1. INTRODUCTION
The share of plant-based food materials is increasing promptly and is gaining popularity in the global market. Plant-based gaining interest because of several factors, such as changes in lifestyle, awareness toward healthy alternative foods, and increased understanding regarding the renewable production of food. Edible plants are a rich source of certain micronutrients such as minerals, vitamins, and phytochemicals [1,2]. Plant phytochemicals carry some important properties such as antioxidants, anticarcinogenic, and antimicrobial activities and have displayed possible protective benefits toward cardiovascular disorders, cancer, hormonal imbalance, and osteoporosis [3,4]. Various plant-based foods such as cereals and legumes contain an appropriate number of oligosaccharides and dietary fibers [5,6]. These oligosaccharides are non-digestible carbohydrates, therefore, possess the ability to improve certain physiological properties. Moreover, oligosaccharides act as prebiotics and help in promoting the growth of gut microbiota selectively. β-glucans add some health benefits by reducing the level of cholesterol and help in improving the sensory traits of the end product [7-9]. Despite all such functional characteristics of plant-based food, a careful assessment discloses that commercial plant-based foods are nutritionally imbalanced as compared to animal origin products. Plant proteins more often display low quality, less digestibility, and unwanted inhibitions in essential amino acids [10,11]. Furthermore, some vitamins such as Vitamin D and B12 are found in low amounts or absent in plant-based raw materials which are ultimately are responsible for vitamin deficiencies in a strict vegetarian diet [12,13].
Plant antinutrients directly interact with the minerals and micronutrients and lead to harmful effects on zinc and iron bioavailability as demonstrated in vitro [14,15] and in vivo conditions [16-18]. Moreover, an inadequate amount of micronutrient level is found in the diet of people who are vegetarian or vegan and they are at higher risk of micronutrient deficiency and associated disorders. Plant-based products, raw, or vegan diets often contain antinutrients associated with them as they are accumulated naturally within the plant tissues [18,19]. The most common antinutrient present in plant-based foods is phytates, tannins, lectins, saponins, enzyme inhibitors, and phenolics [Table 1 and Figure 1].
Moreover, mineral antinutrients also reduce the digestibility of certain macromolecules such as dietary proteins, starch, and carbohydrates by forming insoluble complexes with them. Another reason for decreased mineral bioavailability in the plant-based food system is because of the binding of antinutrients with each other like phytic acid (PA) with dietary fiber [20]. Anti-nutrients can have a toxic effect when consumed above a certain limit and causes various health disorders [Figure 1] [19]. Apart from reduced bioavailability and biodigestibility the other common symptoms exhibited by many antinutrients are nausea, bloating, headaches, rashes, etc. [21]. Furthermore, quality improvement is necessary therefore removal of undesirable food components is desirable. Moreover, at low concentrations, these antinutrients have shown various positive health benefits such as reduced glucose levels, prevents cancer risks, antibacterial and antiviral properties, maintaining proper liver functioning, and highly antioxidants [22].
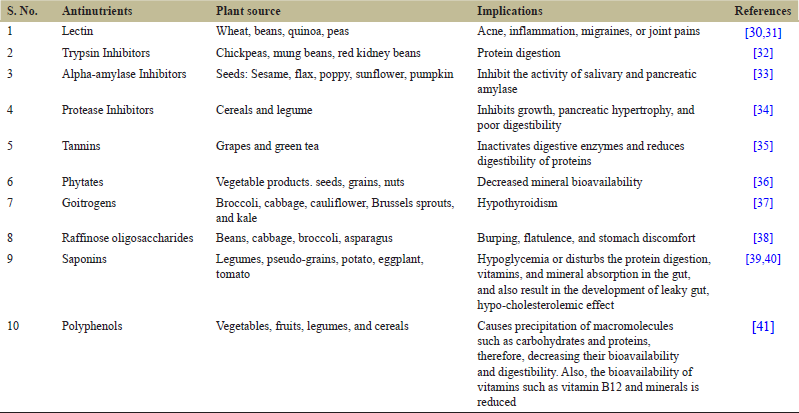 | Table 1: Antinutrients associates with plant-based food sources [Click here to view] |
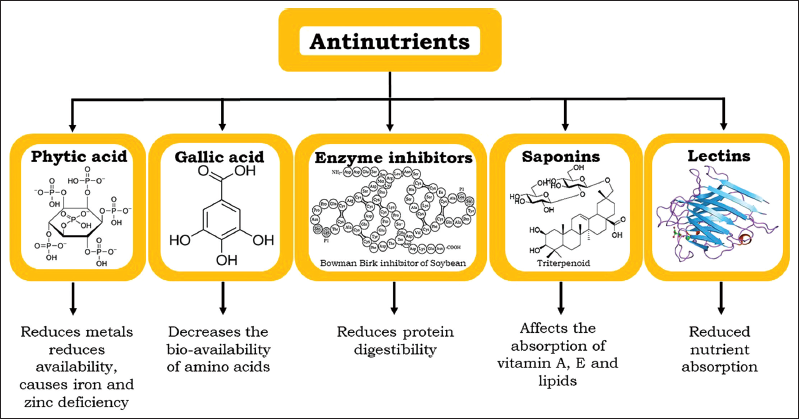 | Figure 1: A brief overview of the types and adverse effects of key anti-nutrients. [Click here to view] |
Oligosaccharides from plants, including raffinose, stachyose, and verbascose, are consumed by gut bacteria through fermentation, resulting in flatulence, diarrhea, etc., [20,23-25]. Unfortunately, the natural flavor of plant-based products displays less acceptance [26]. Plant-based food is to date commonly regarded as products having a displeasing taste, probably due to past experiences with less desirable products available in the market [27]. Plant phenols, terpenes, glucosinolates, and flavonoids based on their molecular weight they produce undesirable changes in organoleptic properties such as bitter taste and pungent smell [28,29].
2. STRATEGIES TO REDUCE ANTINUTRIENTS
Various methods that have been employed to reduce antinutritional factors present in foods are discussed below:
2.1. Soaking
Soaking is a physical process and it is considered one of the easiest ways through which soluble antinutritional factors can be removed and also helps in reducing the cooking time. Soaking also enhances the release of enzymes (e.g., endogenous phytases), which are present in plant foods such as almonds, nuts, grains, and other edible seeds, soaking also reduces the number of enzyme inhibitors to improve both digestibility as well as nutritional value [30-42]. Many of the anti-nutrients are water-soluble in nature, which enhances their removal from foods through leaching. One of the studies reported that the concentration of PA was decreased in chickpea from 47.45% to 55.71% on increasing the time of soaking from 2 to 12 h [43]. Soaking with distilled water, 1% sodium bicarbonate and mixed salt solutions decreased total phenols, ortho-dihydroxy phenols, tannins, and phytates by 33%, 41%, 35%, and 21%, respectively [44]. Soaking of soybean flour has also reduced the total protein, soluble sugar, and tannins [45]. Some drawbacks are also associated with soaking is that it causes leaching of some important water-soluble proteins and minerals [41].
2.2. Heating
The autoclave is an instrument, which is widely used for the application of heat treatments. Autoclaving of plant-based food stimulates the production of endogenous enzymes such as phytase, tannase, and also increases acidity [41]. On consumption of autoclaved plant-based food enhanced health benefits have been observed. Another research showed a significant reduction in anti-nutritional factors in legumes after autoclaving, soaking, and cooking [46]. Most of the previous studies have concluded that autoclaving is the best method for the reduction of various anti-nutritional components relative to other processing methods [47-49].
2.3. Cooking
Cooking helps in reducing various antinutrients such as PA, tannins, and oxalic acid from whole grains, beans, and vegetables. Protease inhibitors are proteinaceous in nature and they get readily denatured by heat treatment [50]. Various studies have shown that reduced antinutrient amounts can be obtained by heating under controlled conditions, that is, when the temperature is needed to be maintained lower than that of boiling point for about 15 min [51]. Boiling of Bambara groundnut seeds for about 1 h greatly decreased the amount of raffinose and also enhanced protein digestibility of seeds was obtained [52]. The cooking of a mixture of sweet potato leaves and lemon together decreased the amount of both phenolic (56%) as well as oxalate components [53].
2.4. Germination
Germination is another process used for the reduction of anti-nutritional factors present in plant-based foods [54]. Germination of seeds typically activates endogenous enzymes such as phytase which hydrolysis the phytate and decreases the amount of PA found in samples. Germination usually brings a change in the nutritional level, biochemical property, and physical characteristics of the food. Germination is the most preferred process and is frequently used for reducing the antinutritional content in cereals [55-57]. Latest studies stated that germination helps in changing the isoflavone profile usually found in soybean due to the activation of an enzyme called β-glucosidases; this helps in improving the nutritional value of soybean as isoflavones display chelating properties [58,59]. Singh et al. [60] stated that on the processing of millet with germination, the polyphenol contents have shown a maximum reduction of about 75% as compared to other processes such as soaking and microwave.
2.5. Fermentation
Fermentation is another alluring option to achieve the goal of reduction of antinutrients from plant-based foods. It is considered a natural solution to food processing since the earlier times of mankind, and nowadays, fermented foods are gaining more popularity than ever before [61]. Fermentation is an ancient and simple technique which is often employed to prevent and improves the safety, nutritional, rheological, and sensorial characteristic of plant-based materials while being natural and economical [62]. Plants can support the growth of a large number of microorganisms [63]. Plant-based fermentation most commonly employs lactic acid bacteria (LAB), bacilli, and yeasts (e.g., Saccharomyces) [64,65]. They have been studied mostly as monocultures; these microbes possess certain proven properties through which they increase necessary nutritional and sensory characteristics. Fermentation has shown considerable improvement in the nutritional quality of cereals. It may also serve to enrich the reservoir of available amino acids, vitamins, and minerals; as a result, fermentation enhances the total digestibility and sensory characteristics of food [65]. Furthermore, fermentation is of immense importance in the degradation of oligosaccharides which are usually found in beans and vegetables and are accountable for digestive difficulties such as flatulence. Fermentation can increase the concentration or bioaccessibility of functional (bioactive) compounds. In a recent report, fermentation of maize flour has been done with a mixture of LAB through the standard method with 12 h intervals to verify the impact of fermentation on the reduction of anti-nutritional components [66]. Significant reduction of antinutrient components (tannin, polyphenol, phytate, etc.) in fermented maize was observed with the increase in fermentation time. Furthermore, it was concluded that fermentation with LAB-consortium reduced more antinutrients than that of spontaneous fermentation [66]. Etsuyankpa et al. [67] performed the microbial fermentation on local cassava products to assess the reduction in anti-nutritional composition present in them. Results showed a significant decrease in the level of cyanide, tannins, phytate, oxalate, and saponins from cassava products due to the microbial fermentation. Fermentation is one of the food processing strategies, which is adopted in Africa to made cereals crops edible and also increases the nutritional properties as well as safety concerns of these foods because cereals are not easily consumed in natural/raw forms. Fermentation of cereals by LAB has been reported to increase free amino acids and their derivatives by proteolysis and by metabolic synthesis. Fermentation has been shown to improve the nutritional value of grains by increasing the content of essential amino acids such as lysine, methionine, and tryptophan.
3. ROLE OF FERMENTATION IN MINERAL AND MICRONUTRIENT BIOAVAILABILITY
Lactic acid fermentation increases the nutritional profile and aids in enhancing the bioavailability of vitamins, minerals, and other nutrients. Therefore, it is considered the most sustainable way for the biological fortification of vitamins, minerals, and essential amino acids in certain food products. This can be achieved in different ways; first, microorganisms apart from containing catabolic property also possess anabolic property in them and can produce certain vitamins and some growth factors. Second, fermentation by LAB, yeast, and mold can hydrolyze coatings and cell walls which are indigestible in nature both chemically as well as physically, and resulting in the release of nutrients trapped within the plants complex structure. Cereals-based foods suffer from low nutritional availability mainly because of the presence of a large amount of anti-nutritional components that impose negative consequences on mineral bioavailability, also in developing countries cereals are the main staple food; therefore, they greatly influence the nutritional status of such area.
However, microbial fermentation can enhance the nutritional value as well as the digestibility of plant foods suffering from low bioavailability [Figure 2]. Microbial enzymes display maximum activity under acidic conditions produced during fermentation and under temperature ranges varying between 22°C and 25°C [68].
Microbial enzymes under an acidic environment including amylases alter the main food components by the degradation of polysaccharides, proteins, phytates, and lipids, respectively. In addition to improving the function of enzymes, fermentation often decreases the number of antinutrients including PA, polyphenols, tannin, trypsin inhibitors, and dietary fibers [19,69] in food that contributes toward enhanced bioavailability of minerals including iron, B vitamin, thiamine, riboflavin, niacin, protein, and simple sugars. Cassava on fermentation has revealed a reduction in the cyanogenic toxin content [70-81] [Table 2].
3.1. PA
PAs also commonly known as myo-inositol-1,2,3,4,5,6-hexakis dihydrogen phosphate and are usually found in plant-based food between the range of 0.1 and 6.0% [82]. PA is a secondary metabolite and is mainly found in legumes, peanuts, cereals, and oilseeds, and other plant sources [83]. The increasing trend of veganism results in the ingestion of a large amount of PA which occurs naturally in plant food [84,85]. According to earlier reports, PA affects the activity of enzymes, required for the hydrolysis of protein within the small intestine and stomach [86]. In general, PA affects the bioavailability of minerals and has a strong impact on infants, pregnant, and lactating women on the consumption of a large amount of cereal-based foods [87,88]. PA form insoluble salts with alkali earth metal and transition metals such as zinc, calcium, manganese, and iron [89]. Strong complexation is obtained between two minerals and phytate. This is commonly documented in the case of zinc and calcium where a strong complex is developed between the calcium-zinc phytate than for any of the minerals [89,90]. Phytases originating from microbial sources can degrade PA. The ideal conditions on which phytase activity depends is on its source: For example, the optimum pH of 4.5–5 is required for the activity of phytases which are found endogenously present in cereals [91,92]. Anastasio et al. [91] demonstrated that the addition of phytase-positive starter culture in sourdough increased the solubility of iron and zinc by 98% and 89.8% in comparison to phytase-negative starter culture where the solubility of both iron and zinc was only about 41% and 60%, respectively. Magala et al. [93] also observed a somewhat similar trend and recorded 89% of degradation of PA in samples of Tarhana and about 100% degradation of PA in beverages made up of rice and oats after fermenting them with Lactobacillus sanfranciscensis CCM 7699 culture.
3.2. Phenolic Compounds (PCs)
Phenolic substances can be defined as the most diverse group of plant components present in plants which range from simple phenolic acid to complex tannic acid. They are abundantly present in vegetables, fruits, legumes, and cereals. PCs bind with macromolecules such as carbohydrates and protein thus causing their precipitation. Their antinutrient property also causes chelation of some minerals with their hydroxyl groups and form insoluble compounds that are not consumed by the human body [20]. To chelate iron PCs should possess either ortho dihydroxy (catechol) or trihydroxy (galloyl) groups in their structure [94,95]. Mladenka et al. [96] and Macakova et al. [97] revealed that complex formation depends upon pH by demonstrating the pH-dependent formation of iron-flavonoid complexes. Iron formed a stable complex with flavonoids at neutral physiological pH but under acidic pH less iron chelation was observed. At acidic pH more fe3+ was reduced to fe2+ due to flavonoids and less at neutral pH [97]. Tannins bind with iron with more affinity than that of PA. Studies making use of the Caco-2-cell model system showed that PC produced maximum inhibition of iron (90%) at a molar ratio of 1:1 iron: Tannin acid as compared to the phytates where 90% iron inhibition took place at a molar ratio of 1:10 iron:PA [98]. The biggest problem associated with PC’s is their well-established therapeutic benefits in the human body. It is therefore important that equilibrium be achieved in such a manner so that PCs are not present at sufficiently high amounts to prevent the absorption of minerals, but at the same time, it should not be present in too low concentrations as a result of which human body would not be benefitted from their antioxidant activity. Metabolism of tannins or other polyphenols by LAB has been characterized only in a few plant fermentations including tempeh [99] and sorghum [92,100]. Among LAB, Lactobacillus plantarum, Lactobacillus paraplantarum, and Lactobacillus pentosus seem to be the only species capable of degrading hydrolyzable tannins through a tannase activity (tannin acyl hydrolase, EC 3.1.1.20) which breaks ester bounds of tannic acid, thus releasing glucose and gallic acid [92,101-103]. Most of the tannase producers were found in fermented vegetables but also human feces. In L. plantarum, tannase is very well characterized. Its activity was demonstrated and characterized [104] and genetic analysis showed it constitutes a novel family of tannases [105]. LAB tannases are intracellular. L. plantarum strain has been reported to produce a very efficient tannase during anaerobic fermentation [106]. Genes involved in tannin degradation are regulated in a coordinated way and are inducible by tannin and other PCs [107]. Characterization of fermented cassava LAB allowed identifying uncommon tannase producers such as Weissella cibaria and Leuconostoc mesenteroides ssp. [108].
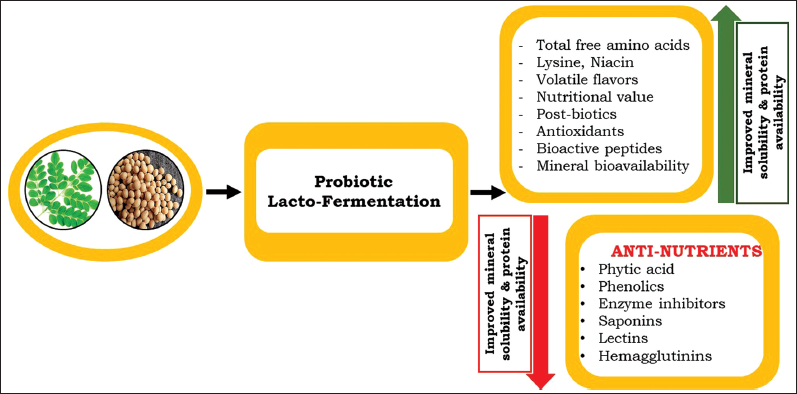 | Figure 2: Graphical overview of the lacto-fermentation of plant based. [Click here to view] |
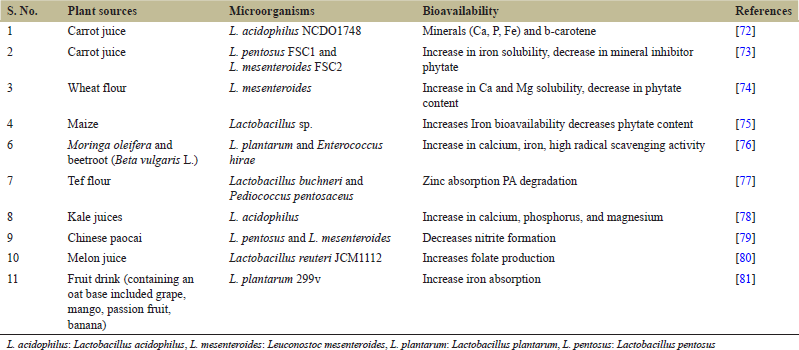 | Table 2: Different lactic acid bacteria strains used for the plant-based fermentation which improved the availability of micronutrients [Click here to view] |
3.3. Dietary Fibers
Dietary fibers mainly include non-digestible carbohydrate and non-carbohydrate components like lignin in them. Dietary fibers are not usually broken down by the human digestive enzymes and therefore these components are not absorbed within the upper alimentary tract [109]. A diet containing a large number of dietary fibers is associated with mineral deficiencies because dietary fibers and other components related to it such as PA and tannins are known to interact with minerals (such as zinc, iron, and calcium) and thus prevent their absorption. The dietary fibers interacts with minerals through electrostatic interaction and adsorption. Dietary fibers possess electrostatic interaction because of the presence of various free carboxylic as well as sulfonated groups that contain a negative charge and therefore they interact with positively charged metal ions resulting in their chelation. pH plays an important role in mineral binding and has been extensively studied. Mineral binding at low pH 4–4.5 has been observed low and maximum at high pH range 5.8–6.5 similar pH range is found in human intestine [110]. The iron-binding was found low at pH 4.0 and at pH 6.5 iron-binding was found high. The decrease in iron-binding could be attributed to the fact that fermentation may have resulted in a decrease in pH due to which mineral binding by fibers will be lowered. In vitro studies have not been able to demonstrate the fermentation of some of the dietary fibers caused by bacterial enzymes that take place within the human intestines. Mineral ions are released on the degradation of various fermentable dietary fibers such as pectin and the released mineral is absorbed eventually [111-113]. Fermentation of kale juice with Lactobacillus acidophilus IFO 3025 showed an increase in calcium, phosphorus, and magnesium [78]. Whole wheat flour contains a large amount of PA naturally. Fermentation of whole wheat flour in presence of L. mesenteroides strain 38, for 9 h established the decrease in PA and the generation of lactic acid leading toward greater calcium and magnesium solubility in comparison to the control medium [114]. The impact of natural fermentation (NF) and controlled fermentation (CF) in the reduction of antinutrient components, α-galactosides, and increase in vitro protein digestibility was examined. The dry bean (Phaseolus vulgaris) flour was used as a raw material for this analysis. Results showed that a reduction in raffinose oligosaccharide, antinutritional constituents, and pH was found in both cases of fermentation. The natural lactic fermentation of ground beans produced a substantial improvement in protein digestibility. Both forms of fermentation decreased the antinutrients and enhanced the nutritional value of the bean flour, and specified the possibility of bean flour to be used as an ingredient for processed foods [115]. Different selected LAB strains in tef fermentations were able to decrease PA content, among different LAB strains Lactobacillus buchneri MF58 displayed the maximum amount of degradation in PA (68%) and is supposed to enhance zinc absorption in humans from tef-injera, and more degradation of PA is possibly required to improve iron absorption [77].
3.4. Saponins
Saponins can be defined as non-volatile phytochemicals, ubiquitous in nature but are mainly present in plants. Saponins are a category of plant composed of water-soluble glycosides are connected to either a lipophilic steroid or triterpenoid. Triterpenoids are generally present in most cultivated crops such as legumes, sunflower seeds, and allium species. However, steroids are usually found in food plants such as oats, yucca, tomato seed, and yam [116,117]. Saponins are distinguished by their bitter taste, a surfactant activity, they can hemolyze red blood cells, they also influence the functioning of the intestinal epithelium, aids in the movement of allergens, and interrupting cell regeneration [118]. They form insoluble complexes with nutrients and inhibiting their absorption along the small intestine [119]. The fermentation with LAB decreases the SC content through the process of glycosylation in which an enzyme glycosyltransferase provides both water solubility as well as chemical stability to the aglycone. Fermentation of quinoa with L. plantarum decreases the saponin content in them up to the permissible level. The pasting, as well as functional properties in quinoa, was also improved. It then brings added benefits to this raw material and offers the potential to obtain a foundation for gluten-free cream soups with somewhat comparable properties to wheat soup [119]. To establish safe dietary supplements, soymilk was fermented for 24 h together with Streptococcus thermophilus 14085 and Bifidobacterium infantis 14603 at 37°C. Results showed that fermentation with LAB reduced the saponin levels with antinutritional activity and increased overall phenolic content and antitumor cell proliferation activity of soymilk against HT-29 and Caco-2 cells [120].
3.5. Lectins and Hemagglutinins
Lectins and hemagglutinins are proteins or glycoproteins that contain at least one non-catalytic domain thus exhibiting reversible binding toward particularly monosaccharides or oligosaccharides. Lectins and hemagglutinins can bind carbohydrate moieties present on the surface of the erythrocyte and agglutinate the erythrocytes, without bringing any change in the characteristics of the carbohydrates [121]. These anti-nutrients are primarily present in foods that are taken in raw forms [122]. Lectins which are glycoprotein are found in seeds such as cereals and beans and in tubers such as potatoes. Lectin hinders absorption of nutrients by getting adhered to cells of the epithelial lining, resulting in damaging the intestinal tract, therefore allowing the bacterial population to come in close contact with the blood stream [123]. The impact of NF on the lectin contents in the seeds of Lens culinaris cultivar Magda 20 was examined. The results were confirmed by ELISA which showed that the lectin component after 72 h of NF was nearly disappeared under the ideal concentration of flour and temperature conditions [124]. Dual solid-state fermentation of soya bean meal (SBM) was performed using the inoculum mixture of two strains of Aspergillus spp. and Bacillus spp. Fermentation resulted in a substantial decrease in lectin levels and enhanced organic acid production particularly lactic acid from non-detectable in SBM to 6.16 ± 0.22% in fermented SBM (FSBM) [125].
3.6. Enzyme Inhibitors
Protease inhibitors are generally found in plants and they commonly act as antinutritional factors. They are capable of suppressing the function of proteolytic enzymes in the gastrointestinal tract of animals. They are gaining importance in the field of research because of their potent way of reducing the activity of enzymes through the formation of protein-protein interactions. They prevent the activity of an enzyme by their catalytic mode of action by blocking the active site of enzymes. Trypsin inhibitor and chymotrypsin inhibitor are two types of protease inhibitors that mainly occur in raw grain legume plants. Trypsin inhibitors hinder the function of the enzymes trypsin and chymotrypsin within the gut, thereby resulting in the inhibition of protein digestion [18]. Cereal seeds primarily possess plant serpins, known as the major protease inhibitor family. They are also known as “suicide inhibitors” and are generally found in other species of plants [126,127]. Serpins act as potent inhibitors, which in particular suppress the activities of trypsin and chymotrypsin by acting on their overlapping reactive sites [128]. Dual solid-state fermentation of soybean meal was done using an inoculum mixture of Aspergillus spp. and Bacillus spp. and a significant reduction in trypsin inhibitors was observed from 2.56 ± 0.42 mg/g in soybean meal to 0.97 ± 0.14 mg/g in FSBM [125]. A significant decrease in trypsin inhibitors was observed from 7.33 to 6.65 when fermentation was done for 24 h using traditionally fermented pearl millet flour for the preparation of lahoh bread [129]. Ejigui et al. [130] recorded about 41.7% decrease in trypsin inhibitory activity found in yellow maize when it was kept under fermentation for about 4 days under a controlled environmental chamber. Likewise, Osman (2011) [131] obtained a 37–58% reduction in TIA after 24 h of fermentation in three sorghum cultivars. The influence of fermentation on amylase inhibitor activity found in pearl millet suggested a substantial reduction in AIA as the fermentation time increased. After 24 h, the amount of AIA decreased from 80.16 to 39.45 (50.8%) [129].
4. CONCLUSION AND FUTURE ASPECTS
Plant-based food products are becoming more popular as they play an important role in sustainable, low-meat, and balanced diets. Antinutritional factors are well-known plant components and pose difficulty for those who principally choose plant-based food as a diet. Antinutrients may produce unwanted impact when taken in a large amount. Therefore, the occurrence of antinutrients (e.g. lectins, PA, saponins, and enzyme inhibitors) in foods can trigger different reactions when the consumer has little awareness about the environmental effect on the detoxification ability of the human organism. Microbial fermentation by bacteria or fungi has the potential to increase the nutritional value. Fermented plant food materials are superior in nutrients to their unfermented counterpart because of the activation of endogenous enzymes in them and they are having the potential of reducing the antinutritional factors. Fermented foods have displayed great antioxidant potential as compared to unfermented ones due to an increase in Vitamin C content and also due to the release of various health-promoting bioactive components due to the weakening of plant matrix during fermentation. Disruption of plant-based food matrices containing different minerals embedded in them helps in improving the bioavailability of minerals.
At present, the bioaccessibility of minerals is primarily assessed by various in vitro methods which provide a standardized, high-throughput screening method and help in forecasting the mineral bioaccessibility. An idea of the bioavailability of nutrients can be provided by extending these methods to relevant cell lines. However, in vivo studies should be taken under consideration while estimating the nutrient bioavailability from food along with the whole range of nutritional, physiological, and ecological factors that may have an impact on absorption. No doubt, in vitro methods may provide better screening methods; however, confirmation by in vivo methods is important. Furthermore, researchers should not rely only on pulses and cereals but they should also focus on fruit and vegetables, which may be good sources of minerals along with some relevant antinutrients.
5. ACKNOWLEDGMENTS
We hereby acknowledge the support provided by the Amity University Rajasthan and National Dairy Research Institute, Karnal.
6. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
Not applicable.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gernand AD, Schulze KJ, Stewart CP, West KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat Rev Endocrinol 2016;12:274-89. CrossRef
2. Jeske S, Zannini E, Arendt EK. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res Int 2018;110:42-51. CrossRef
3. Zhao D, Shah NP. Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT Food Sci Technol 2014;58:454-62. CrossRef
4. Kumar A, Ahmad F, Zaidi S. Importance of bioactive compounds present in plant products and their extraction: A review. Agric Rev 2019;40:249-60. CrossRef
5. Greifová Z, Kohajdová J, Karovi?ová M. Lactic acid fermentation of some vegetable juices. J Food Nutr Res 2006;45:115-9.
6. Albuquerque TG, Nunes MA, Bessada SM, Costa HS, Oliveira MB, et al. Biologically active and health-promoting food components of nuts, oilseeds, fruits, vegetables, cereals, and legumes. Chem Anal Food 2020;2020:609-56. CrossRef
7. Lazaridou A, Biliaderis CG. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J Cereal Sci 2007;46:101-18. CrossRef
8. Othman RA, Moghadasian MH, Jones PJ. Cholesterol-lowering effects of oat β-glucan. Nutr Rev 2011;69:299-309.
9. Millward DJ. The nutritional value of plant-based diets in relation to human amino acid and protein requirements. Proc Nutr Soc 1999;58:249-60. CrossRef
10. Sá AG, Moreno YM, Carciofi BA. Food processing for the improvement of plant proteins digestibility. Crit Rev Food Sci Nutr 2020;60:3367-86. CrossRef
11. Pawlak R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum Vitamin B12: A review of literature. Eur J Clin Nutr 2014;68:541-8. CrossRef
12. Sebastiani G, Barbero AH, Borrás-Novell C, Casanova MA, Aldecoa-Bilbao V, Andreu-Fernández V, et al. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019;11:1-29. CrossRef
13. Andrews M, Briones L, Jaramillo A, Pizarro F, Arredondo M. Effect of calcium, tannic acid, phytic acid and pectin over iron uptake in an in vitro Caco-2 cell model. Biol Trace Elem Res 2014;158:122-7. CrossRef
14. Suliburska J, Krejpcio Z. Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J Food Sci Technol 2014;51:589-94. CrossRef
15. Schlegel P, Nys Y, Jondreville C. Zinc availability and digestive zinc solubility in piglets and broilers fed diets varying in their phytate contents, phytase activity and supplemented zinc source. Animal 2010;4:200-9. CrossRef
16. Luo Y, Xie W. Effect of phytase treatment on iron bioavailability in faba bean (Vicia faba L.) flour. Food Chem 2012;134:1251-5. CrossRef
17. Miyada T, Nakajima A, Ebihara K. Degradation of pectin in the caecum contributes to bioavailability of iron in rats. Br J Nutr 2012;107:1452-7. CrossRef
18. Gemede HF, Ratta N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int J Nutr Food Sci 2014;3:284-9. CrossRef
19. Samtiya M, Aluko RE, Dhewa T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod Proc Nutr 2020;2:1-14. CrossRef
20. Rousseau S, Kyomugasho C, Celus M, Hendrickx ME, Grauwet T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit Rev Food Sci Nutr 2019;60:826-43. CrossRef
21. Essack H, Odhav B, Mellem JJ. Screening of traditional South African leafy vegetables for specific anti-nutritional factors before and after processing. Food Sci Technol 2017;37:462-71. CrossRef
22. Popova A, Mihaylova D. Antinutrients in plant-based foods: A review. Open Biotechnol J 2019;13:68-76. CrossRef
23. Rekha CR, Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and Vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J Appl Microbiol 2010;109:1198-208. CrossRef
24. Onyesom I, Enaholo AT, Mordi J. Effect of processing techniques on the contents of flatulence factors and emulsion properties of cowpea (Vigna unguiculata). J Appl Sci Agric Manag 2005;9:65-72. CrossRef
25. Njoumi S, Amiot MJ, Rochette I, Bellagha S, Mouquet-Rivier C. Soaking and cooking modify the alpha-galacto-oligosaccharide and dietary fibre content in five Mediterranean legumes. Int J Food Sci Nutr 2019;70:551-61. CrossRef
26. Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr 2016;56:339-49. CrossRef
27. Wansink B, Sonka S, Goldsmith P, Chiriboga J, Eren N. Increasing the acceptance of soy-based foods. J Int Food Agribus Mark 2005;17:35-55. CrossRef
28. Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: A review. Am J Clin Nutr 2000;72:1424-35. CrossRef
29. Sha’a KK, Clarkson GP, Artimas SP. Phytochemical analysis, proximate composition and antinutritional factors of Corchorus oliterius plant. Int J Biol Chem Sci 2019;13:2147-57. CrossRef
30. Peumans WJ, Van Damme JM, Barre A, Rougé P. Classification of plant lectins in families of structurally and evolutionary related proteins. Mol Immunol Complex Carbohydr 2001;2:27-54. CrossRef
31. Singh J, Basu PS. Non-nutritive bioactive compounds in pulses and their impact on human health: An overview. Food Nutr Sci 2012;3:1664-72. CrossRef
32. Tibe O, Amarteifio JO. Trypsin inhibitor activity and condensed tannin content in Bambara groundnut (Vigna subterranea (L.) Verdc) grown in Southern Africa. J Appl Sci Environ Manag 2007;11:159-64. CrossRef
33. Grant JE, Thomson LM, Pither-Joyce MD, Dale TM, Cooper PA. Influence of Agrobacterium tumefaciens strain on the production of transgenic peas (Pisum sativum L.). Plant Cell Rep 2003;21:1207-10. CrossRef
34. Adeyemo SM, Onilude AA. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger Food J 2013;31:84-90. CrossRef
35. Chu X, Guo Y, Xu B, Li W, Lin Y, Sun X, et al. Effects of tannic acid, green tea and red wine on hERG channels expressed in HEK293cells. PLoS One 2015;10:1-13. CrossRef
36. Kumar V, Sinha AK, Makkar HP, Becker K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem 2010;120:945-59. CrossRef
37. Abdul-Aziz A, Kadhim KK. Efficacy of the cruciferous vegetable on the thyroid gland and the gonads in rabbits. Adv Anim Vet Sci 2015;3:183-91. CrossRef
38. Kannan U, Sharma R, Gangola MP, Chibbar RN. Improving grain quality in pulses: Strategies to reduce raffinose family oligosaccharides in seeds. Ekin J Crop Breed Genet 2018;4:70-88.
39. El Barky AR, Hussein SA, Alm-Eldeen AE. Saponins and their potential role in diabetes mellitus. Diabetes Manag 2017;7:148.
40. Ikewuchi CC. Hypocholesterolemic effect of an aqueous extract of the leaves of Sansevieria senegambica Baker on plasma lipid profile and atherogenic indices of rats fed egg yolk supplemented diet. EXCLI J 2012;11:346.
41. Ertop MH, Bekta? M. Enhancement of bioavailable micronutrients and reduction of antinutrients in foods with some processes. Food Health 2018;4:159-65. CrossRef
42. Kumari S. The Effect of Soaking Almonds and Hazelnuts on Phytate and Mineral Concentrations. Doctoral Dissertation. Otago: University of Otago; 2018.
43. Erta? N, Türker S. Bulgur processes increase nutrition value: Possible role in in-vitro protein digestability, phytic acid, trypsin inhibitor activity and mineral bioavailability. J Food Sci Technol 2014;51:1401-5. CrossRef
44. Devi R, Chaudhary C, Jain V, Saxena AK, Chawla S. Effect of soaking on anti-nutritional factors in the sun-dried seeds of hybrid pigeon pea to enhance their nutrients bioavailability. J Pharmacogn Phytochem 2018;7:675-80.
45. Agume AS, Njintang NY, Mbofung CM. Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods 2017;6:12. CrossRef
46. Torres J, Rutherfurd SM, Muñoz LS, Peters M, Montoya CA. The impact of heating and soaking on the in vitro enzymatic hydrolysis of protein varies in different species of tropical legumes. Food Chem 2016;194:377-82. CrossRef
47. Shimelis EA, Rakshit SK. Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem 2007;103:161-72. CrossRef
48. Vadivel V, Pugalenthi M, Megha S. Biological evaluation of protein quality of raw and processed seeds of gila bean (Entada scandens Benth.). Trop Subtrop Agroecosyst 2008;8:125-33.
49. Doss A, Pugalenthi M, Vadivel VG, Subhashini G, Subash AR. Effects of processing technique on the nutritional composition and antinutrients content of under-utilized food legume Canavalia ensiformis L. DC. Int Food Res J 2011;18:965-70.
50. Fernando R, Pinto P, Pathmeswaran A. Goitrogenic food and prevalence of goitre in Sri Lanka. Int J Intern Med 2012;1:17-20.
51. Udousoro II, Akpan EB. Anthropometric measurements, changes in anti-nutrients contents of edible vegetables under varied temperature and heating time. Curr Res Nutr Food Sci J 2014;2:146-52. CrossRef
52. Adeleke OR, Adiamo OQ, Fawale OS, et al. Effect of processing methods on antinutrients and oligosaccharides contents and protein digestibility of the flours of two newly developed bambara groundnut cultivars. Int Food Res J 2017;24:1948-55.
53. Mwanri A, Kogi-Makau W, Laswai H. Nutrients and antinutrients composition of raw, cooked and sun-dried sweet potato leaves. Afr J Food Agric Nutr Dev 2011;11:5142-56. CrossRef
54. Nkhata SG, Ayua E, Kamau EH, Shingiro JB. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr 2018;6:2446-58. CrossRef
55. Laxmi G, Chaturvedi N, Richa S. The impact of malting on nutritional composition of foxtail millet, wheat and chickpea. J Nutr Food Sci 2015;5:1-3. CrossRef
56. Oghbaei M, Prakash J. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food Agric 2016;2:1136015. CrossRef
57. Onyango CA, Ochanda SO, Mwasaru MA, Ochieng JK, Mathooko FM, Kinyuru JN. Effects of malting and fermentation on anti-nutrient reduction and protein digestibility of red sorghum, white sorghum and pearl millet. J Food Res 2013;2:41. CrossRef
58. Yoshiara LY, Mandarino JM, Carrão-Panizzi MC, Madeira TB, da Silva JB, de Camargo AC, Ida EI. Germination changes the isoflavone profile and increases the antioxidant potential of soybean. J Food Bioact 2018;3:144-50. CrossRef
59. de Camargo AC, Favero BT, Morzelle MC, Franchin M, Alvarez-Parrilla E, de la Rosa LA, Schwember AR. Is chickpea a potential substitute for soybean? Phenolic bioactives and potential health benefits. Int J Mol Sci 2019;20:1-42. CrossRef
60. Singh A, Gupta S, Kaur R, Gupta HR. Process optimization for anti-nutrient minimization of millets. Asian J Dairy Food Res 2017;36:322-6. CrossRef
61. Adler P, Bolten CJ, Dohnt K, Hansen CE, Wittmann C. Core fluxome and meta fluxome of lactic acid bacteria under cocoa pulp fermentation simulating conditions. Appl Environ Microbiol 2013;79:5670-81. CrossRef
62. Demir N, Bahceci KS, Acar J. The effects of differential initial Lactobacillus plantarum concentrations on some properties of fermented carrot juice. J Food Proc Preserv 2006;30:352-63. CrossRef
63. Sethi S, Tyagi SK, Anurag RK. Plant-based milk alternatives an emerging segment of functional beverages: A review. J Food Sci Technol 2016;53:3408-23. CrossRef
64. Steinkraus KH. Classification of fermented foods: Worldwide review of household fermentation techniques. Food Control 1997;8:311-7. CrossRef
65. Ray M, Ghosh K, Singh S, Chandra K. Folk to functional: An explorative overview of rice-based fermented foods and beverages in India. J Ethnic Foods 2016;3:5-18. CrossRef
66. Ogodo AC, Agwaranze DI, Aliba NV, Kalu AC, Nwaneri CB. Fermentation by lactic acid Bacteria consortium and its effect on anti-nutritional factors in maize flour. J Biol Sci 2019;19:17-23. CrossRef
67. Etsuyankpa MB, Gimba CE, Agbaji EB, Omoniyi I, Ndamitso MM, Mathew JT. Assessment of the effects of microbial fermentation on selected anti-nutrients in the products of four local cassava varieties from Niger state, Nigeria. Am J Food Sci Technol 2015;3:89-96.
68. Hasan MZ. Sultan, and M. Mar-E-Um. Significance of fermented food in nutrition and food science. J Sci Res 2014;6:373-86. CrossRef
69. Jesperson L. Occurrence and taxonomic characteristics of Saccharomyces cerevisae predominant in African indigenous fermented foods and beverages. FEMS Yeast Res 2003;3:191-200. CrossRef
70. Chelule PK, Mokoena MP, Gqaleni N. Advantages of Traditional Lactic Acid Bacteria Fermentation of Food in Africa. In: Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; 2010. p. 1160-7.
71. Terefe NS, Augustin MA. Fermentation for tailoring the technological and health related functionality of food products. Crit Rev Food Sci Nutr 2020;60:2887-913. CrossRef
72. Rakin M, Vukasinovic M, Siler-Marinkovic S, Maksimovic M. Contribution of lactic acid fermentation to improved nutritive quality vegetable juices enriched with brewer’s yeast autolysate. Food Chem 2007;100:599-602. CrossRef
73. Bergqvist SW, Sandberg AS, Carlsson NG, Andlid T. Improved iron solubility in carrot juice fermented by homo-and hetero-fermentative lactic acid bacteria. Food Microbiol 2005;22:53-61. CrossRef
74. Lopez HW, Ouvry A, Bervas E, Guy C, Messager A, Demigne C, et al. Strains of lactic acid bacteria isolated from sour doughs degrade phytic acid and improve calcium and magnesium solubility from whole wheat flour. J Agric Food Chem 2000;48:2281-5. CrossRef
75. Proulx AK, Reddy MB. Fermentation and lactic acid addition enhance iron bioavailability of maize. J Agric Food Chem 2007;55:2749-54. CrossRef
76. Vanajakshi V, Vijayendra SV, Varadaraj MC, Venkateswaran G, Agrawal R. Optimization of a probiotic beverage based on Moringa leaves and beetroot. LWT Food Sci Technol 2015;63:1268-73. CrossRef
77. Fischer MM, Egli IM, Aeberli I, Hurrell RF, Meile L. Phytic acid degrading lactic acid bacteria in tef-injera fermentation. Int J Food Microbiol 2014;190:54-60. CrossRef
78. Kim SY. Production of fermented kale juices with Lactobacillus strains and nutritional composition. Prevent Nutr Food Sci 2017;22:231.
79. Yan PM, Xue WT, Tan SS, Zhang H, Chang XH. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control 2008;19:50-5. CrossRef
80. Santos F, Wegkamp A, de Vos WM, Smid EJ, Hugenholtz J. High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl Environ Microbiol 2008;74:3291-4. CrossRef
81. Hoppe M, Önning G, Berggren A, Hulthén L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br J Nutr 2015;114:1195-202. CrossRef
82. Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol 2015;52:676-84. CrossRef
83. Nissar J, Ahad T, Naik HR, Hussain SZ. A review phytic acid: As antinutrient or nutraceutical. J Pharmacogn Phytochem 2017;6:1554-60.
84. Kwun IS, Kwon CS. Dietary molar ratios of phytate: Zinc and millimolar ratios of phytate× calcium: Zinc in South Koreans. Biol Trace Elem Res 2000;75:29-41. CrossRef
85. Amirabdollahian F, Ash R. An estimate of phytate intake and molar ratio of phytate to zinc in the diet of the people in the United Kingdom. Public Health Nutr 2010;13:1380-8. CrossRef
86. Kies AK, De Jonge LH, Kemme PA, Jongbloed AW. Interaction between protein, phytate, and microbial phytase. In vitro studies. J Agric Food Chem 2006;54:1753-8. CrossRef
87. Chan SS, Ferguson EL, Bailey K, Fahmida U, Harper TB, Gibson RS. The concentrations of iron, calcium, zinc and phytate in cereals and legumes habitually consumed by infants living in East Lombok, Indonesia. J Food Comp Anal 2007;20:609-17. CrossRef
88. Al Hasan SM, Hassan M, Saha S, Islam M, Billah M, Islam S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr 2016;2:1-10. CrossRef
89. Coulibaly A, Kouakou B, Chen J. Phytic acid in cereal grains: Structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am J Plant Nutr Fertil Technol 2011;1:1-22. CrossRef
90. Greiner R, Konietzny U, Jany KD. Phytate-an undesirable constituent of plant-based foods? J Nutr Med 2006;8:18-28.
91. Anastasio M, Pepe O, Cirillo T, Palomba S, Blaiotta G, Villani F. Selection and use of phytate-degrading LAB to improve cereal-based products by mineral solubilization during dough fermentation. J Food Sci 2010;75:M28-35. CrossRef
92. Licandro H, Ho PH, Nguyen TK, Petchkongkaew A, Van Nguyen H, Chu-Ky S, et al. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control 2020;110:1-6. CrossRef
93. Magala M, Kohajdová Z, Karovi?ová J. Degradation of phytic acid during fermentation of cereal substrates. J Cereal Sci 2015;61:94-6. CrossRef
94. Kårlund A, Gómez-Gallego C, Korhonen J, Palo-Oja OM, El-Nezami H, Kolehmainen M. Harnessing microbes for sustainable development: Food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients 2020;12:1020. CrossRef
95. Chvatalova K, Slaninova I, Brezinova L, Slanina J. Influence of dietary phenolic acids on redox staturs of iron: Ferrous iron autooxidation and ferric iron reduction. Food Chem 2008;106:650-60. CrossRef
96. Mladenka P, Macakova K, Filipsky T, Zatloukalova L, Jahodar L, Bovicelli P, et al. In vitro analysis of iron chelating activity of flavonoids. J Inorg Biochem 2011;105:693-701. CrossRef
97. Macakova K, Mladenka P, Filipsky T, Riha M, Jahodar L, Trejtnar F, et al. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem 2012;135:2584-92. CrossRef
98. Glahn RP, Wortley GM. Inhibition of iron uptake by phytic acid, tannic acid and ZnCl2: Studies using an in vitro digestion/Caco2 cell model. J Agric Food Chem 2002;50:390-5. CrossRef
99. Starzy?ska-Janiszewska A, Stodolak B, Mickowska B. Effect of controlled lactic acid fermentation on selected bioactive and nutritional parameters of tempeh obtained from unhulled common bean (Phaseolus vulgaris) seeds. J Sci Food Agric 2014;94:359-66. CrossRef
100. Svensson L, Sekwati-Monang B, Lutz DL, Schieber A, Ganzle MG. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J Agric Food Chem 2010;58:9214-20. CrossRef
101. Osawa RO, Kuroiso K, Goto S, Shimizu A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl Environ Microbiol 2000;66:3093-7. CrossRef
102. Rodríguez H, Curiel JA, Landete JM, de las Rivas B, de Felipe FL, Gómez-Cordovés C, et al. Food phenolics and lactic acid bacteria. Int J Food Microbiol 2009;132:79-90. CrossRef
103. Vaquero I, Marcobal Á, Muñoz R. Tannase activity by lactic acid bacteria isolated from grape must and wine. Int J Food Microbiol 2004;96:199-204. CrossRef
104. Rodríguez H, de las Rivas B, Gómez-Cordovés C, Muñoz R. Characterization of tannase activity in cell-free extracts of Lactobacillus plantarum CECT 748T. Int J Food Microbiol 2008;121:92-8. CrossRef
105. Iwamoto K, Tsuruta H, Nishitaini Y, Osawa R. Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917T. Syst Appl Microbiol 2008;31:269-77. CrossRef
106. Aguilar-Zarate P, Cruz MA, Montañez J, Rodriguez-Herrera R, Wong-Paz JE, Belmares RE, et al. Gallic acid production under anaerobic submerged fermentation by two bacilli strains. Microbial Cell Factories 2015;14:1-7. CrossRef
107. Reverón I, Jiménez N, Curiel JA, Peñas E, de Felipe FL, de las Rivas B, et al. Differential gene expression by Lactobacillus plantarum WCFS1 in response to phenolic compounds reveals new genes involved in tannin degradation. Appl Environ Microbiol 2017;83:e03387-16. CrossRef
108. Kostinek M, Specht I, Edward VA, Pinto C, Egounlety M, Sossa C, et al. Characterisation and biochemical properties of predominant lactic acid bacteria from fermenting cassava for selection as starter cultures. Int J Food Microbiol 2007;114:342-51. CrossRef
109. Tangyu M, Muller J, Bolten CJ, Wittmann C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl Microbiol Biotechnol 2019;103:9263-75. CrossRef
110. Gabaza M, Muchuweti M, Vandamme P, Raes K. Can fermentation be used as a sustainable strategy to reduce iron and zinc binders in traditional African fermented cereal porridges or gruels? Food Rev Int 2017;33:561-86. CrossRef
111. Williams BA, Mikkelsen D, Flanagan BM, Gidley MJ. Dietary fibre: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J Anim Sci Biotechnol 2019;10:1-12. CrossRef
112. Rollan GC, Gerez CL, LeBlanc JG. Lactic fermentation as a strategy to improve the nutritional and functional values of pseudocereals. Front Nutr 2019;6:98. CrossRef
113. Keddari S, Benaoum N, Boufadi Y, Belhocine M, Riazi A. Antioxidant activity and in vitro fermentation of dietary fiber extracts from durum wheat bran. J Food Nutr Res 2016;4:508-14.
114. Lopes GK, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta 1999;1472:142-52. CrossRef
115. Admassu SE, Kumar RS. Effect of processing on antinutrients and in vitro protein digestibility of kidney bean (Phaseolus vulgaris L.) varieties grown in East Africa. Food Chem 2007;103:161-72. CrossRef
116. D’Mello JP, Duffus CM, Duffus JH, editors. Toxic Substances in Crop Plants. Sawston: Woodhead Publishing; 1991. CrossRef
117. Moses T, Papadopoulou KK, Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol 2014;49:439-62.
118. Francis G, Makkar HP, Becker K. Dietary supplementation with a Quillaja saponaria mixture improves growth performance and metabolic efficiency in common carp (Cyprinus carpio L.). Aquaculture 2002;203:311-20.
119. Bolívar-Monsalve J, Ceballos-González C, Ramírez-Toro C, Bolívar GA. Reduction in saponin content and production of gluten-free cream soup base using quinoa fermented with Lactobacillus plantarum. J Food Proc Preserv 2018;42:1-10.
120. Lai LR, Hsieh SC, Huang HY, Chou CC. Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J Biosci Bioeng 2013;115:552-6.
121. Lam SK, Ng TB. Lectins: Production and practical applications. Appl Microbiol Biotechnol 2011;89:45-55.
122. Hamid R, Masood A, Wani IH, Rafiq S. Lectins: Proteins with diverse applications. J Appl Pharm Sci 2013;3:S93-103.
123. Muramoto K. Lectins as bioactive proteins in foods and feeds. Food Sci Technol Res 2017;23:487-94.
124. Cuadrado C, Hajos G, Burbano C, Pedrosa MM, Ayet G, Muzquiz M, et al. Effect of natural fermentation on the lectin of lentils measured by immunological methods. Food Agric Immunol 2002;14:41-9.
125. Ruiz N, Robles-Montes A, van Eys J. Fermentation of soybean meal results in a net reduction of heat-labile and heat-stable antinutritional factors. Poult Sci Assoc 2020;2020:206.
126. Habib H, Fazili KM. Plant protease inhibitors: A defense strategy in plants. Biotechnol Mol Biol Rev 2007;2:68-85.
127. Haq SK, Atif SM, Khan RH. Biochemical characterization, stability studies and N-terminal sequence of a bi-functional inhibitor from Phaseolus aureus Roxb. (Mung bean). Biochimie 2005;87:1127-36.
128. Dahl SW, Rasmussen SK, Hejgaard J. Heterologous expression of three plant serpins with distinct inhibitory specificities. J Biol Chem 1996;271:25083-8.
129. Osman MA. Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. J Saudi Soc Agric Sci 2011;10:1-6.
130. Ejigui J, Savoie L, Marin J, Desrosiers T. Beneficial changes and drawbacks of a traditional fermentation process on chemical composition and antinutritional factors of yellow maize (Zea mays). J Biol Sci 2005;5:590-6.
131. Osman MA. Changes in sorghum enzyme inhibitors, phytic acid, tannins and in vitro protein digestibility occurring during Khamir (local bread) fermentation. Food Chem 2004;88:129-34.