1. INTRODUCTION
The emergence of multi-drug resistant bacteria (MDR) over the last years constitutes nowadays a major issue of national and international concern. This emergence of antimicrobial resistance is due to incorrect and irrational use of antibiotics [1]. Bacterial strains have developed resistance to most useful antibiotic classes by using different strategies such as elimination by efflux pumps or reduction in outer membrane permeability, production of antibiotic-modifying or -hydrolyzing enzymes, and mutation in antibiotic targets [2,3]. The discovery of new antimicrobial agents with a new mechanism of action represents a major challenge.
Medicinal herbs and their related products are usually employed in developing countries to treat a wide variety of diseases.
Essential oils (EOs) are complex odoriferous mixtures of volatile and scent-laden compounds like monoterpenes, sesquiterpenes, and their derivatives such as aldehydes and phenols [4]. The composition differs between species and seasons of the year [5]. They are known for their antibacterial, antifungal, antiviral, insecticidal, and antioxidant properties [6]. Some studies also showed that some EOs may restore the antibacterial efficacy of antibiotics on resistant strains and represent an attractive and commercially interesting alternative in fighting these multi-resistant bacteria [7].
Cymbopogon genus belongs to the Poaceae family, whose species are widely distributed in the tropical and subtropical regions of Africa, Asia, and America where they are used as medicinal drugs in many countries for various diseases [8]. Cymbopogon giganteus (CG) is a grass, which can grow up to 2–3 m, spread in tropical Africa. Several extracts of CG have been used in traditional medicine: EO was used to treat boils, stomach pain, and toothache [9] while aqueous decoction of leaves was used to treat headaches, common cold, conjunctivitis, sickling, cellular diseases, or for tranquilizing epileptic seizures [10]. This EO, also called “Ahibero EO” or “Citronelle de Madagascar” is widely commercialized, most often in external use, for its antiseptic and antifungal properties. The composition of EOCG has been previously investigated. Limonene and p-menthane derivatives were detected as the main components of this EO from various origins [11,12]. Many studies showed the good antimicrobial properties of EOCG against a wide range of reference strains [11.13], but not on clinical strains nor in combination with antibiotics.
The main objective of this study was to analyze the composition of CGEO originating from Benin and collected in the Parakou area and evaluate its antibacterial properties alone or in combination with antibiotics against 11 clinical multi-resistant bacteria and four references strains as well as to determinate its eventual cytotoxicity.
2. MATERIALS AND METHODS
2.1. Antimicrobial Assays
2.1.1. Culture media and bacterial strains
The 11 clinical and references strains tested are listed in Table 1. Minimal inhibitory concentrations (MICs) were determined by broth microdilution method following Clinical and Laboratory Standards Institute recommendations in cation-adjusted Mueller-Hinton broth (CA-MHB, Becton, Dickinson and Company, Franklin Lakes, NJ). Susceptibility was categorized according to the European Committee on Antibiotic Susceptibility Testing (EUCAST version 6.0) interpretive criteria (http://www.eucast.org/clinical_breakpoints/; assessed 12 December 2016). All organisms were maintained in CA-MHB containing 20% (v/v) glycerol at −80°C. Before testing, the suspensions were transferred to Mueller Hinton Agar and aerobically grown overnight at 37°C.
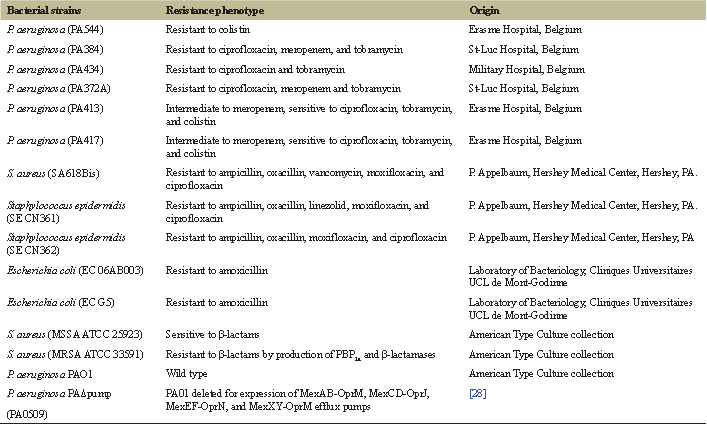 | Table 1: Bacterial strains used in this study. [Click here to view] |
2.1.2. Antibiotics
The following antibiotics were used as microbiological standards (with abbreviations and potencies shown in brackets): colistin sulfate (CST; 79.64%); ampicillin (AMP; 87.99%); oxacillin (OXA; 90%); amoxicillin (AMX; 90%) from Sigma-Aldrich (St Louis MO); moxifloxacin (MXF; 90%); ciprofloxacin (CIP; 85%) from Bayer, Leverkusen, Germany; tobramycin (TOB; 100%) from Teva, Wilrijk, Belgium; meropenem as Meronem (MEM; 74%) from AstraZeneca, Brussels, Belgium; linezolid (LZD; 100%) from Pfizer Inc., New York, and vancomycin (VAN; 100%) was obtained as VANCOCIN from GlaxoSmithKline, Belgium.
2.1.3. Determination of MIC and fractional inhibitory concentration indices (FICI)
The MIC was established using resazurin microdilution assay [14]. For this purpose, EOs were diluted to the highest concentration (1% v/v) with tween 80 (1% v/v) in MHB-CA to enhance EO solubility and then multi-fold dilutions were made to get a concentration range from 1% down to 0.013% v/v in 50 μl of sterile MHB-CA. An aliquot of 50 μl of the inoculum (106 cfu/ml) was added to each well, which contains diluted EO and/or antibiotic. Positive and negative growth controls were performed for each plate. The plates were incubated aerobically at 37°C for 16—20 hours. After that, 30 μl of a 0.02% resazurin (Sigma-Aldrich, St Louis MO) aqueous solution was added in each well, which allows to easily identify conditions in which bacteria had grown (metabolization of blue resazurin into pink resorufin). The MIC was considered as the lowest concentration where the well did not change color to pink. The checkerboard synergy test was done in 96-well plates by multi-fold dilutions of antibiotics horizontally with the highest concentration of 2 × MIC while EOCG was diluted vertically with the highest concentration of 1%. The FICI used to determine the checkerboard test was obtained by calculating the sum of the FICs using this formula: FICI = FIC A + FIC E.
FIC A is the MIC of antibiotic in combination/MIC of antibiotic alone while FIC E is the MIC of EO in combination/MIC of EO alone [15]. Different results can be observed: synergistic for FICI ≤ 0.5, additive for 0.5 < FICI ≤ 1, indifferent for 1 < FICI ≤ 4, and antagonistic for FICI > 4 according to the European Committee for Antimicrobial Susceptibility Testing [16]. All experiments were done in triplicate.
2.2. Plants
2.2.1. Collection
CG Chiov was collected in Parakou areas (9°20′N, 2°37′E) in November 2016. Crops were identified by Herbier National du Bénin (Université Abomey-Calavi) where a voucher specimen was deposited under number AA6680/HNB.
2.2.2. EO and extraction
200 g air-dried leaves were submitted to hydro-distillation using a Clevenger steam-distillation apparatus for 3 hours. EOs were stored at 4°C prior use. The yields were calculated according to the starting weight of the plant material before hydro-distillation (expressed as percentage w/w of the dry vegetable material).
2.2.3. GC/FID Analysis
Gas chromatography analysis was performed on a FOCUS GC (Thermo Finigan; Milan, Italy) equipped with a flame ionization detector and a DB-wax column (30 m × 0.25 mm; 0.25 μm film thickness; Agilent, Palo Alto, CA). Carrier gas: helium in constant flow mode (1.3 ml/min) and the oven temperature program was: 5 minutes at 45°C, 45°C–250°C (3°C/minute) and 5 minutes at 250°C. 1 μl of EO diluted in TBME (1%) was injected at 230°C for the front inlet and at 260°C for detection. The split ratio was 1:50. Calculation of peak area percentage was performed by ChromCard (Interscience Technology) using the normalization method.
2.2.4. GC/MS analysis
The GC/MS (Trace GC 2,000 series Thermo Quest, Rodano, Italy) was interfaced with Trace MS (Thermo Quest) operating in the impact electronic mode at 70 eV and was equipped with DB-wax column (30 m × 0.25 mm; 0.25 μm film thickness; Agilent Palo Alto, CA). Carrier gas: helium in constant flow mode (1.3 ml/minute) and the oven temperature program was: 5 minutes at 45°C, 45°C–250°C (3°C/minute) and 5 minutes at 250°C. 1 μl of EO diluted in TBME (1%) was injected at 230°C for the front inlet in split-less mode. Mass spectra of the resulting peaks were analyzed and term-to-term compared with the NIST/EPA/NIH 98 library. The similarity score must be greater than 700. These spectra were also compared with a home-made mass spectra library of pure compounds under the same conditions. These identifications are also supported by comparison with literature and the GC retention times relative to a mixture of fatty acid methyl esters “C5–C27” on the same DB-wax column [14].
2.3. Cytotoxicity Assay
The evaluation of cytotoxicity was performed using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphényltetrazolium bromide] (Sigma-Aldrich, St Louis MO) test [17], which determined the cell viability by measurement of metabolic activity.
WI38 cells cultured in DMEM medium (5 × 103 cells/ml) were seeded into 96-well plates (180 μl/well) and were incubated for 24 hours. After that, 20 μl of EOs solutions in DMEM medium were added to each well in concentrations ranging from 1–0.008 mg/ml. The 96-well plates were then incubated for 72 hours. Cytotoxicity of Tween 80, which was used to enhance the dispersion of EO in the culture medium was also tested and found to be not cytotoxic at the highest concentration of 0.1 mg/ml. Camptothecin (Sigma-Aldrich, St Louis MO) was used as a positive control. After 72 hours, the medium was rejected and 100 μl of MTT solution in RPMI medium (0.3 mg/ml) was added to each well for 45 minutes of incubation time. After removal of the MTT solution, 100 μl of DMSO was added in each well to dissolve formazan and the optical density was measured at 570 nm with a reference wavelength at 620 nm using a spectrophotometer (SpectraMax-Molecular Devices, Berkshire, UK). All assays were done in triplicates. The IC50 values were obtained with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
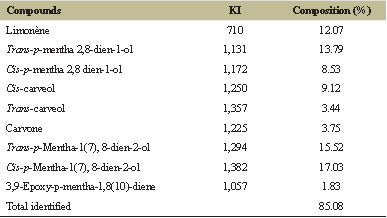 | Table 2. Percentage composition of EOCG obtained by hydrodistillation. [Click here to view] |
3. RESULTS AND DISCUSSION
EO extracted from air-dried leaves of a Beninese sample of C. giganteus was obtained with 0.57% yield. Bassole et al. [11,18,19] reported similar yields: 0.5%, 0.52%, and 0.6%, respectively, with the air-dried leaves of the same species from Burkina-Faso and Benin. The GC-FID and GC-MS analyses (Table 2) allowed to identify about 85% of the composition of this EO. Sixteen components were detected with cis-p-mentha-1(7), 8-dien-2-ol (17.03%), trans-p-mentha-1(7),8-dien-2-ol (15.52%), trans-p-mentha 2,8 dien-1-ol (13.79%), limonene (12.07%), cis-carveol (9.12%), and cis-p-mentha 2,8 dien-1-ol (8.53%) as major constituents. Comparison of the compositions of our sample of EOCG and those described in the literature (from Benin or other countries) showed mostly quantitative differences in major constituents. Indeed, Kpoviessi et al. [20] obtained cis-p-mentha-1(7),8-dien-2-ol (8.9%), trans-p-mentha-1(7),8-dien-2-ol (18.3%), trans-p-mentha 2,8 dien-1-ol (15.5%), limonene (8.3%), cis-carveol (7.3%), and cis-p-mentha 2,8 dien-1-ol (11.3%) while Alitonou et al. [18] reported cis-p-mentha-1(7),8-dien-2-ol (17.34%), trans-p-mentha-1(7),8-dien-2-ol (13.95%), trans-p-mentha 2,8 dien-1-ol (13.91%), limonene (19.33%), and cis-p-mentha 2,8 dien-1-ol (8.10%) as major compounds. However, Bassole et al. [11] reported composition of EOCG from Burkina-Faso with limonene as the dominant compound (42%). It has further been shown that the composition of EOCG (particularly, the limonene content) can differ with the extraction method [21] and collection period or place [20].
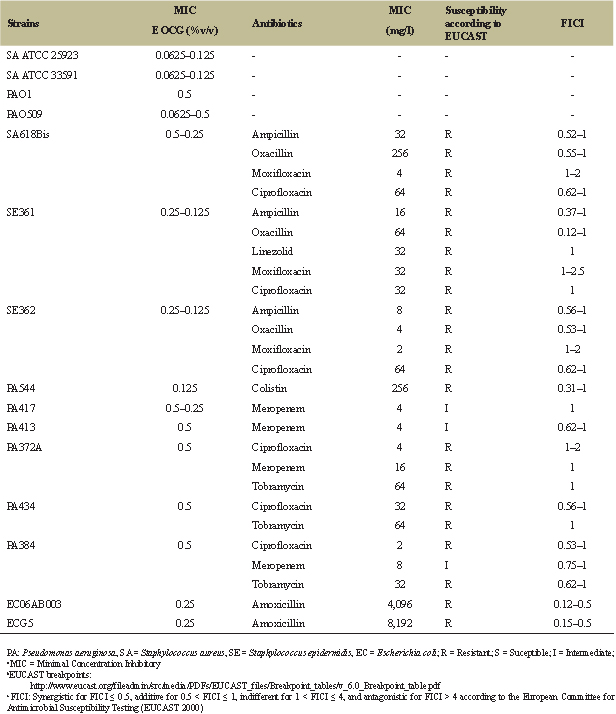 | Table 3: Effect of EOCG alone and in combination with different antibiotics. [Click here to view] |
Table 3 summarizes the MIC of the EOCG against four reference strains (Staphylococcus aureus MSSA ATCC 25923, S. aureus MRSA ATCC 33591, Pseudomonas aeruginosa PAO1, and its deletion mutant PAO509 (which does not express anymore the main RND multidrug efflux pumps) and a series of clinical isolates of S. aureus, S. epidermidis, P. aeruginosa, or Escherichia coli harboring different resistance phenotypes (see Table 1). The results indicate that EOCG was active with an MIC of about 0.0625%–0.125% v/v against S. aureus MSSA ATCC 25923 and S. aureus MRSA ATCC 33591, suggesting that this activity is not modified by the production of β-lactamases and modified PBP target (PBP2a). EOCG showed a greater effect on PA0509 (0.06% v/v) than PAO1 (0.5% v/v) suggesting its sensitivity to efflux pumps, as antibiotics. Against clinical isolates, EOCG showed activity against Gram-positive bacteria (0.125%–0.25% v/v) and Gram-negative bacteria (0.125%–0.5% v/v), whatever their resistance mechanisms. Antimicrobial activity of EOCG was also reported against other reference bacterial strains but not on clinical isolates strains. Indeed, Bassole et al. [11] reported an MIC of 2.1 mg/ml against S. aureus ATCC 9144 with lower activities for P. aeruginosa CRBIP19.249 (70 mg/ml) being the most resistant strain. Several studies have reported that P. aeruginosa is the least sensitive bacteria to EOs [22]. The direct antimicrobial activity observed could perhaps be explained, at least in part, by the presence of a consequent percentage (71.86%) of oxygenated compounds such as p-menthane derivatives, carvone, and carveol possessing antimicrobial activities [23]. Other essentials oils rich in p-mentha-1(7), 8-dien-2-ol which is the major component of EOCG have also demonstrated interesting antibacterial activities [24].
The checkerboard test was used to analyze the combination of EOCG with different classes of antibiotics against MDR bacteria. The improvement of antibacterial activity of these antibiotics can be due to a possible action of the EOCG on the mechanism of resistance to a specific antibiotic, an increased effect due to the combination of different actions on the bacteria, or an improvement of antibiotic concentration at the target site in the presence of EO. The results of FICI values obtained for combinations of EOCG and antibiotics are given in Table 3. We only analyzed combinations between EOCG and antibiotics to which our 11 different strains were resistant, or at least intermediate (ampicillin, oxacillin, vancomycin, linezolid, moxifloxacin, ciprofloxacin, meropenem, tobramycin, colistin, and amoxicillin, see Table 1).
Our result on the multi-resistant staphylococci clinical species (SA618Bis, SECN361, and SECN362) showed that the addition of EOCG does not lower the MIC of moxifloxacin.
All other tested combinations on Staphylococcus species (aureus and epidermidis) indicated some additive effect, except combinations with β-lactams drugs such as ampicillin and oxacillin on SECN361 showing a synergistic/additive effect (FICI: 0.12–1). Nevertheless, this synergistic activity is not sufficient to reverse resistance of this strain to these antibiotics (Table 4).
The results of checkerboard test on multi-resistant P. aeruginosa clinical species reveal in general, some additive effects except for combination with ciprofloxacin on PA372A where it can be considered that no effect was detected, and combination of colistin against PA544, a colistin-resistant strain which shows a synergistic/additive effect (FICI: 0.31–1) with a significant decrease of the MIC of colistin, but not enough to revert resistance (Table 4).
For amoxicillin-resistant E. coli clinical isolates, a synergistic effect was observed with amoxicillin on both strains, reversing resistance to amoxicillin on EC06A003, but not ECG5 (Table 4). Several studies also reported a synergistic action when an EO was combined with β-lactams drugs against E. coli clinical isolated strains [25] but it is the first time that it was shown with EOCG.
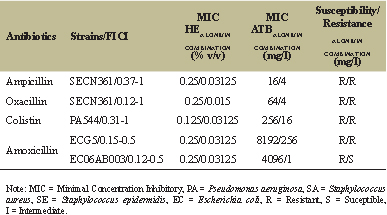 | Table 4: Impact of synergy on MIC of antibiotic and EOs. [Click here to view] |
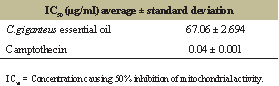 | Table 5: Cytotoxic effect of EOCG. [Click here to view] |
The mechanism of synergy between antibiotics and EOs is not elucidated yet and is difficult to establish due to the possible multitarget actions of EOs and their complex compositions [26]. Furthermore, EOs could act non-specifically, affecting membrane integrity which could improve the antibiotics uptake [27]. The best improvement of antibiotic efficacy was obtained with a combination of EOCG and amoxicillin on amoxicillin-resistant clinical isolates of E.coli reducing amoxicillin MIC from 32 to about 4,000-fold.
We also analyzed the cytotoxicity of EOCG on a human non-cancer fibroblast cell line (WI38) by the MTT tests and showed its low cytotoxicity (Table 5). This result was in accordance with the report of Kpoviessi et al. [20] who found an IC50 higher than 50 μg/ml on Chinese hamster ovary cells and the WI38 cell line.
4. CONCLUSION
In conclusion, we showed synergistic effects between EOCG and amoxicillin against two amoxicillin-resistant Escherichia coli strains, synergistic/additive effects between EOCG and colistin and oxacillin/ampicillin, respectively, against P. aeruginosa PA544 and Staphylococcus epidermidis SE361. However, in order to assess the potential of these combinations, further work will be essential to understand the mode of action of EOCG and/or its constituents, its toxicity/safety profile, and the molecular mechanisms of observed synergy.
ACKNOWLEDGMENT
The authors are grateful to Mr. Agbani (Botanist of University of Abomey-Calavi, Cotonou, Benin) for plant collections. We wish to thank Marie-Christine Fayt for skillful technical assistance in GC and all the members of the Pharmacognosy group and Cellular and Molecular Pharmacology Group from LDRI, UCL, Belgium, for helpful scientific advice and support.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
FUNDING
This work received funding from ARES-CCD (PRD project: VALTRAMED).
REFERENCES
1. WHO. Antimicrobial resistance: global report on surveillance. World Health Organization, 2014.
2. Olaitan AO, Morand S, Rolain J-M. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 2014;5:643. CrossRef
3. Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol 2014;12:35–48. CrossRef
4. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol 2008;46:446–75. CrossRef
5. Hajlaoui H, Mighri H, Noumi E, Snoussi M, Trabelsi N, Ksouri R, et al. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: a high effectiveness against Vibrio spp. strains. Food Chem Toxicol 2010;48:2186–92. CrossRef
6. Scazzocchio F, Garzoli S, Conti C, Leone C, Renaioli C, Pepi F, et al. Properties and limits of some essential oils: chemical characterisation, antimicrobial activity, interaction with antibiotics and cytotoxicity. Nat Prod Res 2016;30:1909–18. CrossRef
7. Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008;15:639–52.
8. Avoseh O, Oyedeji O, Rungqu P, Nkeh-Chungag B, Oyedeji A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules 2015;20:7438–53. CrossRef
9. Sahouo GB, Tonzibo Z, Boti B, Chopard C, Mahy J, N’guessan YT. Anti-inflammatory and analgesic activities: chemical constituents of essential oils of Ocimum gratissimum, Eucalyptus citriodora and Cymbopogon giganteus inhibited lipoxygenase L-1 and cyclooxygenase of PGHS. Bull Chem Soc Ethiop 2003;17. CrossRef
10. Adjanohoun ÉJ. Contribution aux études ethnobotaniques et floristiques en République Populaire du Bénin: Agence de coopération culturelle et technique, 1989.
11. Bassole IH, Lamien-Meda A, Bayala B, Obame LC, Ilboudo AJ, Franz C, et al. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 2011;18:1070–4. CrossRef
12. Boti JB, Muselli A, Tomi F, Koukoua G, N’Guessan TY, Costa J, et al. Combined analysis of Cymbopogon giganteus Chiov. leaf oil from Ivory Coast by GC/RI, GC/MS and 13 C-NMR. C Re Chim 2006;9:164–8. CrossRef
13. Jirovetz L, Buchbauer G, Eller G, Ngassoum MB, Maponmetsem PM. Composition and antimicrobial activity of Cymbopogon giganteus (Hochst.) chiov. essential flower, leaf and stem oils from Cameroon. J Essent Oil Res 2007;19:485–9. CrossRef
14. Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007;42:321–4. CrossRef
15. Bonapace CR, Bosso JA, Friedrich LV, White RL. Comparison of methods of interpretation of checkerboard synergy testing. Diagn Microbiol Infect Dis 2002;44:363–6. CrossRef
16. EUCAST. Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect 2000;6:503–8. CrossRef
17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63. CrossRef
18. Alitonou GA, Avlessi F, Sohounhloue DK, Agnaniet H, Bessiere JM, Menut C. Investigations on the essential oil of Cymbopogon giganteus from Benin for its potential use as an anti-inflammatory agent. Int J Aromather 2006;16:37–41. CrossRef
19. Menut C, Bessiere J, Samate D, Djibo A, Buchbauer G, Schopper B. Aromatic plants of tropical west Africa. XI. chemical composition, antioxidant and antiradical properties of the essential oils of three Cymbopogon species from Burkina Faso. J Essent Oil Res 2000;12:207–12. CrossRef
20. Kpoviessi S, Bero J, Agbani P, Gbaguidi F, Kpadonou-K, Poviessi B, et al. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J Ethnopharmacol 2014;151:652–9. CrossRef
21. Bossou AD, Ahoussi E, Ruysbergh E, Adams A, Smagghe G, De Kimpe N, et al. Characterization of volatile compounds from three Cymbopogon species and Eucalyptus citriodora from Benin and their insecticidal activities against Tribolium castaneum. Ind Crops Prod 2015;76:306–17. CrossRef
22. Dorman H, Deans S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000;88:308–16. CrossRef
23. Höferl M, Buchbauer G, Jirovetz L, Schmidt E, Stoyanova A, Denkova Z, et al. Correlation of antimicrobial activities of various essential oils and their main aromatic volatile constituents. J Essent Oil Res 2009;21:459–63. CrossRef
24. El-Kamali H, Hamza M, El-Amir M. Antibacterial activity of the essential oil from Cymbopogon nervatus inflorescence. Fitoterapia 2005;76:446–9. CrossRef
25. Yap PSX, Lim SHE, Hu CP, Yiap BC. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013;20:710–3. CrossRef
26. Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 2009;16:97–110. CrossRef
27. Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 2014;40:76–94. CrossRef
28. Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol 2007;189:7600–9. CrossRef