1. INTRODUCTION
Plant breeding is an important science for the improvement of all types of agricultural crops which has begun in very ancient along with the human culture. It mainly uses techniques like selection, hybridization, cross-breeding, mutation breeding, and even development of transgenic plants. Among all techniques, mutation breeding has become popular among the breeders and scientists as it can create genetic variation in plants naturally as well as through mutagens (physical or chemical). Although the first spontaneous mutant plants in cereal crops were found in China nearly 2,300 years before and even reported in 1,590 and 1968 by some scientist, the first publication on induced mutation was published by Muller [1] and Stadler [2–3]. The first commercial mutant cultivar was produced in Nicotiana tabacum using inducing mutations. After this event, mutation breeding has found a niche in plant breeding because of several advantages and has been applied actively to quickly adopt advances in the technology [4,5]. The Food and Agriculture Organization of the United Nations/International Atomic Energy Agency-Mutant Variety Database data [6] reported that nearly 3,364 accessions from 226 species have been developed and officially released as mutants (Fig. 1). Some of these mutant cultivars have revolutionized agriculture not only in densely populated developing countries but also in agriculturally advanced countries like the United States, United Kingdom, Japan, France, etc. (Fig. 2). As per available data, India has also released nearly 344 varieties of different crops (Fig. 2).
Several studies have proved that induced mutagenesis is able to modify many morphological and biochemical plant traits like plant height, maturity, seed shattering resistance, disease resistance, oil quality and quantity, malting quality, size and quality of starch granules in many crops [7,8]. This mutagenesis has also become an alternative tool to develop resistance against harmful pathogens in many susceptible crops, e.g., ginger and coriander, to their improvement in yield and quality traits [9] and (Table 1). It is well known that transgenic technology has been significantly used in improving crops with substantial commercial value, but this technology has some technical limitations in many crops as these plants are highly recalcitrant to genetic transformation and regeneration. Further, the other important method of improvement is the development of transgenic crops but this technology is very much doubtful for the unpredictable risk of environment and food safety [10]. For these kinds of problems, mutagenesis can be used without restrictions like legislative constraints, licensing costs, and societal opposition of transgenic technology [11]. Mutagenesis with high-resolution screening can be able to supply a very good complement to recombinant DNA technologies for the improvement of crops for better adoption in changing environment conditions for increasing global population [12]. Mutants are now effectively used for studying gene expression and gene regulation in many crops and also able to assign a functional role to genes. Induced mutations enhance the rate of genetic variability, introduce many new traits, and identify trait specific genes [13]. Mutation breeding has been proved as a supplement and an effective substitute to conventional breeding where only specific improvement in a variety is required without losing its original acceptable phenotype.
 | Figure 1: The Pie chart representing distribution of officially released mutants from different plant categories from all over world. The Mutant Variety Database contains more 3,364 entries. The above grouping is based on the plant type group. The top classes consist of cereals, flowering plant, and legume/pulses. Data collected from MVD 2021 (https://mvd.iaea.org/#!Search) accessed on Jan 20, 2021. [Click here to view] |
 | Figure 2: Country wise distribution of officially released mutants. Only top 30 countries were taken to make this bar diagram. Data collected from MVD 2021 (https://mvd.iaea.org/#!Search) accessed on Jan 20, 2021. [Click here to view] |
1.1. Types of Mutagens Used in Mutation Breeding
Mutations are responsible for creating genetic variability in several crops naturally or artificially induced. Several physical and chemical mutagens are being used to induce artificial mutations [14,15]. Physical mutagens include various types of radiations like X-rays, gamma rays, neutrons, beta particles, alpha particles, proton or deuterons, UV rays, etc. (Fig. 3). The chemical mutagenesis is caused by several chemicals like alkylating agents [ethyl methane sulfonate (EMS), Methyl methanesulfonate, nitrogen mustard, etc..], base analogues [Bromo uracil (5BU), aminopurine (2AP), Tetramethyl Urea (TMU), etc..], Acridine dyes (Acriflavine, acridine orange, ethidium bromide, etc.). Another category for tool for mutagenesis is the use of biological agents such as transposons, retrotransposons, and T-DNA [16,17]. The genome editing systems like transcription activator-like effector nucleases (TALENS) and clustered regularly interspaced short palindromic repeat (CRISPR) induce target mutations and can be used as an alternative mutagen [18] (Fig. 3). These chemicals have chromosome damaging effects on plants by oxygen derived radicals; these effects can occur both spontaneously and artificially following induction through mutagens [9]. There are several mutagens available for crop improvement and each has its own important role as positive or negative effect on crops [19].
1.2. Steps Involved in Mutation Breeding
Several steps are involved in the process of induced mutagenesis (Fig. 4). The first step involves irradiation or chemical treatment of the seeds or any tissue of the parent variety and this material is designated as M0. These Mutagenic treatments can induce some chromosomal rearrangements or can make some changes in genes at the nucleotide level to develop other allelic forms. This alteration may be responsible for the change in one or more specific characters in the M1 generation which is grown from mutagenized seed or tissue (M0). The population of M1 generation is homogenous type as the plants may have a different effect of allelic mutation. Genetically, Ml mutant plants are heterozygous in nature as in most of the cases only one allele is affected by one mutation during treatment. The occurrence of having mutation in both alleles of a gene is extremely low and very rare. Moreover, only dominant mutations can be identified in M1, while it is impossible to identify a recessive mutation. Therefore, the selection is not done in M1 generation and plant breeders attempt screening mutations in subsequent generations where segregations occur. Now the seeds of M1 generation are used to create M2 generation [20]. The seeds of M2 generation are sown in experimental plots to obtain segregating M2 population which is subjected to various screening procedures for the selection of plants with desired characters. The selected plant material of M2 is further grown on as the M3 generation. Usually, M3–5 generations are used to screen the quantitatively inherited characters, homozygosity, and stability in field performance on a line or a single plant basis. These selected promising homozygotic mutants can multiply directly in the field in subsequent generations M6–8 and comparative analysis is also done with checks in different locations. After the multiplication of seed and different location testing, the mutants can go for official testing before release as mutant variety (Fig. 4). Further, these lines can be used as breeding material in a cross breeding program [20,21].
 | Table 1: List of improvements in medicinal and aromatic crops through Mutation breeding. [Click here to view] |
1.3. Improvement in Medicinal and Aromatic Plants Using Mutagenesis
The conventional plant breeding methods are very effective in many crops including cereals and pulses and have been using since long to improve the yield and other characters in these crops. But these conventional breeding methods are not very much successful and take a long period for improvement in high yield of biomass and increase in the active principles in medicinal and aromatic crops due to low genetic variability. Hence, induced mutagenesis has been proved as an important approach for creating the genetic variation and diversity in these crops to overcome from these bottleneck conditions. Induced mutagenesis can utilize the potential of plant genetic resource to create variability which is available to plant breeders as raw material to generate new crop variety and these varieties can provide a significant contribution for sustainable crop production. The contribution of mutation breeding in medicinal and aromatic crops is less than one percent which is comparatively very less as compared to cereals (48%), flowers (22%), and legumes (15%) [22] (Fig. 1). There is scope for improvement in medicinal and aromatic crops through mutation breeding in terms of enhancement of important features like new colors and shapes of flower, plant morphology, flowering times, postharvest quality, biotic and abiotic stress tolerance, and alkaloid content in short time with low cost. The mutation breeding was successfully done in several spices, flower species, grasses, and wild species that come under medicinal and aromatic plants to improve several characters like yield, alkaloid contents, disease resistance, etc. (Table 1). In this review, some cases out of those several mutagenesis studies in medicinal and aromatic plants are discussed.
Farooqi and Shriramu [23] released a Henbane (Hyoscyamus niger L.) variety, Aela, belong to the family Solanaceae using irradiated culture. The developed variety had more than double vigorous growth with a yield of 73 q/ha as compared to its parent (50 q/ha), and also records a higher alkaloid level 0.545% as compared to 0.167% in the parent plant. This mutant can be identified by its yellow flowers with a slight purple tinge at the base of the petals. In black cumin (Nigella sativa L.), some superior mutants lines were developed by induced mutagenesis through X-rays and gamma radiations and proper selection to improve the many yield attributing characters like lax branching, feathery leaf mutant, bushy, male sterile, crumpled leaf, early flowering, brown seed coat, etc. [24]. The gamma irradiation and EMS treatment on dry, filled, and black seeds of cumin were able to produce five dark reddish brown, one yellowish brown, and one peach color seeded plants out of 7,956 plants progenies [25]. A mutant line RZ-223 of Cuminum cyminum developed through mutation breeding using gamma rays was found to resistant to wilt and blight diseases, superior in yield and seed quality over its parental line [26]. In Coriandrum sativum a mutant variety “RCr 684” was developed for resistant to stem gall and less susceptible to the powdery mildew [22].
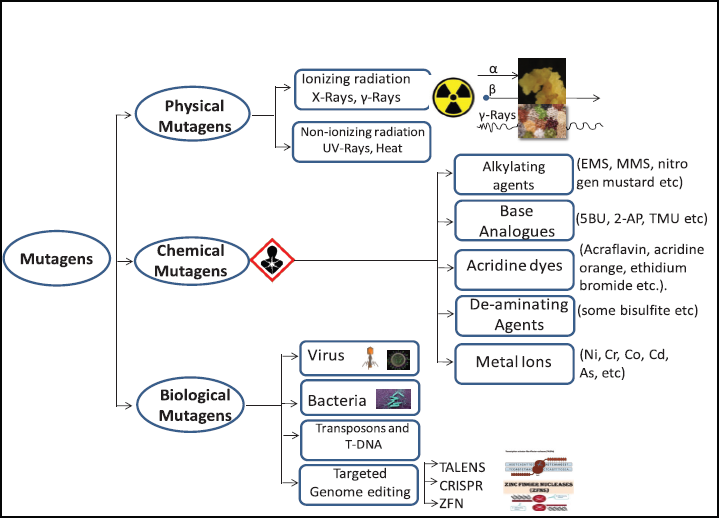 | Figure 3: Diagram representing the three different kinds of mutagens used in mutagenesis of different crops. Abbreviations: EMS = Ethyl methyl sulphonate; MMS = Methyl methanesulfonate; 5BU = Bromo uracil; 2AP = aminopurine; TMU = Tetramethyl Urea; TALENS = transcription activator-like effector nucleases; CRISPR = clustered regularly interspaced short palindromic repeat; ZFN = Zinc Finger Nucleases. [Click here to view] |
Kulkarni and Baskaran [27] used Catharanthus seeds for chemical mutagens treatment to develop a high alkaloid producing variety “Dhawal”. Another Catharanthus mutant with leafless inflorescence, increased flower frequency, and salt tolerant was also developed using chemical mutagenesis [28]. A mutant variety of periwinkle with higher total root and leaf alkaloids including anticancer alkaloids, vincristine and vinblastine, was also developed through EMS induced mutation [29]. A dwarf mutant of Catharanthus roseus was developed to evaluate morphological parameters and the antibacterial activity of the aqueous and ethanol leaf extracts against five medically important bacterial strains [30].
Basu et al. [31] reported that the mutation breeding approach in Fenugreek (Trigonella foenum-graecum L.) seeds using different dose of EMS for different times and mutated plants were selected for new breeding material with several improved characters. In another report, several mutant lines were selected for higher yield and diosgenin content as compared to their parental lines based on morphological performance from M2 to M4 generation after treated with different mutagens like gamma rays, EMS, and ethylene imine (EI) with different dose and time [32]. The mutation breeding approach was also applied in narcotic opium to make non-narcotic and alkaloid free opium variety “Sujata” using gamma rays and EMS [33]. Five improved opium poppy mutant lines showing higher seed yield and high morphine content over control varieties of commercial values were developed from the progeny of a known poppy variety using gamma rays of additionally with EMS [34]. A variety of induced mutation using EMS, diethylsulfate (DES), gamma rays either alone or in combination was applied in Thorn apple (Datura inoxia Mill.) which resulted in development of several homozygous lines of the mutants with higher alkaloid scopolamine yield with altered agronomic performance [35].
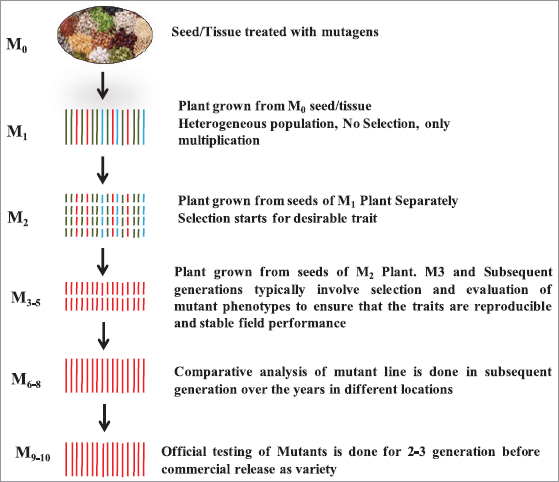 | Figure 4: Flow chart of traditional mutation breeding scheme. Each row describes the steps for a specific generation. The generation nomenclature starts with M0. Subsequent generations are designated as M1, M2, etc. [Click here to view] |
An important medicinal plant Coleus forskohlii member of Lamiaceae family grows wildly in the subtropical climate. The roots and leaves have been useful in the treatment of various allergic disease, viz., psoriasis, eczema, skin infections, leucorrhea, and asthma. The tuberous roots are found to be a rich source of the labdane diterpenoid forskolin (22). Srinivasappa et al. [36] used tissue cultured plant, root cuttings, and un-rooted cutting for gamma irradiation to improve various quantitative and qualitative traits like leaf shape, plant height, leaf color, tuber yield, and forskolin content. In another attempt, a variety Suphala was developed for high yield (15.93 t/ha) and can be cultivated throughout the year.
Solanum viarum is a perennial shrub with a prickly stem and prickly leaves which yields solasodine, an active ingredient of the contraceptive pill. A mutant Arka Sanjeevani a Spineless variety of S. viarum was also developed through mutation breeding for high alkaloid content [37]. The strains such as Glaxo BARC, RRL-20-2, and RRL-SL-6 were developed with very high yield potential for alkaloids through mutation breeding [38,39]. Isabgol (Plantago ovata L. Forsk) is an important indigenous drug plant of India. The seed and husk are used in ayurvedic medicines to treat many stomach disorders such as diarrhea, ulcers, constipation, and piles. Several investigators have worked on ionizing radiation for inducing mutation in psyllium and released some varieties like Niharika, Mayuri, and Nimisha for high seed yield up to 10–11 q/ha [40–43]. Mayuri was a distinct early maturing, high seed yielding potential of about 13 q/ha seeds of psyllium with the distinct pigment marker which can indicate the right stage of panicles maturity for harvesting to avoid the seed shattering in psyllium. In chamomile, two improved mutant lines were developed using gamma irradiation with changes in plant height, flower number, and oil yield in both positive and negative directions. These two lines also showed changes in some key enzymes responsible for the biosynthesis of quality components and subsequently two varieties, “Vallary” and Ujjawla, are released for commercial cultivation with Dry flower yield 7.0 q/ha and Oil yield 6.0 kg/ha [44,45]. Mutation breeding resulted in the development of another variety CIM-Sammohak with a higher yield of dry flowers (7.5 q/ha) and oil yield of 6–7 kg/ha containing important alkaloids like 12% chamazulene, 20% bisabolol oxide-A, and 11% bisabolol oxide-B (22).
A widely adapted peppermint (Mentha piperita) variety “Kukrail” and “Pranjal” with erect plant habit was developed for higher oil yield and disease resistant against Spilarctia obliqua (Bihar hairy caterpillar). Another peppermint variety “Tushar” has also been developed through mutation breeding for higher oil produce up to 85–90 kg/ha oil [22]. A high yielding and disease resistant (against Puccinia menthae) peppermint variety “multimentha” was also developed through induced mutation. This variety showed improved features like high essential oil content and resistance against pathogens [22,46]. Various studies showed that irradiation by gamma rays in M. piperita L. resulted in various mutations that may be used for the plant breeders to create new varieties of mentha. These mutated lines may be very useful for pharmaceutical companies and industries due to high essential oil constituents such as Menthol (48%) by the dose of 8 k-Rad gamma rays and Menthone (21%) by the dose of 6 k-Rad [47].
Improvement in the vegetatively propagated crops like ginger and turmeric can also be done using induced mutagenesis. In ginger, six potential mutants were screened for resistance against Ralstonia solanacearum and Pythium sp. after mutagenesis [48]. Other researchers were also found that the different doses of gamma rays and EMS can cause significant variations in the number of mother, primary, secondary, and tertiary rhizomes in ginger and turmeric [49–52]. In Curcuma longa, some varieties like BSR1 and BSR2 were also developed using mutagens for higher curcumin content (up to 4.2%) in rhizomes and crop yields up to 32 t/ha in a shorter crop duration of 240–250 days while another variety CO.1 has bold and orange yellow rhizomes, suitable for drought affected areas [22].
The improvement in genus Cymbopogon using mutagenesis has been carrying out since long. A methyl-eugenol deficient mutant of Cymbopogon flexuosus was developed using X-rays radiation which made it a good substitute for citronella oil [53]. Gamma irradiation on the seed of Palmarosa (Cymbopogon martini) resulted in a higher yield and better quality of the essential oil [54]. Several varieties of Cymbopogon winterianus like Bhanumati, Bibhuti, Niranjan, Phullara, Sourar, and Subir have been developed through mutation breeding with genetic divergence and better oil composition for different regions and climatic conditions at North Indian and South Indian [55]. CIM-Jeeva, a variety of C. winterianus with herb yield 215 q/ha and oil yield 285 kg/ha has also been developed through mutation [22]. Treatment of UltraViolet-B exposure was able to stimulate the production of oil cells in changing the quality and percentage of essential oil contents of lemongrass, i.e., Cymbopogon citratus [56]. Gamma irradiation was able to create changes in physical characteristic in C. citratus at the early growth phase in M1 generation [57]. Five improved mutant clones of Jamrosa were isolated with variation in quality/quantity of essential oil using various doses of gamma-rays in dormant vegetative slips [58].
1.4. Limitations and Challenges for Mutagenesis
As the other plant breeding methods, mutation breeding is also having some limitations. Among them, the most important is non-availability of sequence information of these medicinal plants like other cereals and pulses. These plants also have distinct characteristics such as the phylogenetic distance from other model plants. Therefore, both forward and reverse genetics are restricted in medicinal plants due to the unavailability of genomic. The gene information of many alkaloids and other essential biochemical information are very limited for medicinal plants. Regeneration and transformation methods of several medicinal plants are rarely explored. The development of gene editing and mapping approaches has enabled the progressions in the improvement of medicinal and aromatic plants. The implication of a combination of approaches of improved genomics and breeding methods can help to overcome the above-mentioned limitations towards mutagenesis research in medicinal and aromatic plants [59]. Successful application of mutagenesis on medicinal plants can be proved as a basic model system for the application and guidelines for future planning of induced mutagenesis in several other important and orphan crops for their improvement [60].
2. CONCLUSION
Mutational breeding is considered as an important and alternative approach to plant breeders for increasing crop productivity. Creation of variation and diversity in a given crop is the biggest advantage of mutation breeding. Mutagenesis coupled with in vitro selection and plant biotechnology approaches allow plant breeders to screen for characters that were tough to obtain in breeding. Therefore, the researchers are able to break yield plateau and enhance tolerance using variability by a new combination of genes created in mutagenesis. The superior lines developed through mutation breeding can be used directly as improved lines or can be used as breeding material for developing new population for improved varieties. These newly developed crop varieties through mutation breeding can significantly contribute to global food and nutritional security. Further, several agents for mutagenesis (target or random) are proven as revolutionary tools for plant breeding. The contribution of mutation breeding in medicinal and aromatic crops is less than the other cereals and pulse crops due to unavailability of genomic information and phylogenetic relationship with model plants. Therefore, there is huge scope for improvement in alkaloid contents, oil yield, and some other biochemical properties of medicinal and aromatic crops through mutation breeding. Considering these aspects, it can be said that mutagenesis certainly has a great impact on crop genetics and breeding in past, present, and future.
3. ACKNOWLEDGEMENTS
The authors are thankful for DBS, global, Dehradun for providing internal project funding and support for research work and publication.
4. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
5. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
6. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
REFERENCES
1 Muller HJ. Artificial transmutation of the gene. Science 1928;66:84–7. CrossRef
2. Stadler LJ. Mutations in barley induced by X-rays and radium. Science 1928;68:186–7. CrossRef
3. Stadler LJ. Genetic effects of X-rays in maize. Proc Natl Acad Sci U S A. 1928;14:69–75. CrossRef
4. Pathirana R. Plant mutation breeding in agriculture. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 2011;6(032):1–20. CrossRef
5. Beyaz R, Yildiz M. The use of gamma irradiation in plant mutation breeding, plant engineering, Snježana Jurić, IntechOpen, 2017; doi:10.5772/intechopen.69974 CrossRef
6. The Joint FAO/IAEA mutant variety database. Available via https://mvd.iaea.org/#!Home (Accessed 20 Januarey 2021).
7. Goyal S, Khan S. Induced mutagenesis in Urd bean Vigna mungo (L.) Hepper]: a review. Int J Bot 2010;6:194–206. CrossRef
8. Singh, DP, Sharma SP, Lal M, Ranwah BR, Sharma V. Induction of genetic variability for polygentraits through physical and chemical mutagens in cowpea (Vigna unguiculata). Legume Res 2013;36:10–4.
9. Sharma AK, Sharma R. Chemical mutagens and their mode of action. Crop improvement and mutation breeding, Scientific Publishers, Delhi, India, 2014.
10. Xiong JS, Ding J, Li Y. Genome-editing technologies and their potential application in horticultural crop breeding. Hortic Res 2015:2:15019. CrossRef
11. Parry MAJ, Madgwick PJ, Bayon C, Tearall K, Lopez AH, Baudo M, et al. Mutation discovery for crop improvement. J Exp Bot 2009;60:2817–25. CrossRef
12. Sikora P, Chawade A, Larsson M, Olsson J, Olsson O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int J Plant Genome 2011;2011:1–13. CrossRef
13. Kumar S, Katna G, Sharma N. Mutation breeding in chickpea. Adv Plants Agric Res 2019;9:355‒62.
14. Wei FJ, Droc G, Guiderdoni E, Hsing YC. International consortium of rice mutagenesis: resources and beyond. Rice 2013;6:39. CrossRef
15. Oladosu Y, Rafii MY, Abdullah N, Hussin G, Ramli A, Rahim HR, et al. Principle and application of plant mutagenesis in crop improvement: a review. Biotech Biotechnol Equip 2016;30:1–16. CrossRef
16. Serrat X, Esteban R, Guibourt N, Moysset L, Nogués S, Lalanne E. EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods 2014;10:5. CrossRef
17. Kim SH, Kim SW, Ryu J, Kang SY, Kang BC, Kim JB. Dark/light treatments followed by γ-irradiation increase the frequency of leaf-color mutants in Cymbidium. Plants (Basel) 2020;9(4):E532. CrossRef
18. Viana VE, Pegoraro C, Busanello C, Costa de Oliveira A. Mutagenesis in rice: the basis for breeding a new super plant. Front Plant Sci 2019;10:1326. CrossRef
19. Fahad AQ, Khan S. Mutagen effect of sodium azide and its application in crop improvement. World Appl Sci J 2001;6:1589–601.
20. Jankowicz-Cieslak J, Mba C, Till BJ. Mutagenesis for crop breeding and functional genomics. In: Jankowicz-Cieslak J, Tai T, Kumlehn J, Till B (eds.). Biotechnologies for plant mutation breeding. Springer, Cham, Switzerland, pp 3–18, 2017; doi:10.1007/978-3-319-45021-6_1 CrossRef
21. Maluszynski M, Ahloowalia BS, Sigurbjornsson B. Application of in vivo and in vitro mutation techniques for crop improvement. Euphytica 1995;85:303–15. CrossRef
22. Kolakar SS, Nadukeri S, Jakkeral SA, Lakshmana D, Hanumanthappa M, Gangaprasad S. Role of mutation breeding in improvement of medicinal and aromatic crops: review. J Pharmacogn Phytochem 2018;SP3:425–9.
23. Farooqi AA, Sreeramu BS, Cultivation of medicinal and aromatic crops. Universities press (India) Ltd, Hyderabad, India, pp 145–51, 2001.
24. Datta SK. Mutation breeding for crop improvement: a review. Role of classical mutation breeding in crop improvement. Daya Publishing House, Delhi, India, pp 20–35, 2005.
25. Rang SK, Datta AK. Mutation in seed-coat colour in black cumin (Nigella sativa L.). Indian J Genet 2001;61:80–1.
26. Parashar M, Jakhar ML, Malik CP. A review on biotechnology, genetic diversity in Cumin (Cuminum cyminum). Life Sci 2014;4:L17–34.
27. Kulkarni RN, Baskaran S. Inheritance of resistance to Pythium dieback in the medicinal plant, periwinkle. Plant Breed 2003;122:184–7. CrossRef
28. Kumar S, Rai SP, Kumar SR, Singh D, Srivastava S, Mishra RK. Plant variety of Catharanthus roseus named ‘lli’. United States Patent, p 18315, 2007.
29. Baskaran K, Srinivas KVNS, Kulkarni RN. Two induced macro-mutants of periwinkle with enhanced contents of leaf and root alkaloids and their inheritance. Ind Crops Prod 2013;43:701–3. CrossRef
30. Verma AK, Singh RR. Induced dwarf mutant in Catharanthus roseus with enhanced antibacterial activity. Indian J Pharm Sci 2010;72:655–7. CrossRef
31. Basu SK, Surya N, Acharya Thomas JE. Genetic improvement of fenugreek (Trigonella foenum-graecum L.) through EMS induced mutation breeding for higher seed yield under western Canada prairie conditions. Euphytica 2008;160:249–58. CrossRef
32. Floria F, Ichim MC. Valuable fenugreek (Trigonella foenum-graecum L.) mutants induced by gamma rays and alkylating agents. Plant Mutat Rep 2006;1:30–1.
33. Sharma JR, Lal RK, Gupta AP, Misra HO, Pant V, Singh NK, et al. Development of non-narcotic (opiumless and alkaloid-free) opium poppy, Papaver somniferum. J Plant Breed 1999;118:449–52. CrossRef
34. Floria F, Ichim MC. High yielding opium poppy (Papaver somniferum L.) mutant lines. Plant Mutat Rep 2006;1:28.
35. Floria F. Induced red purple mutants (RP-R) in Datura innoxia Mill. By Ethyl Methanesulphonate. Plant Mutat Rep 2006;1(2):29.
36. Srinivasappa KN, Kathiresan C, Farooqi A, Vasundhara A, Nutan M, Raju B. Crop improvement studies in Coleus forskohlii Briq. through mutation breeding. Biomed 2010;5:90–7.
37. Dasar GV, Manjunatha GO, Gangadharappa PM, Sarangamath PA. Effect of different levels of NPK on solasodine content of Solanum viarum in paddy fallows. Karnataka J Hortic 2005;1(2):49–54.
38. Krishnan R, Nanda Kumar D, Subhas Chander M. Recombinant lines for less-spininess in steroid-bearing Solanum viarum using induced mutants as parents. IAEA, Vienna, Austria, 1988.
39. Singh SP, Khanna KR, Shukla S. Breeding of Solanum viarum: current status as steroid bearing plant. J Med Aromat Plant Sci 1998;20(2):423–31.
40. Bhagat NR. Hardas MW. Studies in induced and natural polygenic variation in Plantago ovato L. Forsk. Indian Drugs 1980;8:376–80.
41. Sareen S, Kaul V, Kaul AK. Resource allocation in induced variants of [Plantago ovata (L.) Forsk.]. Crop Improv 1999;26:38–45.
42. Lal RK. Sharma JR. Induction of gamma irradiation (60Co), characterization and utilization of mutants for economic traits in isabgol (Plantago ovata), J Med Aromat Plant Sci 2002;24:689–94.
43. Jain DK, Nagar RK. Induced variability by gamma irradiation in Isabgol (Plantago ovata Forsk). Int J Agric Sci Res 2018;8:73–8. CrossRef
44. Lal RK, Khanuja SPS. Induced genetic variability and their exploitation in chamomile (Chamomilla recutita [L.] rauschert). Acta Hortic 2007;749:103–9. CrossRef
45. Lal RK, Chanotiya CS, Singh VR, Dhawan SS, Gupta P, Shukla S, Mishra A. Induced polygenic variations through γ-rays irradiation and selection of novel genotype in chamomile (Chamomilla recutita [L.] Rauschert). Int J Radiat Biol 2019;95:1242–50. CrossRef
46. Murray MJ, Todd WA. Registration of Todd’s Mitcham peppermint. Crop Sci 1972;12:128. CrossRef
47. Mohamed WW, El-Shim I. The effect of gamma rays on the pharmaceutical products of essential oils for peppermint (Mentha piperita L.). Int J Farm Allied Sci 2014;3:884–94.
48. Prabhukumar K, Thomas V, Sabu M, Prasanth A, Mohanan K. Induced mutation in ornamental gingers (Zingiberaceae) using chemical mutagens viz. Colchicine, acridine and ethylmethanesulphonate. J Hortic 2015;19:18–27.
49. Jayachandran BK. Induced mutations in ginger. Ph.D. Thesis submitted to Kerala Agricultural University, Trissur, India, 1989.
50. Natarajan ST. Studies on the yield components and gamma ray induced variability in turmeric (Curcuma longa L.). M.Sc. (Ag.) Thesis submitted to faculty of horticulture, TNAU, Coimbatore, India, 1975.
51. Usha NDH. Induction of mutagenesis in turmeric (Curcuma longa L.) through gamma rays for variability and quality improvement. Ph.D. Thesis submitted to Tamil Nadu Agricultural University, Coimbatore, India, 2006.
52. Choudhary DK, Kaul BL. Radiation induced methyl-eugenol deficient mutant of Cymbopogon flexuosus (Nees ex Steud) Wats. Proc Indian Acad Sci 1979;88:225–8.
53. Jayachandran BK, Mohankumaran N. Effects of gamma ray irradiation in ginger. South Indian Hortic 1992;40:280–8.
54. Srivastava HK, Tyagi BR. Effects of seed irradiation on yield and quality of essential oil in palmarosa (Cymbopogon martini Stapf.). Euphytica 1986;35:369–80. CrossRef
55. Maluszynski M, Nichterlein K, Van Zanten L, Ahloowalia BS. Officially released mutant varieties – the FAO/IAEA. Mutat Breed Rev 2000;12:84.
56. Rima K, Agrawal SB, Abhijit S. Evaluation of changes in oil cells and composition of essential oil in lemongrass [Cymbopogon citratus (D.C.) Stapf.] due to supplemental ultraviolet-B irradiation. Curr Sci, 2009; 97:1137–42.
57. Herman S, Mahir AM, Mohamad O, Bakhendri S, Ramadan G, Sobri J. Dosimetry study using gamma rays on lemongrass. In: Abdul Latif AZ, Nor Raizan, AH (eds.). Abstract of the national conference on agrobiodiversity conservation and sustainable utilization-saving lives by saving agrobiodiversity. Kuching, Malaysia, 2006.
58. Kak SN, Bhan MK, Rekha K. Development of improved clones of Jamrosa [Cymbopogon nardus (L.) Rendle var. Confertiflorus (Steud.) Bor. x C. jwarancusa (Jones) Schult.] through induced mutations. J Essent Oil Res 2000;12:108–10. CrossRef
59. Chaudhary J, Alisha A, Bhatt V, Chandanshive S, Kumar N, Mir ZA, et al. Mutation breeding in tomato: advances, applicability and challenges. Plants (Basel) 2019;8(5):128. CrossRef
60. Datta SK. Induced mutations: technological advancement for development of new ornamental varieties. Nucleus 2020;63:119–20. CrossRef