1. INTRODUCTION
Citrus fruits are highly preferable products that are widely consumed either fresh or processed products such as juices, jams, and liqueurs due to their nutritional values and characteristic flavor [1]. Results from the chemical analysis showed that citrus fruits are rich in phytochemicals (e.g., flavonoids, coumarins, and limonoids), exhibiting health-promoting effects such as the prevention of cancers, diabetes, or cardiovascular diseases [2]. Pomelo (Citrus grandis [L.] Osbeck), belonging to the family Rutaceae, is also the most commercially vital fruit of the citrus group [3]. Phytochemical compounds in pomelo juice were reported to have a positive effect on the prevention of chronic diseases [4].
The increasing interest in phytochemical compounds has prompted the development of innovative products from pomelo fruit. Conventionally, the fermentation processes are applied in dairies, meat products, or used to produce alcoholic beverages. Currently, there has been increasing attention toward the production of fermented fruit juice due to the health-promoting effects of fermented foods [5]. Alcoholic fermentation is the anaerobic conversion of fermentable carbohydrates to ethanol by the use of a starter to initiate the fermentation process, usually yeasts namely Saccharomyces [2]. Numerous fermentation processes have been applied to produce fermented juice from apple, orange, mulberry, and pomegranate [6]. It has been found that the resulting product from the fermentation process revealed a higher concentration of bioactive phytochemicals than the respective substrates [1,6,7]. Oliveras-López et al. [7] reported that flavonoids and provitamin A content were considerably higher than those in the orange juice on the 11th day of the fermentation process and the melatonin content increased continuously until the end of the fermentation process (15th day). Escudero-López et al. [8] stated that the process of alcoholic fermentation was found to enhance the bioaccessibility and bioavailability of bioactive compounds as a result of structural changes. Besides, the moderate alcohol content of fermented juice promotes health-beneficial effects on lipid profile, coagulation system, and atherosclerotic process as well as decreases risks of cardiovascular diseases [8].
In the Mekong Delta of Vietnam, the pomelo variety, namely Da Xanh, contributes to the large fruit production segment, mostly consumed as fresh. The development of pomelo-derived innovative products is scarcely in Vietnam. Developing fermented pomelo juice could create a commercial beverage with a diversity of beneficial effects from its bioactive constituents and increase the economic value of this pomelo cultivar. Therefore, this study investigated the process parameters in developing fermented pomelo juice from the Da Xanh cultivar. The revealed result could enhance the commercialization ability of the Da Xanh pomelo and provide the consumer with a new potentially healthful beverage, diversifying the supply of other similar drinks related to alcohol content.
2. MATERIALS AND METHODS
2.1. Pomelo Juice Preparation and Chemical Reagents
The Da Xanh pomelo (Citrus grandis (L.) Osbeck) was harvested in the months between April and July in Ben Tre province, Vietnam. The visual appearance of the Da Xanh variety is characterized by an oval shape and green peel with reddish fruit flesh as shown in Figure 1. Pomelos were then washed, and the peels were removed to obtain the pomelo flesh to be prepared for further experiments. Yeast (Saccharomyces cerevisiae BV818) and pectinase were supplied from Angel Yeast Co., Ltd, Hubei, China. Yeast was activated by dissolving in sugar and water at the ratio of yeast: sugar:water of 1:2:10 at 37°C for 15 min.
 | Figure 1: Visual appearance of the Da Xanh pomelo and its flesh. [Click here to view] |
The dichromate reagent was prepared by dissolving 20 g of potassium dichromate (Merck KGaA, Darmstadt, Germany) in 100 mL of 5 M sulfuric acid solution. The solution of 3,5-dinitrosalicylic acid (DNS) reagent (Merck KGaA, Darmstadt, Germany) was prepared by mixing 10 g of DNS, 16 g of sodium hydroxide, and 300 g of sodium potassium tartrate. The mixture solution was filled with distilled water to a final volume of 1000 mL. Standard solution (glucose and ethanol) was purchased from Merck KGaA, Darmstadt, Germany. Other analytical chemicals were from standard commercial supplies.
2.2. Extraction of Pomelo Juice
The prepared pomelo flesh was subjected to different extraction methods. First, the pomelo fruit flesh was mechanically pressed to obtain the juice and mixed with water at a ratio of 1:1 (v/v). Second, the pomelo flesh was blended with water at a ratio of 1:1 (v/v) using a blender (MX-V310KRA, Panasonic Corporation, Japan). Pectinase at 0.2% (w/v) was included in the mixture and allowed to incubate for 2 h at 50°C [9]. In the third group, pomelo sacs were mixed with water at a ratio of 1:1 (w/w). Three pomelo juice groups were then mixed with citric acid and white granulated sugar to obtain a pH of 5.0 and total soluble solids (TSS) of 20° Brix [2].
2.3. Fermentation of Pomelo Juice
Pomelo juice obtained from the selected extraction method was mixed with the water at different ratios (from 1:1 to 1:4). The mixture solution was mixed with citric acid and white granulated sugar to achieve the pH of 5.0 and 20° Brix. The NaHSO3 was then added to the mixture solution (140 mg/L) and allowed to stand for 2 h to kill microorganisms before fermentation. Each 100 mL of sample was fermented with an addition of 0.04% yeast and allowed to incubate at 28–33°C for different periods from 2 to 5 days. The fermentation was continued till the ethanol concentration of 5% was obtained.
2.4. Physicochemical Characteristics
The total soluble solid was determined by using a refractometer (MASTER-α, Brix: 0.0 to 33.0%, AtagoCo., Ltd., Japan). pH was measured by using a pH meter (Mettler Toledo, USA). Total acid was determined by titration method using 0.1 N NaOH with phenolphthalein as a color indicator.
2.5. Reducing Sugar
Reducing sugar content was determined using a DNS assay. Briefly, a reaction mixture composed of 3 mL of DNS reagent and 1.5 mL of sample was boiled in a water bath for 5 min and cooled to room temperature. An aliquot (0.2 mL) was taken out and diluted with 2.5 mL of distilled water. The absorbance was read at 540 nm and glucose served as a standard solution with a concentration range of 3.7–12.4 mM [10].
2.6. Vitamin C
The vitamin C content was assessed by using the 2,6-dichlorophenol-indophenol method according to the Official Methods of Analysis of AOAC International [11]. An equal volume (5 mL) of sample was mixed with 20 mL of 1% HCl, and 1% oxalic acid was added to the mixture to obtain a total volume of 100 mL. The mixture was filtrated using filter paper (Whatman No 1, UK) and 10 mL of filtrate was titrated with 0.001 N DCPIP solutions until the appearance of persistent pale pink color. The vitamin C content was expressed as mg per L of pomelo juice.
2.7. Ethanolic Content
Briefly, 1 mL of TBP and 1 mL of pomelo juice sample were vigorously vortexed in a 2 mL microtube until the appearance of two-phase separation (very clear upper-phase and turbid lower phase). An aliquot (500 μL) from the upper phase was mixed with 500 μL of dichromate reagent in the 2 mL microtube and vortexed vigorously for 10 min until obtaining two-phase separation. Dichromate reagent (100 μL) at the lower phase (blue–green color) was transferred into the test tube and 10-times diluted for optical density measurement. The standard solution was absolute ethanol. Both the standard solution and sample were measured at 595 nm using a 722-Visible spectrophotometer (China Yangzhou Wandong Medical Co., Ltd, China) [12].
2.8. Statistical Analysis
Each experimental data were in three replicates. One-way analysis of variance and Tukey’s HSD test to compare the mean values were analyzed using SPSS software 20 (IBM Corp., Armonk, New York, USA) at the level of 5% (P < 0.05).
3. RESULTS AND DISCUSSION
3.1. Effects of Extraction Methods on the Properties of Fermented Pomelo Juice
The properties of fermented pomelo juice were found to be influenced by different extraction methods as shown in Table 1. The pectinase-assisted-blending extraction method was found to achieve the highest ethanol content of 5.15% compared to the others. The production of ethanol is the conversion of sugars in fruit juice into ethanol by yeast cells [13]. Pectinase plays a role in breaking down the glycosidic bonds between the galacturonic acid monomers, leading to a decrease in water-holding capacity and making pomelo juice susceptible to the fermentation process by yeasts [14]. Jiang et al. [13] also noted that pectinase treatment probably promoted a synergistic effect on fermented juice yield which was 16% higher than the control sample without the pectinase treatment. Pectinase-treated palmyra fruit wine showed the highest alcohol content (9.6 %) compared to untreated wine (6.8%) [15]. The lowest content of reducing sugars and Brix value (5.06 g/100 mL and 8.5 °Brix) obtained from the pectinase-assisted blending extraction method indicated more reducing sugars that were consumed for the production of ethanol during fermentation [16]. This is highly correlated to the highest production of ethanol from the fermented pomelo juice by the blending-pectinase extraction method. The vitamin C content of fermented pomelo juice extracted by blending assisted with pectinase (596 mg/L) was considerably lower than the others. This could possibly be accredited to the oxidation of vitamin C during hot pectinase treatment at 50°C [17]. On the contrary, the total acid content was found to be insignificant (P > 0.05) between the treatment groups. This result was consistent with a study by Nikhanj et al. [18]. In this study, the extraction method of blending assisted with pectinase appeared to have higher efficacy in producing ethanol. Besides, the clarification effect of pectinase contributing to the sensorial properties of fermented juice has been widely discussed in a previous report [14]. Therefore, the extraction of pomelo juice by blending assisted with pectinase treatment was selected for further experiments.
Table 1: Properties of fermented pomelo juice obtained from different extraction methods.
| Extraction method | Brix | Vitamin C (mg/L) | Total acid (g/L) | Total reducing sugar (g/100 mL) | Total ethanol (%) |
|---|---|---|---|---|---|
| Pressing | 10±0.50b | 743±12b | 5.76±0.24a | 6.42±0.54c | 4.63±0.23b |
| Pectinase assisted-blending | 8.5±0.35a | 596±21a | 5.76±0.56a | 5.06±0.21a | 5.15±0.17c |
| Pomelo sacs | 9.7±0.25b | 787±36b | 5.90±0.23a | 5.52±0.17b | 4.04±0.16a |
Data are expressed as mean±SD. Superscripts (a, b, c) are used to differentiate mean values within the same column (P<0.05). SD: Standard deviation
3.2. Effect of Juice: Water Ratios on the Properties of Fermented Pomelo Juice
The properties of fermented pomelo juice affected by juice: water ratios are presented in Figure 2. Increasing juice: water ratios were observed to cause the reduction of alcohol production at the end of the fermentation period, from 4.43% to 1.80%. This was ascribed to the diluted factor causing the nutritional stress on the growth of yeast cells. Although the total soluble solids in each fermentation batch were 20 °Brix, other essential nutritional compounds such as nitrogen, and lipid in the fermentation medium at higher ratios of water addition were inadequate for the growth of yeast cells, leading to significant cellular stresses for yeast growth [19,20]. This led to the ineffective utilization of sugar for ethanol production. The reducing sugar content and Brix value of the fermented pomelo juice at the 1:1 juice: water ratio were the lowest with respect to 11 °Brix and 39.52 g/100 mL, indicating the effective sugar consumption of yeast for ethanol production. Fermented pomelo juice at 1:1 juice: water ratio showed the highest vitamin C and total acid content, 318 mg/L and 6.63 g/L, respectively. These values were found to decrease at higher juice: water ratios. In this study, 1:1 pomelo juice: water ratio was found to facilitate the alcoholic fermentation of yeast cells, thereby this ratio was selected for another analysis.
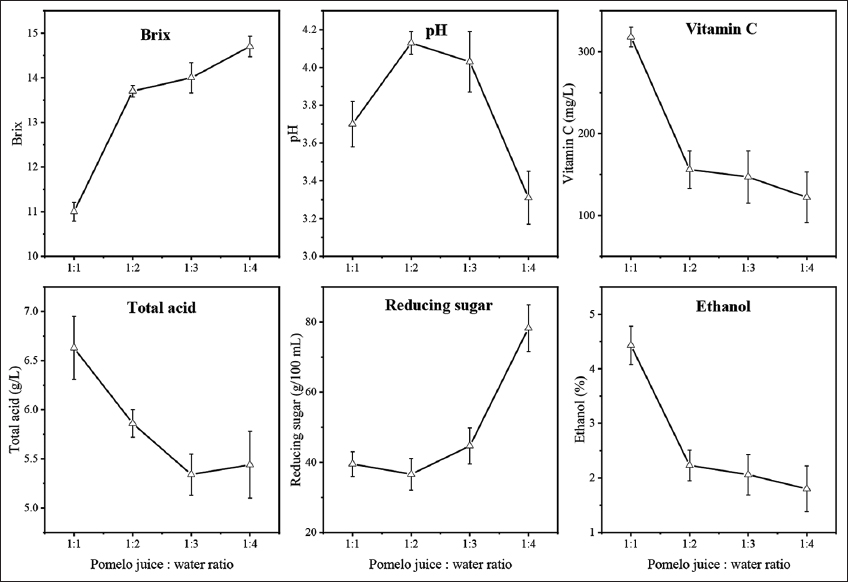 | Figure 2: Effect of juice:water ratios on the properties of fermented pomelo juice. [Click here to view] |
3.3. Properties of Fermented Pomelo Juice at Different Fermentation Times
The effects of fermentation time on the properties of fermented pomelo juice are presented in Figure 3. The Brix values and reducing sugars experienced a decreasing trend due to the conversion of sugars into ethanol production during the fermentation period [13]. The ethanol content, which achieved 5% after 2 days of fermentation, continuously increased to 8.23% on the 5th day of the fermentation process. The pH of fermented pomelo juice was found to slightly reduce during the fermentation period (from 4.09 to 3.46). This was possibly due to the formation of organic acids along with ethanol during fermentation [2]. The changes in total acid content were observed with an increase on the 3rd day due to the formation of organic acids [2]. Besides, naturally occurring lactic acid bacteria in the mixture could possibly produce lactic acid and acetic acid in the presence of carbohydrate sources [21,22]. However, it started declining in later days due to the consumption of lactic acid by acetobacter and its conversion to ethyl lactate during acetic acid fermentation. This phenomenon was consistent with a study done by Chen et al. [2]. In terms of vitamin C content, it was found to significantly decrease when exposed to a long time of fermentation from 716 mg/L to 442 mg/mL. Vitamin C, which is unstable to heat and oxygen, is easily oxidized to nonantioxidant compounds. Therefore, the extended fermentation process could possibly reduce the vitamin C content as it was decomposed when exposed to oxygen, light, or heat in the mixture [23,24]. According to the properties of fermented juice and the desired ethanol concentration of our work, the fermentation process for 2 days was appropriate to obtain the ethanol concentration of 5% while it retained the high vitamin C content and other organic acids.
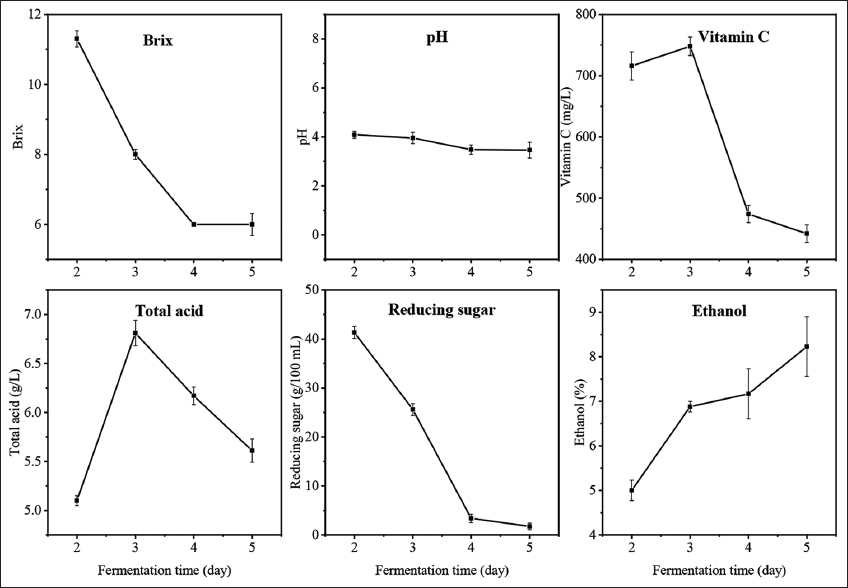 | Figure 3: Properties of fermented pomelo juice at different fermentation days. [Click here to view] |
4. CONCLUSION
In this study, the fermented pomelo juice with 5% of ethanol was successfully developed. The blending extraction method assisted with pectinase treatment showed higher ethanol content of fermented juice than the others. The 1:1 pomelo juice: water ratio was an appropriate medium for yeast growth to produce ethanol production. Fermentation time for 2 days was selected to obtain the ethanol content of 5% as desired for the final fermented pomelo juice and it also retained the high vitamin C content and produced organic acids. This result could facilitate the development of fermented juice from pomelo fruit to further applications on an industrial scale.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
The present work was financially supported by Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam and Can Tho University, Can Tho City, Vietnam.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Multari S, Guzzon R, Caruso M, Licciardello C, Martens S. Alcoholic fermentation of citrus flavedo and albedo with pure and mixed yeast strains:Physicochemical characteristics and phytochemical profiles. LWT 2021;144:111133. [CrossRef]
2. Chen Y, Huang Y, Bai Y, Fu C, Zhou M, Gao B, et al. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT 2017;84:753-63. [CrossRef]
3. Tocmo R, Pena-Fronteras J, Calumba KF, Mendoza M, Johnson JJ. Valorization of pomelo (Citrus grandis Osbeck) peel:A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr Rev Food Sci Food Saf 2020;19:1969-2012. [CrossRef]
4. Gupta AK, Koch P, Mishra P. Optimization of debittering and deacidification parameters for Pomelo juice and assessment of juice quality. J Food Sci Technol 2020;57:4726-32. [CrossRef]
5. Sanlier N, Gökcen BB, Sezgin AC. Health benefits of fermented foods. Crit Rev Food Sci Nutr 2019;59:506-27. [CrossRef]
6. Cerrillo I, Escudero-López B, Hornero-Méndez D, Martín F, Fernández-Pachón MS. Effect of alcoholic fermentation on the carotenoid composition and provitamin A content of orange juice. J Agric Food Chem 2014;62:842-9. [CrossRef]
7. Oliveras-López MJ, Cerezo AB, Escudero-López B, Cerrillo I, BernáG, Martín F, et al. Changes in orange juice (poly)phenol composition induced by controlled alcoholic fermentation. Anal Methods 2016;8:8151-64. [CrossRef]
8. Escudero-López B, Cerrillo I, Herrero-Martín G, Hornero-Méndez D, Gil-Izquierdo A, Medina S, et al. Fermented orange juice:Source of higher carotenoid and flavanone contents. J Agric Food Chem 2013;61:≅-82. [CrossRef]
9. Ni H, Yang YF, Chen F, Ji HF, Yang H, Ling W, et al. Pectinase and naringinase help to improve juice production and quality from pummelo (Citrus grandis) fruit. Food Sci Biotechnol 2014;23:739-46. [CrossRef]
10. Teixeira RS, da Silva AS, Ferreira-Leitão VS, da Silva Bon EP. Amino acids interference on the quantification of reducing sugars by the 3, 5-dinitrosalicylic acid assay mislead carbohydrase activity measurements. Carbohydr Res 2012;363:33-7. [CrossRef]
11. Horwitz W. AOAC Official Method 967.21. Ascorbic Acid in Vitamins Preparations and Juices, 2, 6-Dichloroindophenol Titrimetric Method. Vol. 17. Ch. 45. Maryland:AOAC International;2000. 1-16.
12. Miah R, Siddiqa A, Tuli JF, Barman NK, Dey SK, Adnan N, et al. Inexpensive procedure for measurement of ethanol:Application to bioethanol production process. Adv Microbiol 2017;7:743-8. [CrossRef]
13. Jiang XH, Lu Y, Liu SQ. Effects of pectinase treatment on the physicochemical and oenological properties of red dragon fruit wine fermented with Torulaspora delbrueckii. LWT 2020;132:109929. [CrossRef]
14. Verma H, Narnoliya LK, Jadaun JS. Pectinase:A useful tool in fruit processing industries. Nutr Food Sci Int J 2018;5:555673. [CrossRef]
15. Sobini N, Wickramasinghe I, Subajini M. Development of Wine from Pectinase Treated Palmyrah Fruit Pulp by Using Toddy Yeast. Jaffna University International Research Conference Proceedings;2018. 1-4.
16. Casabar JT, Unpaprom Y, Ramaraj R. Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Convers Biorefin 2019;9:761-5. [CrossRef]
17. Gani G, Naik HR, Jan N, Bashir O, Hussain SZ, Rather AH, et al. Physicochemical and antioxidant properties of pear juice prepared through pectinase enzyme-assisted extraction from William Bartlett variety. J Food Meas Charact 2021;15:743-57. [CrossRef]
18. Nikhanj P, Kocher GS, Boora RS. Fermentative production of guava wine from pectinase treated and untreated juice of 'punjab pink'cultivar of Psidium guajava L. Agric Res J 2017;54:244-7. [CrossRef]
19. Tesnière C. Importance and role of lipids in wine yeast fermentation. Appl Microbiol Biotechnol 2019;103:8293-300. [CrossRef]
20. Gobert A, Tourdot-Maréchal R, Sparrow C, Morge C, Alexandre H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol 2019;83:71-85. [CrossRef]
21. Szutowska J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices:A systematic literature review. Eur Food Res Technol 2020;246:357-72. [CrossRef]
22. Du Plessis H, Du Toit M, Nieuwoudt H, Van der Rijst M, Hoff J, Jolly N. Modulation of wine flavor using Hanseniaspora uvarum in combination with different Saccharomyces cerevisiae, lactic acid bacteria strains and malolactic fermentation strategies. Fermentation 2019;5:64. [CrossRef]
23. Otegbayo BO, Akwa IM, Tanimola AR. Physico-chemical properties of beetroot (Beta vulgaris L.) wine produced at varying fermentation days. Sci Afr 2020;8:e00420. [CrossRef]
24. CaritáAC, Fonseca-Santos B, Shultz JD, Michniak-Kohn B, Chorilli M, Leonardi GR. Vitamin C:One compound, several uses. Advances for delivery, efficiency and stability. Nanomed Nanotechnol Biol Med 2020;24:102117. [CrossRef]