1. INTRODUCTION
Anthropogenic climate change and overexploitation of natural resources, stemming from the continuous growth of the global population and industrial development, encourage the adoption of more sustainable and environmentally friendly processes. Regarding this, biofuels have been a part of the agenda for mitigating emissions and decreasing the dependence on fossil fuels. The global bioethanol production has increased by 30% in the last decade, reaching a value of 111–120 billion liters in 2023 [1]. Such bioethanol production might generate up to 1 trillion liters of vinasse worldwide, considering that the production rate of vinasse is variable, depending on the raw material, distillation methods, and distillation bottom handling [1,2].
In Colombia, the sugar industry developed several sugarcane varieties that are among the most productive in the world, harvested mainly in the upper valley of Cauca River (the second largest in the country). In this region, 456 million liters of ethanol are produced annually, generating up to 1.4 trillion liters of vinasse, a waste stream that imposes environmental pressures in industrial areas, primarily due to its handling and disposal [3,4]. In Colombia, bioethanol lies on final molasses after sucrose crystallization (comprising 60–70% of the raw material), along with a portion of B molasses (30–40%) [4]. Vinasse can be characterized by the high chemical oxygen demand (COD) ranging from 70 to 120 gO2/L, an average pH of 4.0, significant amounts of suspended solids, and honey/malt-like taste and smell [5,6]. Due to its composition, vinasse is considered to be approximately 100 times more contaminating than domestic wastewater [5,6]. Colombia lacks specific legislation regarding vinasse treatment, reutilization, and disposal. Vinasse is commonly employed for fertigating sugarcane, avoiding direct discharges into water bodies, but such application in improper doses may lead to lixiviation of high-organic-content liquids, soil salinization, and ion leaching, as remarkable environmental risks [3,4].
In contrast to the leading bioethanol producer in Latin America (Brazil), in Colombia, lower volumes of vinasse are produced by following an evaporation process to facilitate handling and transportation. Locally, 1–3 l of concentrated vinasse is generated per liter of anhydrous ethanol. The composition of this concentrated vinasse ranges between 20 and 50 °Brix, with high levels of potassium (0.6–3.0 kg K2O/m3), nitrogen (0.57–1.2 kg/m3), and phosphorus (0.1–0.34 kg/m3) [4]. The evaporation of dilution water in vinasse is difficult; hence, vinasse is reused in biotechnological applications such as composting or anaerobic digestion, making their subsequent utilization more challenging [7]. In addition, the color of vinasse is of huge concern because some dyes are not only recalcitrant to biodegradation but are also inhibitors of biological activities [8,9].
Since fertigation of sugarcane crops may lead to soil saturation and contamination of water bodies, several methods have been explored for the treatment and utilization of vinasse, such as photocatalytic oxidation with ultraviolet light [10,11], the Fenton process based on the combination of iron salts with hydrogen peroxide, and the oxidation of recalcitrant compounds like melanoidins with ozone, all intended to decrease the COD [12]. Vinasse, due to its high carbon content, also represents a viable alternative for obtaining biogas for energy recovery through anaerobic digestion to produce methane and hydrogen as gases for combustion [13,14]. Despite the promising research, a significant and economically feasible reduction in the pollutant components has not been achieved, and, therefore, its final disposal in crop irrigation continues to be implemented as the only method [15,16].
Vinasses can be considered a valuable nutrient source for microbial growth given the high content in carbohydrates [17]. Growth of microalgae Micracctinium sp. Embrapa LBA32 and C. biconvexa Embrapa LBA40 was tested by varying the vinasse concentration and supplementing the medium with 46–76% of carbon source [18]. Filamentous fungi of the basidiomycete ligninolytic family have shown great potential for degrading recalcitrant compounds by diluting vinasse up to 25% v/v [19]. Lactic acid bacteria (LAB) are grown using a mixture of vinasse of low sugar concentration (1.57% w/w) and sugar beet molasses with a high content of carbohydrates (53.16% w/w). In this case, vinasse was used as a solvent in the medium preparation [20].
LAB represent a significant group of microorganisms with wide industrial applications and are utilized extensively in the food and beverage industry, hence producing valuable products like lactic acid (LA), bacteriocins, and exopolysaccharides [20,21]. In recent years, there has been a notable focus on fermentative LA production, driven by the growing demand due to its various applications in different industries such as chemical, food, pharmaceutical, cosmetic, and polymer [22]. LA has multiple uses, serving as an acidifying and flavoring agent in food products, an antimicrobial substance and preservative in the cosmetic and food industries, and a precursor of the biodegradable polylactides in the pharmaceutical and plastics industries [23].
The global production of LA is approximately 270,000 tons per year, with 90% being produced through fermentation with pure substrates [24,25]. Despite various feedstocks utilized for LA production, significant challenges persist in achieving an economically viable production. Raw material substrates and fermentation processes constitute approximately 40–70% of production costs, and currently, LA production relies on costly sugars, often competing with food resources such as refined sugars (glucose) or starches [21]. Therefore, employing biorefinery platforms for waste materials generates high-value bioproducts while simultaneously addressing waste remediation. To address this issue, the use of low-cost substrates is strongly advocated for LA production [21].
While the adaptation of microorganisms to high concentrations of specific sugars or fermentation inhibitors has been proposed to improve bioethanol and xylitol production, data regarding the use of LAB for LA production using substrates rich in sugars and inhibitors, like in the case of vinasse, are still lacking [20]. The present study explores the pretreatment and subsequent use of vinasse as a substrate for the growth of LAB as an alternative valorization method for this residue with high pollution potential and low demand in the market. Specifically, a consortium of LAB formed by Lactococcus lactis subsp. Cremoris (ATCC 19257) and Lactococcus lactis subsp. Lactis (ATCC 7962) was considered for lactic fermentation of vinasse for LA production.
3. RESULTS AND DISCUSSION
3.1. Effect of Pretreatment on Vinasse Inhibitor Concentration
Table 1 presents the results of vinasse characterization obtained from the last evaporation cycle in which vinasse by-product of the bioethanol distillation is concentrated up to 32.7 ± 0.1 °Brix. For utilization in microbiological cultures, vinasse was autoclaved as described earlier. It is important to highlight that vinasse in the distillery has been subjected at temperatures close to those arisen in an autoclave. Since the vinasse has suffered an extensive thermal process, it is expected that most of the temperature-sensitive compounds have already reacted, and, therefore, the composition before and after autoclaving remained almost constant. In this case, the registered variation in the total sugar concentration and total dissolved solids were lower than 5.0%.
Table 1: Crude and autoclaved vinasse composition.
| Component | Crude | Autoclaved |
|---|
| Total ash (% m/m) | 8.16 | 8.04 |
| Total nitrogen content (% m/m) | 0.50 | 0.49 |
| Total carbohydrates (% m/m) | 27.73 | 28.587 |
| Ethanol (mg/L) | 0.64 ± 0.32 | 0.56 ± 0.41 |
| Glycerol | 8.39 ± 0.50 | 8.72 ± 0.22 |
| Iron (mg/L) | 108.56 ± 3.66 | 106.40 ± 2.98 |
| Total organic carbon (g C/L) | 129.84 | 128.32 |
| COD (gO2/L) | 341 ± 4 | 339 ± 4 |
| Total solids (% m/m) | 29.90 | 30.23 |
| Total polyphenols (g/L) | 18.84 ± 2.36 | 18.98 ± 2.70 |
| Density (g/L) | 1143 ± 1.0 | 1160 ± 1.0 |
| Acidity (pH) | 4.74 ± 0.05 | 4.71 ± 0.05 |
| Dissolved solids (Brix) | 32.7 ± 0.1 | 33.11 ± 0.1 |
As observed in Table 2, vinasse from the sugar industry contains important amounts of inhibitory compounds for the microbial activity, such as metals (mainly ferrous iron, Fe2+) and polyphenols [8,34]. To mitigate the impact of these species, a preliminary treatment stage was conducted to vinasse diluted 1:3, that is, simulating the composition at the outlet of the distillation tower. In the pretreatment, vinasse was aerated from the bottom with an upward airflow; oxygen, upon contact with ferrous ions (Fe2+), oxidizes them to ferric species (Fe3+), which, combined with oxygen, results in iron oxide (Fe2O3), an insoluble species under basic pH. In addition, hydroxyl ions (OH-) trap free ferric ions in the solution, forming iron oxyhydroxide (FeO(OH)), another compound insoluble under these conditions, facilitating its removal by decantation [35]. The wine lees consisted mostly of organic matter 4.96 ± 1.20 g/kg, which includes yeast and remaining sugars. It also contains residual amounts of inorganics (in g/kg): nitrogen 0.70 ± 0.20, phosphorus 0.90 ± 0.30, potassium 11.70 ± 1.40, magnesium 1.10 ± 0.20, and iron 0.19 ± 0.04. Such composition gives certain nutritional value to the lees for exploration of further valorization alternatives.
Table 2: Summary of general kinetic parameters of LAB batch fermentation with vinasse (17% v/v) and MRS medium.
| Parameter | Symbol (Units) | Value |
|---|
| Fermentation time | t (h) | 12 |
| Substrate concentration | S (g/L=L) | 23.40 |
| Maximum biomass concentration | Xmax (g/L) | 2.20 |
| Maximum specific growth rate | μmax (h−1) | 0.28 |
| Maximum product (lactic acid) concentration | Qmax (g/L) | 16.01 |
| Lactic acid yield | PQ/S (g/g) | 0.59 |
| Ratio of products | Q/X (g/g biomass) | 7.27 |
| Overall yield | PP/S (g/g) | 0.67 |
The Fe2+ concentration dynamics is presented in Figure 1. The dynamics of the pH serves as a proxy of the extent of reaction, as observed in the time course of ferrous ion in connection with the pH change. The aeration of the medium decreases the pH of the solution as the oxidation of the ferrous species advances, generating hydronium ions (H3O+) in the aqueous solution that are easily measurable [35]. Thus, the dynamics of the treatment can be followed online by pH measurement instead of sampling for Fe2+ (or Fe3+) determination. According to the reaction dynamics, the optimum treatment time is estimated in 2.2 h as the rate of change slows down after 2 h of aeration.
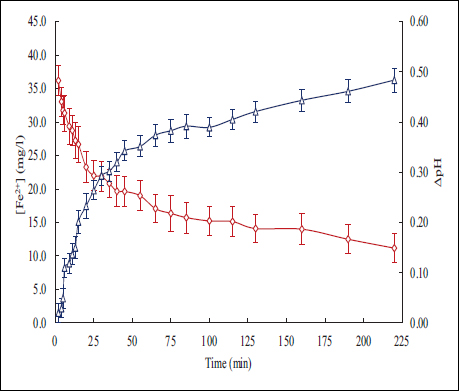 | Figure 1: Ferrous ion concentration (diamonds) and ΔpH (triangles) time course during vinasse (33% v/v) pretreatment with airflow of 0.27 l of air/(l of vinasse.s).
[Click here to view] |
It has been reported that the oxidation of Fe2+ proceeds more efficiently in a basic medium above pH 8.0 since it favors the kinetics of oxidation and increases the stability of the formed molecule [36]. In this case, to enhance the oxidation of aqueous iron, the pH was adjusted every 8 min for controlling between 8.0 and 9.0 by adding 20% w/v NaOH. This facilitated the formation and precipitation of ferric ions as iron oxide (Fe2O3) [36]. The application of aeration as pretreatment led to a 26% decrease in oxidizable compounds in solution, consequently reducing the inhibitory load of vinasse before its use as a substrate for fermentation with LAB.
Aeration reduced the Fe2+ content in the vinasse down to 68%, one of the main inhibitors of the microbial activity in the vinasse. Since polyphenols are organic compounds difficult to oxidize by aeration, the addition of a subsequent ozonation stage was proposed to increase the proportion of inhibitors eliminated from the raw diluted vinasse. The ozonation stage contributed to a 22% reduction in polyphenols from 6.28 ± 1.35 to 4.90 ± 1.84 g/L, leading to a total decrease of 48% in the concentration of oxidizable species in vinasse.
LAB cultures in media prepared with pretreated vinasse and MRS components were compared at concentrations ranging from 1 to 10% v/v of final vinasse concentration to visualize the effect of treatment in the maximum biomass accumulation. As shown in Figure 2, the differences in biomass concentrations obtained in each medium ranged from 0.1 to 0.4 g/L, increasing the biomass as the fermentable sugar concentration increases as a result of higher proportion of vinasse in the final medium.
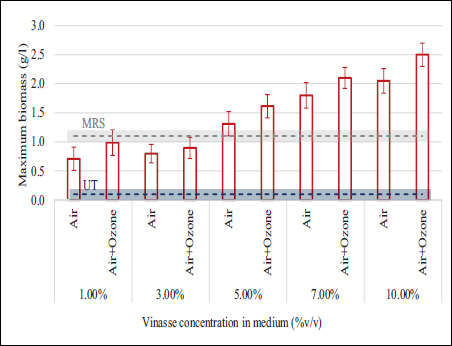 | Figure 2: Biomass (bars) of lactic acid bacteria cultivated in pretreated vinasse (1–10% v/v) with MRS medium. Positive control: pure MRS medium (gray line). Negative control: untreated vinasse (blue line).
[Click here to view] |
The high concentration of oxidable inhibitors in the crude diluted vinasse did not allow any appreciable growth of LAB at all dilutions (negative control). As expected, in all cases the maximum biomass was observed in the medium with lower inhibitor concentration (vinasse aerated and ozonized). The average biomass production using vinasse treated with air/ozone is 11% higher than the vinasse treated only with air. Therefore, both pretreatments improve the conditions for using vinasse as a substrate or supplement for LAB fermentation. Nevertheless, the cost of applying ozonation is considerably higher compared to the simple aeration [37]. This vinasse has a low content of ethanol, as shown in Table 1. The content of ethanol is 0.56 ± 0.41 mg/L, and the variability in ethanol composition did not correlate with the biomass variability in control experiments (Pearson correlation test, p-value = 0.2191). Since biomass values did not show significant differences between pretreatments at all dilutions, it was decided to consider only aeration as the most economically feasible pretreatment. This is advantageous for application in the industrial scale, where the less sophisticated and cheap waste treatments are preferred. Thus, the simple aeration is a promising technique for decreasing the inhibitor composition in the distillation effluent for further utilization of vinasse in biological processes.
3.2. Effect of Vinasse on Lactic Acid and LAB Growth
Ethanol producers in the region of Valle del Cauca perform concentration of distillation bottoms to reduce the overall volume of vinasse in the plant, resulting in an approximate ratio of 1–3 l of vinasse per liter of anhydrous bioethanol for fuel mixtures [3]. Without concentration, this ratio reaches up to 10 l of vinasse per liter of distilled ethanol [38]. Under these considerations, LAB biomass and LA production were explored using pretreated vinasse for medium preparation with final concentrations ranging from 0 to 33% v/v. Figure 3 displays the LA and biomass maximum concentrations attained at 24 h of batch cultivation between 0 and 20% v/v vinasse concentration in the medium. Vinasse concentrations over 20% totally inhibited the LAB growth (data not shown).
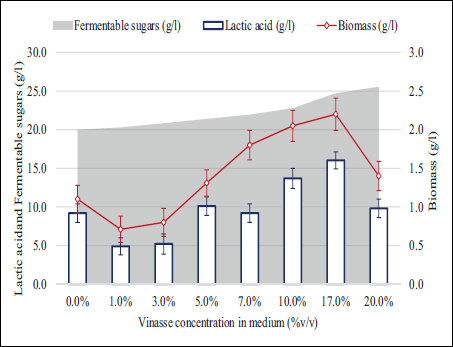 | Figure 3: Lactic acid, fermentable sugars, and biomass maximum concentrations in 24 h batch cultivation using media prepared with pretreated vinasse.
[Click here to view] |
The data summarized in Figure 3 evidence a maximum point of biomass and LA accumulations when cultivated using a medium prepared with vinasse up to 17% v/v, doubling the value obtained when using standard MRS medium. However, as the vinasse concentration increases beyond 17% v/v, biomass and LA production decreases, suggesting that inhibitory compounds became significant at less at 20% v/v and higher. Since the purpose is to treat the vinasse at the highest viable concentration promoting high LAB growth and LA accumulation, the 17% v/v dilution was established as the most suitable concentration for further application. Moreover, by dealing with this concentration, it does not necessarily imply the use of fresh water for dilution since the concentration of vinasse varies according to the fermented molasses fed to the distillation tower. Vinasses can be obtained from the first, second, or third centrifugation stage as A-, B-, and C-type molasses, respectively, conferring variable characteristics of vinasse leaving the distiller [39]. In addition, evaporation processes would be unnecessary in a biological treatment scenario, reducing operating costs related to the required thermal energy.
To analyze bacterial growth under the established culture conditions as experimental optimum, biomass and LA were monitored during the complete fermentation. Samples were taken and pH was adjusted by adding 20% w/v NaOH. The results show that maximum biomass is reached at 12 h with a maximum specific growth rate (μmax) of 0.28 h−1, which aligns with the reported range in previous studies estimating a time between 10 and 12 h [21,40]. The maximum LA accumulation was recorded between 14 and 20 h.
The growth curve of LAB [Figure 4] confirms the kinetics of biomass, substrate uptake, and metabolite production. The secretion in the fermentation medium causes a decrease in pH, which is related to the concentration of acid and its conjugate base through the proton mass balance (H3O+) [41,42]. This also can act as an inhibitor of the bacterial growth, so the pH control can extent the stationary phase of the culture. Therefore, the progress of the culture can be easily followed online through two main variables (biomass and pH), providing certainty about the direction of the fermentation process and metabolite production.
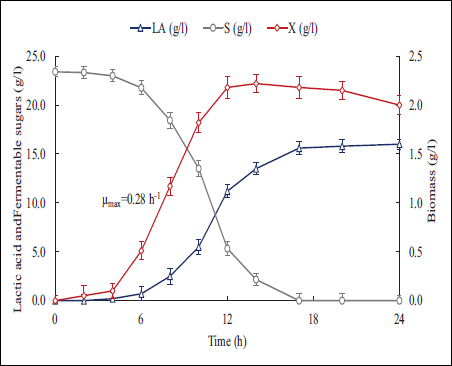 | Figure 4: Lactic acid (LA), fermentable sugars (S), and biomass (X) time course of concentrations in LAB cultures with 17% pretreated vinasse.
[Click here to view] |
LAB can ferment not only the lactose used as carbon source in the MRS medium but also may use the sugars present in the vinasse to produce LA and biomass as main products given their homofermentative nature. In addition to lactose, these strains can use other sugars such as glucose, galactose, and sucrose as carbon sources, as well as peptides and amino acids as nitrogen and energy source, expanding their potential in various biotechnological applications [43,44]. The results in Figures 3 and 4 confirm that increases in the fermentable sugars in the media lead to more LA and biomass being produced, approaching the theoretical yield of 1.0 g LA/g of fermentable sugars in the medium [45].
Table 2 summarizes the kinetic constants of batch cultivation of LAB key aspects of the fermentation process. Initially, it was observed that the total duration of fermentation is 12 h. During this process, it starts with an initial concentration of available sugars of 31 g/L, resulting in an overall yield of 0.67. This value is very similar to previous reports on pure cultures with LAB [46]. For comparison, a yield of approximately 78% in a standard medium L. lactis is reported. The difference between these two values is only 14%, supporting the good performance obtained, considering that vinasses are residual and complex substrate for the culture of any microorganism, given the significant imbalance in salts content and oxidable compounds of inhibitory nature.
Given the interest in mitigating the environmental impact associated with vinasse management, evaluations of variables related to discharge and post-treatment disposal were conducted [47]. In the first stage of pretreatment, a significant 14% reduction in COD was achieved, starting from a relatively high value of 341 ± 4 gO2/L of solution. This decrease had a significant effect on the growth of microorganisms, as described previously, suggesting that different microorganisms present in the environment may find favorable conditions to consume nutrients and degrade the pollutants in the distillation effluent pretreated with simple aeration. After the combined oxidative and biological treatment, this value decreased to 156 ± 4 gO2/L. In addition, the pH was adjusted to 6.50, complying with environmental regulations established in Resolution 0883 of 2018 issued by the Ministry of Environment and Sustainable Development of Colombia [48], which set a range between 6.0 and 9.0 pH units for effluent discharge. This progress is significant, considering that the initial pH of the vinasse is 4.74 ± 0.05. Another crucial aspect of the process was the complete removal of total suspended solids in the final stage. These results indicate significant progress in treating vinasse as a contaminant, favoring the possibility of using it more safely and sustainably. Further improvements are needed as the implementation of a purification strategy to obtain D-lactic acid with high purity degree, and the possible utilization of this effluent in anaerobic digestion and composting, in order to get valuable products like hydrogen and biogas. After such a combination of treatments, the pollution potential of the vinasse will be likely reduced almost completely.
3.3. Setup of Fed-batch LAB Cultivation Using vinasse
Considering the high volume of vinasse generated in the region, fed-batch operation was proposed as an alternative to increase the utilization of this residue as substrate for LAB cultivation. For this purpose, vinasse was added after 12 h of fermentation in pulses to observe the response of LAB to vinasse addition. The results presented in Figure 5 evidence a sustained growth over 48 h, in contrast to that observed in batch operation [Figure 4]. The dilution effect due to medium addition after the 24th h decreases the biomass concentration slightly [Figure 5A]. Nevertheless, the biomass concentration remains almost constant during the fed-batch phase as the dilution is approximately equal to the growth rate.
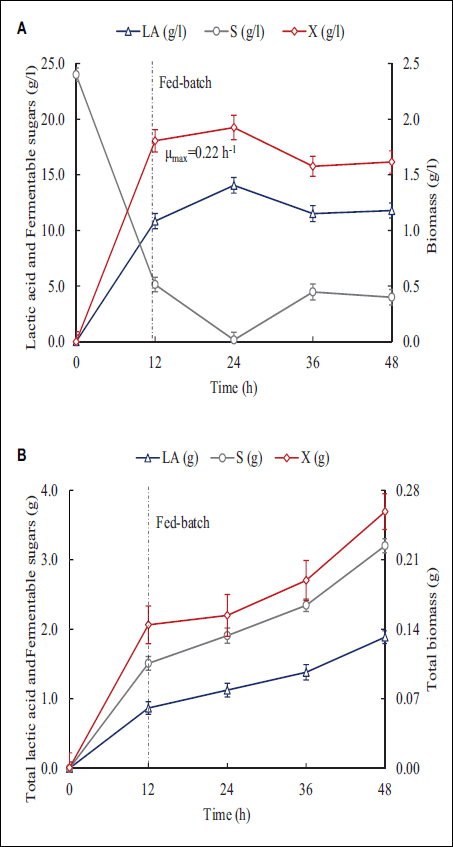 | Figure 5: Lactic acid (triangles), fermentable sugars (circles), and biomass (diamonds) in LAB cultures with 17% pretreated vinasse. (A) Time course of concentrations. (B) Time course of substrate, biomass, and product accumulation.
[Click here to view] |
In Figure 5B, the total accumulation of LA, biomass, and fermentable sugars is presented. During the first 12 h, a significant increase in biomass characteristic of the exponential growth (μmax = 0.22 h−1) phase is noted, where the nutritional factors supplied by the components of the MRS medium promote the acceleration of biomass production. The addition of vinasse as a supplement in the fed-batch stage provides fermentable sugars but also increases the composition of inhibitors, but those apparently do not affect the growth trend of LAB, in which the specific growth rate is 0.02 h−1 during the deacceleration phase. The contribution of carbohydrates in the vinasse serves as a carbon source to sustain a growth phase with a constant supply of those nutrients available in the 40.0 ± 0.5 mL of 17% v/v vinasse in each addition.
These results suggest that it is feasible to carry out fed-batch operation in the cultivation of LAB using a medium that includes vinasse as a supplement. The findings of this research offer promising prospects linked to the production of LA and biomass of LAB. In addition, as seen in other studies, the valorization of waste streams yields good performances in relation to LA production [46,49]. Currently, the commercial production of LA is mainly carried out through bacterial fermentation of glucose derived from sources such as sugarcane or beet molasses, corn starch, rice, wheat, potatoes, barley, and cassava, posing competition for the food production as they are also used for human and animal consumption [24].
The possibilities of using pretreated vinasse with a simple and economical method like aeration add to other materials with potential, such as lignocellulosic materials (sawdust, poplar, sugarcane bagasse), and spent brewery grains, among others, which are considered promising raw materials for LA production. This is due to their abundance, potential cost-effectiveness, high carbohydrate content, and noncompetition with the food chain [19]. In addition, the biomass obtained in the process, when supplemented with organic sources like breadcrumbs or lentil residues, has the potential to be used as a probiotic in animal farming.
dat
1. Devi A, Bajar S, Sihag P, Sheikh ZUD, Singh A, Kaur J, et al. A panoramic view of technological landscape for bioethanol production from various generations of feedstocks. Bioengineered. 2023;14(1):81-112.
2. Parsaee M, KianiDehKiani M, Karimi K. A review of biogas production from sugarcane vinasse. Biomass Bioenergy. 2019;122:117-25.
3. Ospina León LJ, Manotas-Duque D, Ramirez Malule HD. Desafíos y oportunidades de la Vinaza de caña de azúcar. Un análisis bibliométrico. Ing Compet. 2023;25(1):e-30412144.
4. Rueda-Ordóñez DA, Leal MRLV, Bonomi A, Cortez LAB, Cavalett O, Rincón JM. Simulating scenarios for compost and vinasse use to improve the economics and environmental aspects of representative Colombian sugarcane production systems. Renew Agric Food Syst. 2020;35(5):579-93.
5. Santana H, Cereijo CR, Teles VC, Nascimento RC, Fernandes MS, Brunale P, et al. Microalgae cultivation in sugarcane vinasse: selection, growth and biochemical characterization. Bioresour Technol. 2017;228:133-40.
6. Christofoletti CA, Pedro-Escher J, Fontanetti CS. Assessment of the genotoxicity of two agricultural residues after processing by diplopods using the Allium cepa assay. Water Air Soil Pollut. 2013;224(4):1523.
7. Cesaro A, Belgiorno V. Combined Biogas and bioethanol production: opportunities and challenges for industrial application. Energies (Basel). 2015;8(8):8121-44.
8. Arimi MM, Zhang Y, Götz G, Kiriamiti K, Geißen SU. Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int Biodeterior Biodegradation. 2014;87:34-43.
9. Li G, Xu F, Yang T, Wang X, Lyu T, Huang Z. Microbial behavior and influencing factors in the anaerobic digestion of distiller: a comprehensive review. Fermentation. 2023;9(3):199.
10. Campos CR, Mesquita VA, Silva CF, Schwan RF. Efficiency of physicochemical and biologicaltreatments of vinasse and their influence on indigenous microbiota for disposal into the environment. Waste Manag. 2014;34:2036-46.
11. de Souza RP, Ferrari-Lima AM, Pezoti O, Santana VS, Gimenes ML, Fernandes-Machado NRC. Photodegradation of sugarcane vinasse: evaluation of the effect of vinasse pre-treatment and the crystalline phase of TiO2. Acta Sci Technol. 2016;38(2):217-26.
12. Lucas MS, Peres JA, Li Puma G. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep Purif Technol. 2010;72(3):235-41.
13. Janke L, Leite A, Nikolausz M, Schmidt T, Liebetrau J, Nelles M, et al. Biogas production from sugarcane waste: assessment on kinetic challenges for process designing. Int J Mol Sci. 2015;16(9):20685-703.
14. Barrera A, Gómez-Ríos D, Ramírez-Malule H. Assessment of a low-cost hydrogen sensor for detection and monitoring of biohydrogen production during sugarcane straw/vinasse co-digestion. Agriengineering. 2024;6:479-90.
15. Martinez EJ, Rosas JG, Gonzalez R, Garcia D, Gomez X. Treatment of vinasse by electrochemical oxidation: evaluating the performance of boron-doped diamond (BDD)-based and dimensionally stable anodes (DSAs). Int J Environ Sci Technol (Tehran). 2018;15(6):1159-68.
16. Arreola AR, Tizapa MS, Zurita F, Morán-Lázaro JP, Valderrama RC, Rodríguez-López JL, et al. Treatment of tequila vinasse and elimination of phenol by coagulation–flocculation process coupled with heterogeneous photocatalysis using titanium dioxide nanoparticles. Environ Technol. 2020;41(8):1023-33.
17. Djukic-Vukovic AP, Mojovic LV, Vukašinovic-Sekulic MS, Rakin MB, Nikolic SB, Pejin JD, et al. Effect of different fermentation parameters on l-lactic acid production from liquid distillery stillage. Food Chem. 2012;134(2):1038-43.
18. Rodrigues CV, Santana KO, Pires LO, Brienzo M, Maintinguer SI. Biohydrogen generation: concepts and applications on Brazilian agro-industrial wastewaters. Series: Chemistry Research and Applications, Publisher: Nova science publishers In: Biohydrogen Production, Applications and Technology. 2017. p. 1-68.
19. Tsapekos P, Alvarado-Morales M, Baladi S, Bosma EF, Angelidaki I. Fermentative production of lactic acid as a sustainable approach to valorize household bio-waste. Front sustain (Lausanne). 2020;1:4.
20. Mladenovic D, Pejin J, Kocic-Tanackov S, Djukic-Vukovic A, Mojovic L. Enhanced lactic acid production by adaptive evolution of Lactobacillus paracasei on agro-industrial substrate. Appl Biochem Biotechnol. 2019;187(3):753-69.
21. Ahmad A, Banat F, Taher H. A review on the lactic acid fermentation from low-cost renewable materials: recent developments and challenges. Environ Technol Innov. 2020;20:101138.
22. Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, et al. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol. 2021;9:612285.
23. Li X, Lin Y, Liu M, Meng L, Li C. A review of research and application of polylactic acid composites. J Appl Polym Sci. 2023;140(7):e53477.
24. Alexandri M, Schneider R, Mehlmann K, Venus J. Recent advances in D-lactic acid production from renewable resources. Food Technol Biotechnol. 2019;57(3):293-304.
25. Alves de Oliveira R, Komesu A, Vaz Rossell CE, Maciel Filho R. Challenges and opportunities in lactic acid bioprocess design—from economic to production aspects. Biochem Eng J. 2018;133:219-39.
26. Djukic-Vukovic AP, Mojovic LV, Jokic BM, Nikolic SB, Pejin JD. Lactic acid production on liquid distillery stillage by Lactobacillus rhamnosus immobilized onto zeolite. Bioresour Technol. 2013;135:454-8.
27. García Martínez EM, Fernández Segovia I, Fuentes López A. Determinación de polifenoles totales por el método de Folin-Ciocalteu; 2015 [cited 2023 Aug 26]; Available from: https://riunet.upv.es:443/handle/10251/52056
28. Saska M, Figueroa EA, Zossi BS, Marcelo-Ruiz R. Determinación de azúcares reductores en azúcar crudo por el método de Luff-Schoorl; 2021 [cited 2023 Sep 10]; Available from: http://www.scielo.org.ar/pdf/riat/v99n1/v99n1a08.pdf
29. Standard Methods Committee of the American Public Health Association, American Water Works Association, Water Environment Federation. 3111 Metals by flame atomic absorption spectrometry. In: Lipps WC, Baxter TE, Braun-Howland E, editors. Standard Methods for the Examination of Water and Wastewater. Washington (DC): APHA Press; 2017.
30. Instituto Colombiano de Normas Técnicas y Certificación. NTC 4754:2000. Calidad del agua - Determinación de hierro - Método de la fenantrolina. Bogotá, Colombia: ICONTEC; 2000.
31. Mæhre H, Dalheim L, Edvinsen G, Elvevoll E, Jensen IJ. Protein determination—method matters. Foods. 2018;7(1):5.
32. Avramidis P, Bekiari V. Application of a catalytic oxidation method for the simultaneous determination of total organic carbon and total nitrogen in marine sediments and soils. PLoS One. 2021;16(6):e0252308.
33. Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA. The potential of microplastics as carriers of metals. Environ Pollut. 2019;255(3):113363.
34. Bird LJ, Coleman ML, Newman DK. Iron and copper act synergistically to delay anaerobic growth of bacteria. Appl Environ Microbiol. 2013;79(12):3619-27.
35. Alicilar A, Meriç G, Akkurt F, Sendil, O. Air oxidation of ferrous iron in water. Int J Environ Sci Technol (Tehran). 2008;3:409-14.
36. Morgan B, Lahav O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution – basic principles and a simple heuristic description. Chemosphere. 2007;68(11):2080-4.
37. Sgroi M, Snyder SA, Roccaro P. Comparison of AOPs at pilot scale: energy costs for micro-pollutants oxidation, disinfection by-products formation and pathogens inactivation. Chemosphere. 2021;273:128527.
38. Moraes BS, Zaiat M, Bonomi A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: challenges and perspectives. Renew Sustain Energy Rev. 2015;44:888-903.
39. Incauca.com [Internet]. Procesos de Incauca S.A.S Ingenio del Cauca; 2016 [updated 2016 Jun 30; cited 2023 Nov 21]. Available from: https://www.incauca.com/es/procesos/
40. Tian X, Liu X, Zhang Y, Chen Y, Hang H, Chu J, et al. Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresour Technol. 2021;323:124549.
41. Rodriguez E, Calzada J, Arqués JL, Rodriguez JM, Nuñez M, Medina M. Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 in cheese. Int Dairy J. 2005;15(1):51-7.
42. Esper RC, Córdova JRC, Córdova LDC. Modelo fisicoquímico del equilibrio ácido-base. Conceptos actuales (3ª de tres partes). Rev Fac Med Univ Nac Auton Mex. 2008;51(5):193-5.
43. Zacharof MP, Lovitt RW. Modelling and simulation of cell growth dynamics, substrate consumption, and lactic acid production kinetics of Lactococcus lactis. Biotechnol Bioprocess Eng. 2013;18(1):52-64.
44. Pérez-Guzmán AE, Victoria TC y, Cruz-Camarillo R, Hernández-Sánchez H. Purification and characterization of x-prolyl-dipeptidyl aminopeptidase from Lactococcus lactis subsp. cremoris NRRL 634. World J Microbiol Biotechnol. 2006;22(9):953-8.
45. Abedi E, Hashemi SMB. Lactic acid production - producing microorganisms and substrates sources-state of art. Heliyon. 2020;6(10):e04974.
46. Serna-Cock L, Rodriguez-de-Stouvenel A. Lactic acid production by a strain of Lactococcus lactis subs lactis isolated from sugar cane plants. Electron J Biotechnol. 2006;9(1):40-5.
47. Singh SN, Srivastava G, Bhatt A. Physicochemical determination of pollutants in wastewater in Dheradun. Curr World Environ. 2012;7(1):133-8.
48. Vertimientos puntuales a cuerpos de agua. Resolución 0883, Ministerio de Ambiente y Desarrollo Sostenible [Internet], 2018 May 18 [cited 2024 Feb 18] (Colombia). Available from: https://www.minambiente.gov.co/wp-content/uploads/2021/08/resolucion-0883-de-2018.pdf.
49. Song L, Yang D, Liu R, Liu S, Dai L, Dai X. Microbial production of lactic acid from food waste: latest advances, limits, and perspectives. Bioresour Technol. 2022;345:126052.




