1. INTRODUCTION
Cardiovascular diseases (CVDs) are responsible for the highest number of deaths worldwide. According to estimates, around 17.9 million people die every year due to CVDs, which account for 32% of all global deaths [1]. One of the major contributing factors to CVDs is hypercholesterolemia, a condition where the level of cholesterol in the blood is abnormally high. Hypercholesterolemia has become a significant risk factor for CVDs and is responsible for 2.6 million deaths annually [2]. Therefore, managing hypercholesterolemia is crucial to prevent CVDs.
Monascus purpureus (Ascomycetes fungus) is a species of red mold that has been used for centuries in Asian countries, where rice fermented with this fungus is primarily used as food and food colorant [3]. M. purpureus fermented rice is known as a red yeast rice (RYR) and it is known to produce anti-hypercholesterolemic, anti-hypertensive metabolite, etc. M. purpureus is primarily cultivated to produce various secondary metabolites, such as lovastatin (monacolin k), mevastatin, monacolin J, and pravastatin [4]. Lovastatin is the primary active compound in M. purpureus fermented food, that is, RYR [5]. The first commercial anti-cholesterolemic drug authorized by US Food and drug administration was lovastatin [6]. It lowers blood cholesterol levels by competitively inhibiting 3-hydroxy-3-methylglutaryl Co-A reductase enzyme during cholesterol biosynthetic pathway [7]. In addition to lowering cholesterol, lovastatin also has antimicrobial, anticancer, and neuroprotective effects [8].
Over the past 30 years, lovastatin, as a hypocholesterolemic drug commonly produced by Aspergillus terreus, has been effectively developed and widely utilized throughout the world [9]. Nonetheless, lovastatin produced by A. terreus is typically employed as a clinical drug, as this strain is not usually acknowledged as safe. M. purpureus contains high lovastatin content and has less adverse effects and a range of other biological functions as compared to lovastatin from A. terreus. Along with statins, the fermented rice of M. purpureus (RYR) also includes lipid-reducing components like β-sitosterol, stigmasterol, and campsterol. Sterol helps in lowering levels of low-density lipids in the blood, hence lowering total cholesterol without influencing high-density lipoproteins and triglycerides levels [10]. In the therapeutic use of anti-hypercholesterolemia, the combination of statin and sterols is more effective in decreasing cholesterol levels than the use of statin alone [11].
Eleusine coracana L. (Finger millet [FM]) is a member of the Poaceae family. In India, it is commonly known as ragi, in South Africa as rapoko, and in Ethiopia as dagusa. FM is grown in various regions of India and Africa and is a staple food crop for a large proportion of the population in these countries [12]. It is an agriculturally sustainable crop because it can be cultivated at high altitudes, on small lands, and it also can easily tolerate high water and salt stress. In addition, it requires very less water for irrigation and fertilizers, but it still manages to provide optimal yields [13,14]. Nutritionally, FM is highly rich in minerals and has a greater micronutrient quantity than the world’s two most important cereals, wheat and rice. Specifically, it has up to 10 times more calcium than rice, maize, and wheat, and 3 times more than milk. Aside from calcium, it is an excellent source of iron, amino acids such as methionine, slowly digesting starch, and phytochemicals such as polyphenols [15].
Fungi are responsible for producing a variety of biotechnologically important products, such as enzymes and secondary metabolites. Interestingly, most of these products are obtained from fungi that were grown on solid substrates. In recent times, solid-state fermentation (SSF) has become increasingly popular for the production of secondary metabolites. This method provides a more conducive environment for fungi to thrive, resulting in a higher yield of products. In addition, using agricultural and industrial residues as a substrate has several advantages, such as better fermentation output, less-catabolic suppression, and reduced water requirements [16].
Optimization process is a time-consuming procedure due to the involvement of multiple process factors. During this procedure, relevant factors are initially screened, and these selected factors are subsequently optimized using various methodologies. Therefore, a statistical tool optimization consisting a three-factorial design developed in response surface methodology (RSM) that shows the correlation between independent input factors and one or more dependent responses [17,18]. RSM provides several advantages, including fewer experiments, adaptability for multivariable experiments, the search for factor-factor relationships, and the identification of the optimal condition and anticipated response [19].
Many experiments in the field of biomedicine and functional foods have focused on maximizing the yield of anti-hypercholesterolemic metabolites through the fermentation process [20]. This research aims to analyze and validate variables that contribute to the maximum production of statin and sterol from M. purpureus MTCC 369 using FM as a substrate in the SSF fermentation process. To the best of our knowledge, this is the first research report on the optimization of anti-hypercholesterolemic metabolites production from Monascus purpureus MTCC 369 fermented FM.
2. MATERIALS AND METHODS
2.1. Microorganism
M. purpureus MTCC 369 culture was procured from the Institute of Microbial Technology (IMTECH), Chandigarh, India. The fungus strain was maintained and stored on potato dextrose agar (PDA) slants at 4°C and sub-cultured at every 30-day intervals.
2.2. Chemicals and Substrate
Standards of cholesterol and lovastatin were purchased from Sigma Chemical Co. Bangalore, India. PDA, methanol, chloroform, and other solvents were of highest pure and analytical quality from Himedia Ltd., India. Eleusine coracana (FM) for SSF was procured from the Centre of Excellence in Millets, Tamil Nadu Agricultural University, Athiyandal, Tamil Nadu, India.
2.3. Inoculum Preparation
The spore suspensions (5.7 × 103 spores/mL) of 15% were transferred into 250 mL Erlenmeyer flasks containing 100 mL of basal medium (100 g dextrose, 10 g C13H24O4, 2 g NH4H2PO4, 2 g KNO3, 0.5 g MgSO47H2O, and 0.1 g CaCl2 in 1L of dH2O, pH 6) [19]. Finally, each fungus culture was incubated at 30°C for 48 h at 120 rpm in a shaker incubator.
2.4. Preparation of Substrate
The wet filter paper was put in the petri dish and then seeds of FM were evenly dispersed, and water was sprayed on them at a rate of 2:1 w/v. The petri dishes were incubated at 30°C for 72 h, then the germinated seeds of FM were dried for 24 h in a drier at 40°C. The germinated FM was coarsely ground and used as substrate [21].
2.5. Solid-state Fermentation
In a 500 mL conical flask, 35 mL of dH2O was added to 20 g of FM seeds. The substrates were then autoclaved and cooled overnight at room temperature (27 ± 2°C). After that, the FM medium was inoculated with a culture of M. purpureus MTCC 369 [21]. RSM was used to design the fermentation process conditions, including fermentation period, temperature, inoculum size, and pH, for independent experimental runs at various levels.
2.6. Optimization of Culture Conditions by Central Composite Design (CCD)
CCD is considerable the most useful and popular second-order design. Based on the literature survey we have selected the following four physical parameters, namely: (a) Fermentation time period, (b) temperature, (c) pH, and (d) inoculum volume for the optimization process using CCD approach. Different ranges of these independent variables used in 30 experiment runs given by design expert 13.0 (Stat-Ease Inc., USA) software are presented in Tables 1 and 2. All the experiments were performed in triplicates and the mean value was expressed as response. The 3D surface response was studied for determination of correlation between independent variables on the production of metabolites. Statistically significant factors were evaluated through analysis of variance (ANOVA).
Table 1: Experimental levels of process parameters (independent variables).
| Factors | Name | Units | Min. | Max. | Coded Low | Coded High |
|---|---|---|---|---|---|---|
| A | Time | Days | 8.00 | 20.00 | −1↔11.00 | +1↔17.00 |
| B | Temperature | °C | 25.00 | 35.00 | −1↔27.50 | +1↔32.50 |
| C | pH | 3.00 | 7.00 | −1↔4.00 | +1↔6.00 | |
| D | Inoculum Volume | mL | 3.50 | 7.50 | −1↔4.50 | +1↔6.50 |
Table 2: Central composite design for process parameters with statin and total sterols concentration (actual and predicted values).
| Runs | A: Time (Days) | B: Temperature °C | C: pH | D: Inoculum volume (mL) | Statin (mg/g) | Total Sterol (mg/g) | ||
|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | |||||
| 1 | 14 | 25 | 5 | 5.5 | 0.20 | 1.40 | 0.1 | 0.17 |
| 2 | 17 | 27.5 | 4 | 6.5 | 4.2 | 4.17 | 0.26 | 0.25 |
| 3 | 14 | 30 | 5 | 5.5 | 9.43 | 10.32 | 0.44 | 0.52 |
| 4 | 17 | 27.5 | 6 | 4.5 | 4.63 | 4.42 | 0.28 | 0.27 |
| 5 | 14 | 30 | 3 | 5.5 | 6.78 | 6.24 | 0.47 | 0.41 |
| 6 | 11 | 32.5 | 4 | 6.5 | 2.41 | 3.21 | 0.067 | 0.11 |
| 7 | 17 | 32.5 | 6 | 4.5 | 1.82 | 2.59 | 0.19 | 0.2 |
| 8 | 14 | 30 | 5 | 5.5 | 11.12 | 10.32 | 0.5 | 0.52 |
| 9 | 11 | 32.5 | 6 | 6.5 | 3.22 | 3.77 | 0.09 | 0.09 |
| 10 | 11 | 32.5 | 6 | 4.5 | 3.70 | 4.32 | 0.07 | 0.11 |
| 11 | 20 | 30 | 5 | 5.5 | 0.23 | −0.47 | 0.13 | 0.10 |
| 12 | 14 | 30 | 5 | 5.5 | 10.29 | 10.32 | 0.61 | 0.52 |
| 13 | 14 | 30 | 5 | 7.5 | 9.13 | 8.51 | 0.33 | 0.32 |
| 14 | 11 | 32.5 | 4 | 4.5 | 3.02 | 3.43 | 0.062 | 0.09 |
| 15 | 14 | 30 | 5 | 5.5 | 10.54 | 10.32 | 0.55 | 0.52 |
| 16 | 11 | 27.5 | 6 | 6.5 | 5.55 | 5.47 | 0.28 | 0.26 |
| 17 | 14 | 35 | 5 | 5.5 | 0.12 | −2.02 | 0.04 | −0.08 |
| 18 | 17 | 32.5 | 6 | 6.5 | 1.98 | 2.59 | 0.17 | 0.20 |
| 19 | 8 | 30 | 5 | 5.5 | 2.12 | 1.88 | 0.09 | 0.07 |
| 20 | 17 | 27.5 | 4 | 4.5 | 4.20 | 4.01 | 0.28 | 0.26 |
| 21 | 17 | 32.5 | 4 | 4.5 | 1.63 | 2.29 | 0.11 | 0.17 |
| 22 | 17 | 27.5 | 6 | 6.5 | 4.30 | 4.25 | 0.26 | 0.22 |
| 23 | 11 | 27.5 | 4 | 4.5 | 5.20 | 5.18 | 0.33 | 0.33 |
| 24 | 11 | 27.5 | 6 | 4.5 | 6.64 | 6.19 | 0.38 | 0.34 |
| 25 | 14 | 30 | 5 | 5.5 | 10.24 | 10.32 | 0.54 | 0.52 |
| 26 | 14 | 30 | 7 | 5.5 | 7.62 | 7.22 | 0.38 | 0.39 |
| 27 | 14 | 30 | 5 | 3.5 | 9.21 | 8.89 | 0.38 | 0.34 |
| 28 | 17 | 32.5 | 4 | 6.5 | 1.82 | 2.63 | 0.19 | 0.22 |
| 29 | 11 | 27.5 | 4 | 6.5 | 5.20 | 4.79 | 0.32 | 0.3 |
| 30 | 14 | 30 | 5 | 5.5 | 10.31 | 10.32 | 0.52 | 0.52 |
2.7. Experimental Model Validation
The experimental model and regression equation were carried out at the software-based predicted optimum value of independent variables in the fermentation medium. The flask containing fermentation medium with CCD optimized condition of fermentation temperature 29.44°C, fermentation time of 13.8 days, inoculum volume 5.37 mL, and pH 5.0 was performed. By taking the response in triplicates, the predicted response model was validated.
2.8. Extraction and Quantification of Statin
Fermented FM was dried for 24 h at 50°C and ground into a powder. After that, 1.0 g of dried material was mixed with methanol: Water (50:50) and then incubated at 28°C in a shaker up to 2 h at 150 rpm to extract statin. Then, the mixture was centrifuged at 10,000 rpm for 15 min followed by filtration through 0.45-μm size membrane filter [22]. 1 mL of supernatant was taken with 1 mL of 1% tri-fluoro acetic acid and incubated for 10 min for lactonization of statin. 0.5 mL solution was taken from the above and 10 times diluted with methanol and absorbance was recorded at 238 nm using UV-Vis spectrophotometer (Agilent Technologies, California, USA) [23].
2.9. Extraction and Quantification of Total Sterol
1g of grounded fermented material was taken with 20 mL of 2.5 N NaOH and autoclaved. After the autoclave, an equal amount of ether was mixed and shaken for 1 h in an incubator [24]. At 4°C, the solution was allowed for separation. An ether layer was formed having sterol separated by a separating funnel. A small quantity of Na2SO4 was added to the substance, which was then dried with N2 gas to remove moisture. The dried compound was mixed with chloroform (1 mL) and then filtered by a membrane filter (0.45 μm). The solvent containing filtrate was evaporated by placing it in the oven at 50°C. The residue of cholesterol was resuspended with chloroform (1 mL) and absorption of sterol was taken on a UV-VIS spectrophotometer (Agilent Technologies, California, USA) at 640 nm [25].
3. RESULTS
3.1. Process Optimization for Hyperproduction of Anti-hypercholesterolemic Metabolites using CCD Approach
To optimize the process variables affecting the synthesis of statins and sterols, we performed SSF in an Erlenmeyer flask with FM and M. purpureus MTCC 369. Four independent variables (fermentation time, temperature, pH, and inoculum volume,) were selected for investigation because these parameters affect fungal growth and secondary metabolite production in SSF. A 30-run experimental design with five central points was performed using CCD for the four independent variables. The effects of each of these parameters and how they work together were studied by letting the fermentation be carried out at multiple levels of all four factors that were chosen at random [Table 1]. The response of every single run was measured in terms of statin and sterol production.
The results of predicted values and actual values of statin and sterol are presented in Table 2. Production of statin and sterol in each run was analyzed by Design Expert 13 (Stat-Ease Inc., USA) software. A quadratic model was chosen to study the response based on the fit summary suggested by Design Expert software [Tables 3 and 4]. The model’s goodness of fit was assessed by correlation coefficient (R2), which indicates correlation variability and their interactions. The R2 value (close to 1) implies a greater and more predictive model.
Table 3: Fit summary of statin.
| Source | Sequential P-value | Lack of fit P-value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | 0.7177 | 0.0002 | −0.0700 | −0.2225 | |
| 2FI | 1.0000 | <0.0001 | −0.4043 | −0.6630 | |
| Quadratic | <0.0001 | 0.0745 | 0.9291 | 0.8068 | Suggested |
| Cubic | 0.2207 | 0.0747 | 0.9508 | −0.1106 | Aliased |
Table 4: Fit summary of sterol.
| Source | Sequential P-value | Lack of fit P-value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | 0.4785 | 0.0070 | −0.0139 | −0.1417 | |
| 2FI | 0.9906 | 0.0035 | −0.2809 | −0.5511 | |
| Quadratic | <0.0001 | 0.3907 | 0.8634 | 0.6757 | Suggested |
| Cubic | 0.3749 | 0.3671 | 0.8817 | −0.3849 | Aliased |
In Table 3, the fit summary of statin shows that the R2 value is 0.9633, indicating that 96.33% of the correlation between independent variables and response for statin production. The predicted R2 value of 0.8068 is reasonably close to the adjusted R2 value of 0.9291, with a difference of <0.2. Similarly, in Table 4, the fit summary of total sterol shows an R2 value of 0.9294, which indicates that 92.94% of the total correlation is attributed to the independent variables and sterol production response. The adjusted R2 value of 0.8634 is also reasonably close to the predicted R2 value of 0.6757. The difference between the two values is <0.2. The correlation coefficient of the given model showed a significant agreement between observed and predicted responses of statin and total sterol production from SSF, thus suggesting a highly significant model. To analyze the effects of four factors on statin and sterol production, we used a multiple non-linear quadratic model. The model generated 3D response surface plots for each response variable, as shown in Figures 1 and 2 for statin and sterol, respectively. These plots illustrate the optimal levels of the factors for maximizing the responses.
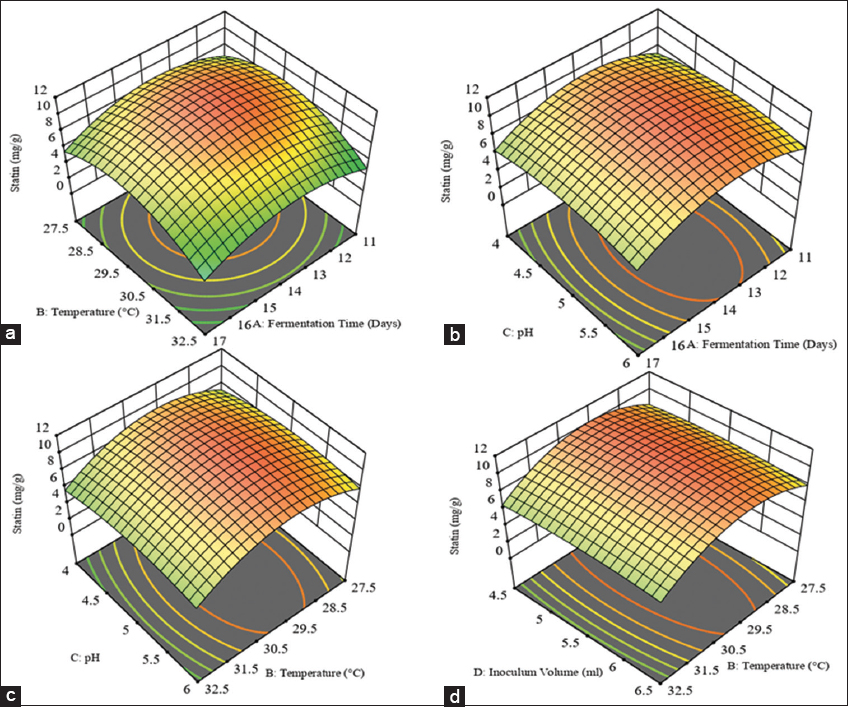 | Figure 1: (a-d) 3D response surface plots illustrating the corelative effects of different independent factors on the production of statin. [Click here to view] |
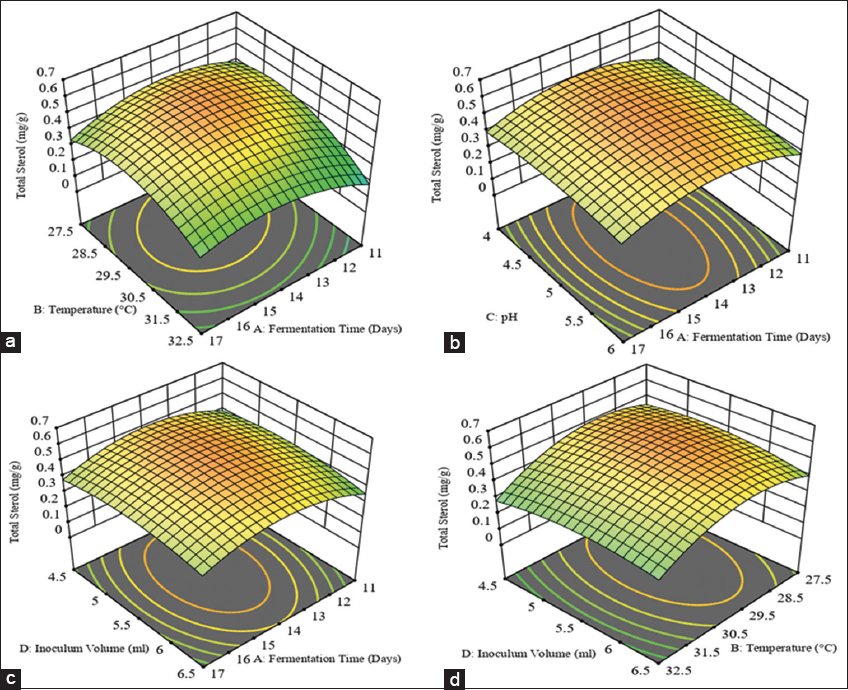 | Figure 2: (a-d) 3D response surface plots illustrating the correlative effects of different independent factors on the production of sterol. [Click here to view] |
3.2. Regression Analysis
Statistical experimental design techniques could be helpful in enhancing the product formation and give a better specification of the output to nominal and low expenditure. This speculative statistical model technique is also used for multiple regression analysis using accessible data. The ANOVA of the quadratic model for statin and sterol production is presented in Tables 5 and 6, respectively. The P-values were used as a tool to check the significance of each coefficient, which also indicated the interaction strength between each independent variable. In statin, P < 0.0001 and f-value is 28.13 implies that the model is fit. In general, an F test was employed to evaluate the statistical significance of the quadratic polynomial. The F-value obtained from the analysis is very large, indicating a significant effect of the independent variable on the dependent variable. The probability of getting such a high F-value by chance alone is extremely low, only 0.01%. This means that the results are unlikely to be due to noise or random variation. The lack of fit (LOF) is statistically non-significant, with an F-value of 3.86.
Table 5: Analysis of variance of the calculated model for statin.
| Sum of Squares | Df | Mean Square | F-value | P-value | ||
|---|---|---|---|---|---|---|
| Model | 340.75 | 14 | 24.34 | 28.13 | <0.0001* | Significant |
| A-Fermentation time | 8.33 | 1 | 8.33 | 9.63 | 0.0073* | |
| B-Temperature | 17.48 | 1 | 17.48 | 20.20 | 0.0004* | |
| C-pH | 1.42 | 1 | 1.42 | 1.64 | 0.2194 | |
| D-Inoculum volume | 0.2243 | 1 | 0.2243 | 0.2592 | 0.6181 | |
| AB | 0.0016 | 1 | 0.0016 | 0.0018 | 0.9663 | |
| AC | 0.3600 | 1 | 0.3600 | 0.4161 | 0.5286 | |
| AD | 0.3025 | 1 | 0.3025 | 0.3496 | 0.5631 | |
| BC | 0.0144 | 1 | 0.0144 | 0.0166 | 0.8991 | |
| BD | 0.0289 | 1 | 0.0289 | 0.0334 | 0.8574 | |
| CD | 0.1089 | 1 | 0.1089 | 0.1259 | 0.7277 | |
| A² | 158.57 | 1 | 158.57 | 183.27 | <0.0001* | |
| B² | 193.80 | 1 | 193.80 | 223.99 | <0.0001* | |
| C² | 22.12 | 1 | 22.12 | 25.57 | 0.0001* | |
| D² | 4.51 | 1 | 4.51 | 5.22 | 0.0374* | |
| Residual | 12.98 | 15 | 0.8652 | |||
| Lack of fit | 11.49 | 10 | 1.15 | 3.86 | 0.0745 | Not Significant |
| Pure error | 1.49 | 5 | 0.2976 | |||
| Cor Total | 353.72 | 29 |
* The P<0.05 in the tables represents significant values, Df: Degree of freedom.
Table 6: Analysis of variance of the calculated model for sterol.
| Sum of Squares | Df | Mean Square | F-value | P-value | ||
|---|---|---|---|---|---|---|
| Model | 0.7745 | 14 | 0.0553 | 14.10 | <0.0001* | Significant |
| A-fermentation time | 0.0020 | 1 | 0.0020 | 0.5186 | 0.4825 | |
| B-temperature | 0.1015 | 1 | 0.1015 | 25.87 | 0.0001* | |
| C-pH | 0.0003 | 1 | 0.0003 | 0.0663 | 0.8004 | |
| D-inoculum volume | 0.0011 | 1 | 0.0011 | 0.2891 | 0.5987 | |
| AB | 0.0226 | 1 | 0.0226 | 5.75 | 0.0299* | |
| AC | 0.0000 | 1 | 0.0000 | 0.0057 | 0.9406 | |
| AD | 0.0007 | 1 | 0.0007 | 0.1756 | 0.6811 | |
| BC | 0.0004 | 1 | 0.0004 | 0.1045 | 0.7510 | |
| BD | 0.0035 | 1 | 0.0035 | 0.8795 | 0.3632 | |
| CD | 0.0019 | 1 | 0.0019 | 0.4877 | 0.4956 | |
| A² | 0.3282 | 1 | 0.3282 | 83.63 | <0.0001* | |
| B² | 0.3909 | 1 | 0.3909 | 99.62 | <0.0001* | |
| C² | 0.0257 | 1 | 0.0257 | 6.56 | 0.0217 | |
| D² | 0.0636 | 1 | 0.0636 | 16.19 | 0.0011* | |
| Residual | 0.0589 | 15 | 0.0039 | |||
| Lack of Fit | 0.0429 | 10 | 0.0043 | 1.35 | 0.3907 | Not Significant |
| Pure Error | 0.0159 | 5 | 0.0032 | |||
| Cor Total | 0.8334 | 29 |
* The P<0.05 in the tables represents significant values, Df: Degree of freedom.
In total sterol, P < 0.0001 and an F-value of 14.10 imply that the model is fit. The LOF is statistically non-significant, with an F-value of 1.35 shows that the suggested model equation fits well with the experimental results. The optimal values of the variables for maximum statin and sterol production were determined using the point prediction tool of the design expert program. Using multiple regression analysis on the experimental data, we obtained the following quadratic polynomial equations for statin (eqn. 1) and total sterol production (eqn. 2), where A, B, C, and D are the coded independent variables.
Statin (mg/g) = +10.32 − 0.5892A − 0.8533B + 0.2433C − 0.0967D + 0.0100AB − 0.1500AC + 0.1375AD − 0.0300BC + 0.0425BD − 0.0825CD − 2.40A² − 2.66B² − 0.8981C² − 0.4056D² (1)
Sterol (mg/g) = +0.5267 + 0.0092A − 0.0650B − 0.0033C − 0.0069D + 0.0376AB + 0.0012AC + 0.0066AD + 0.0051BC + 0.0147BD − 0.0109CD − 0.1094A² − 0.1194B² − 0.0306C² − 0.0481D² (2)
3.3. Quantitative Analysis and Model Validation
The optimal fermentation conditions for producing statin and sterol from FM were found to be: temperature of 29.44°C, 13.8 days of incubation time, 5.37 mL of inoculum volume, and 5.0 pH. Under these conditions, the predicted yields were 10.43 mg/g of statin and 0.53 mg/g of sterol. A validation experiment in three Erlenmeyer flasks confirmed these results with an average yield of 9.78 mg/g of statin and 0.46 mg/g of sterol, which is 93.4% of the predicted value.
4. DISCUSSION
The traditional substrate used for M. purpureus cultivation is rice, although it can also be cultivated on different substrates such as wheat, corn, soya, potatoes, peanut flour [26], buckwheat flour, tapioca flour [27], barley, sorghum [28], millet [29], and FM [21]. Recent studies suggest that millet can be used as a gluten-free substitute for cultivating M. purpureus [29,30]. Millet has a smaller particle size, larger growth surface area, higher aeration, and lower viscosity compared to rice and other cereals, which makes it feasible for fast colonization by M. purpureus, thereby shortening the production cycle and potentially producing a high amount of statin and other secondary metabolites [30]. Other contributing factors for the production of secondary metabolites from Monascus are fermentation time period, inoculum volume, and solid medium pH, which have the greatest impact on fungal growth and the formation of secondary metabolites.
Kamal et al. [31] used rice straw as a substrate to statistically optimize several factors such as moisture, inoculum volume, temperature, and pH for increased lovastatin production from A. terreus. The optimum yield was reported to be 2140 μg/g. Temperature, inoculum volume, and pH were discovered to be significant variables. Al-Saman et al. [32] optimized the different fermentation process factors such as inoculum volume, fermentation temperature, fermentation time, pH, initial moisture content, and nitrogen source were screened for higher lovastatin production. It was found that pH was the most significant factor in the production of statin.
Panda et al. [17] studied the optimization of different process parameters such as temperature, time, inoculum size, and pH using Box-Behnken’s design (BBD) of RSM for higher production of lovastatin by M. purpureus. A maximum lovastatin production of 3.422 mg/g was achieved by day 14.43 of fermentation in a rice-based medium of pH 6 when fermented at a temperature of 29.46°C, an inoculum size of 5.11 mL. Panda et al. [33], optimized the conditions for fementation and coculture the Monascus ruber MTCC 1880 and M. purpureus MTCC 369 for lovastatin production. It was found that SSF of rice with pH 6.03 at 29.46°C for 13.89 d, yielded 2.80 mg/g of lovastatin.
Vankateshwaran and Vijayalakshmi [21] evaluated the anti-hypercholesterolemic metabolites i.e., statin and total sterol production from M. purpureus MTCC 410 using different substrates such as sorghum, njavara, wheat, rice, parboiled rice, FM and maize. The total statin production from germinated FM was 5.2 g/kg, which is significantly greater than the range of 1.04–4.41 g/kg seen in other substrates. Along with statin, germinated FM produced 0.053 g/kg dry weight of dietary sterol, which is 7.6 times more than the control. Zhang et al. [29] efficiently produced lovastatin by solid-state fermentation of M. ruber using millet as a substrate. In this study, rice, corn, millet, barley, and wheat, were employed as raw substrates for the SSF of M. ruber. Millet was found to be the best of these substrates for higher production of lovastatin, with a yield of 7.12 mg/g. Maric et al. [30] compared the lovastatin production of six M. purpureus strains cultivated on rice and millet. Only M. purpureus “MOPU GS1” strain produces a small amount of monacolin k 1.3 and 1.6 mg/g for rice and millet, respectively. In comparison to other grain substrates studied thus far, millet appears to be a better alternative for statin production. Till now limited research is available on the production of statin and sterol by using millet as a substrate.
Based on the literature survey, fermentation time period, temperature, pH, and inoculum volume were selected for the optimization process for maximum production of statin and sterol from different M. purpureus strains [17,21,32-34]. Many findings suggest that M. purpureus produces a good amount of lovastatin at near 30°C temperature in a medium of pH 5–6 and time 12–14 days, results are consistent with the results of previous reports. In relation to the substrate, FM was used as a substrate, as FM might represent a good gluten-free substitute for the cultivation of M. purpureus. In the present study, the optimization of fermentation process parameters and interaction of the factors on the production of anti-hypercholesterolemic metabolites in fermented FM by M. purpureus MTCC 369 were investigated. Experimental runs were designed by CCD of RSM and statistical analysis using Design Expert versus 13 software.
Fermentation process factors such as fermentation time period and temperature were significant positive factors, while pH and inoculum volume were found to be insignificant factors. The interaction between two factors has been shown from proposed nonlinear polymeric equation of quadratic model. In the case of statin, fermentation time interacted positively with inoculum volume and temperature and negatively with pH. Temperature interacted positively with inoculum volume and negatively with pH. In sterol, fermentation time interacted positively with temperature, pH and inoculum volume, and time with pH and inoculum volume. Fermentation time interacted positively with inoculum volume and pH and only inoculum volume and pH interacted negatively.
5. CONCLUSION
In this current research work, we have performed SSF in an Erlenmeyer flask with FM and M. purpureus MTCC 369 to optimize the process variables affecting the synthesis of statins and sterols. Four independent variables (fermentation time, temperature, pH, and inoculum volume,) were optimized, in which fermentation time and temperature were key parameters. The highest amount of 9.78 mg/g of statin and 0.46 mg/g of total sterol production were found at 29.44°C temperature, 13.8 days, and inoculum volume of 5.37 with a pH of 5.0. The statin and sterols produced through SSF by M. purpureus MTCC 369 using FM indicate that M. purpureus is an excellent producer of anti-hypercholesterolemic metabolites and it has a great potential to be utilized as the source of statins in future. This study highlights the fact that the FM as a substrate and M. purpureus as a fungus can be utilized for higher production of statins under the optimum condition stated in this research.
6. ACKNOWLEDGMENTS
The Department of Botany, Maharshi Dayanand University, Rohtak and Council of Scientific and Industrial Research (CSIR), New Delhi, and DST-FIST, New Delhi are acknowledged for providing the requisite facilities for the preparation of this manuscript.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
10. ETHICAL APPROVALS
This research does not involve any animal or human subjects for ethical approval.
11. DATA AVAILABILITY
All data generated and analyzed are included within this research article.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
13. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Organization WHO. Cardiovascular Disease (CVDs);2021. Available from:https://www.who.int/cardiovasculardiseases/aboutcvd/en [Last accessed on 2023 Aug 26].
2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng R, et al. Heart disease and stroke statistics-2018 update:A report from the American heart association. Circulation 2018;137:e67-492. [CrossRef]
3. Gum SI, Nguyen PA, Lee JR, Han YH, Cho MK. The physico-chemical alteration of lovastatin and enhanced antioxidant effect of Bacillus subtilis fermented-red yeast rice product. Food Chem 2017;232:203-9. [CrossRef]
4. Zhu B, Qi F, Wu J, Yin G, Hua J, Zhang Q, Qin L. Red yeast rice:A systematic review of the traditional uses, chemistry, pharmacology, and quality control of an important Chinese folk medicine. Front Pharmacol 2019;10:1449. [CrossRef]
5. Xiong Z, Cao X, Wen Q, Chen Z, Cheng Z, Huang X, et al. An overview of the bioactivity of monacolin K/lovastatin. Food Chem Toxicol 2019;131:110585. [CrossRef]
6. Manzoni M, Rollini M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol 2002;58:555-64. [CrossRef]
7. Xie L, Zhu G, Shang J, Chen X, Zhang C, Ji X, Zhang Q, et al. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell Signal 2021;87:110122. [CrossRef]
8. Monu M, Sehrawat KD, Singh A, Chaudhary G, Bamal D, Sehrawat AR. An overview on the therapeutic potential and anticancer mechanism of monacolin K/Lovastatin. Pharmacol Res Modern Chin Med 2022;100187. [CrossRef]
9. Feng Y, Shao Y, Zhou Y, Chen F. Monacolin K production by citrinin-free Monascus pilosus MS-1 and fermentation process monitoring. Eng Life Sci 2014;14:538-45. [CrossRef]
10. Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W, et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014;232:346-60. [CrossRef]
11. Han S, Jiao J, Xu J, Zimmermann D, Actis-Goretta L, Guan L, et al. Effects of plant stanol or sterol-enriched diets on lipid profiles in patients treated with statins:systematic review and meta-analysis. Sci Rep 2016;6:31337. [CrossRef]
12. Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber:A review. J Food Sci Technol 2014;51:1021-40. [CrossRef]
13. Kumar A, Metwal M, Kaur S, Gupta AK, Puranik S, Singh S, et al. Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front Plant Sci 2016;7:934. [CrossRef]
14. Singh A, Sehrawat KD, Sehrawat A, Singh M, Sehrawat AR. Assessing the genetic diversity of finger millet (Eleusine coracana) germplasm for agronomic traits. Indian J Agric Sci 2023;93:489-94. [CrossRef]
15. Puranik S, Kam J, Sahu PP, Yadav R, Srivastava RK, Ojulong H, et al. Harnessing finger millet to combat calcium deficiency in humans:Challenges and prospects. Front Plant Sci 2017;8:1311. [CrossRef]
16. Subhagar S, Aravindan R, Viruthagiri T. Response surface optimization of mixed substrate solid state fermentation for the production of lovastatin by Monascus purpureus. Eng Life Sci 2009;9:303-10. [CrossRef]
17. Panda BP, Javed S, Ali M. Statistical analysis and validation of process parameters influencing lovastatin production by Monascus purpureus MTCC 369 under solid-state fermentation. Biotechnol Bioprocess Eng 2009;14:123-7. [CrossRef]
18. Al Mamun MA, Mian MM, Saifuddin M, Khan SN, Hoq MM. Optimization of fermenting medium by statistical method for production of alkaline protease by Bacillus licheniformis MZK05M9. J App Biol Biotechnol 2019;5:24-8.
19. Sayyad SA, Panda BP, Javed S, Ali M. Optimization of nutrient parameters for lovastatin production by Monascus purpureus MTCC 369 under submerged fermentation using response surface methodology. App Microbiol Biotechnol 2007;73:1054-8. [CrossRef]
20. Liu X, Sun A, Li Q, Du Y, Zhao T. A systematic study of the production of Monacolin K by solid state fermentation of Monascus ruber. AMB Exp 2022;12:29. [CrossRef]
21. Venkateswaran V, Vijayalakshmi G. Finger millet (Eleusine coracana)-an economically viable source for antihypercholesterolemic metabolites production by Monascus purpureus. J Food Sci Technol 2010;47:426-31. [CrossRef]
22. Mouafi FE, Ibrahim GS, Elsoud MM. Optimization of lovastatin production from Aspergillus fumigatus. J Genet Eng Biotechnol. 2016;14:253-9. [CrossRef]
23. Raghunath R, Radhakrishna A, Angayarkanni J, Palaniswamy M. Production and cytotoxicity studies of lovastatin from Aspergillus niger PN2 an endophytic fungi isolated from Taxus baccata. Int J App Bio Pharm Tech 2012;3:342-51.
24. Kieber RJ, Payne WJ, Appleton GS. The sterol content of Fungi:I. Methods for disrupting cells, extracting and determining sterols. App Microbiol 1955;3:247. [CrossRef]
25. Sabir SM, Hayat I, Gardezi SD. Estimation of sterols in edible fats and oils. Pak J Nutr 2003;2:178-81. [CrossRef]
26. Ogbonna CN. Review:Production of food colourants by filamentous fungi. Afr J Microbiol Res 2016;10:960-71. [CrossRef]
27. Dikshit R, Tallapragada P. Comparative study of Monascus sanguineus and Monascus purpureus as potential sources for red pigment production. Int J Pharm Bio Sci 2012;3:885-95.
28. Ivanis^tova E, Rajtar M, Franc^a´kova´H, Toka´r M, Dra´b S^, Kluz M, Kac^a´niova´M. Characterization of bioactive compounds from Monascus purpureus fermented different cereal substrates. Potr Slovak J Food Sci 2017;11:183-9. [CrossRef]
29. Zhang BB, Xing HB, Jiang BJ, Chen L, Xu GR, Jiang Y, et al. Using millet as substrate for efficient production of monacolin K by solid-state fermentation of Monascus ruber. J Biosci Bioeng 2018;125:333-8. [CrossRef]
30. MaricA, Skocaj M, Likar M, SepcicK, CigicIK, Grundner M, et al. Comparison of lovastatin, citrinin and pigment production of different Monascus purpureus strains grown on rice and millet. J Food Sci Technol 2019;56:3364-73. [CrossRef]
31. Kamal S, Akhter N, Khan SG, Kiran S, Farooq T, Akram M, Shah S, et al. Enhanced production of Lovastatin by filamentous fungi through solid state fermentation. Pak J Pharm Sci 2018;31:1583-9.
32. Al-Saman MA, Helmy MA, Abdella A, Wilkins MR, Gobba NA, Mahrous H. Optimization of lovastatin production by Aspergillus terreus ATCC 10020 using solid-state fermentation and its pharmacological applications. Biocatal Agric Biotechnol 2021;31:101906. [CrossRef]
33. Panda BP, Javed S and Ali M. Optimization of fermentation parameters for higher lovastatin production in red mold rice through co-culture of Monascus purpureus and Monascus ruber. Food Bioproc Tech 2010;3:373-8. [CrossRef]
34. Ravuri M, Shivakumar S. Statistical optimization of production parameters for enhanced lovastatin production from endophytic Aspergillus terreus. Biocatal Agric Biotechnol 2020;12:101787. [CrossRef]