1. INTRODUCTION
Bioenergy plants have been extensively explored as an aspect of the search for sustainable and renewable energy sources. Elephant grass, sometimes referred to as Napier grass (P. purpureum Schumach), is one of the prospective feedstocks for the generation of bioethanol. Due to its high productivity, low cost of inputs, and adaptability to a wide variety of environments, it is a potential source of bioethanol for the bioenergy industry [1]. Recently, it has acquired popularity in the scientific community. Numerous studies have been carried out to evaluate the potential of this species, including research on pretreatment techniques [2,3] enzymatic hydrolysis [4,5] fermentation [1], and coproduction of ethanol and other biofuels [6]. Recent research in the field of processing of P. purpureum has resulted in the advancement of new technologies and processes that improve the efficiency of the bioethanol production process. Taufikurahman et al. used simultaneous saccharification and fermentation to produce bioethanol using crude cellulase enzyme extract from P. purpureum [5], Wu et al. evaluated the coproduction of ethanol and methane from P. purpureum using NaOH pretreatment and ensiling-NaOH pretreatment [3].
Despite the promising results of using P. purpureum for bioethanol production, there are still challenges that need to be addressed including the need for more cost-effective and sustainable processing methods. One challenge is the low sugar content in P. purpureum, which hampers the efficiency of ethanol production. In addition, the high lignin content in P. purpureum poses difficulties in extracting fermentable sugars during the pretreatment process. Enzymatic hydrolysis of P. purpureum is challenging due to its complex structure, necessitating harsher pretreatment methods that may result in the formation of inhibitors, which can interfere with ethanol production. Moreover, scaling up the production of bioethanol from P. purpureum proves challenging due to the substantial amounts of feedstock required and the need for specialized equipment and facilities.
Despite these challenges, the potential of P. purpureum as a sustainable bioenergy crop for bioethanol production remains significant, especially considering the growing demand for renewable and sustainable bioenergy sources [2,7]. The present review explores recent advancements in various aspects of bioethanol production and underscores the potential of P. purpureum as a sustainable bioethanol source while identifying the challenges and opportunities in its large-scale commercial production.
2. ETHANOL PRODUCTION FROM NAPIER GRASS
Numerous investigations have been conducted on the production of ethanol from Napier grass, and the results of these studies have been compared to one another. An example of this would be a study that was carried out by Tsai et al. [8] that investigated various pretreatment techniques of Napier grass to produce bioethanol [8]. The study’s primary emphasis was on optimizing enzymatic saccharification and ethanol output. When compared to other pre-treatment techniques such as hot water, alkaline, and steam explosion, the findings demonstrated that the sugar and ethanol outputs were greatest with the dilute acid pre-treatment. According to the findings of the research, treating Napier grass with a dilute acid before using it to produce bioethanol is a potential technique for increasing the effectiveness, with which the grass functions as a lignocellulosic (LC) feedstock (Ref). According to the findings of the research, it is essential to produce greater yields of fermentable sugars and ethanol from LC biomass by optimizing the circumstances under which the pretreatment is carried out. Compared to the other pretreatment techniques, the dilute acid pretreatment procedure produced the largest sugar yield (57.6%) and ethanol production (22.6 g/L). Comparative analyses of the effectiveness of various preprocessing methods, about the saccharification of Napier grass, have also been conducted in another research. In one study, Ismail et al. [9] studied the pretreatment of extruded Napier grass for ethanol production utilizing the hydrothermal method with diluted sulfuric acid and fermentation using yeast that can hydrolyze cellulose and absorb xylose [9]. In this study, ethanol was produced using yeast that can hydrolyze cellulose and absorb xylose. The ethanol yield was 0.26 g/g of biomass, and the fermentation efficiency was stated to be 60.5%.
In a study conducted by Kongkeitkajorn et al., the viability of employing Napier grass in the fermentation process to produce bioethanol was examined [10]. The findings of the study suggested that the Napier grass has a lot of potential as a LC raw material and can support a fermentation that can yield a lot of ethanol. Fermentation of enzymatic hydrolysate by S. cerevisiae through distinct hydrolysis and fermentation resulted in the highest ethanol production of 187.4 g/kg of Napier grass at a concentration of 44.7 g/L. Investigators employed a range of fermentation techniques to ascertain what constitutes a suitable procedure for the manufacture of batch bioethanol in a different study by Kongkeitkajorn et al. [11]. After the cultivation process, the ethanol concentrations produced by both the process, simultaneous high-fructose fermentation (SHF), and the simultaneous saccharification and fermentation (SSF) fermentation produced a comparable yield of 30.6 g/L in the SHF and 28.5 g/L in the SSF. The finding suggests that the choice of fermentation process and the quantity of enzyme supplied can have a significant impact on the amount of bioethanol generated.
Furthermore, numerous studies have explored the impact of different microbial species on enhancing the quantity of bioethanol obtainable from Napier grass. For instance, in a research project of [12], the possibility of Kluyveromyces marxianus CCT 7735 for the generation of bioethanol utilizing Napier grass as a feedstock was investigated. The yeast was discovered to be capable of successfully metabolizing the sugars present in the Napier grass hydrolysate, resulting in the formation of 0.44 g/g of ethanol. In their study, they determined that the most successful method for free fermentable carbohydrates from their cellular structures was acid pretreatment followed by enzymatic breakdown. According to the observations, K. marxianus appears to be a potential organism for the generation of bioethanol from Napier grass and other LC feedstocks, particularly in tropical areas. Another research that was carried out by Mueansichai et al. concentrates on the production of LC bioethanol from Napier grass using a coculture fermentation technique that included Trichoderma reesei and Saccharomyces cerevisiae [13]. The authors altered the parameters of the fermentation process to produce the maximum possible yield of ethanol, which came out to 7.8 g of ethanol/L. Although this method showed potential, the amount of ethanol that could be produced by it was significantly less than the amount that could be produced by the method used in the prior research, which was pretreatment with dilute acid. The writers emphasized how critical it is for the production of bioethanol to make use of feedstocks that are both affordable and environmentally friendly.
Overall, these research findings indicate that Napier grass has a lot of potential as a bioenergy crop for producing bioethanol. However, to increase production yields and decrease production costs, further research is needed to optimize the various stages of bioethanol production.
3. PRE-TREATMENT STRATEGIES TO OPTIMIZE SACCHARIFICATION OF NAPIER GRASS
The rate and output of enzymatic hydrolyses are usually enhanced by pre-treatment, which reduces the lignin content in the biomass, increases the surface area that provides more sites for enzyme attachment, [Figure 1] and decreases crystallinity [14].
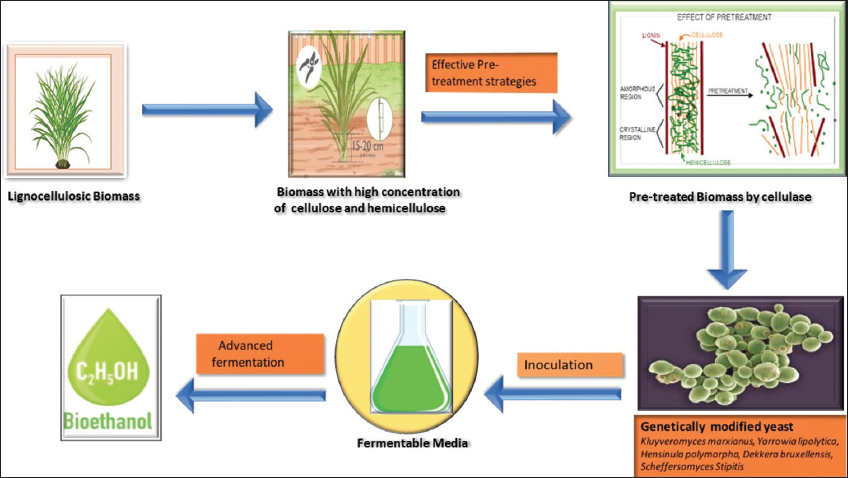 | Figure 1: Diagrammatic representation of pre-treatment strategies [Click here to view] |
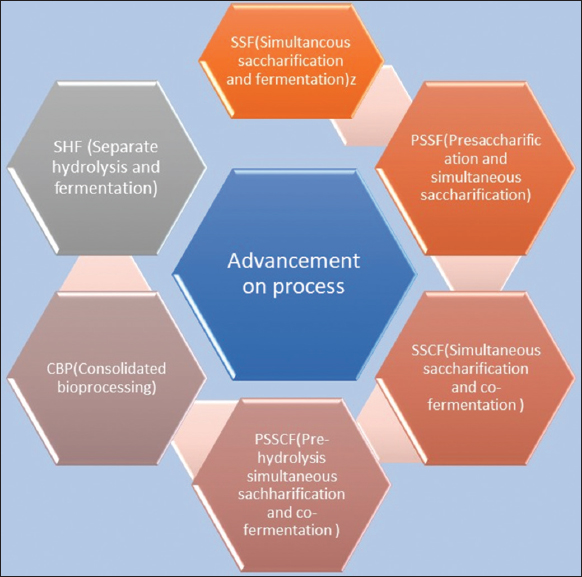 | Figure 2: Advancement in pre-treatment process [Click here to view] |
3.1. Physical Methods
The physical method of pre-treatment increases cellulose’s accessible surface area while decreasing particle size and crystallinity. However, this method is not very efficient in removing lignin and hemicellulose, and it consumes a lot of energy [15]. This size reduction reduces the heat and mass transfer limitations caused by the biomass’s large particle size, as well as an increase in bulk density, thus, allowing the pre-treatment of more concentrated feedstock [16]. There are several methods of physical pre-treatment techniques such as grinding, mechanical comminution (mechanical reduction in biomass particulate size), freezing, and radiation that are used to pre-treat LC waste.
3.1.1. Mechanical methods
Comminution is a crucial stage in the pre-treatment of various materials, including LC biomass, to make it simpler to manage in subsequent production steps. Different comminution methods are available, such as dry, wet, and vibratory ball milling, compression milling, grinding, and chipping. The primary objective of comminution is to reduce the size of the material to increase its surface area and enhance its accessibility to enzymes. Milling is typically the first comminution stage in the pre-treatment process, and several varieties of milling can be employed, including hammer milling, knife milling, ball milling, and disk milling [17]. A major drawback of milling is that it consumes large amounts of energy. Chipping is commonly used for large waste materials, straws, wood chips, etc. It usually brings down the particle size of biomass to 10–30 mm. However, this technique is not as effective as milling and grinding, which reduces the particle size to 0.2 mm. Size reduction alone is not very effective and hence is often used in combination with chemical processes for pre-treatment [18].
3.1.2. Microwave
The microwave irradiation technique is a traditional technique that differs from the simple heating method as it only changes the ultrastructure of cellulose and selectively degrades the lignin and hemicellulose components while leaving the cellulose intact. It also improves reducing sugar enzymatic sensitivity by disrupting the waxy surface. This method is quite beneficial as it is highly uniform and selective, and it requires less time and energy compared to simple heating [17].
3.1.3. Pyrolysis
LC biomass is processed using pyrolysis, an endothermic process, at temperatures above 300°C. Compared to other endothermic reactions, it consumes less energy. During this process, the cellulose in the biomass breaks down into the gaseous substance’s hydrogen, carbon monoxide, and char. The residue is then subjected to a gentle acid treatment before being washed with water. The main component of water leachate is glucose, which can be utilized to make biofuels because it can serve as a source of carbon.
3.1.4. Extrusion
Extrusion is a physical pretreatment that combines mechanical and thermal effects in a single machine. To achieve the appropriate screw profile, it uses extruders with one or two axes and movable screws. In a temperature-controlled, constrained barrel, the screws rotate. Continuously pushed through the machine’s restricted space, the biomass creates strong shearing pressures that mechanically change the structure of the fibers, opening up additional surface area for enzymatic hydrolysis [19]. Extrusion can also be used in combination with other pre-treatment methods to further enhance their effectiveness. During the extrusion process, the biomass might be given an alkali or acid treatment to improve sugar recovery. However, it is essential to carefully control the extrusion parameters, such as temperature, pressure, and screw profile, to achieve the desired modifications in the biomass structure without causing excessive degradation or damage [20].
3.2. Chemical Methods
The primary purpose of chemical pre-treatments is the internal degradation or rupturing of lignin and hemicelluloses, which is accomplished by rupturing the underlying lignin and hemicellulose linkages. These pre-treatments typically involve the use of acids, alkalis, ionic liquids, and organic acids as catalysts, such as oxalic acid, acetylsalicylic acid, as well as salicylic acid [16].
3.2.1. Alkaline treatment
In industrial applications, alkaline pre-treatments offer the necessary reaction settings, including the employment of less corrosive chemicals, lower reaction temperature, and pressure, the reuse of residual alkali, and simpler reactors [16]. Compared to acid processing, alkaline processing results in less sugar degradation and higher recovery of caustic salts [21]. The removal of lignin using an alkali method is the most typical pre-treatment for lignocelluloses [22]. In the experiment study carried out by Tsai et al., the biomass (insoluble fraction) was collected and rinsed in flowing water until a neutral pH was obtained to boost productivity [8]. Sticks of Napier grass were treated with 10% NaOH at a solid-to-liquid ratio of 1:20 (w/v) after an hour at 90°C. The pre-treated biomass was kept at 4°C in sealed plastic bags after the free water was removed using a dehydrator. The feedstock was dried in an oven for 24 h at 65°C. Before pre-treatment, dried grass stalks contained 25.0% lignin, 41.8% cellulose (glucan), and 23.2% hemicellulose. Lignin and hemicellulose were removed from the raw material as a result of drying, resulting in a significantly increased cellulose content of 62.1% w/w in the biomass, indicating that around 70.3% of the glucan was preserved [8].
3.2.2. Dilute acid treatment
LC biomass is also treated with concentrated acids. The hydrolysis of the hemicellulose by the dilute acid pre-treatment is quite effective [24]. Sulfuric acid and hydrochloric acid are the two most widely used acids. About 19.4% lignin, 20.9% hemicellulose, and 22.6% cellulose make up the non-pre-treated biomass [2]. A 2% sulfuric acid solution and 100 g of LC material were combined, with a solid-to-liquid ratio of 1 g biomass/mL solution. The suspension was swirled for 90 min at 90°C. The mixture was filtered to remove the black liquor from the pre-treated material. The solid substance was dried and put in the freezer after being rinsed with water until it had a pH of neutral. Following the pre-treatment, it was discovered that the cellulose content had grown to 24.0% (wt.% based on dry biomass) [Table 1]. Acid hydrolysis of this grass caused solubilization, which decreased the lignin and hemicellulose contents to 13.4% and 13.5%, respectively, in the solid phase [2].
Table 1: Percentage c omposition of Napier grass after chemical pre-treatments (wt.%, based on the dry matter).
| Pre-treatment strategy | Time | Temp. | Cellulose (wt. %) | Hemi-cellulose (wt. %) | Lignin (wt. %) | Ref. |
|---|---|---|---|---|---|---|
| Alkaline treatment | 1 h | 90°C | 62.1 | 16.1 | 19.5 | [25] |
| Dilute acid treatment | 90 min. | 90°C | 24.0 | 13.5 | 13.4 | [26] |
| Two-stage treatment | 1 h (1% H2SO4) 6 h (2% NaOH) | 120°C (H2SO4) 80°C (NaOH) | 67.6 | 4.0 | 17.0 | [25] |
3.2.3. Two-stage pre-treatment
Sticks of Napier grass were subjected to an hourlong incubation in the reactor containing 1% H2SO4 at a solid-to-liquid ratio of 1:10 (w/v) and a temperature of 120°C. After the biomass had been treated, the insoluble component was collected and then washed with running water. After that, the particles were heated to 80°C for 6 h while being treated with 2% sodium hydroxide in a solid-to-liquid ratio of 1:10. After being dehydrated, the pre-treated biomass was put into plastic sacks, given a good seal, and kept at a temperature of 4°C. After drying the pre-treated biomass in an oven at 65°C for 24 h, its weight after drying was determined. Due to the combined procedure applied to Napier grass, approximately 93.4% of the hemicellulose and 74.1% of the lignin were removed, while 61.4% of the glucan content was preserved in the dried raw material [8].
3.3. Biological Methods
The biological process of pre-treatment is an efficient, safe, environmentally sustainable, and cost-effective method of removing lignin from LC biomasses. Due to their lignin-degrading abilities, microorganisms such as brown- and white-rot fungi are used to degrade hemicellulose and lignin in agricultural waste. Brown-rot and white-rot fungi attack different compounds with brown-rot fungus primarily attacking cellulose and white-rot fungus attacking cellulose and lignin. The most effective basidiomycetes pre-processing of LC substrates are white-rot fungi. Because many white-rot fungi break down lignin, they are used to produce ligninase and degrade lignocellulose [26].
3.3.1. Fungal pre-treatment
Micro-organisms like brown- and white-rot fungi are employed to break down lignin and hemicellulose in biomass due to their capacity to degrade lignin [26]. The pre-treatment circumstance used in the study is presented below [Table 2]. The Aspergillus niger and P. chrysosporium biological pre-treatments were conducted in the SSF system, and the pre-treatment concentration was established through preliminary analysis. Sterilization was accomplished by a low-temperature pasteurization procedure. 4 g of sterilized Napier grass and an extra nutrition solution were added to a roux bottle to obtain an 89% moisture level. The nutrient solution served as the SSF system’s medium for fungal culture. The substrate was incubated in a 35°C oven after inoculation for 9 days. The pre-treated biomass was agitated at 130 rpm for 30 min while being rinsed with an acetate buffer (0.2 M, pH 4.5). A. niger treatment produced a lower lignin: cellulose ratio (around 25%), whereas biological pretreatment with P. chrysosporium produced a high lignin: cellulose ratio (31%). The pre-treatment was successful if there was low lignin: cellulose ratio [5]. A. niger performs better when cellulose, hemicellulose, and reducing sugar content are taken into account when comparing the two fungal species for superior delignification ability under ideal conditions [5].
Table 2: Fungal strains used in pretreatment for bioethanol production from Napier grass.
| Fungal strain | Type of fungus | Enzymes produced | Pre-treatment mechanism | Reference (s) |
|---|---|---|---|---|
| Postia placenta | Brown-Rot Fungus | Notable for selective degradation of cellulose | Cellulose and hemicellulose degradation, increased accessibility | [11] |
| Phanerochaete chrysosporium | White-Rot Fungus | Lignin degradation, improvement of biomass digestibility | [26] | |
| Gloeophyllum trabeum | Brown-Rot Fungus | Notable for selective degradation of cellulose | Cellulose and hemicellulose degradation, increased accessibility | [11] |
| Aspergillus spp. | Cellulolytic Fungus | Cellulases, hemicellulases | Direct hydrolysis of cellulose and hemicellulose | [5] |
| Candida shehatae | Yeast | Fermentation of xylose | Xylose fermentation for bioethanol production | [11] |
3.3.2. Enzymatic delignification
An alternative to the biological pre-treatment procedure that aims to speed up reaction time and improve delignification effectiveness is enzyme-mediated delignification of grasses. The use of raw or pure enzymes obtained from hydrolytic or ligninolytic enzymes is a component of enzyme treatment. According to reports, the amount of lignin removed from LC biomass using enzymatic pre-treatment may be comparable to that removed using fungal pre-treatment [27]. In addition to allowing for substrate specificity, this approach shortens the processing time without the usage of carbohydrates from weeks to only a few hours. Delignification enzymes can be acquired from commercial enzymes including concentrated and purified enzymes or from ligninolytic microbe culture supernatant. P. chyrosporium, C. subvermispora, Pyconoporus cinnarbarinus, Cyathus stercolerus, and Ceriporiala cerata are the primary producers of laccase, mannase, and peroxidases. As laccase doses were raised, it was discovered that the lignin level was much lower following the enzymatic sequence. Following treatment, ethanol content reached 2 g/L, enzymatic hydrolysis increased by 12%, and delignification by 50%.
3.4. Physico-chemical Pre-treatment
While each preliminary treatment method contributes significantly, a pre-treatment method, alone, does not produce effective outcomes without a few drawbacks. As a result, the combined pre-treatment techniques could reduce the downsides while still achieving the desired result. The most popular steam-based physiochemical pre-treatments for natural fiber include superheated steam, hydrothermal steam, and steam explosion. Natural fibers are typically subjected to physiochemical pre-treatment to get rid of lignin and hemicellulose [28].
3.4.1. Hydrothermal process
Physical and chemical pre-treatment processes are combined in hydrothermal pretreatment, which takes place in water environments. Several instances include steam explosion, autohydrolysis, liquid hot water, and subcritical water hydrolysis. Steam explosion has become one of the most popular physicochemical pre-treatments, and it is carried out at high steam pressure and temperature. This treatment is frequently used in industry due to its low cost, great effectiveness without altering the cellulose structure, disrupting H-bonds, swelling of the biomass, and little preparation and handling requirements. For the entire procedure, it only required water that had been heated to a high degree [28]. Hydrothermal pre-treatment (HTP) was carried out in batch mode with a solid-to-liquid ratio of 1:10 (w/w) and 200 g (dry weight) of processed Napier grass sample. At 125, 150, 175, and 200°C, the HTP was performed three times. Every experiment involved heating the vessel to the required temperature for varied lengths of time, holding it there for 15 min, and then allowing it to cool to about 80°C before raising the lid. The solid and liquid fractions were separated using a 200-mesh screen following HTP. As the HTP increased in temperature, it was seen that the amount of hemicellulose in the solids fraction fell noticeably. At 200°C, total hemicellulose liquefaction took place, meaning that no hemicellulose was found in the solids fraction. On the other hand, the amount of cellulose and lignin in the biomass increased as the HTP temperature increased. Lignin increased more visibly than cellulose at higher HTP temperatures as a result of its stronger resistance to pre-treatment conditions [29].
3.4.2. Steam explosion
The most popular technique for pre-processing LC biomass is steam explosion. Several LC materials have been effectively converted into ethanol by steam explosion. This approach involves applying saturated steam to biomass when it is under intense pressure. The components explode when the combination is held for a while to encourage hemicellulose hydrolysis [30,31]. Before the materials are exposed to air pressure, steam explosions are conducted at high pressures ranging from 0.2 to 5 MPa and temperatures between 160 and 260°. The potential for cellulose hydrolysis is increased by the high temperature’s effects on lignin transformation and hemicellulose degradation. It uses less energy than mechanical comminution and has no negative environmental effects. Traditional mechanical procedures take 70% more energy than a steam explosion to reduce particle size by the same amount [32].
3.4.3. Ammonia-assisted pre-treatment
The ammonia-assisted pre-treatment procedure is of particular interest because ammonia creates an alkaline environment conducive to delignification [33]. The lack of inhibitor formation, high sugar yield recovery (up to >90% over theoretical values), and recyclable nature of this technology are just a few of its benefits [27,34]. Before hydrolyzing the biomass with different enzymes, the researchers treated the biomass with ammonia. This low-temperature technique uses concentrated ammonia (0.3–2 kg ammonia/kg dry weight) as a catalyst. In a high-pressure reactor, ammonia is added to biomass; after 5–45 min of cooking, the pressure is rapidly released. The temperature employed in this technique is typically approximately 90°C. According to [31], AFEX operates on a similar principle to that of a steam explosion.
4. MICROBE-MEDIATED BIOETHANOL PRODUCTION FROM NAPIER GRASS
4.1. Kluyveromyces Marxianus
K. marxianus is a species of yeast that is emerging as a powerful tool in the fermentation industry. It is generally isolated from a wide range of habitats out of which the most common ones are fermented dairy products, and sugar industry sewage [35]. The increased interest in the use of K. marxianus in bioethanol production is due to its potential advantages over the conventional yeast S. cerevisiae. One of the most captivating properties of this species is its thermotolerant nature; the yeast can survive even in high temperatures ranging from 50 to 52°C contrary to S. cerevisiae, which gets impaired at high growth temperatures which leads to the repetitive use of coolants resulting in higher cost of production [36]. Now, another benefit of this thermotolerance power is that most of the cellulosic enzymes possess a stable working temperature of 40–50°C, leading to enhanced production from Napier grass. Another advantage is that this species works on a broad range of substrates to produce industrially important enzymes, vaccines, and probiotics and can utilize a broad spectrum of carbon sources. The different types of substrates include xylose, lactic acid, malic acid, and fructose.
K. marxianus has been used in a variety of research to produce ethanol from different substrates [Table 3].
Table 3: Products obtained using different substrates and microbes.
| Microbe name | Substrate used | Product reported | Yeild obtained | References |
|---|---|---|---|---|
| Kluyveromyces marxianus | Sterilized organic whey | Ethanol | 0.50g ethanol/g lactose | [7] |
| Non-sterilized organic whey | Ethanol | 2.5-4.5g/l/h | [7] | |
| Taro peels | Ethanol | 48.98g/l | [58] | |
| Yarrowia lipolytica | Defatted biomass | Ethanol | 13.39g/l | [59] |
| Sugarcane bagasse hydrolysate | Lipid | 6.68g/l | [60] | |
| Hansenula polymorpha | Substrate rich in xylose | Ethanol | 9.8g/l | [12] |
| Dekkera bruxellensis | Substrate rich in Arabinose (80g/l) and Xylose (60g/l) | Ethanol | 1.9g/l | [6] |
| Substrate rich in Xylose and Glucose (4:1) | Ethanol | 5.9g/l | [6] | |
| Pichia stipitis | Sugarcane bagasse | Ethanol | 8.4 g/l | [2] |
In a study organic whey from cheese production was used to produce ethanol under the action of K.marxianus which concluded K. marxianus was very much suitable for industrial ethanol production. The study showed high production of ethanol from unsterilized whey (2.5–4.5 g/l/h) when the dilution rate was 0.2/h in continuous mode. When sterilized whey was used the productivity decreased to (0.50 g ethanol/g lactose) in the batch mode [37]. Another study utilized the effluents of the dairy industry, that is, Whey and Scotta to produce ethanol. Whey has already been viewed as a potential source to produce bioethanol and can be used for other uses, such as animal feeding. On the other hand, Scotta is considered a waste as it has no protein left in it. Different modes of fermentation with varying temperature ranges were performed that showed that the semicontinuous fermentation at low-temperature ranges (28°C) gives fairly good productivity and the waste can be used for bioethanol production [38]. Another initiative was taken to convert Taro waste into bioethanol using 5% inoculum of K. marxianus. At 40°C after 22 h of cultivation maximum yield was observed (48.98 g/l). An upscaling experiment was also conducted to implement this process on an industrial scale, and it yielded fairly good results when compared to the previous one [39].
4.2. Yarrowia Lipolytica
Y. lipolytica, yeast belonging to the Dipodascaseae family is a dominant species in industrial microbiology due to its versatile substrate requirements. It can feed on conventional substrates such as fruit and vegetable peels as well as non-conventional ones like hydrocarbons. The species name “lipolytica” comes from the ability to hydrolyze lipids efficiently and synthesize a large number of essential nutrients [40]. Moreover, this species can deal with extreme temperatures and alkaline conditions which is a preferred property of strains used in fermentation processes. The easily available substrate and higher rates of production lead to lower production costs and highly renewable energy sources. Another characteristic that makes it convenient in the fermentation processes is its ecofriendly nature and non-pathogenicity due to which it can be used in the food industry and can be found living in cheese and meat [41]. Furthermore, its safe nature and easy accessibility from a variety of habitats like protein-rich environments (yogurt and cheese) make it a potential weapon to be used for biofuel production. As the Napier grass comprises 9.9–17.5% protein of crude protein, the Y. lipolytica has the potential to convert most of it into bioethanol [42].
Studies have been conducted for using Y. lipolytica as an alternative for S. cerevisiae in the fermentation process of bioethanol and to broaden the range of substrates so that we get a more economical and feasible method. A study was performed where defatted biomass was used to produce bioethanol using Y. lipolytica and the maximum growth noted was 13.39 g/l. If un-defatted biomass was used, yield was not significantly improved [43]. In addition, the introduction of genes with the help of gene editing technologies is also viewed as a promising source in enhancing production. This can help to get all the desirable properties in one species. One more study investigated the use of detoxified sugarcane bagasse hydrolysate instead of a carbon source for biodiesel production. Two different nitrogen sources (Peptone and ammonium nitrate) were taken for the experiment. The lipid yield was found to be 6.68 g/l in the presence of peptone in comparison to D-glucose, and D-xylose [43]. Hence, confirming the extraordinary action of Y. lipolytica [Table 3].
4.3. Hansenula Polmorpha
H. polymorpha, yeast that belongs to the methylotrophic family, means it can consume methanol as a sole energy source. This yeast has gained interest in the field of recombinant DNA technology. It has been used for more than three decades for heterologous gene expression and the production of crucial enzymes and vaccines like Hepatitis B. It is also thermotolerant and can survive in high temperatures of more than 50°C. Due to this thermotolerance power, it has been studied for use as a host for ethanol fermentation processes. Furthermore, it possesses the ability to ferment xylose, cellobiose, and glucose efficiently at higher temperatures [44]. It has been looked like a potential host due to its property of not only metabolizing glucose but xylose to ethanol also which is the second most abundant sugar in the world. These properties gave it an upper hand over the conventional first generation hosts [Table 3] [45].
Till now different strains of H. polymorpha have been studied to get a greater yield of bioethanol. Nearly all strains are known to produce ethanol from xylose at elevated temperatures with no additional cooling requirements. Although the yield from xylose exceeds the conventional ones, the final concentration was found less than the expected yield. Out of many strains tested, the yield from the best mutant was recorded to be 9.8 g ethanol/l at 45°C [46,47].
4.4. Dekkera Bruxellensis
D. bruxellensis is a very eminent name in the wine and bioethanol production industries. It has been looked up as an alternative to the conventional yeast S. cerevisiae due to its distinguishable properties among yeasts. D. bruxellensis has long been considered spoilage yeast, attributed to the off-flavor taste it imparts in wine and dairy products over the years [48]. However, according to several new studies, scientists have investigated the beneficial impact of this species in bioethanol production. This discovery shows the ability of this yeast to consume nitrate and other nitrogen sources simultaneously without affecting the yield [49]. Furthermore, it possesses the ability to cellobiose and dextrin as carbon sources which give a broader range for the substrates. Another feature that may confer an advantage industrially is the tolerance of this species against high ethanol and pH concentrations which makes it suitable to be used in a fermentation process. In comparison with S. cerevisiae, D. bruxellensis is more efficient in consuming glucose in sugar-limited conditions. Furthermore, studies suggest that it can be due to its high metabolism or high affinity towards glucose [50].
A study conducted in 2018 by a group of scientists showed that there is not much difference in the yield of S. cerevisiae and D. bruxellensis. The differences noted after the experiments were that the sugar was mostly converted by D. bruxellensis into lactic and acetic acid and the low capacity to digest sucrose, which is the main reason behind not using it as fermentation yeast [51]. There was another experiment where the growth rate of yeast and ethanol yield was checked at different temperatures and carbon sources (maltose and glucose). The results revealed that temperature does not have any noticeable effect, but the yield varies among maltose and glucose, with maltose having a slightly lower yield [52]. In one more experiment, the ethanol yield was studied with different sugars to check the ability of D. bruxellensis, the results demonstrated that the yeast is capable of giving maximum yield with xylose and glucose in comparison with xylose and arabinose which is quite the same in ethanol yield and hence can be a good alternative for fermentation processes [Table 3] [53].
4.5. Pichia Stipitis
P. stipitis is among the crucial yeasts used in the fermentation industry. It can thrive in aerobic conditions as well as in low oxygen requirements with temperature requirements of 25–37°C. According to the studies after 40°C, the growth of the microbe ceases. This yeast is of high significance due to its extraordinary natural ability to ferment xylose robustly and convert it to ethanol [54]. According to the studies reported, when working on LC material, a yield of 41g ethanol/l was observed. This is the most desirable property to produce bioethanol, as the main problem that arises is to degrade all the sugars present in the substrate that this yeast offers. Due to this, the yeast is greatly implied to ferment grain and sugarcane to produce ethanol with high economic benefit [Table 3].
P. stipitis has been used to ferment several substrates to produce bioethanol. In a study, sugarcane bagasse was used as a substrate with a temperature of 30°C, pH 5.5 for 24 h and the yield noticed was 8.4 g/l [55]. One more study was conducted where P. stipitis and S. cerevisiae were used together to ferment the glucose and xylose present in rice straw. Here, both the yeasts were applied sequentially one after the other. At the time of the addition of P. stipitis, S. cerivisiae cells were inactivated. The yield of ethanol observed was 21.1 g/l from 10% (w/w). There were certain advantages of this process at the industrial level [56]. Xylose fermentation efficiency was seen at 50°C. Another study in which cofermentation was performed like the previous one, used Prosopis juliflora hydrolysate also showed positive results towards this cofermentation process, determining its feasibility which can be used industrially [57].
5. ADVANCEMENTS IN BIOETHANOL PRODUCTION
5.1. Advancement in New Technology
There are three primary phases in the metabolic conversion of biomass to bioethanol. Pre-treatment, hydrolysis, and fermentation are the names of these procedures [58-60]. These steps might be finished separately or simultaneously with different technical advancements. It has been used to tune the temperatures of the enzymatic hydrolysis and fermentation separately from one another (Separate hydrolysis and fermentation, or SHF) [61,62]. However, it has been demonstrated that this approach has several disadvantages. The disadvantages of this strategy include hydrolyzing enzymes, pretreatment techniques, high costs for different reactors, the creation of inhibitors, and a higher concentration of sugars in the hydrolyzate that can put fermentative yeast cells under osmotic stress. By employing a different technique, these drawbacks can be eliminated. As an alternative to SHF, simultaneous saccharification and fermentation (SSF) has been proposed. SSF has several benefits over SHF, including lower startup costs and a reduced risk of enzyme inhibition due to hexose synthesis and contamination [22].
Microorganisms cannot thrive at temperatures above 37°C, which is a need for hydrolysis and fermentation in the SSF cycle. This is one of the most important limits of the SSF process. Candida brassicae, C. lusitaniae, Fabospora fragilis, S. uvarum, and K. marxianus are only some of the thermotolerant microorganisms that have been studied and tested for prospective usage in SSF to address these issues. The pre-hydrolysis and simultaneous saccharification and fermentation process, often known as the PSSF process, is an additional blending method that is an improvement on the SSF method in which the enzymatic reaction takes place at temperatures close to the 50°C optimum enzymatic hydrolysis condition [63,64]. When the yeast is added during the PSSF procedure, the temperature stays the same in the pot even though it has dropped. Another advanced technology known as simultaneous saccharification and cofermentation (SSCF) has been developed. With this method, both hexoses and pentoses can go through the process of cofermentation in the same vessel with the help of genetically engineered yeast and bacteria [65-67].
When compared to the methods that were previously addressed, SSCF is thought to be a better method for completing saccharification, hexose fermentation, and pentose fermentation in a single reaction [23]. Liu and Chen showed that SSCF is an effective method for converting glucan and xylan in maize stovers to 82.0% and 82.1%, respectively, at a solid load of 20%. S. cerevisiae and modified Escherichia coli KO11 each play important roles in this process [68]. This method makes use of yeast and bacteria that have been genetically engineered to be thermotolerant [69]. The process of producing bioethanol from LC biomass can be completed in one pot thanks to consolidating bioprocessing, or CBP, a relatively recent advancement in the world of biotechnology. In addition, this approach allows for simultaneous fermentation and hydrolyzing enzyme synthesis. E. coli is a critical part of the CBP because it produces a range of enzymes that are required for the CBP’s numerous bioethanol production pathways [61,70,71].
5.2. Advancement on Feedstock Raw Materials
The potential implications of research on feedstock raw materials for biofuel production for the long-term economic and environmental viability of biofuel production have elevated the importance of this area of study in recent years. Research has been done on a variety of alternative feedstocks, including microalgae, waste products, and LC biomass in recent years. Due to their high productivity, tolerance to a wide range of environmental conditions, and ability to create a variety of biofuels, microalgae have been identified as a potentially useful feedstock. Waste resources including municipal solid refuse and agricultural leftovers have been explored as viable feedstocks due to their cheap pricing and abundant availability. LC biomass has become an important feedstock for biofuel manufacturers due to its accessibility and potential to lower greenhouse gas emissions [72,73]. The use of LC biomass in the creation of biofuels has been constrained, nevertheless, by technological and financial challenges. Enzymatic hydrolysis and the high cost of pre-treatment are just two examples of the issues that can occur.
The ideal crop for producing bioenergy produces a high concentration of C6-sugars in polysaccharides such as cellulose, callose, galactan, and mixed glucose while minimizing the concentration of fermentation inhibitors such as pentoses and acetate [74]. LC biomass is widely available and has the potential to reduce greenhouse gas emissions, making it a key feedstock for the manufacture of biofuel. Dedicated energy crops, forestry residues, and agricultural residues are all included in this [73]. Although LC biomass has a lot of potential as a source of biofuel, numerous technical and financial barriers make it difficult to use. Enzymatic hydrolysis and the high cost of pre-treatment are just two examples of the issues that can occur.
The majority of LC feedstocks are made up of woody plants such as poplar and perennial, non-food grasses such as switchgrass and Miscanthus. Deconstructing biomass to increase surface area, decrystallizing cellulose to emphasize the enzymatic hydrolysis, and deconstructing biomass are all necessary steps in the conversion of LC biomass to sugars for the development of fuels because the crystalline cellulose core of the cell walls is so resistant to chemical and biological degradation. This is the case because the complicated cell wall structures themselves are so resistant to chemical and biological disintegration, as well as because the crystalline cellulose at the center of the cell walls is [75]. In addition, fermenting LC biomass into fuels is more difficult than fermenting hexoses due to the high pentose concentration of this biomass.
Plant biomass may be a better feedstock for the generation of biofuel if it comes from plants that have been genetically engineered to have lower amounts of xylan in their secondary walls [76]. Researchers are looking at certain sectors to create and improve feedstocks for the effective production of bioethanol by altering the structure of the cell walls so that they can be broken down into their component sugars in a more time and energy-efficient way. These sugars may provide the optimal chemical makeup for making fuel. In addition, it is attractive as a possible future bioethanol feedstock because large harvests may be generated even on marginal soils of low quality and without the usage of fertilizer [77].
According to several studies, nitrogen fertilizer addition may enhance rice varieties’ ability to saccharify biomass for the manufacture of bioethanol [78]. Genetic modifications, such as synthetic genetic circuits (SGC) and clustered regularly interspaced short palindromic repeats (CRISPR), focus on lacking undesired components and boost bioethanol production [7]. The high cost and low yield associated with the manufacture of bioethanol can be decreased by altering the biomass feedstock, which can be accomplished in cereal plants through transgenesis, cisgenesis, mutagenesis, and traditional breeding [79,80].
Recent research highlights the potential of altering cell wall polysaccharide structure, lignin composition, and total cell wall content as advanced strategies in biomass technology. These modifications aim to reduce chemical complexity, enhance cellulose content, and facilitate efficient hydrolysis for increased fermentation sugar yields, potentially mitigating carbon dioxide emissions associated with biofuel manufacturing [80]. In general, the economic and environmental feasibility of biofuel production may be enhanced by investigating methods to enhance the quality of feedstock materials. Ongoing research focuses on refining the cultivation, harvesting, and conversion of alternative feedstocks into biofuels, to develop novel and improved technologies in this field.
5.3. Advancement in Genetic Approach
Recent research has demonstrated that genetic manipulation, gene coexpression profiling, and correlation analysis between OsGH9 and OsCESA can be used to modify the genetic cell wall in genetic mutants and transgenic herbaceous plants and food crops to increase biomass yield and enzymatic saccharification [81,82]. Some rice genes, such as OsGH9A3 and B5, have been linked to cellulose production, while others, including OsGH9B1, 3, and 16, were found to be involved in modifying the crystallinity of lignocellulose also put out a novel cell wall model with grooves that enhance the amorphous regions of natural cellulose microfibrils [83,84]. This framework provides a methodical approach to enhancing the growth of bioenergy crops and the production of biofuels. The same team of scientists led by Li et al., investigated whether genetic modification may increase the output of plant biomass from bioenergy crops as well as the production of biofuels. This study focused on the extraordinary mutants Osfc17 and Osfc30. These two mutants showed both typical plant growth and high biomass enzymatic digestibility, both in situ and in vitro. Numerous XAT cellulose genes and GH9B candidate genes for enhancing hemicellulosic Arabinose and decreasing crystalline index (CrI), respectively, were found. These two effects were both noted. The results suggested that by simultaneously overexpressing GH9B and XAT genes in rice, cell wall changes might be achieved to increase the enzymatic digestibility of biomass and the plant’s resistance to lodging [85].
The enhancement of biomass enzymatic digestibility is a likely outcome of these genetic adjustments. Researchers further investigated the impact of these modifications on cell wall characteristics and biomass saccharification in genetically modified rice plants [81]. OsGH9B1 and OsGH9B3 are two genes of the GH9B subfamily that were studied by Hunag et al. 2019. Their innovative approach led to a remarkable increase in cellulase activity in vitro compared to the wild type (WT), suggesting the potential for enhanced enzymatic biomass degradation. The cellulose microfibrils of the transgenic lines displayed increased amounts of reducing ends of β-1,4-glucans. Composition of wall polymers (cellulose, hemicellulose, and lignin) as well as cellulose polymerization was all found to have undergone minor changes. The crystalline index (CrI) of lignocellulose also decreased by 21–22%. High ethanol yields at a dry matter concentration of 22.5% were obtained from the modified lines after moderate alkali pretreatments and subsequent enzymatic hydrolysis. Twelve OsGH9 and OsGH10 genes were investigated for possible roles in transgenic rice lines by [86]. These genes control how cellulose and hemicellulose are changed, as well as how the cell wall is remodeled. The target genes OsGH9 and OsGH10 were discovered to be highly expressed in rice, which significantly increased the enzymatic digestibility of the biomass and demonstrated that rice transgenic plants carrying the CesA8:SUS3 gene had considerably enhanced biomass saccharification and lodging resistance [86]. To do this, the cellulose’s crystallinity and cell wall thickness were also reduced. The discovery points to a genetic method for bioenergy crop cell wall modification. This research has brought attention to the value of genetic approaches in enhancing biomass yield and quality for the generation of bioenergy.
5.4. Advancement on Pretreatment Condition
The time-consuming and expensive process of pre-treatment for LC materials is known as biochemical conversion. The intricate plant cell wall network has now been dissociated from the main polymer components. Many pre-treatment techniques have been developed, including biological, chemical, physicochemical, and physical methods. Although the majority of these techniques continue to be unaffordable, the bioethanol sector views them as investments in the future [87]. Due to this, scientists are focusing on developing effective and affordable pre-treatment technologies for use in the biofuel business. Recent advances in pre-treatment have focused mostly on breaking down the complex bonds between cellulose, hemicellulose, and lignin, the three main components of LC biomass. One such technique is pre-treatment with liquid hot water (LHW), which involves both high pressure and high temperature. To improve hydrolysis yields, we came up with a novel combination pre-treatment of deacetylation and LHW on biomass [88].
The ammonia fiber explosion (AFEX) is another method, in which liquid ammonia is added to the biomass under certain physical and chemical circumstances. Over the last decade, ammonia-based pre-treatments have been the subject of many studies, and they now stand as one of the most promising pre-treatment methods for lignocellulose biorefining. Raghavi et al. studied a novel sugarcane waste pre-treatment technique, and they found that it was superior to acid and alkali pre-treatment in terms of hemicellulose and lignin removal [89-90].
Numerous pre-treatment strategies have been attempted and evaluated to maximize the quantity of bioethanol that may be generated from organic feedstocks. Among the many available pre-treatment strategies, the alkaline reagent approach stands out as one of the most promising process alternatives. Kim et al. discovered that pre-treatment with aqueous ammonium hydroxide may remove up to 97.2% of lignin from maize stover and hybrid poplar [90], resulting in ethanol production of more than 75%. Pre-treatment with 1% NaOH and 8% NH4OH at 50°C for 48 h removes 63.6% of lignin from maize stover, as reported by [91]. Wi et al. found that pre-treatment with hydrogen peroxide and acetic vinegar at 80°C for 2 h could remove 97.2% of lignin from a range of biomass feedstocks [92]. Kim et al. found that the greatest glucose output from reed was 326.5 g/kg biomass when hydrodynamic cavitation was utilized with 3.0% NaOH at a solid-to-liquid ratio of 11.8% for 41.1 min [14]. Verardi et al. found that sugarcane bagasse steam pre-treatment at temperatures of 200°C for 5min and 210°C for 15 min may provide a glucose output of 100% [93].
Maize stover was pre-treated with magnesium bisulfide for 60 min at 170°C and a pH of 5.2 by Yu et al. [94]. They were able to extract almost 90% of the lignin and 80% of the hemicellulose. In 2018, Matsakas et al. created a unique birch pre-treatment using a hybrid organosolv. The ethanol and hydrogen sulfide pre-treatment temperature was set at 200°C, and the ratio of ethanol to hydrogen sulfide was 60% by volume and 1% by weight. There was 77.9% dry-weight cellulose, 8.9% dry-weight hemicellulose, and 7.0 dry-weight lignin content/86.2% delignification in the final product [95]. A new alkaline organosolv approach (a sodium-hydroxide-methanol solution) was shown to be effective in treating corn stover, as reported by the work of Yuan et al. [96]. The result was 97.5% retention of glucan and an 83.5% retention of xylan. At a low temperature (100°C) for 2 h, the redox couple catalyst of FeCl3/NaNO3 converted 71.53 and 70.66% of the cellulose in hardwood and softwood, respectively, to sugar in the research by [97]. Hydrothermal pre-treatment of sugarcane bagasse at 190°C 10 min results in yields of glucose recovery in the range of 0.28–0.30 g/g and 0.14 g/g of ethanol, according to research by Ambye-Jensen et al. [98].
More recently, Zhang et al. [99] investigated whether or not pre-treatment with alkaline hydrogen peroxide and bio-additives could boost the performance of high-solids enzymatic hydrolysis of sugarcane bagasse to produce succinic acid [99]. Researchers found that sugarcane bagasse’s enzymatic hydrolysis efficiency was greatly enhanced by the addition of bio-additives to an alkaline hydrogen peroxide pretreatment. The output of fermentable sugars and succinic acid increased as a consequence. This study suggests a method for effectively turning sugarcane bagasse into useful biochemicals, which, if adopted, might help foster the growth of a sustainable bioeconomy.
The biochemical conversion of LC materials relies heavily on pre-treatment, making the development of efficient and cost-effective pre-treatment technologies crucial if the bioethanol industry is to fulfill future needs. Pre-treatment strategies that have shown promise in recent studies include alkaline reagent, LHW, and ammonia-based approaches; these processes warrant additional investigation for usage in commercial settings.
5.5. Advancement in Hydrolysis
The extraction of fermentable sugars from LC biomass using hydrolysis is an essential step in the production of bioethanol that plays a substantial role in determining the overall effectiveness of the process. The hydrolysis method can be generally broken down into two different strategies: The chemical method, and the enzymatic method. Enzymatic hydrolysis has emerged as a more viable choice for the production of bioethanol in recent years, even though chemical hydrolysis is capable of releasing fermentable carbohydrates. Chemical hydrolysis is notorious for being hazardous, corrosive, and expensive [100]. Researchers are presently looking into a wide variety of approaches to enhance enzymatic hydrolysis. Some of these approaches include increasing enzyme yield while decreasing enzyme costs. Among these various methods, enzyme immobilization through the use of calcium alginate is a promising approach that has the potential to increase biocatalytic productivity as well as enzyme stability, which will eventually lead to a reduction in costs [101].
Coimmobilization of enzymes is another technique that, in comparison with free enzymes, results in increased stability and storage time. This is because multiple enzymes can be immobilized together in this process [101] To increase sugar yield, significant research has been conducted on cellulase cocktails. These cocktails comprise multiple enzymes and are designed to overcome low enzyme activity as well as product inhibition. Tein engineering and directed evolution are two additional methods that can be used to boost enzyme production [102]. In the manufacturing of bioethanol, the use of industrial enzymes such as Cellic® Ctec2 and Cellic® Htec2 from Novozymes in Denmark has become increasingly common [103].
In addition, businesses such as Sigma have been marketing cellulase blends (SAE0020) that contain other enzymes to generate fermentable sugar from hemicellulose and increase sugar output [104]. The production of beta-glucosidase, a crucial enzyme in the manufacture of biofuels, has been improved through the application of genetic engineering [105]. A promising approach for increasing cellulase production in T. reesei involves combining genes that have been mutated in strains that are hyper-cellulolytic. There was also research conducted on enzyme systems that included cellulase, amylase, and hemicellulase. These enzymes were generated by a mixed culture of Aspergillus niger and T. reesei [106]. In general, the improvement of bioethanol production holds a lot of promise, and this can be seen in the development of novel enzyme cocktails as well as the optimization of enzymatic hydrolysis.
5.6. Advances in Fermentation
When it comes to increasing overall ethanol production, the bioethanol industry confronts several obstacles. Conventional strains of the S. cerevisiae yeast that are used in distilleries are unable to convert pentoses into ethanol, which presents a substantial challenge. There are a limited of natural micro-organisms that are capable of metabolizing xylose into ethanol [71,106]. Yeast species such as Candida shehatae, Pichia (Scheffersomyces) stipitis, and Pachysolen tannophilus are among these natural microorganisms. The production of ethanol is now being increased through a variety of various strategies. These include selecting strains and isolating them from their natural or commercial habitats, improving nutrition, engineering metabolism, and evolving in an adaptive laboratory environment [107].
Another difficulty is determining the temperature at which saccharification and fermentation can occur most effectively. The majority of the bacteria that ferment ethanol prefer temperatures in the range of 28–37°C, whereas the optimal temperature for cellulolytic enzyme activity is approximately 50°C. The simultaneous saccharification and fermentation (SSF) cycle may be improved using genetically modified microorganisms that can grow at higher temperatures (50–55°C) and do not require additional enzyme charges during the enzymatic saccharification process [108].
The goal of the research is to develop a single engineered bacterium that can digest plant material and secrete sufficient enzymes to efficiently hydrolyze cellulose and hemicellulose. This is one of their goals. In addition, researchers are investigating the upper bounds of ethanol tolerance among other conditions that stress yeast species [109]. Identifying candidate genes that are involved in ethanol tolerance in S. cerevisiae has been made easier thanks to the development of novel network-based techniques [110].
6. MODEL FOR FUTURE PERSPECTIVES
The world’s consumption of fossil fuels is being driven by the growing population and developing economy. However, this trend is causing concern for environmental well-being, and the need for long-lasting solutions has become imperative. As being technologically, commercially, and environmentally viable, alternative fuels such as bioethanol, are viewed as a potential source of energy in the future. Although some nations view bioethanol as the greatest replacement for conventional fuels, there are still issues with raw resources, cost, technology, marketing, and prioritization that could make the switch to biofuels challenging shortly.
More research is necessary to advance the technology used for bioethanol production, to evaluate alternative raw materials, and to explore ways to establish bioethanol as a sustainable alternative to fossil fuels. Researchers are currently exploring genetic engineering techniques to enhance the conversion of biomass to bioethanol by improving saccharification and increasing the potential yield of biofuels. Moreover, the pilot-scale production of bioethanol using various feedstocks has been successful, with examples including the use of the PSSF technique to produce ethanol from Eucalyptus grandis and the production of bioethanol from rice straw in India Companies such as Praj have also achieved success in producing second-generation bioethanol [111,112].
Bioethanol production from renewable resources and its potential as a greener alternative to traditional fuels has driven significant scientific progress in recent years. However, further research and development are needed to overcome the challenges related to bioethanol production and establish it as a sustainable alternative to fossil fuels.
7. CONCLUSION
The potential of P. purpureum as a bioenergy crop for bioethanol production has been demonstrated through various studies. However, there is still a need for continued research and development efforts to fully maximize its potential. This includes further studies on agronomic practices, such as optimal planting density, fertilization, and irrigation, to increase biomass production and quality. In addition, research on the genetic improvement of P. purpureum for traits such as increased biomass yield, higher cellulose and hemicellulose content, and improved stress tolerance could further enhance its suitability as a bioenergy crop. Advances in genetic engineering could also lead to the development of P. purpureum varieties that are more efficient in converting LC biomass into bioethanol. Furthermore, the development of cost-effective and efficient technologies for bioethanol production from P. purpureum is crucial for its commercialization. This includes exploring different pretreatment and saccharification methods to increase the efficiency of the conversion process and reduce production costs. Developing innovative approaches to valorize the lignin-rich residue generated during bioethanol production could also increase the economic viability of P. purpureum as a bioenergy crop. Overall, the utilization of P. purpureum as a bioenergy crop for bioethanol production could contribute to the production of renewable and sustainable energy while reducing greenhouse gas emissions. Therefore, it is essential to continue research and development efforts to fully realize the potential of this grass species as a bioenergy crop.
8. ACKNOWLEDGMENT
The authors of this paper thank the Hon’ble President, Prof. Kamal Ghanshala, Graphic Era (Deemed to be University), Dehradun, Uttarakhand, India, for motivation and support for this work.
9. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
10. FUNDING
There is no funding to report.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
15. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Aiyejagbara M, Aderemi B, Ameh A, Ishidi E, Ibeneme EF, Olakunle MS. Production of bioethanol from elephant grass (Pennisetum purpureum) stem. Int J Innov Math Stat Energy Policies 2016;4:1-9.
2. Eliana C, Jorge R, Juan P, Luis R. Effects of the pretreatment method on enzymatic hydrolysis and ethanol fermentability of the cellulosic fraction from elephant grass. Fuel 2014;118:41-7. [CrossRef]
3. Wu P, Kang X, Wang W, Yang G, He L, Fan Y, et al. Assessment of coproduction of ethanol and methane from Pennisetum purpureum:Effects of pretreatment, process performance, and mass balance. ACS Sustain Chem Eng 2021;9:10771-84. [CrossRef]
4. Soares IB, Marques OM, Benachour M, Abreu C. Ethanol production by enzymatic hydrolysis of elephant grass. J Life Sci 2011;5:157-61.
5. Taufikurahman, Sherly, Jessica, Delimanto WO. Production of bioethanol from Napier grass:Comparison in pre-treatment and fermentation methods. IOP Conf Ser Earth Environ Sci 2020;520:012005. doi:10.1088/1755-1315/520/1/012005 [CrossRef]
6. Balat M, Balat H, Oz C. Progress in bioethanol processing. Prog Energy Combust Sci 2008;34:551-73. [CrossRef]
7. Mohapatra S, Ray R, Ramachandran S. Bioethanol from biorenewable feedstocks:Technology, economics, and challenges. In:Bioethanol Production from Food Crops. United States:Academic Press;2019. 3-27. [CrossRef]
8. Tsai MH, Lee WC, Kuan WC, Sirisansaneeyakul S, Savarajara Akaracharanya A. Evaluation of different pre-treatments of Napier grass for enzymatic saccharification and ethanol production. Energy Sci Eng 2018;6:683-92. [CrossRef]
9. Ismail KS, Matano Y, Sakihama Y, Inokuma K, Nambu Y, Hasunuma T, et al. Pretreatment of extruded Napier grass by hydrothermal process with dilute sulfuric acid and fermentation using a cellulose-hydrolyzing and xylose-assimilating yeast for ethanol production. Bioresour Technol 2022;343:126071. [CrossRef]
10. Kongkeitkajorn MB, Sae-Kuay C, Reungsang A. Evaluation of Napier grass for bioethanol production through a fermentation process. Processes 2020;8:567. [CrossRef]
11. Kongkeitkajorn MB, Yaemdeeka R, Chaiyota I, Hamsupo K, Oraintara A, Reungsang A. Bioethanol from Napier grass employing different fermentation strategies to evaluate a suitable operation for batch bioethanol production. Energy Convers Manage X 2021;12:100143. [CrossRef]
12. Campos BB, Diniz RH, Da Silveira FA, Ribeiro Junior I, Fietto LG, Machado JC, et al. Elephant grass (Pennisetum purpureum Schumach) is a promising feedstock for ethanol production by the thermotolerant yeast Kluyveromyces marxianus CCT 7735. Braz J Chem Eng 2019;36:43-9. [CrossRef]
13. Mueansichai T, Rangseesuriyachai T, Thongchul N, Assabumrungrat S. Lignocellulosic bioethanol production of Napier grass using Trichoderma reesei and Saccharomyces cerevisiaeco-culture fermentation. Int J Renew Energy Dev 2022;11:423-33. [CrossRef]
14. Kim I, Lee I, Jeon SH, Hwang T, Han JI. Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour Technol 2015;192:335-39. [CrossRef]
15. Manokhoon P, Rangseesuriyachai T. Effect of two-stage sodium hydroxide pretreatment on the composition and structure of Napier grass (Pakchong 1) (Pennisetum purpureum). Int J Green Energy 2020;17:864-71. [CrossRef]
16. Mohapatra S, Mishra C, Behera SS, Thatoi H. Application of pre-treatment, fermentation, and molecular techniques for enhancing bioethanol production from grass biomass-A review. Renew Sust Energ Rev 2017;78:1007-32. [CrossRef]
17. Kumari D, Singh R. Pretreatment of lignocellulosic wastes for biofuel production:A critical review. Renew Sust Energ Rev 2018;90:877-91. [CrossRef]
18. Rodriguez C, Alaswad A, Benyounis KY, Olabi AG. Pretreatment techniques used in biogas production from grass. Renew Sust Energ Rev 2017;68:1193-204. [CrossRef]
19. Mankar AR, Pandey A, Modak A, Pant KK. Pretreatment of lignocellulosic biomass:A review on recent advances. Bioresour Technol 2021;341:125235. [CrossRef]
20. Gallego-Garcia M, Moreno AD, Manzanares P, Negro MJ, Duque A. Recent advances on physical technologies for the pretreatment of food waste and lignocellulosic residues. Bioresour Technol 2023;369:128397. [CrossRef]
21. Chen BY, Chen SW, Wang HT. Use of different alkaline pretreatments and enzyme models to improve low-cost cellulosic biomass conversion. Biomass Bioenergy 2012;39:182-91. [CrossRef]
22. Yasuda M, Nagai H, Takeo K, Ishii Y, Ohta K. Bio-ethanol production through simultaneous Saccharification and Co-Fermentation (SSCF) of a low-moisture anhydrous ammonia (LMAA)-pretreated Napiegrass (Pennisetum purpureum Schumach). Springerplus 2014;3:333. [CrossRef]
23. Yasuda M, Ishii Y, Ohta K. Napier grass (Pennisetum purpureum Schumach) as raw material for bioethanol production:Pre-treatment, saccharification, and fermentation. Biotechnol Bioprocess Eng 2014;19:943-50. [CrossRef]
24. Camesasca L, Ram'rez MB, Guigou M, Ferrari MD, Lareo C. Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of Napier grass for fuel ethanol production. Biomass Bioenergy 2015;74:193-201. [CrossRef]
25. Limayem A, Ricke SC. Lignocellulosic biomass for bioethanol production:Current perspectives, potential issues, and future prospects. Prog Energy Combust Sci 2012;38:449-67. [CrossRef]
26. Liong YY, Halis R, Lai OM, Mohamed R. Conversion of lignocellulosic biomass from grass to bioethanol using materials pretreated with alkali and the white rot fungus, Phanerochaete chrysosporium. Bioresources 2012;7:5500-13. [CrossRef]
27. Sankaran R, Parra Cruz RA, Pakalapati H, Show PL, Ling TC, Chen WH, et al. Recent advances in the pretreatment of microalgal and lignocellulosic biomass:A comprehensive review. Bioresour Technol 2020;298:122476. [CrossRef]
28. Norrrahim MN, Huzaifah MR, Farid MA, Shazleen SS, Misenan MS, Yasim-Anuar TA, et al. Greener pretreatment approaches for the valorisation of natural fibre biomass into bioproducts. Polymers (Basel) 2021;13:2971. [CrossRef]
29. Phuttaro C, Sawatdeenarunat C, Surendra KC, Boonsawang P, Chaiprapat S, Khanal SK. Anaerobic digestion of hydrothermally pretreated lignocellulosic biomass:Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour Technol 2019;284:128-38. [CrossRef]
30. Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 2005;96:673-86. [CrossRef]
31. Iqbal NA, Riaz I, Karadag A, Tabatabaei M. Different pretreatment methods of lignocellulosic biomass for use in biofuel production. In:Biomass for Bioenergy - Recent Trends and Future Challenges. London:IntechOpen;2019. 1-24.
32. Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Indust Eng Chem Res 2009;48:3713-29. [CrossRef]
33. Baksi S, Saha D, Saha S, Sarkar U, Basu D, Kuniyal JC. Pre-treatment of lignocellulosic biomass:Review of various physio-chemical and biological methods influencing the extent of biomass depolymerization. Int J Environ Sci Technol 2023;20:1-28. [CrossRef]
34. Chiaramonti D, Prussi M, Ferrero S, Oriani L, Ottonello P, Torre P, et al. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012;46:25-35. [CrossRef]
35. Bilal M, Ji L, Xu Y, Xu S, Lin Y, Iqbal HM, et al. Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Front Bioeng Biotechnol 2022;10:562. [CrossRef]
36. Abdel-Banat BM, Hoshida H, Ano A, Nonklang S, Akada R. High-temperature fermentation:How can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast?Appl Microbiol Biotechnol 2010;85:861-7. [CrossRef]
37. Christensen AD, Kádár Z, Oleskowicz-Popiel P, Thomsen MH. Production of bioethanol from organic whey using Kluyveromyces marxianus. J Ind Microbiol Biotechnol 2011;38:283-9. [CrossRef]
38. Zoppellari F, Bardi L. Production of bioethanol from effluents of the dairy industry by Kluyveromyces marxianus. N Biotechnol 2013;30:607-13. [CrossRef]
39. Wu WH, Hung WC, Lo KY, Chen YH, Wan HP, Cheng KC. Bioethanol production from taro waste using thermo-tolerant yeast Kluyveromyces marxianus K21. Bioresour Technol 2016;201:27-32. [CrossRef]
40. Sun T, Yu Y, Wang K, Ledesma-Amaro R, Ji X. Engineering Yarrowia lipolyticato produce fuels and chemicals from xylose:A review. Bioresour Technol 2021;337:125484. [CrossRef]
41. Mamaev D, Zvyagilskaya R. Yarrowia lipolytica:A multitalented yeast species of ecological significance. FEMS Yeast Res 2021;21:foab008. [CrossRef]
42. Miller KK, Alper HS. Yarrowia lipolytica:More than an oleaginous workhorse. Appl Microbiol Biotechnol 2019;103:9251-62. [CrossRef]
43. Tsigie YA, Wang CY, Truong CT, Ju YH. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour Technol 2011;102:9216-22. [CrossRef]
44. Suwannarangsee S, Oh DK, Seo JW, Kim CH, Rhee SK, Kang HA, et al. Characterization of alcohol dehydrogenase 1 of the thermotolerant methylotrophic yeast Hansenula polymorpha. Appl Microbiol Biotechnol 2010;88:497-507. [CrossRef]
45. Radecka D, Mukherjee V, Mateo RQ, Stojiljkovic M, Foulquié-Moreno MR, Thevelein JM. Looking beyond Saccharomyces:The potential of non-conventional yeast species for desirable traits in bioethanol fermentation. FEMS Yeast Res 2015;15:fov053. [CrossRef]
46. Kurylenko OO, Ruchala J, Hryniv OB, Stojiljkovic M, Moreno MR, Thevelein JM. Metabolic engineering and classical selection of the methylotrophic thermotolerant yeast Hansenula polymorpha for improvement of high-temperature xylose alcoholic fermentation. Microb Cell Factor 2014;13:122. [CrossRef]
47. Olena PI, Voronovsky AY, Stasyk OV, Gayda GZ, Gonchar MV, Abbas CA, et al. Overexpression of pyruvate decarboxylase in the yeast Hansenula polymorpha results in increased ethanol yield in high-temperature fermentation of xylose. FEMS Yeast Res 2008;8:1164-74. [CrossRef]
48. Steensels J, Daenen L, Malcorps P, Derdelinckx G, Verachtert H, Verstrepen KJ. Brettanomyces yeasts-from spoilage organisms to valuable contributors to industrial fermentations. Int J Food Microbiol 2015;206:24-38. [CrossRef]
49. Neto AG, Pestana-Calsa MC, de Morais MA, Calsa T Jr. Proteome responses to nitrate in bioethanol production contaminant Dekkera bruxellensis. J Proteom 2014;104:104-11. [CrossRef]
50. Blomqvist J, Passoth V. Dekkera bruxellensis-spoilage yeast with biotechnological potential, and a model for yeast evolution, physiology and competitiveness. FEMS Yeast Res 2015;15:fov021. [CrossRef]
51. Pereira LF, Lucatti E, Basso LC, de Morais MA Jr. The fermentation of sugarcane molasses by Dekkera bruxellensis and the mobilization of reserve carbohydrates. Antonie Van Leeuwenhoek 2014;105:481-9. [CrossRef]
52. Blomqvist J, Eberhard T, Schnürer J, Passoth V. Fermentation characteristics of Dekkera bruxellensis strains. Appl Microbiol Biotechnol 2010;87:1487-97. [CrossRef]
53. Codato CB, Martini C, Ceccato-Antonini SR, Bastos RG. Ethanol production from Dekkera bruxellensis in synthetic media with pentose. Braz J Chem Eng 2018;35:11-7. [CrossRef]
54. Agbogbo FK, Coward-Kelly G. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol Lett 2008;30:1515-24. [CrossRef]
55. Buaban B, Inoue H, Yano S, Tanapongpipat S, Ruanglek V, Champreda V, et al. Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis. J Biosci Bioeng 2010;110:18-25. [CrossRef]
56. Li Y, Park JY, Shiroma R, Tokuyasu K. Bioethanol production from rice straw by a sequential use of Saccharomyces cerevisiae and Pichia stipitis with heat inactivation of Saccharomyces cerevisiaecells prior to xylose fermentation. J Biosci Bioeng 2011;111:682-6. [CrossRef]
57. Naseeruddin S, Desai S, Venkateswar Rao L. Co-culture of Saccharomyces cerevisiae(VS3) and Pichia stipitis (NCIM 3498) enhances bioethanol yield from concentrated Prosopis juliflora hydrolysate. 3 Biotech 2021;11:21. [CrossRef]
58. Dien BS, Anderson WF, Cheng MH, Knoll JE, Lamb J, O'Bryan PJ, et al. Field productivities of Napier grass for production of sugars and ethanol. ACS Sustain Chem Eng 2020;8:2052-60. [CrossRef]
59. Liu YK, Chen WC, Huang YC, Chang YK, Chu IM, Tsai SL, et al. Production of bioethanol from Napier grass via simultaneous saccharification and co-fermentation in a modified bioreactor. J Biosci Bioeng 2017;124:184-8. [CrossRef]
60. Amnuaycheewa P, Rodiahwati W, Sanvarinda P, Cheenkachorn K, Tawai A, Sriariyanun M. Effect of organic acid pretreatment on Napier grass (Pennisetum purpureum) straw biomass conversion. KMUTNB Int J Appl Sci Technol 2017;10:107-17.
61. Choudhary J, Singh S, Nain L. Thermotolerant fermenting yeasts for simultaneous saccharification fermentation of lignocellulosic biomass. Electron J Biotechnol 2016;21:82-92. [CrossRef]
62. Kumar A. Assessment of different pretreatment technologies for efficient bioconversion of lignocellulose to ethanol. Front Biosci 2018;10:350-71. [CrossRef]
63. Takano M, Hoshino K. Bioethanol production from rice straw by simultaneous saccharification and fermentation with statistical optimized cellulase cocktail and fermenting fungus. Bioresour Bioprocess 2018;5:16. [CrossRef]
64. Olofsson K, Bertilsson M, Gunnar L. A short review on SSF-an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 2008;1:7. [CrossRef]
65. Dahnum D, Tasum SO, Triwahyuni E, Nurdin M, Abimanyu H. Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch. Energy Proc 2015;68:107-16. [CrossRef]
66. Manzanares P, Negro MJ, Oliva JM, Saéz F, Ballesteros I, Ballesteros M, et al. Different process configurations for bioethanol production from pretreated olive pruning biomass. J Chem Technol Biotechnol 2011;86:881-7. [CrossRef]
67. Nielsen F, Zacchi G, Galbe M, Wallberg O. Sequential targeting of xylose and glucose conversion in fed-batch simultaneous saccharification and co-fermentation of steam-pretreated wheat straw for improved xylose conversion to ethanol. Bioenergy Res 2017;10:800-10. [CrossRef]
68. Liu ZH, Chen HZ. Simultaneous saccharification and co-fermentation for improving the xylose utilization of steam exploded corn Stover at high solid loading. Bioresour Technol 2016;201:15-26. [CrossRef]
69. Qin L, Zhao X, Wen-Chao L, Zhu JQ, Liu L, Bing-Zhi L, Yuan YJ. Process analysis and optimization of simultaneous saccharification and co-fermentation of ethylenediamine-pretreated corn stover for ethanol production. Biotechnol Biofuels 2018;11:118. [CrossRef]
70. Bokinsky G, Peralta-Yahya PP, George A, Holmes BM, Steen EJ, Dietrich J, et al. Synthesis of three advanced biofuels from ionic liquid-pretreated switch grass using engineered Escherichia coli. Proc Natl Acad Sci 2011;108:19949-54. [CrossRef]
71. Zabed H, Sahu JN, Suely A, Boyce AN, Faruq G. Bioethanol production from renewable sources:Current perspectives and technological progress. Renew Sustain Energy Rev 2017;71:475-501. [CrossRef]
72. Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation:A review. Bioresour Bioprocess 2018;5:1-15. [CrossRef]
73. Saini JK, Saini R, Tewari L. Lignocellulosic agriculture wastes as biomass feed stocks for second-generation bioethanol production:Concepts and recent developments. 3 Biotech 2015;5:337-53. [CrossRef]
74. Brandon AG, Scheller HV. Engineering of bioenergy crops:Dominant genetic approaches to improve polysaccharide properties and composition in biomass. Front Plant Sci 2020;11:282. [CrossRef]
75. Blanch HW. Bioprocessing for biofuels. Curr Opin Biotechnol 2012;23:390-5. [CrossRef]
76. Petersen PD, Lau J, Ebert B, Yang F, Verhertbruggen Y, Kim JS, et al. Engineering of plants with improved properties as biofuels feedstocks by vessel-specific complementation of Xylan biosynthesis mutants. Biotechnol Biofuels 2012;5:84. [CrossRef]
77. Mortimer JC. Plant synthetic biology could drive a revolution in biofuels and medicine. Exp Biol Med 2019;244:323-31. [CrossRef]
78. Zahoor Z, Sun D, Li Y, Wang J, Tu Y, Wang Y, et al. Biomass saccharification is largely enhanced by altering wall polymer features and reducing silicon accumulation in rice cultivars harvested from nitrogen fertilizer supply. Bioresour Technol 2017;243:957-65. [CrossRef]
79. Rocha-Meneses L, Ferreira JA, Mushtaq M, Karimi S, Orup~old K, Kikas T. Genetic modification of cereal plants:A strategy to enhance bioethanol yields from agricultural waste. Indust Crop Prod 2020;150:112408. [CrossRef]
80. Furtado A, Lupoi JS, Hoang NV, Healey A, Singh S, Simmons BA, et al. Modifying plants for biofuel and biomaterial production. Plant Biotechnol J 2014;12:1246-58. [CrossRef]
81. Huang J, Xia T, Li G, Li X, Li Y, Wang Y, et al. Overproduction of native endo-b1, 4-glucanases leads to largely enhanced biomass saccharification and bioethanol production by specific modification of cellulose features in transgenic rice. Biotechnol Biofuels 2019;12:11. [CrossRef]
82. Fan C, Hua Y, Qin S, Yongli L, Alam A, Changzhen X, et al. Brassinosteroid overproduction improves lignocellulose quantity and quality to maximize bioethanol yield under green-like biomass process in transgenic poplar. Biotechnol Biofuels 2020;13:9. [CrossRef]
83. Xie G, Yang B, Xu Z, Li F, Guo K, Zhang M, et al. Global identification of multiple OsGH9 family members and their involvement in cellulose crystallinity modification in rice. PLoS One 2013;8:e50171. [CrossRef]
84. Wang Y, Fan C, Huizhen H, Ying L, Sun D, Wang Y, et al. Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol Adv 2016;34:997-1017. [CrossRef]
85. Li F, Zhang M, Guo K, Hu Z, Zhang R, Feng Y, et al. High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol J 2015;13:514-25. [CrossRef]
86. Fan C, Feng S, Huang J, Wang Y, Wu L, Li X, et al. AtCesA8-driven OsSUS3 expression leads to largely enhanced biomass Saccharification and lodging resistance by distinctively altering lignocellulose features in rice. Biotechnol Biofuels 2017;10:221. [CrossRef]
87. Hosseini Koupaie E, Dahadha S, Bazyar Lakeh AA, Azizi A, Elbeshbishy E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J Environ Manag 2019;233:774-84. [CrossRef]
88. Jiang W, Chang S, Yongshui Q, Zhang Z, Jian X. Changes in structural properties of biomass pretreated by combined deacetylation with liquid hot water and its effect on enzymatic hydrolysis. Bioresour Technol 2016;220:448-56. [CrossRef]
89. Raghavi S, Sindhu R, Binod P, Gnansounou E, Pandey A. Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour Technol 2016;199:202-10. [CrossRef]
90. Kim TH, Gupta R, Lee YY. Pretreatment of biomass by aqueous ammonia for bioethanol production. Methods Mol Biol 2009;581:79-91. [CrossRef]
91. Zuo Z, Tian S, Chen Z, Li J, Yang X. Soaking pretreatment of corn Stover for bioethanol production followed by anaerobic digestion process. Appl Biochem Biotechnol 2012;167:2088-102. [CrossRef]
92. Wi SG, Cho EJ, Lee DS, Lee SJ, Lee YJ, Bae HJ. Lignocellulose conversion for biofuel:A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol Biofuels 2015;8:228. [CrossRef]
93. Verardi A, Blasi A, De Bari I, Calabr`o V. Steam pretreatment of Saccharum officinarum L. Bagasse by adding impregnating agents for advanced bioethanol production. Ecotoxicol Environ Saf 2016;134:293-300. [CrossRef]
94. Yu H, Ren J, Liu L, Zheng Z, Zhu J, Yong Q, et al. A new magnesium bisulfite pretreatment (MBSP) development for bio-ethanol production from corn stover. Bioresour Technol 2016;199:188-93. [CrossRef]
95. Matsakas L, Nitsos C, Raghavendran V, Yakimenko O, Persson G, Olsson E, et al. A novel hybrid organosolv:Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol Biofuels 2018;11:1-14. [CrossRef]
96. Yuan W, Gong Z, Wang G, Zhou W, Liu Y, Wang X, et al. Alkaline organosolv pretreatment of corn stover for enhancing the enzymatic digestibility. Bioresour Technol 2018;265:464-70. [CrossRef]
97. Gogoi P, Zhang Z, Geng Z, Liu W, Hu W, Deng Y. Low-temperature, low-energy, and high-efficiency pretreatment technology for large wood chips with a redox couple catalyst. ChemSusChem 2018;11:1121-31. [CrossRef]
98. Ambye-Jensen M, Balzarotti R, Thomsen ST, Fonseca C, K'ad'ar Z. Combined ensiling and hydrothermal processing as efficient pretreatment of sugarcane bagasse for 2G bioethanol production. Biotechnol Biofuels 2018;11:1-11. [CrossRef]
99. Zhang J, Li K, Liu S, Huang S, Xu C. Alkaline hydrogen peroxide pretreatment combined with bio-additives to boost high-solids enzymatic hydrolysis of sugarcane bagasse for succinic acid processing. Bioresour Technol 2022;345:126550. [CrossRef]
100. Dubey AK, Gupta PK, Garg N, Naithani S. Bioethanol production from waste paper acid pretreated hydrolyzate with xylose fermenting Pichia stipitis. Carbohydr Polym 2012;88:825-9. [CrossRef]
101. Sankar K, Muthuvelu RR, Kumar MN, Sivakumar U. Development of co-immobilized tri-enzyme biocatalytic system for one-pot pretreatment of four different perennial lignocellulosic biomass and evaluation of their bioethanol production potential. Bioresour Technol 2018;269:227-36. [CrossRef]
102. Contreras F, Subrata Pramanik I, Rozhkova IN, Zorov OK, Sinitsyn AP, Schwaneberg U, et al. Engineering robust cellulases for tailored lignocellulosic degradation cocktails. Int J Mol Sci 2020;21:1589. [CrossRef]
103. Phuong NT, Hoang PH, Dien LQ, Doan TH. Optimization of sodium sulfide treatment of rice straw to increase the enzymatic hydrolysis in bioethanol production. Clean Technol Environ Policy 2017;19:1313-22. [CrossRef]
104. Baksi S, Ball AK, Sarkar U, Banerjee D, Wentzel A, Preisig HA, et al. Efficacy of a novel sequential enzymatic hydrolysis of lignocellulosic biomass and inhibition characteristics of monosugars. Int J Biol Macromol 2019;129:634-44. [CrossRef]
105. Singhania RR, Patel AK, Pandey A, Ganansounou E. Genetic modification:A tool for enhancing beta-glucosidase production for biofuel application. Bioresour Technol 2017;245:1352-61. [CrossRef]
106. Berlowska J, Pielech-Przybylska K, Balcerek M, Dziekonska-Kubczak U, Patelski P, Dziugan P, et al. Simultaneous saccharification and fermentation of sugar beet pulp for efficient bioethanol production. BioMed Res Int 2016;2016:3154929. [CrossRef]
107. Robak K, Balcerek M. Review of second-generation bioethanol production from residual biomass. Food Technol Biotechnol 2018;56:174-87. [CrossRef]
108. Yanase S, Hasunuma T, Yamada R, Tanaka T, Ogino C, Fukuda H, et al. Direct ethanol production from cellulosic materials at high temperature using the thermotolerant yeast Kluyveromyces marxianus displaying cellulolytic enzymes. Appl Microbiol Biotechnol 2010;88:381-8. [CrossRef]
109. Watcharawipas A, Watanabe D, Takagi H. Enhanced sodium acetate tolerance in Saccharomyces cerevisiaeby the Thr255Ala mutation of the ubiquitin ligase Rsp5. FEMS Yeast Res 2017;17. doi:10.1093/femsyr/fox083 [CrossRef]
110. Kasavi C, Eraslan S, Arga KY, Oner ET, Kirdar B. A system based network approach to ethanol tolerance in Saccharomyces cerevisiae. BMC Syst Biol 2014;8:90. [CrossRef]
111. Banerjee R. Pilot Scale Production of 2G Ethanol Utilizing Rice Straw:An Integrated Bio-Refinery Approach. Berlin, Germany:Published in Proceedings of 11th World Bioenergy Congress and Expo;2018.
112. Narinthorn R, Choorit W, Chisti Y. Alkaline and fungal pretreatments for improving methane potential of Napier grass. Biomass Bioenergy 2019;127:105262. [CrossRef]