India is considered to be the second-highest rice producer in the world and rice is consumed conventionally as a staple food. However, there is a prevalence of the extensive tradition of making unique fermented rice products with a variety of tastes and textures that are associated with ethnic diversity. This fermented rice product is traditionally prepared by rural women using village art and is popular in Goalpara district, Assam, also, known locally as pachoi in the North Eastern Region of India. In the present investigation, fermentative production of pachoi was carried out by optimizing the fermentation parameters, viz. time, temperature, and initial pH by using response surface methodology (Box-Behnken approach), and nutritional constituents were evaluated under optimized process conditions. Experimental results revealed that carbohydrate, protein, and fat were found 52.671%, 11.89%, and 1.23%, respectively, under optimum conditions of 25ºC temperature, 24 h time, and pH 5.49 and higher than the value of carbohydrate, protein, and fat of 32.9%, 12.4%, and 1.1% carried out by conventional natural fermentation FP (N). There is no such difference in phenolic, flavonoid, and DPPH free radical scavenging activity in both FP (L) and FP (N).
Khatun L, Ray S, Brahma R, Boruah DC. Optimization of fermentation conditions of fermented rice production through response surface methodology: Effect on nutritional and bio-functional properties. J Appl Biol Biotech 2025;13(6):88-95. https://doi.org/10.7324/JABB.2025.237412
In Southeast Asian nations, rice (Oryza sativa) is a widely utilized cereal crop. It belongs to the Poaceae family and exhibits impressive genetic variation, with numerous cultivars growing throughout Asia. In comparison to its equivalents such as wheat, rye, oats, maize, etc., rice differs from other cereals due to its greater amount of carbohydrates, energy, and a lower percentage of fat [1]. The food-processing process uses a variety of technologies to transform perishable, mostly inedible raw materials-which are also somewhat bulky-into more useful, pleasant foods or consumable liquids with longer shelf lives. Food processing improves the availability and market value of many ethnic foods, reducing waste and loss, and promoting food safety [2]. Since ancient times, rice has been used to make a variety of traditional and ethnic fermented meals and beverages. In addition to regular rice, pigmented rice types possess the ability to be employed as the primary ingredient in rice-based fermented foods due to their phytochemical levels, which are multiplied by many during fermentation. Traditional fermented rice-based foods are becoming less and less popular as a result of urbanization and lifestyle changes [1]. For people with celiac disease, rice flour is preferable since it has qualities including being gluten-free, having a lot of readily digestible carbohydrates, having a bland flavor, being colorless, having low sodium levels, and being hypoallergenic [3].
Rice is the most widely used and easily accessible food item since antiquity, it is employed in the manufacturing of a variety of fermented meals and beverages. The fermented rice matrix is dominated by a variety of probiotics, making it an ideal environment for the development and maintenance of probiotics [4]. In addition, fermentation improves rice’s nutritional composition by adding more vitamins, minerals, and amino acids, which raises the product’s nutrient value, energy level, and medicinal potential [5]. Fermentation improves the nutrient content and functional qualities of food by transforming substances because of food and microbes’ relationship and producing final outcomes that are bioactive or bioavailable, which is good for human health [6]. For a very long time, starter cultures have been used in the production of fermented foods and drinks. When a starting culture is added, the product’s taste alters in a consistent, desired, and predictable way, improving its sensory attributes and nutrient content [7]. Fermented items made from cereal have a better nutritional content than nonfermented cereal-based items [8]. Starting cultures for fermented food production are traditionally prepared by backslopping, combining a little quantity of old ferment, utilizing an appropriate vessel, and incorporating particular natural items that contain effective bacteria [9]. These age-old techniques, which are still used in small- to mid-scale production units, especially in the creation of household-type products, made it easier to prepare individual or different kinds of fermented meals and beverages [10]. The accessibility of nutritional fibers, indigestible carbohydrates, polyphenolic content, vitamins, and minerals is increased through fermentation. The majority of these substances that are generated during fermentation are known to have prebiotic qualities and to encourage the growth and development of probiotic microbes [10].
According to a recent report [11], rice-fermented food based on Asparagus racemosus is an excellent resource for lactic acid bacteria, yeast, Bifidobacterium spp., and other microorganisms. It is also rich in minerals, water-soluble vitamins, oligosaccharide (G3-maltotriose), unsaturated fatty acids, and a variety of essential and non-essential amino acids [11]. The external covering of rice grains that breaks down during the milling process is called rice bran. A significant percentage of rice bran 90% is utilized as animal feed, and the remaining portion is removed to make rice bran oil [12]. Rice bran is appropriate to be employed as a substrate in the preparation of high-protein foods since it not only contains macronutrients such as protein, fat, and dietary fiber but also known to include micronutrients such as minerals and vitamin E [12].
Pachoi is a traditional fermented rice product commonly consumed by the Bengali Muslim community in Assam, India. It is primarily consumed during the seasons of autumn and summer and is renowned for its flavor and taste. No literature has been reported on pachoi preparation and on its nutritional effects till now, as per my study, so this study focused on the nutritive values of pachoi and optimizing the fermentation parameters (time, temperature, and initial pH), as well as assessing its nutritional characteristics similar with the pachoi in natural fermentation.
Response surface methodology (RSM), a combination of statistical and mathematical techniques for determining the ideal conditions of factors for desirable responses, is a commonly used optimization technique [13]. RSM’s Box-Behnken design is simple to use since it can fully explore an experimental domain, making variables easier to optimize.
Ranjit raw rice variety and Ahu Kalogoria grain cultivar were considered in this study and purchased from local farmers of Goalpara district, Assam, NER, India. Ahu Kalogoria paddy germinated and sundried according to the method described by Veluppillai et al. [14]. The breakdown of germinated part and the husk is done in dheki, and the separation of the husk and germinated part from rice is done. The rice is then powdered into flour and are kept for the further fermentation process. The Ranjit raw rice was cooked for 10 min in induction using a 1:2 ratio of water. After cooking, it is kept to cool down.
A Box-Behnken central fractional factorial design was used to optimize the process conditions, viz., temperature, time, and initial pH in order to the enrichment of nutrition in pachoiwere observed and the responses chosen were carbohydrate, fats and proteins. To achieve the initial pH for optimization, lactic acid and sodium bicarbonate were used. Finally, for the optimization purpose, the same protocol was followed as indicated in natural fermentation with the only exception where the beaker was covered with aluminum foil. For pachoi preparation 100 g of Ranjit rice (cooked) was taken then 5 g of flour, 0.5 g of salt, and 7 g of sugar were thoroughly combined. Next, 30 mL of water was used to immerse 8 g of husk with germinated roots and shoots for 30 min, after which the mixture was filtered. 3 mL of pasteurized liquid milk Amul Taza (bought from the local market in the Goalpara district of Assam, NER, India) were added to the mixture along with the filtered water. Following complete mixing, the mixture was placed in a 500 mL beaker, covered with aluminum, and allowed to ferment in the incubator in different temperatures, time, and different pH. Then, amount of carbohydrates, fats, and proteins, was measured.
The Kjeldahl method can be used to convert the total nitrogen content into the total protein content. The conversion factor is 6.25 [15].
Carbohydrate analysis was carried out using the phenol sulphuric acid method by Guo et al. [16] with little modification. In this method, D-glucose was used as a standard solution. 500 mg sample was taken in 5 mL of 2.5 N HCL and heated in the water bath for 3 h then make up the volume up to 50 mL with distilled water. Standard was taken in five test tubes in different concentrations and 5% phenol, then 96% sulphuric acid was added. Different test tubes with different samples were taken, followed by the addition of phenol and sulfuric acid. Absorbance was taken in 490 nm using spectrophotometer, and the graph was prepared. From the graph, carbohydrate concentration in the sample was calculated.
Lipid content was estimated by extracting the sample with petroleum ether in Soxhlet apparatus for 8 h, and the amount of lipid was determined after the removal of petroleum ether [17]. The W1 is the weight of the flask (initial weight). W2 is the weight of the flask after evaporation of the ether, i.e., the final weight.
Fat% = W2–W1/sample weight × 100
RSM was used to find the optimum condition for the production of fermented pachoi. A Box-Behnken design with three factors and three levels was chosen to evaluate the combined effect of three independent variables, i.e., fermentation temperature, fermentation Time, and initial pH, coded as A, B, and C, respectively. After preliminary fermentation trials, the upper and lower limits for the independent variables were established. Temperature, time, and pH levels are 25–30°C, 6–24 h, and 4–7, respectively. Three levels of each variable were chosen, and 12 fermenting trials [Table 1] were performed for the evaluation of the optimized condition. A statistical tool (Design-Expert Version 7.0) was used to perform the computations, which included the choice of experimental points, randomization, analysis of variance (ANOVA), fitting of the models, and graphical displays. ANOVA was performed on the data to find variations across formulations that were statistically significant (P < 0.05).
Table 1: Experimental design for preparation of pachoi.
| Standard | Run | Block | A: Temp (ºC) | B: Time (h) | C: pH | Response (Fat%) | Response (Carbohydrate%) | Response (Protein%) |
|---|---|---|---|---|---|---|---|---|
| 2 | 1 | Block 1 | 30 | 6 | 5.5 | 1.01 | 69.14 | 10.94 |
| 7 | 2 | Block 1 | 25 | 15 | 7 | 0.91 | 74.94 | 9.32 |
| 1 | 3 | Block 1 | 25 | 6 | 5.5 | 1.1 | 70.94 | 10.21 |
| 11 | 4 | Block 1 | 27.5 | 6 | 7 | 1.01 | 73.29 | 9.02 |
| 14 | 5 | Block 1 | 27.5 | 15 | 5.5 | 1.15 | 64.78 | 11.73 |
| 10 | 6 | Block 1 | 27.5 | 24 | 4 | 1.23 | 61.54 | 10.64 |
| 15 | 7 | Block 1 | 27.5 | 15 | 5.5 | 1.19 | 54.12 | 10.96 |
| 12 | 8 | Block 1 | 27.5 | 24 | 7 | 1.12 | 68.76 | 9.81 |
| 5 | 9 | Block 1 | 25 | 15 | 4 | 1.02 | 59.34 | 10.79 |
| 9 | 10 | Block 1 | 27.5 | 6 | 4 | 1.041 | 67.14 | 9.19 |
| 3 | 11 | Block 1 | 25 | 24 | 5.5 | 1.41 | 36.94 | 13.36 |
| 13 | 12 | Block 1 | 27.5 | 15 | 5.5 | 1.21 | 45.12 | 11.12 |
| 4 | 13 | Block 1 | 30 | 24 | 5.5 | 1.43 | 34.74 | 13.32 |
| 8 | 14 | Block 1 | 30 | 15 | 7 | 0.94 | 71.14 | 9.14 |
| 6 | 15 | Block 1 | 30 | 15 | 4 | 1.14 | 61.34 | 10.21 |
About 100 g of cooked Ranjit rice was mixed well with 5 g of flour, 0.5 g of salt, and 7 g of sugar. Then, 8 g of husk with germinating roots and shoots were soaked in 30 mL of water for 30 min and filtered it. The filtered water was added to the mixture along with 3 mL of pasteurized liquid milk purchased from the local market of Goalpara district, Assam, NER, India. After mixing it thoroughly, the mixture was transferred into a beaker (500 mL), covered by the Petri plate, kept for fermentation, and carbohydrate, fats, proteins, and alcohol were estimated. fat, carbohydrates, and proteins are estimated using the method explained in the subsection of method and materials section.
Quantitative estimation of ethanol was performed using the potassium dichromate method [18]. 10–50 μL of absolute alcohol was taken in different test tubes as standard then the volume was made up to 500 μL by adding distilled water. 30 μL of test samples (juice) was taken and the volume makeup to 500 μL by adding distilled water. 1 mL of potassium dichromate reagent was added in each test tube, and then 2 mL of NaOH solution was added in each test tube and incubated at 50°C for 30 min. After incubation absorbance was measured at 600 nm wavelength by spectrophotometer. Moreover, a graph was drawn for the calculation of the ethanol quantity.
Folin ciocalteu (FC) was used to estimate the TPC described by Dewanto et al. [19]. Each extract 120 μL was taken and followed by 2.5 mL of FC reagent (10%) added, and in between 8 min, 2 mL of sodium carbonate (Na2CO3 [7.5%]) was mixed. The samples were vortexed immediately and incubated in the dark for 30 min at 40 °C. Using a Shimadzu ultraviolet (UV)-1800 spectrometer (Shimadzu Inc., Kyoto, Japan), the absorbance of the blue color was measured at 760 nm. The TPC was reported as mg of gallic acid equivalents (GAE)/100 g of sample dry weight, using gallic acid as the standard (DW).
The method of Khatun et al. [18] with little modification was used. Crude extract sample preparation in 1 mg/mL methanol stock. A sample of 200 microliters was taken from stock. Make up the volume up to 1 mL in all the test tubes with methanol. Followed by the addition of 0.5 mL of 5% NaNO2 and 0.5 mL of 10% AlCl3. After 5 min of reaction, 2 mL of NaOH (4%) solution was added and incubated at room temperature for 15 min and read the absorbance at 510 nm using a UV-VIS spectrophotometer. Quercetin is used as a standard solution. Blank preparation using methanol (1 mL) and all chemicals except the sample solution. Moreover, the total flavonoid content result is expressed using the graph as quercetin equivalent mg/100 g.
This method was described by Dewanto et al. [19] with some modifications. DPPH gives strong absorbance at 517 nm (deep violet color) due to its unpaired electron. When this radical pairs off in the presence of a free radical scavenger, the absorption vanishes, resulting in decoloration or yellowish color. 200-μL sample extract and make up the volume up to 1 mL with methanol, and control as 1 mL methanol and 3 mL DPPH (0.004%).
DPPH scavenged% = Acon–Atest/Acon × 100
Acon–is the absorbance of the control reaction
Atest–is the absorbance of the test sample.
A widely used technique for assessing microbial levels in food is the TPC method, which measures the population of viable bacteria. In this process, 25 mL of a well-blended food sample is mixed with 225 mL of sterile water in a flask to form a uniform suspension. From this mixture, 1 mL is taken and added to 9 mL of sterile water in a test tube, then thoroughly mixed using a vortex mixer. This dilution is labeled as 10–1. The serial dilution process continues similarly, with each subsequent dilution clearly marked.
From each dilution level, 1 mL of the sample is dispensed into sterile Petri dishes. Then, 15 mL of nutrient agar, previously cooled to 45°C, is poured into each dish. The mixture is gently swirled to ensure uniform dispersion of microorganisms throughout the medium and is allowed to solidify. Once solidified, the plates are incubated upside-down at 37°C for 24–48 h to facilitate bacterial growth. After incubation, only those plates showing 30–300 colonies are selected for enumeration, as this range provides statistically reliable data and avoids errors due to excessive or insufficient colony numbers. Finally, the number of colony-forming units (CFU) per gram or milliliter of the food sample is calculated from the colony counts, providing a quantitative measure of microbial contamination [20].
log10 CFU/g = log10 (Number of colonies/volume plated × dilution factor).
Three-dimensional response surface plots were produced to elucidate the relationships between the responses and the experimental levels of each independent variable. The optimum level of each variable for maximum nutritional content was resolved using the response optimizer tool of the software. The following quadratic equation based on linear coefficients of independent variables calculates the optimal point of the given model.
X = C0 + A* + B* + C* + AB* + AC* + BC* + A2* + B2* + C2*
Where A is Temperature, B is Time and C is pH.
The fat content range is between the range of 0.91–1.43%, as shown in [Table 1]. R2 value of 0.89 indicates 89% of the goodness of fit. [Figure 1] shows that the points are close to the fitted line, indicating good fit [Figure 2] indicates the 3D surface of the fat content. The ANOVA data for the fat content is tabulated in Table 2.
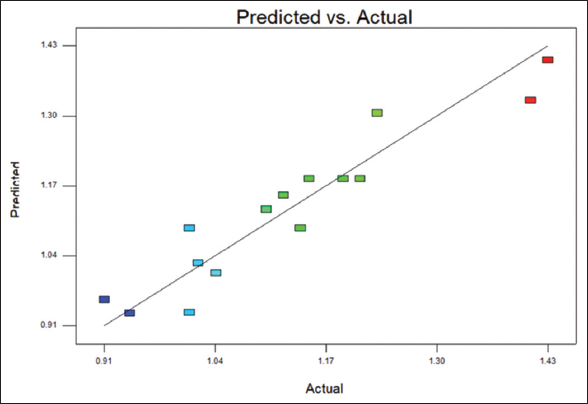 | Figure 1: Scatter plot of predicted value versus actual value of fat content from response surface methodology design. [Click here to view] |
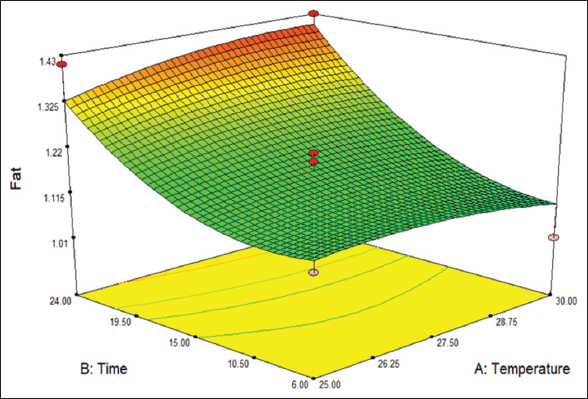 | Figure 2: 3D surface graphical representation of fat content. [Click here to view] |
Table 2: Analysis of variance table for fat content.
| Source | Sum of square | df | Mean square | F value | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 0.29 | 9 | 0.032 | 4.62 | 0.0534 | Not significant |
| A-Temperature | 8.000E-004 | 1 | 8.000E-004 | 0.12 | 0.7480 | |
| B-Time | 0.13 | 1 | 7.55 | 19.07 | 0.0072 | |
| C-ph | 0.025 | 1 | 0.025 | 3.66 | 0.1138 | |
| AB | 3.025E-003 | 1 | 3.025E-003 | 0.44 | 0.5384 | |
| AC | 2.025E-003 | 1 | 2.025E-003 | 0.29 | 0.6123 | |
| BC | 1.560E-003 | 1 | 1.560E-003 | 0.22 | 0.6554 | |
| A2 | 1.753E-003 | 1 | 1.753E-003 | 0.25 | 0.6366 | |
| B2 | 0.021 | 1 | 0.021 | 3.07 | 0.1402 | |
| C2 | 0.093 | 1 | 0.093 | 13.45 | 0.0145 | |
| Lack of fit | 0.033 | 3 | 0.011 | 11.73 | 0.0796 | Not significant |
| R-square value | 0.896 | |||||
Carbohydrates are in the range of 39.3–74.25% as shown in [Table 1]. [Figure 3] shows that the points are not close to the fitted line that indicating there is average fit [Figure 4] indicates the 3D surface of the carbohydrate content. The ANOVA data for the carbohydrate content are tabulated in Table 3.
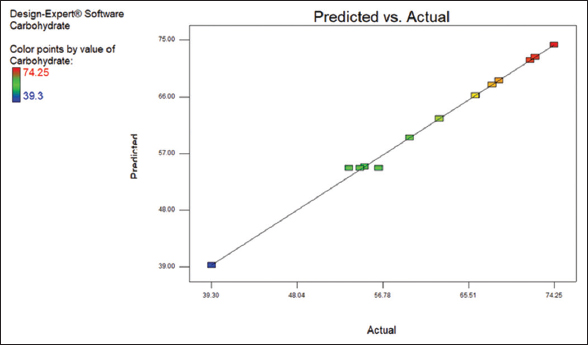 | Figure 3: Scatter plot of predicted value versus actual value of carbohydrate content from response surface methodology design. [Click here to view] |
| Figure 4: 3D surface graphical representation of carbohydrate content. [Click here to view] |
Table 3: Analysis of variance table for carbohydrate content.
| Source | Sum of square | df | Mean square | F-value | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 1214.94 | 12 | 101.25 | 43.39 | 0.0227 | Significant |
| A-Temperature | 34.57 | 1 | 34.57 | 14.82 | 0.0613 | |
| B-Time | 52.56 | 1 | 52.56 | 22.53 | 0.0416 | |
| C-ph | 30.80 | 1 | 30.80 | 13.20 | 0.0681 | |
| R-square value | 0.9962 | |||||
The protein content estimated by the Kjeldahl method and the protein range was 9.02–13.32%, as shown in [Table 1]. R2 value of 0.91 indicates 91% of the goodness of fit. [Figure 5] shows that the points were close to the fitted line indicating a good fit. [Figure 6] indicates the 3D surface of the protein content. The ANOVA data for the protein content are tabulated in Table 4.
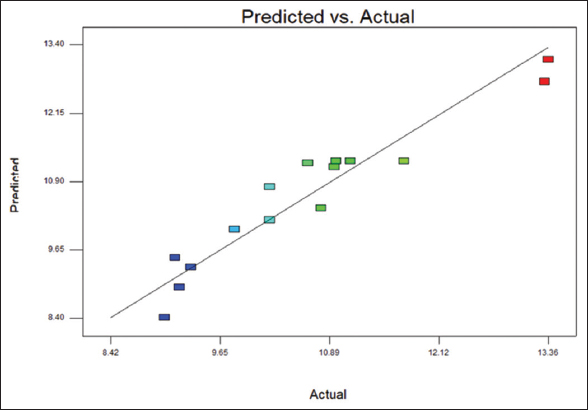 | Figure 5: Scatter plot of predicted value versus actual value of protein content from response surface methodology design. [Click here to view] |
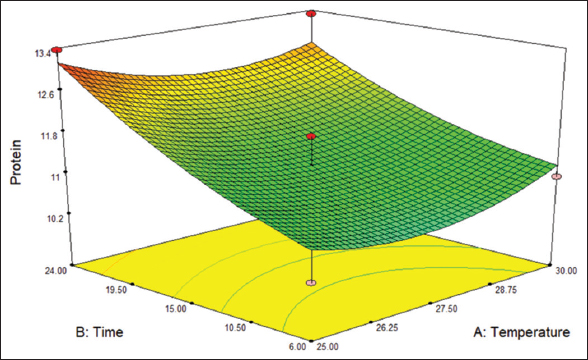 | Figure 6: 3D surface graphical representation of protein content. [Click here to view] |
Table 4: Analysis of variance table for protein content.
| Source | Sum of square | df | Mean square | F-value | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 23.75 | 9 | 2.64 | 5.88 | 0.0327 | Significant |
| A-Temperature | 6.125E-004 | 1 | 6.125E-004 | 1.366E-003 | 0.9720 | |
| B-Time | 7.55 | 1 | 7.55 | 16.83 | 0.0093 | |
| C-ph | 1.57 | 1 | 1.57 | 3.49 | 0.1206 | |
| AB | 0.15 | 1 | 0.15 | 0.33 | 0.5903 | |
| AC | 0.040 | 1 | 0.040 | 0.089 | 0.7772 | |
| BC | 0.11 | 1 | 0.11 | 0.24 | 0.6431 | |
| A2 | 0.73 | 1 | 0.73 | 1.62 | 0.2589 | |
| B2 | 0.22 | 1 | 0.22 | 0.49 | 0.5155 | |
| C2 | 12.62 | 1 | 12.62 | 28.14 | 0.0032 | |
| Lack of fit | 1.91 | 3 | 0.64 | 3.86 | 0.2125 | Not significant |
| R-square value | 0.91 | |||||
After considering all the 3 responses; the sample with 1.32904% fat, carbohydrate of 51.7548%, and protein of 13.1355% was selected as the best sample with a desirability score of 0.686, as shown in Table 5. With a protein content of 13%, it can be claimed as highly nutritious for human health. Figure 7 shows the 3D surface graphical representation of desirability. According to Prakash Tamang et al. [21], during the fermentation procedure, the metabolic activity of bacteria produces a significant quantity of free amino acids. The amount of protein is directly correlated with the density of fermentative bacteria. Increased microbial growth results in higher protein content and nutritional content of the product altered by microbial density.
Table 5: Best sample based on maximum carbohydrate, protein, fat.
| No. | Temperature | Time | pH | Fat | Carbohydrate | Protein | Desirability | |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 24 | 5.49 | 1.32904 | 51.7548 | 13.1355 | 0.686 | Selected |
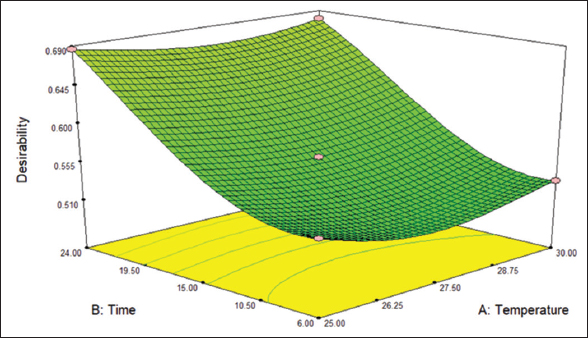 | Figure 7: 3D surface graphical representation of desirability. [Click here to view] |
Table 6 mentioned above indicates that there is little difference between the actual and observed values of fat, protein, and carbohydrates in relation to the values predicted by the software.
Table 6: Predicted versus observed value of carbohydrate, fat and protein in pachoi.
| Parameter | Predicted | Observed |
|---|---|---|
| Carbohydrate | 51.7548 | 52.671 |
| Protein | 13.1355 | 11.89 |
| Fat | 1.32904 | 1.23 |
The pH of pachoi was dropped after 24 h of fermentation, from 5.9 to roughly 4.1. It has been documented that various fermented foods experience a pH drop during cereal fermentation. Lactic acid bacteria use the free sugars in cereal fermentation to make lactic acid, which causes the reduction of pH. pH fall increases the activity of microbial enzymes, which gives extra benefits of fermented meals. Furthermore, meals with low pH helps to preserve food by delaying the growth of several other potentially dangerous microbes [21-23]. The total carbohydrate content was analyzed using phenol sulfuric method. The carbohydrates content found in naturally fermented pachoi was 32.9%. Depending on the type of rice and herbs used in various fermented foods, the quantity of carbohydrates varies [24]. The total protein content of pachoi was analyzed by using the Kjeldahl method and found to be 12.4%. Protein content of traditional Indian fermented breakfast idli and dosa was found as 7.2 ± 1.10 g and 6.6 ± 0.25 g, respectively, and significantly less than pachoi [25]. However, according to the study of Krishnamoorthy et al. [25], no such change was found in the protein content of fermented rice product idli and non-fermented rice product Khaman.
The fat content of the naturally fermented rice sample was estimated at 1.1%. According to the study of Rajalakshmi and Vanaja [26], traditional Indian breakfast idli and dosa were prepared by using rice and millet mix separately through overnight fermentation. Experimental results revealed that fat content in rice-based idli and millet mix idli were 0.84 ± 0.35 g and 5.2 ± 0.28 g, respectively. However, the fat content in rice-based dosa and millet mix dosa was 1.96 ± 0.26 g and 9.8 ± 0.10g, respectively. According to the study of Hannah and Jyoti [27], the fat content of selroti, an ethnic fermented food, was increased after fermentation.
The total alcohol content estimated in pachoi was found to be 1.3% v/v. Alcohol is produced during the fermentation process due to microbial activity. There is a variation in alcohol concentration during the fermentation. According to the study of Ghosh et al. [28], the total alcohol content of the rice beer chuwak in Tripura was found to be 26–35% v/v.
The TPC of FP (L) and FP (N) is 563.66 ± 1.15 and 579 ± 1.15 (mg GAE/100 g). The total flavonoid content and DPPH Radical Scavenging content of both FP (L) and FP (N) are 467 ± 1, 478.66 ± 1.15 (mg QE/100 g) and 68.33 ± 1.52, 71.33 ± 0.57% respectively [Table 7]. According to Saharan et al. [29], phenolic and antioxidant content increases during fermentation this could be because of the important role that enzymes (including glucosidase, xylanase, and amylase) play in the breakdown of insoluble bound phenolics during fermentation. In rice, 46.98 ± 1.02 μM/g GAE was noted, and on the 5th day of incubation, TPC increased up to eight or nine times more during fermentation. Antioxidant phenol compounds are created throughout the process of fermentation by microbes via a secondary metabolic process or are liberated from the matrix of the substrate by extracellular enzymatic action [30]. According to Ghosh et al. [28] 63.42 mg GAE/g total phenol and total Flavonoids 45.36 mg QE/g was observed in Haria a rice fermented food. The fermented rice had a significantly greater number of flavonoids and phenolics. One explanation for this could be that the strain’s production of microbial enzymes and acids helped to liberate the flavonoids and phenolics from their complex in dietary fiber and into a form that was easily soluble. On the 4th day of fermentation, the rice-fermented food had a significant level of free radical scavenging activity. It is associated with fermented rice that has greater levels of flavonoids and phenolics [31]. Ghosh et al. [28] discovered that Haria had 82.54% antioxidant activity against DPPH free radicals.
Table 7: Phenolic, flavonoid and DPPH radical scavenging activity of two sample FP (L) and FP (N).
| Sample ID | Total phenolic content (TPC; mg GAE/100 g) | Total flavonoid content (TFC; mg QE/100 g) | DPPH radical scavenging activity % |
|---|---|---|---|
| FP (L) | 563.66±1.15 | 467±1 | 68.33±1.52 |
| FP (N) | 579±1.15 | 478.66±1.15 | 71.33±0.57 |
The values are expressed as means±SD of triplicate assays, and the values with different superscripts indicate that they are significantly different (P<0.05). TPC: Total phenolic content.
The TPC at the initial stage, i.e., 0 h of fermentation, is 2.07 and 1.02 in FP (N) and FP (L) and then increases after 24 h of fermentation, as shown in Figure 8. The rise in TPC observed during rice fermentation is a typical and predictable outcome, indicating the expansion of microbial populations due to the presence of abundant nutrients and favorable environmental factors. This microbial activity significantly contributes to altering the sensory qualities-such as the consistency, taste, and shelf life-of the resulting fermented product. According to the study of [32], there is a considerable increase in microbial counts during traditional rice fermentation processes. As microorganisms break down the nutrients found in rice, they alter the surrounding environment-producing factors such as a mildly acidic pH, higher carbon dioxide levels, and various metabolic substances-that support the expansion of a wide range of microbial species, especially in the initial phases of fermentation. The starting ingredients, such as rice and water, naturally contain indigenous microorganisms. When fermentation begins, these native microbes become active, utilizing available resources and reproducing quickly, particularly when there are no chemical preservatives or antimicrobial agents present to restrict their growth [33]. When examining other traditional rice-based fermented drinks, clear variations in the levels of total bacterial populations were evident. For example, Haria, a customary fermented rice drink from West Bengal, showed a markedly elevated count of aerobic bacteria at the initial phase of fermentation, measuring 10.51 log10 CFU/g. These aerobic microorganisms remained predominant throughout the early fermentation period, sustaining their high numbers until the 3rd day [34]. On the other hand, Xaj Pani, a traditional starter used for rice wine preparation in Assam, showed a relatively reduced count of aerobic mesophilic bacteria, with values between 1.2 and 3.1 log10 CFU/g [35].
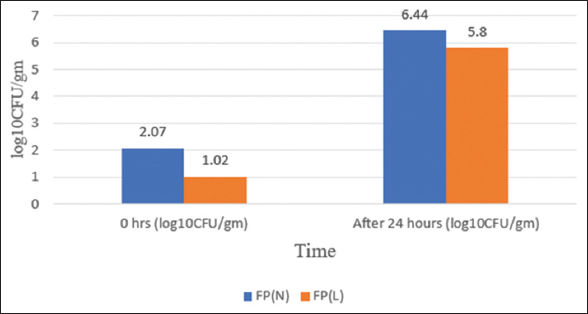 | Figure 8: Total plate count of FP (n) and FP (l). [Click here to view] |
Almost every community in the globe has a distinct food tradition that reflects its ethnic background, social structure, and cultural heritage. In this study, the fermented rice-based food product very popular in Goalpara district, Assam, NER of India, has been established as a fermented rice variety of significant nutritional value in comparison to its natural counterpart. Therefore, it can be considered as the first account of the fermented cuisine of the Goalpara district. According to the study’s findings, the ideal conditions for preparing this food product are 25°C, 24 h, and 5.49 pH. In comparison to FP (L), FP (N) has somewhat higher levels of phenolic, flavonoid, and DPPH free radical scavenging activities. The enhanced nutritional importance under optimized fermentation conditions and considerably low alcohol content has been established its acceptability as a fermented food in this region of India.
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
This study received no specific financial support.
The authors report no financial or any other conflicts of interest in this work.
The Ethical Committee of the Central Institute of Technology Kokrajhar, Assam, India has granted approval for this study on 31May 2025 (Ref. No. CITK/IEC/24).
All data underlying the results is available as part of the article.
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
1. Mishra S, Aravind SM, Charpe P, Ajlouni S, Ranadheera CS, Chakkaravarthi S. Traditional rice-based fermented products:Insight into their probiotic diversity and probable health benefits. Food Biosci. 2022;50:102082. [CrossRef]
2. Tang H, Ma JK, Chen L, Jiang LW, Kang LZ, Guo YY, et al. Characterization of key flavor substances and their microbial sources in traditional sour bamboo shoots. Food Chem. 2024;437:137858. [CrossRef]
3. Kittisuban P, Ritthiruangdej P, Suphantharika M. Optimization of hydroxypropylmethyl cellulose, yeast b-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT Food Sci Technol. 2014;57:738-48. [CrossRef]
4. Acharya PP, Panda PK, Kiran D, Sahu AK. A review articles of Clinical outcomes, and the origin, transmission, Immunologic aspects of characteristics and public health response to novel corona virus (COVID19). Asian J Pharm Res Dev. 2020;8(5):129-37. [CrossRef]
5. Ray S, Bagyaraj DJ, Thilagar G, Tamang JP. Preparation of Chyang, an ethnic fermented beverage of the Himalayas, using different raw cereals. J Ethnic Foods. 2016;3:297-9. [CrossRef]
6. Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, FolignéB, et al. Health benefits of fermented foods:Microbiota and beyond. Curr Opin Biotechnol. 2017;44:94-102. [CrossRef]
7. Jha PK, Le-Bail A, Rawson A, Parua S, Mohapatra PK, Mondal KC. Indigenous fermented beverages of the Indian subcontinent:Processing methods, nutritional, and nutraceutical potential. In:Food Bioactives. Florida:Apple Academic Press;2019. pp. 323-58. [CrossRef]
8. Safrakidou P, Michaelidou AM, Biliaderis CG. Fermented cereal-based products:Nutritional aspects, possible impact on gut microbiota and health implications. Foods. 2020;9(6):734. [CrossRef]
9. Mondal KC, Ghosh K, Mitra B, Parua S, Das Mohapatra PK. Rice-based fermented foods and beverages:Functional and nutraceutical properties. In:Ray RC, Montet D, editors. Fermented Foods:Part II:Technological Interventions. 1st ed. Boca Raton:CRC Press;2016. pp. 150-76. [CrossRef]
10. Hor PK, Pal S, Halder SK, Ghosh K, Chakrabarti S, Dwivedi SK, et al. Antiobesity, antihyperglycemic, and antidepressive potentiality of rice fermented food through modulation of intestinal microbiota. Front Microbiol. 2022;13:794503. [CrossRef]
11. Hor PK, Goswami D, Ghosh K, TakóM, Halder SK, Mondal KC. Preparation of rice fermented food using root of Asparagus racemosus as herbal starter and assessment of its nutrient profile. Syst Microbiol Biomanuf. 2022;2:147-56. [CrossRef]
12. Tan BL, Norhaizan ME, Chan LC. Rice bran:From waste to nutritious food ingredients. Nutrients. 2023;15(11):2503. [CrossRef]
13. Aydar AY. Utilization of response surface methodology in optimization of extraction of plant materials. In:Statistic Approaches with Emphasis on Design of Experiments Applied to Chemical Processes. London:Intecopen;2018. pp. 157-69. [CrossRef]
14. Veluppillai S, Nithyanantharajah K, Vasantharuba S, Balakumar S, Arasaratnam V. Biochemical changes associated with germinating rice grains and germination improvement. Rice Sci. 2009;16:240-2. [CrossRef]
15. Oliveira MD, Feddern V, Kupski L, Cipolatti EP, Badiale-Furlong E, Souza-Soares LA. Physico-chemical characterization of fermented rice bran biomass Caracterizaciónfisico-química de la biomasa del salvado de arroz fermentado. CyTA J Food. 2010;8:229-36. [CrossRef]
16. Guo Q, Cui SW, Kang J. Classical Methods for Food Carbohydrate Analysis. United States:Wiley;2014. [CrossRef]
17. AOAC. Official Methods of Analysis of the Association of Analytical Chemists International. 18th ed. Maryland, USA:AOAC Gaithersburg;2005.
18. Khatun L, Ray S, Baruah DC. Impact of germination versus non-germination on nutritional and functional potentials of two rice varieties (Ranjit and Ahu Kalogoria) available in three different districts of Assam, India. J Food Technol Res. 2023;10(4):103-119. [CrossRef]
19. Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010-4. [CrossRef]
20. Mangang KC, Das AJ, Deka SC. Comparative shelf life study of two different rice beers prepared using wild-type and established microbial starters. J Inst Brew. 2017;123(4):523-30. [CrossRef]
21. Prakash Tamang J, Thapa S. Fermentation dynamics during production of BhaatiJaanr, a traditional fermented rice beverage of the Eastern Himalayas. Food Biotechnol. 2006;20:251-61. [CrossRef]
22. Magala M, KohajdováZ, KarovicováJ, GreifováM, HojerováJ. Application of lactic acid bacteria for production of fermented beverages based on rice flour. Czech J Food Sci. 2016;33:458-63. [CrossRef]
23. Goswami G, Baruah H, Boro RC, Barooah M. Fermentation reduces anti-nutritional content and increases mineral availability in poitabhat. Asian J Chem. 2016;28(9):1929-32. [CrossRef]
24. Borah VV, Choudhury M, Phanjom P. Preparation and health benefits of rice beverages from ethnomedicinal plants:Case Study in North-East of India. In:Plant-Based Functional Foods and Phytochemical. Florida:Apple Academic Press;2021. 36. [CrossRef]
25. Krishnamoorthy S, Kunjithapatham S, Manickam L. Traditional Indian breakfast (Idli and Dosa) with enhanced nutritional content using millets. Nutr Diet. 2013;70:241-6. [CrossRef]
26. Rajalakshmi R, Vanaja K. Chemical and biological evaluation of the effects of fermentation on the nutritive value of foods prepared from rice and grams. Brit J Nutr. 1967;21(2):467-73. [CrossRef]
27. Hannah Y, Jyoti PT. Microbiology and nutritional value of Selroti, an ethnic fermented cereal food of the Himalayas. Food Biotechnol. 2010;24(3):227-47. [CrossRef]
28. Ghosh SK, Rahaman L, Kaipeng DL, Deb D, Nath N, Tribedi P, et al. Community-wise evaluation of rice beer prepared by some ethnic tribes of Tripura. J Ethn Foods. 2016;3:251-6. [CrossRef]
29. Saharan P, Sadh PK, Duhan JS. Comparative assessment of effect of fermentation on phenolics, flavanoids and free radical scavenging activity of commonly used cereals. Biocatal Agric Biotechnol. 2017;12:236-40. [CrossRef]
30. Dey TB, Chakraborty S, Jain KK, Sharma A, Kuhad RC. Antioxidant phenolics and their microbial production by submerged and solid-state fermentation process:A review. Trends Food Sci. 2016;53:60-74. [CrossRef]
31. Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50(7):2161-8. [CrossRef]
32. Omemu AM, Bankole MO. Microbiological and physicochemical changes during fermentation of rice (Oryza sativa) for the production of rice gruel. Afr J Food Sci. 2015;9(5):247-53.
33. Melaku A, Satheesh N, Awol SJ, Tadesse A, Solomon T, Bultosa G, et al. The effect of fermentation temperature and duration on the physicochemical, microbial, and sensory attributes of Kocho. Discov Food. 2024;4:170. [CrossRef]
34. Ghosh K, Ray M, Adak A, Dey P, Halder SK, Das A, et al. Microbial, saccharifying and antioxidant properties of an Indian rice based fermented beverage. Food Chem. 2015;168:196-202. [CrossRef]
35. Keot J, Bora SS, Das Kangabam R, Barooah M. Assessment of microbial quality and health risks associated with traditional rice wine starter Xaj-pitha of Assam, India:A step towards defined and controlled fermentation. 3 Biotech. 2020;10(2):64. [CrossRef]
Year
Month
Effect of Wild and Mutant Strain of Lasiodiplodia Pseudotheobromae Mass Produced on Rice Bran as a Potential Bioherbicide Agents for Weeds under Solid state Fermentation
Adetunji C. O.a, Oloke J.KbMedium Formulation and its optimization for increased protease production by Penicillium sp. LCJ228 and its potential in blood stain removal
V. Benluvankar, S. Evelyne Priya, J. Joel GnanadossProduction and Characterization of Collagenase by Penicillium sp. UCP 1286 Isolated From Caatinga Soil
Maria Carolina de Albuquerque Wanderley , Jose Manoel Wanderley Duarte Neto, Carolina de Albuquerque Lima, Sara Isabel da Cruz Silverio, Jose Luiz de Lima Filho, Jose Antonio Couto Teixeira, Ana Lucia Figueiredo PortoScreening, Selection and Optimization of the Culture Conditions for Tannase Production by Endophytic Fungi Isolated from Caatinga
Rayza Morganna Farias Cavalcanti, Pedro Henrique de Oliveira Ornela, João Atílio Jorge, Luís Henrique Souza GuimarãesProteome and fermentative parameters of Saccharomyces cerevisiae CAT-1 under Very High Gravity Fermentation (VHGF) using sugarcane juice
Gabriela de Sá Azarias, Heloisy Suzes Barbosa, Cynthia Barbosa Rustiguel, José Cesar Rosa, José Roberto Ernandes, Luis Henrique Souza GuimarãesProduction, characterization and optimization of fermented tomato and carrot juices by using Lysinibacillus sphaericus isolate
Naga Sivudu Seelam, Harika Akula, Umamahesh Katike, Vijaya Sarathi Reddy ObulamFermentative Production of Polyhydroxyalkanoates (PHAs) from Glycerol by Zobellella taiwanensis Azu-IN1
Mohamed Ali Abdel-Rahman, Said El-Sayed Desouky, Mohamed Salah Azab, Mahmoud E. EsmaelBiochemical and molecular characterization of a new pullulan producer Rhodosporidium paludigenum PUPY-06
RS Singh, Navpreet KaurBiocatalysis of agro-processing waste by marine Streptomyces fungicidicus strain RPBS-A4 for cellulase production
Rajanikanth Akurathi, Damodharam ThotiInulinase production in shake-flask fermentations from Mucor circinelloides BGPUP-9
Ram Sarup Singh, Kanika Chauhan, Randeep Kaur, Ramanpreet KaurLaccase production by Myrothecium gramineum and its optimization under solid state fermentation using cowpea pod as substrate
V. Daphne Vivienne Gnanasalomi, J. Joel GnanadossScreening and identification of amylase producing strains of Bacillus
Kumar Pranay, Shree Ram Padmadeo, Vijay Jha, Birendra PrasadAnalysis of physicochemical and sensory parameters of wine produced from Carica papaya
Suhail Cholassery, Vidhu Krishna, SreethuVeliparambil Sethuraj,Shabnam Shajahan Rehina, Vandana Ranganathan, Lisha Chanassery Dileep, Kuzhunellil Raghavanpillai Sabu, Bhama Ramachandran Rajesh, Ramachandran Pratap ChandranEnhanced biobutanol production by optimization of the medium from Clostridium acetobutylicum MTCC 11274 using macroalgae Gracilaria edulis
Shelly Rana, Kashyap Dubey, Ashwani DhingraMedia optimization studies and production of adenosylcobalamin (Vitamin B12) by environment friendly organism Rhizobium spp
Neha Nohwar, Rahul V. Khandare, Neetin S. DesaiAmylase production by Aspergillus niger in submerged cultivation using cassava
Muralikandhan Kamaraj, Dhanasekaran SubramaniamProduction and purification of extracellular fungal cellulases using agricultural waste
Abishna Burugu, Dheerendra Kumar Suman, Chandrasekhar ChandaEnhancement of Vitamin D2 content through ultraviolet-B irradiation in submerged cultivated Pleurotus eryngii mycelia using response surface methodology
Umesh Singh, Ashwani Gautam, Satyawati SharmaRole of lacto-fermentation in reduction of antinutrients in plant-based foods
Mehak Manzoor, Deepti Singh, Gajender Kumar Aseri, Jagdip Singh Sohal, Shilpa Vij, Deepansh SharmaImpact of diverse processing treatments on nutritional and anti-nutritional characteristics of soybean (Glycine max L.)
Priyanka Thakur, Krishan Kumar, Naseer Ahmed, Ajar Nath Yadav, Sunil Kumar, Qurat Ul Eain Hyder Rizvi, Divya Chauhan, Sumaira JanEffect of diverse fermentation treatments on nutritional composition, bioactive components, and anti-nutritional factors of finger millet (Eleusine coracana L.)
Sumaira Jan, Krishan Kumar, Ajar Nath Yadav, Naseer Ahmed, Priyanka Thakur, Divya Chauhan, Qurat-Ul-Eain Hyder Rizvi, Harcharan Singh DhaliwalSolid-state fermentation of groundnut (Arachis hypogaea) shell using Trichoderma sp., tape yeast, and tempeh yeast to produce cellulase
Muhammad Yusuf Abduh, Chalil Rizqullah Ramadhan, Alfanny Putri Fadhlilah, Siti Dhiffah Nabilah Abdul, Khairul Hadi BurhanIdentification and characterization of acidosis on in vitro rumen fermentation with feeds based on grass, rice bran, concentrate, and tofu pulp
Darwin, Tiya Humaira, Ami MuliawatiImpact of soaking, germination, fermentation, and roasting treatments on nutritional, anti-nutritional, and bioactive composition of black soybean (Glycine max L.)
Divya Chauhan, Krishan Kumar, Naseer Ahmed, Priyanka Thakur, Qurat Ul Eain Hyder Rizvi, Sumaira Jan, Ajar Nath YadavExtraction of a novel bacteriocin from Lacticaseibacillus casei VITCM05 and its antibacterial activity against major food-borne pathogens
Jannatul Firdous Siddique, Mohanasrinivasan VaithilingamIsolation, characterization and optimization of keratinolytic bacteria from chicken feather waste dumping site
Thiyagarajan Amuthavalli, Cyril RaviHigh cell density fermentation strategies for the production of bovine lactoferrin in Pichia pastoris
Thuy Thi Thu Trinh, Huong Thu Ngo, Phong Quoc TruongIncreasing polyphenol antioxidant in Orthosiphon stamineus Benth leaves with fermentation extraction by Saccharomyces cerevisiae ATCC-9763
Muhammad Aria Chandra, Khaswar Syamsu, Laksmi Ambarsari, Nurul Fatimah, Waras Nurcholis,A novel fibrinolytic enzyme producer from mangrove soil sediments: Screening, isolation, strain improvement, and fermentation
Bhavana Sompalli, Alok Malaviya,,Effects of process parameters on the alcoholic fermentation of pomelo (Citrus grandis (L.) Osbeck) juice
Huynh Xuan Phong, Tran Thi Yen Nhi, Nguyen Ngoc Thanh, Le Dang TruongRecent advances in the processing of Napier grass (Pennisetum purpureum Schumach) as a potential bioenergy crop for bioethanol production
Priya Chamoli, Samiksha Jhildiyal, Palak Agrawal, Navin Kumar, Pallavi SinghOptimizing solid-state fermentation for metabolite enrichment by Aspergillus tamarii on rice bran and wheat
Syaefudin Suminto,, Arthur Alba Huang, Uswatun Hasanah, Waras Nurcholis,Statistical optimization of process variables for anti-hypercholesterolemic metabolites production from Monascus purpureus MTCC 369 fermented finger millet
Monu Kumar, Gaurav Chaudhary, Anita Rani Sehrawat, Anoop Singh, Guddu Kumar GuptaInvestigation of optimal parameters for aspartic protease production from Aspergillus terreus using solid-state fermentation
Sadeq Fenjan Hasnawi,, Alaa Jabbar Abd Al-Manhel, Zena Kadhim AL-YounisThe fermentation conditions of low alcoholic three-leaved (Cayratia trifolia (L.) Domin) cider using Saccharomyces cerevisiae HG1.3
Tien Thi Kieu Doan, Mi Thi Ngoc Huynh, Thu Thi Minh Tran, Thanh Huu Nguyen, Son Thi Bich Le, Thanh Ngoc Nguyen, Phong Xuan HuynhSustainable improvement of nutrition quality and biological activity from cassava residue and okara through solid-state fermentation by Pleurotus citrinopileatus mycelium
Hang Nguyen Thi Bich, Cuong Chi Doan, Uyen Nguyen Khanh Phan, Khanh Trang Vu Le, Thang Duc Bui, Munehiro Tanaka, Minh Van VoMutational enhancement of Aspergillus niger Tiegh. for higher cellulase production comparable to Trichoderma species in solid-state fermentation
Harjeet Singh, Komal Janiyani, Ajit GangawaneOptimization of Justicia gendarussa Burm.f. fermentation by Aspergillus oryzae based on total phenolic, total flavonoid, and antioxidant capacity responses
Syaefudin Suminto,, Chandra Daniel Setiawan, Waras Nurcholis,, Uswatun Hasanah,, Trivadila TrivadilaThe application of natural Saccharomyces cerevisiae HG 1.3 and commercial Saccharomyces cerevisiae RV002 yeast strains in mixed fermentation to improve dragon fruit beverage ethanol content and sensory properties
Tien Thi Kieu Doan, Hoa T. TruonghuynhEnhanced fibrinolytic protease production by Serratia marcescens RSPB11 through Plackett-Burman and response surface methodological approaches
Paruchuru Lakshmi Bhargavi, Reddy Shetty PrakashamStatistical optimization of culture conditions for enhanced mycelial biomass production using Ganoderma lucidum
Pooja Shah, Hasmukh ModiStatistical optimization of chitinase production by Box–Behnken design in submerged fermentation using Bacillus cereus GS02
Garima Dukariya, Anil KumarOptimization and statistical modeling of microbial cellulase production using submerged culture
Pratibha Maravi, Anil KumarStatistical optimization of fermentation media for beta lactamase inhibitor kalafungin production from marine Streptomyces sp. SBRK1
Thankaraj Rajam Jabila Mary, Rajaretinam Rajesh Kannan, Appadurai Muthamil Iniyan, Samuel Gnana Prakash VincentOptimization of process and conditions for enhanced xylanase production under SSF using inexpensive agro-industrial waste
Vimalashanmugam Kanagasabai, Karuppaiya MaruthaiOptimization of extraction conditions of phytochemical compounds in “Xiem” banana peel powder using response surface methodology
Ngo Van Tai, Mai Nhat Linh, Nguyen Minh ThuyOptimization of ingredient levels of reduced-calorie blackberry jam using response surface methodology
Nguyen Minh Thuy, Huynh Manh Tan , Ngo Van TaiMedia optimization for the production of alkaline protease by Bacillus cereus PW3A using response surface methodology
Gururaj B. Tennalli, Soumya Garawadmath, Lisa Sequeira, Shreya Murudi, Vaibhavi Patil, Manisha N. Divate, Basavaraj S. HungundOptimization of active antioxidative defatted Canarium indicum L. (Canary) protein hydrolysate production
Cintya Nurul Apsari,, Ilma Nugrahani, Sukrasno, Tutus GusdinarOptimization of pasteurization process of the ready-to-drink beverage from Hong Quan (Flacourtia jangomas) fruit by response surface methodology
Tan Duy Nguyen,, Tuyen Thi Xuan Vo,, Khang Nghia Tran,Optimization for nutritional fortification of wheat–millet composite flour mixture by response surface methodology
Gaurav Chaudhary, Monu Kumar, Anita Rani Sehrawat, Sandeep Kumar, Sachidanand TripathiOptimization of biomethane production from pet coke through anaerobic digestion using microbial inoculum and Fe2O3 nanoparticles: A response surface methodology approach
Ravikumar Rajarathinam, Mazen Yousif