. Front Agron 2020;1:3; doi:10.3389/fagro CrossRef
2. Ranga Rao GV, Kumar BR, Sahrawat KL, Wani SP. Integrated pest management (IPM) for reducing pesticide residues in crops and natural resources. In: Chakravarthy AK, (eds.). New horizons in insect science: towards sustainable pest management. Springer, New Delhi, India, pp 397–412. CrossRef
3. Gharde Y, Singh PK, Dubey RP, Gupta PK. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot 2018;107:12–8. CrossRef
4. Kaushik MS, Kumar A, Abraham G, Singh PK. Tolerance of wetland rice field’s cyanobacteria to agrochemicals in cultural condition. Biocatal Agric Biotechnol 2018;13:236–43. CrossRef
5. Chen Z, Juneau P, Qiu B. Effects of three pesticides on the growth, photosynthesis and photoinhibition of the edible cyanobacterium Ge-Xian-Mi (Nostoc). J Aquat Toxicol 2007;81:256–65. CrossRef
6. Aktar MW, Sengupta D, Chowdhury A. Impact of pesticide use in agriculture: their benefits and hazards. Interdiscip Toxicol 2009;2:1–12. CrossRef
7. Geiseller D, Scow KM. Long term effects of mineral fertilizers on soil microorganisms-a review. Soil Biol Biochem 2014;75:54–63. CrossRef
8. Vermaas WJF. Photosynthesis and respiration in cyanobacteria. In: Encyclopedia of life sciences. John Wiley & Sons Ltd, London, UK, pp 1–7, 2001. Available via www.eLS.net CrossRef
9. Singh RK, Tiwari SP, Rai AK, Mohapatra TM. Cyanobacteria: an emerging source for drug discovery. J Antibiot 2011;64(6):401–12. CrossRef
10. Dash NP, Kaushik MS, Kumar A, Abraham G, Singh PK. Toxicity of biocides to native cyanobacteria at different rice crop stages in wetland paddy field. J Appl Phycol 2017;30:483–93. CrossRef
11. Yadav S, Rai R, Shrivastava AK, Singh PK, Sen S, Chatterjee A, et al. Cyanobacteria biodiversity and biotechnology: a promising approach for crop improvement. In: Prasad R, Gill, SS, Tuteja N, (eds.). Crop improvement through microbial biotechnology. Elsevier, Amsterdam, Netherlands, pp 195–219, 2018. CrossRef
12. Chittapun S, Limbipichai S, Amnuaysin N, Boonkerd R, Charoensook M. Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: a pot experiment. J Appl Phycol 2018;30:79–85. CrossRef
13. Chittora D, Meena M, Barupal T, Swapnil P, Sharma K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Rep 2020;22:100737; doi:10.1016/j.bbrep.2020.100737 CrossRef
14. Singh DP, Khattar JIS, Kaur G, Singh Y. Toxicological impact of herbicides on cyanobacteria. Ann Res Rev Biol 2016;9:1–39. CrossRef
15. Swatch GK, Singh DP, Khattar JIS, Mohapatra PK. Interaction of pretilachlor with PS-II activity of the cyanobacterium Desmonostoc muscorum PUPCCC 405.10. J Basic Microbiol 2020;60(6):532–42. CrossRef
16. Lam SS, Peng W, Sonne C. Support Austria’s glyphosate ban. Science 2020;367(6475):257–68. CrossRef
17. Tripathi A, Sundaram S, Tripathy BC, Tiwari BS, Rahman A. Activity and stability of herbicide treated cyanobacteria as potential biomaterials for biosensors. Res J Environ Sci 2011;5:479–85. CrossRef
18. Ahmad MI, Zafeer MF, Javed M, Ahmad M. Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci Rep 2018;8(1):1–9. CrossRef
19. El-Nahhal Y, El-Hams S. Effects of bromacil, malathion and thiabendazole on cyanobacteria mat growth. Int J Appl Sci Res Rev 2017;4:1–9; doi:10.21767/2349-7238.100053 CrossRef
20. Drzyzga D, Lipok J. Glyphosate dose modulates the uptake of inorganic phosphate by freshwater cyanobacteria. J Appl Phycol 2018;30:299–309. CrossRef
21. Wu L, Qiu Z, Zhou Y, Du Y, Liu C, Ye J, et al. Physiological effects of the herbicide glyphosate on the cyanobacterium Microcystis aeruginosa. Aquat Toxicol 2016;178:72–9. CrossRef
22. Ahmed H, Singh V, Panday A, Kannaujiya VK, Rajneesh, Pathak J, et al. Combined effects of UV radiation, photosynthetically active radiation and 2, 4-dichlorophenoxyacetic acid on pigmentation, proteins and antioxidant enzymes in the cyanobacterium Scytonema geitleri strain HKAR-12. J.S.M. Environ Sci Ecol 2017;5(3):1048.
23. Mounika T, Asheervadam T, Malathi T, Rao BD. Effect of herbicides on two species of fresh water cyanobacteria. Int J Biol Res 2018;3:327–31.
24. Singh DP, Khattar JIS, Kaur K, Sandhu BS, Singh Y. Toxicological impact of anilofos on some physiological processes of a rice field cyanobacterium Anabaena torulosa. Toxicol Environ Chem 2012;94:1304–18. CrossRef
25. Singh DP, Khattar JIS, Kaur M, Kaur G, Gupta M, Singh Y. Anilofos tolerance and its mineralization by the cyanobacterium Synechocystis sp. strain PUPCCC 64. PLoS One 2013;8(1):53445. CrossRef
26. Singh DP, Khattar JIS, Kaur G, Gupta M, Singh Y, Gulati A. Effect of pretilachlor on nitrogen uptake and assimilation by the cyanobacterium Desmonostoc muscorum PUPCCC 405.10. Acta Physiol Plant 2015;37(9):1–14. CrossRef
27. Kumar J, Patel A, Tiwari S, Tiwari SA, Srivastava PK, Prasad SM. Pretilachlor toxicity is decided by discrete photo-acclimatizing conditions: physiological and biochemical evidence from Anabaena sp. and Nostoc muscorum. Ecotoxicol Environ Saf 2018;156:344–53. CrossRef
28. Ma JY, Wang SF, Wang PW, Ma LJ, Chen XL, Xu RF. Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotoxicol Environ Saf 2006;63:456–62. CrossRef
29. Lin JC, Qiao X, Haung L, Wang D. Evolution of toxicity upon hydrolysis of fenoxaprop-p-ethyl. J Agric Food Chem 2007;55:7626–9. CrossRef
30. Aktar M, Sengupta D, Purkait S, Chowdhury A. Vertical migration of some herbicides through undisturbed and homogenized soil columns. Interdiscip Toxicol 2008;1(3–4):231–335. CrossRef
31. Pinto AP, Serrano C, Pires T, Mestrinho E, Dias L, Teixeira DM, et al. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci Total Environ 2012;435:402–10. CrossRef
32. Zhang CC, Zhou CZ, Burnap RL, Peng L. Carbon/nitrogen metabolic balance: lessons from cyanobacteria. Trends Plant Sci 2018;223(12):1116–30. CrossRef
33. Safferman RS, Morris ME. Growth characteristics of the blue green algal virus LPP-1. J Bacteriol 1964;88:771–5. CrossRef
34. Allen MB, Arnon DI. Studies on nitrogen fixing blue green algae. I. Growth and nitrogen fixation by Anabaena cylindrical lemm. Plant Physiol 1955;30:366–72. CrossRef
35. Singh DP, Khattar JIS, Rajput A, Chaudhary R, Singh R. Hyperproduction of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. PLoS One 2019;14(9):0221930. CrossRef
36. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–75. CrossRef
37. Holm G. Chlorophyll mutations in barley. Acta Agric Scand 1954;4:457–71. CrossRef
38. Myers J, Kratz WA. Relationship between pigment content and photosynthetic characteristics in a blue green algae. J Gen Physiol 1955;39:11–21. CrossRef
39. Kaushal S, Singh Y, Khattar JIS, Singh DP. Phycobiliprotein production by a novel cold desert cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1. J Appl Phycol 2017;29:1819–27. CrossRef
40. Bennett A, Bogorad L. Complementary chromatic adaption in a filamentous blue-green alga. J Cell Biol 1973;58:419–35. CrossRef
41. Robinson JBD, Allen de MV, Gacoka P. The determination of soil nitrates with a brucine reagent. Analyst 1959;84:635–40. CrossRef
42. Nicholas DJ, Nason A. Determination of nitrate and nitrite. Methods Enzymol 1957;3:981–4. CrossRef
43. Solarzano L. Determination of ammonia in natural waters by the phenol hypochlorite method. Limnol Oceanogr 1969;14:799–801. CrossRef
44. Herrero A, Flores E, Guerrero MG. Regulation of nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119 and Nostoc sp. strain 6719. J Bacteriol 1981;145:175–80. CrossRef
45. Shapiro BM, Stadtman ER. Glutamine synthetase of Escherichia coli. In: Tabor H, Tabor CW, (eds.). Methods of enzymology. Academic Press, New York, NY, Vol. 17A, pp 910–22, 1970. CrossRef
46. Lee LJ, Ngim J. A first report of glyphosate-resistant goosegrass [Eleusine indica (L) Gaertn] in Malaysia. Pest Manag Sci 2000;56(4):336–9. CrossRef
47. Saleh MK, Oyinbo O. Rice production under different weed management technologies adopted by rice farmers in Katsina State, Nigeria. J Agric Ext 2017;21(1):149–52. CrossRef
48. Pan L, Yu Q, Han H, Mao L, Nyporko A, Fan L, et al. Aldo-keto reductase metabolizes glyphosate and confers glyphosate resistance in Echinochloa colona. Plant Physiol 2019;181(4):1519–34. CrossRef
49. Ibrahim WM, Karam MA, El-Shahat RM, Adway AA. Biodegradation and utilization of organophosphorus pesticide malathion by cyanobacteria. Biomed Res Int 2014;2014:1–6; doi:10.1155/2014/392682 CrossRef
50. Rajendran UM, Kathirvel E, Narayanaswamy A. Effects of a fungicide, an insecticide, and a biopesticide on Tolypothrix scytonemoides. Pestic Biochem Physiol 2007;87:164–71. CrossRef
51. Kumar JIN, Kumar RN, Bora A, Amb MK. Photosynthetic, biochemical and enzymatic investigation of Anabaena fertilissima in response to an insecticide hexachloro-hexahydromethano-benzodioxathiepine-oxide. J Stress Physiol Biochem 2009;5:4–12.
52. Kumar N, Bora A, Kumar R, Amb MK. Differential effects of agricultural pesticides endosulfan and tebuconazole on photosynthetic pigments, metabolism and assimilating enzymes of three heterotrophic, filamentous cyanobacteria. J Biol Environ Sci 2012;6:67–75.
53. Ware MA, Belgio E, Ruban AV. Photoprotective capacity of non-photochemical quenching in plants acclimated to different light intensities. Photosynth Res 2015;126:261–74. CrossRef
54. Shikha, Singh DP. Influence of glyphosate on photosynthetic properties of wild type and mutant strains of cyanobacterium Anabaena doliolum. Curr Sci 2004;86:571–6.
55. Bagchi SN, Pistorius EK, Michel KP. A Synechococcus sp. PCC7942 mutant with a higher tolerance towards bentazone. Photosynth Res 2003;75:171–82. CrossRef
56. Zimmermann K, Heck M, Frank J, Kern J, Vass I. Herbicide binding and thermal stability of photosystem II isolated from Thermosynechococcus elongatus. Biochem Biophys Acta 2006;1757:106–14. CrossRef
57. Broser M, Glöckner C, Gabdulkhakov A, Guskov A, Buchta J. Structural basis of cyanobacterial photosystem II inhibition by the herbicide terbutryn. J Biol Chem 2011;286:15964–72. CrossRef
58. Watzer B, Spat P, Neumann N, Koch N, Sobotka R, Macek B, et al. The signal transduction protein PII controls ammonium, nitrate and urea uptake in cyanobacteria. Front Microbiol 2019;10:1428; doi:10.3389/fmicb.2019.01428 CrossRef
59. Lochab S, Kumar PA, Raghuram N. Molecular characterization of nitrate uptake and assimilatory pathway in Arthrospira platensis reveals nitrate induction and differential regulation. Arch Microbiol 2014;196:385–94. CrossRef
60. Maeda S, Murakami A, Ito H, Tanaka A, Omata T. Functional characterization of the FNT family nitrite transporter of marine picocyanobacteria. Life 2015;5:432–46. CrossRef
61. Flores E, Erias JE, Rubio LM, Herrero A. Photosynthetic nitrate assimilation in cyanobacteria. Photosynth Res 2005;84:117–33. CrossRef
62. Mishra SK, Dwivedi N. Effects of insecticides malathion on growth, biochemical compounds and some enzymes of Fischerella muscicola NDUPC001. Int J Adv Sci Res Mang 2019;4:12–5.
63. Muro-Pastor MI, Reyes JC, Florencio J. Ammonium assimilation in cyanobacteria. Photosynth Res 2005;83:135–50. CrossRef
64. Debnath M, Mandal NC, Ray S. Effect of fungicides and insecticides on growth and enzyme activity of four cyanobacteria. Indian J Microbiol 2012;52:275–80. CrossRef