1. INTRODUCTION
Aquaculture, commonly known as aqua-farming, is regarded as the “agriculture of the oceans.” It is the cultivation (growing, rearing, breeding, and maintenance) and harvesting of algae, aquatic plants, fish, crustaceans, mollusks, and other organisms in varied aquatic environments that include ponds, lakes, rivers, and estuaries [1,2]. Aquaculture has significant socioeconomic value [3-5]. The FAO reports that in 2018, the total export value of fish traded internationally was US $ 164 billion [6]. India ranks second in aquaculture and third in fisheries in the world. The fishing industry employs millions of people and is a significant contributor to the country’s food security [7-9]. Fish and fish products contribute significantly to our diet as they are crucial sources of high-quality proteins and essential amino acids. They also consist of polyunsaturated fatty acids and micronutrients, such as vitamins and minerals [10].
Freshwater aquaculture involves the breeding and raising of aquatic organisms which include fishes, prawns, shellfish, and crabs and aquatic plants. They are reared in rivers, reservoirs, ponds, lakes, and other inland waterways – which include brackish water for economic purposes. Freshwater aquaculture plays a pivotal role in the aquaculture industry [11]. India’s fish production comprises nearly 66% (two-thirds) of the country’s total fish production from both culture and capture sources, with an estimated total fish production of 6.24 million metric tons in 2018. In the fish farming sector, freshwater aquaculture plays a key role and is a major contributor, as marine finfish culture is rarely practiced on a large scale. About 12.8% of the total animal protein consumed in India, comes from freshwater fishes [12] [Figure 1].
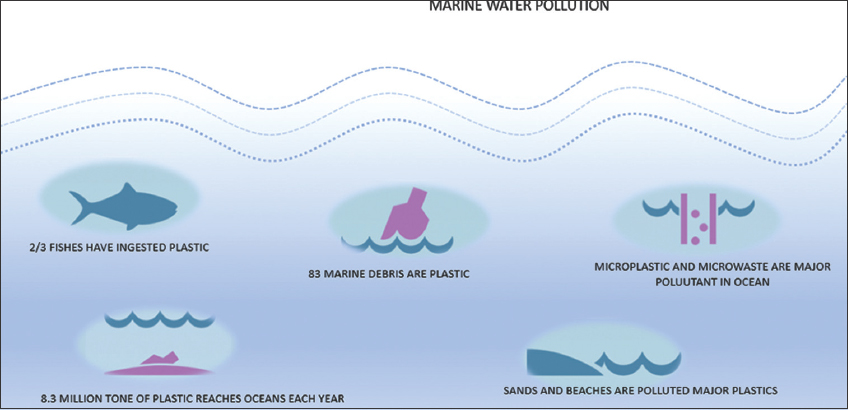 | Figure 1: Major pollutants of marine and ocean water. [Click here to view] |
Our planet is limping toward an impending environmental catastrophe caused by our addiction to single-use or disposable plastic. If this trend continues, by 2050, 20% of the world’s total oil consumption will be accounted for by the plastic industry [13]. The great Pacific patch which was discovered in 1997, by Captain Charles Moore, was the beginning of what Captain Moore quoted as “The Ocean is downhill from everywhere – the principal repository for vagrant plastic waste [14].” Of all the plastic waste generated, the majority (79%) is dumped into landfills, while 12% is incinerated and a measly 9% is recycled. A recent global study found that the most common plastic waste consists of cigarette butts (filters contain tiny plastic fibers), beverage containers, bottle caps, grocery bags, food wrappers, straws, stirrers, etc. If these alarming trends persist, our oceans are estimated to contain more plastic waste and microplastic particles than fish and other aquatic organisms by 2050. Developing efficient and cost-effective initiatives to counter the widespread damage caused by plastic waste to the environment are the need of the hour [15,16].
Although plastic debris has many shapes and sizes, those <5 mm long (the same approximate size as a sesame seed) are regarded as “Microplastics.” Microplastics are the most prevalent plastic debris found in our marine environment (oceans, rivers, and lakes, including the Great Lakes). Certain microplastic particles are derived from primary sources, that is, they are deliberately produced as microplastics. Other varieties emanate from secondary sources; they are the byproduct of weathering and fragmentation of bigger plastic objects [17-19]. As of 2018 [20], plastics constitute 12.20 % of municipal solid waste of the world. India is one among the few countries that have policies to minimize microplastic waste, although currently the major bans focus on larger plastics [21].
Microplastic research has been more focused on the oceans and seas. Studies relating to freshwater microplastics are <4% [22]. The distribution of microplastics is highly heterogeneous, and its abundance in freshwater is comparable to that of the marine environment as well [23,24]. Wastewater treatment plants (WWTPs) are one of the prominent and primary underlying sources of microplastics, while terrestrial sources contribute to the rest [25-30]. WWTPs are capable enough to remove up to 95% of microplastics [31] and tertiary treatment has a removal efficiency of up to 90% of finer particles of size larger than 10 mm [32]. In spite of this, a considerable amount of microplastics are released into the natural water system through WWTPs.
Primary and secondary microplastics are widespread in sediment, water, and biota of both marine and coastal environments. Microplastic fragments are analogous to the size of feed elements and closely resemble the appearance of phytoplankton, zooplanktons, and suspended particles [33]. This leads to the ingestion of synthetic microparticles by invertebrates. The suspension feeders and benthic organisms feed on the indistinguishable microplastics from the contaminated water and bottom sediments, mistaking them to be food particles. Organisms seen to ingest microplastic fibers and fragments include barnacles, lugworms, amphipods, and tiny organisms such as filter-feeding zooplankton which are placed at the bottom of the food chain [34-36]. More than 1401 marine species come in contact with marine plastic debris in numerous ways [37] [Figure 2].
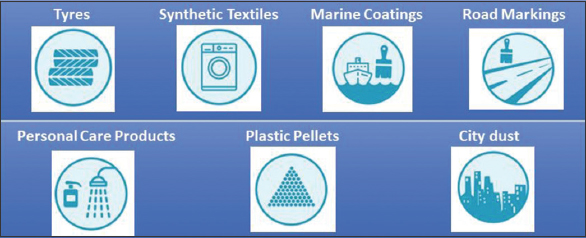 | Figure 2: Primary source of microplastics. [Click here to view] |
Besides the physical risk from plastic litter in the marine environment, there is also the risk of marine organisms ingesting the chemicals that are not only in the plastics but also on the surface water. The plastic particles in the ocean have been found to have the ability to attract organic non-dissolving chemicals that contain known toxic substances. They also have the tendency to absorb Plaster of Paris and heavy metals [38,39]. This has paved the way for increasing studies, exploring plastics’ actions concerning marine organisms and toxic chemicals. This is a result of rapid urbanization and encroaching residential complexes. The biodiversity of a region is highly impacted by its water resources. The lakes that exist now are heavily polluted with plastic debris and also with industrial and urban run-offs. This has an adverse effect on the aquatic fauna as they are subjected to toxic exposure and end up consuming microplastics due to fragmentation [Figure 3]. This review aims to shed light on the impact of plastic and microplastic pollution in the entire marine and freshwater environment, with particular attention to aquatic fauna. The central question is to ultimately understand how this plastic debris would travel up along the food chain and eventually end up on our plates. The World Environmental Day theme of 2021 was on ecosystem restoration, “Reimagine. Recreate. Restore.” which is also much relevant to the present review. Microplastic pollution and studies on how to curb it is the need of the hour.
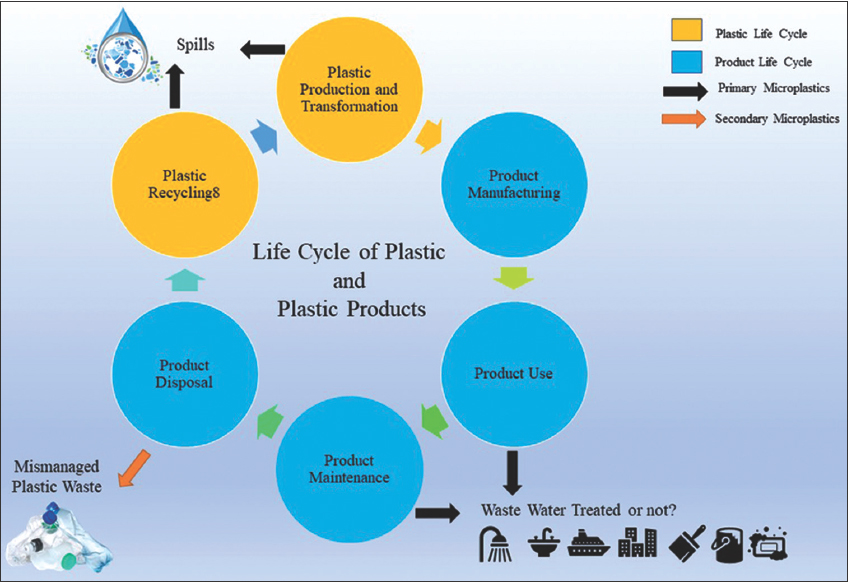 | Figure 3: Lifecycle of plastic and plastic products. [Click here to view] |
2. MICROPLASTICS IN FRESHWATER SOURCES
The United Nations has declared plastic pollution as one among the critically emerging environmental issues of our times [40]. The major share (nearly 80%) of plastic waste in the ocean is carried there by rivers [41,42]. Only 13% of studies focus on plastic pollution in the freshwater environment [43]. In a study conducted at 4 lakes of the Great Lakes system, plastic particles were documented down to the smallest size ever reported of 106–333 mm [44]. A study conducted at Vembanad lake which is a Ramsar site in India, on microplastic pollution in the sediments, revealed a mean abundance of 252.80 ± 25.76 m-2 (96–496 particles m-2) of MPs from all 10 sample sites studied [45]. A research work by Julien et al., to determine the microplastic stocks and fluxes in the Lake Geneva Basin, taking into account two very distinctive approaches: (a) A top-down approach which fixates on modeling plastic fluxes based on the socioeconomic activities and (b) a bottom-up approach which would utilize field measurements to estimate the plastic fluctuations. The release evaluations based on top-down modeling reported a mean value of 49 tons/year, and the bottom-up approach reported a mean value of 59 tons/year of MPs being released into Lake Geneva with an average of 55 tons of microplastics being released Lake Geneva every year [46]. A study conducted at the Mvudi River, in Limpopo province of South Africa focused on the river ecosystems, which are a key component of the global water cycle. The samples were collected from an intermittent stream in the Mvudi River during three seasons, at five different sites. The various study periods were cool-dry season (June 2019), hot-dry season (September 2019), and hot-wet season (February 2020). In this study, a total of 2406 microplastic particles were collected. The particles collected varied with the seasons, with 199 items collected during cool-dry season, 217 items during hot-dry season, and 1990 items collected during the hot-wet season. Fibers and microbeads (white color in abundance) majorly constituted the samples. The mean MPs density was highest during the hot-wet season and lowest during the cool-dry season. Due to the high prevalence of raw sewage in the entire river, the effect of wastewater and sewage was not evident [47].
3. MICROPLASTICS IN CRUSTACEANS AND ZOOPLANKTONS
Microplastics are constantly taken up all aquatic organisms and easily travel up the food chain. In a study led by Ping Yu, on Eriocheir sinensis (Chinese Mitten Crab) exposed to microplastics, the activities of glutathione, glutamic-oxaloacetic transaminase, glutathione peroxidase, and superoxide dismutase (SOD) increased in the specimens exposed to low concentrations of polystyrene microplastics (40 and 400 mg/L) when compared to control. In organisms exposed to higher concentrations of 4000 and 40,000 mg/L microplastics, the levels decreased accordingly [48]. Another research done on the freshwater shrimp Neocaridina palmata to study the retention time and egestion of microplastics revealed that the shrimp had ingested fluorescent polyethylene beads, polystyrene beads, and fluorescent polyvinyl chloride fragments in a concentration-dependent manner. Mostly ingestion of smaller beads 38–45 mm was detected. The shrimp had egested 59% of beads and 18% of fragments within a span of 4 h [49].
Even though most zooplankton evacuates microparticles in a matter of hours, some have been discovered to have retained the undigested particles for as long as 7 days [34]. The nutritional intake and reproductive output of zooplankton were found to have decreased significantly due to their ingestion of polystyrene particles. The zooplankton can usually survive on up to 40% less real food [50,51]. Besides receiving no energy from non-nutritional microplastics, zooplankton and other organisms deal with food shortages. They are also unable to instinctively reduce their metabolic rate during periods of starvation, which directly results from a diet of microplastic beads [50].
The ingestion of synthetic microbeads by zooplankton limits its feeding, growth, and survival by causing heavy hindrance in the digestive tract [35,51,52]. A work by Castro focused on the effect of polyethylene microplastics of the size 40–48 mm on Daphnia magna newborns. The mobility of organisms was not altered due to exposure, but D. magna promptly ingested polyethylene MPs in the first 24 h of exposure. The microplastics did not have any influence on the molting process, but the availability of feed did intervene with the number of molts produced [53]. A study focusing on the effects of food supply and temperature in the ingestion of microplastics by two model organisms Daphnia magna and Daphnia pulex was conducted by Hoffschröer. An increase in temperature increased the MPs uptake in the organisms. In response to elevated temperatures, low oxygen availability, and low food supply, the complex regulation pattern of Daphnia’s filtration current increased the frequency of leg movement, which led to increased ingestion of MPs [54].
4. MICROPLASTIC STUDIES IN MOLLUSKS AND AMPHIBIANS
Pastorino and his team investigated microplastic pollution in Lake Iseo using the Zebra mussel as a bioindicator. It was found that the zebra mussels retained particles >149 mm. The retention efficiency of MPs by mussels was 6% for 0.5 mm microspheres and 29% for 1.0 μm microspheres, respectively. It was higher, 87–100% for all larger microspheres. The most prominent color of MPs in samples was blue (60%), and the most prevalent chemical composition of MPs in samples was 45% of polyethylene terephthalate [55]. A study was conducted using Anodontites trapesialis – a freshwater mussel, as a potential sentinel in determining freshwater microplastic pollution in the South American Pantanal region. The MPs accumulated more in the gills (78%) than the gut (72%) of the bivalves. The average MPs size found in bivalves was around 17–88 μm. The organisms eliminated from 0 to 3300 particles during the study [56]. Research work was conducted by Weber and his team to study the effect of microplastics and thermal stress on Dreissena polymorpha, a freshwater mussel. Throughout the study, no significant interaction was observed between polystyrene microplastics and thermal stressors. However, thermal stress had a greater impact on mussels, which reveals their highly sensitive stress to temperature. In this study, dreissenid mussels did not react much to MPs in a controlled environment, but that might not be the case in an outside environment that is more polluted due to anthropogenic activity [57].
A study by Kolenda was conducted in Southwest Poland from May to June in 2017 and 2018 to analyze microplastic ingestion in tadpoles. A total of 201 tadpoles were collected from eight ponds. The tadpoles belonged to belonging to five different species. The particles were fibers majorly (69 items – 97%) followed by fragments (2 items – 3%). The mean length of fibers reached 2.2 μm. Diverse colors of MPs were seen. Spectroscopy revealed that all MPs particles had an anthropogenic origin and included a majority of nylon (90%), followed by polyisoprene (6%), polyurethane (2%), and 1,2-polybutadiene (2%). This study confirmed that pond-breeding amphibians are exposed to MPs [58].
A study that focused on estuarine gastropods was conducted by Zaki along the Klang River estuary. Ninety-five gastropod samples were collected from 12 sampling sites. All the samples had microplastic present in them. The MPs size ranged from 0.50 to 1.75 particles/g of wet weight tissue or 0.25 to 0.88 particles/individual. The mean MPs sizes ranged from 30 to 1850 μm with a mean size of 538.30 μm. Black was the most dominant MPs color observed in the samples. Fourier-transform infrared (FTIR) spectroscopy analysis confirmed the presence of three different polymers: Polyethylene-propylene-diene (PEPDM) (48%), polyester (27%), and polyurethane (3%) [59].
5. MICROPLASTIC STUDIES ON ZEBRAFISH
A study found that polystyrene microplastics increased the activities of catalase and SOD in Danio rerio. It also revealed that treatment with microplastics induced oxidative stress [60]. A study conducted by Lili Lei on Zebrafish Danio rerio, demonstrated that ingestion of microplastic particles leads to intestinal damage. Further, histological analysis revealed cracking of villi and splitting of enterocytes [61]. Danio rerio when exposed to naturally aged polystyrene microplastics at the intermediate development stage showed a considerable increase in parameters related to oxidative stress [62]. A unique study to check the transgenerational effect of MPs on the fish, Danio rerio, was conducted by Qiang. The polystyrene microspheres accumulated in the intestines of the fish and showed critical fluctuations in mRNA expression levels; however, the offspring was unaffected, and the transgenerational effects were negligible even at high concentrations [63].
6. UPTAKE OF MICROPLASTICS BY FRESHWATER FISHES
Microplastic pollution is majorly influenced by anthropogenic activity. Studies on the influence of microplastics in aquatic organisms are constantly on the rise. Di Sun, in 2020, researched to analyze and characterize the spatial distribution of microplastics in two economically valuable fishes, Tilapia and Mud Carp. Samples were collected from 25 sites of the north and west rivers of Guangdong province. It was found that 20/25 sampling sites had microplastics prevalence. In 76 samples of fish, a total of 160 MPs were found. In the samples, both of Tilapia (80%) and Mud Carp (77.8%) contained MPs. In Tilapia, MPs of three shapes such as pellets, fragments, and fibers were present, whereas, in Mud Carp, only fragments and fibers were found. Based on color, white was highly prevalent, and fragments were the most common shape of MPs found. In the samples, more than 74% of MPs were less than 1 mm in size, which suggests that the smaller the particle size, the higher the uptake of MPs [64].
In another study on riverine systems, the microplastics content in the brown trout Salmo trutta Linnaeus, 1758, was analyzed. A total of 58 fish from six sampling sites were collected for the study. A total of 92 microplastic particles were retrieved from the fishes, which consisted of 11 different polymers. Seventy-one particles were removed from the gastrointestinal tracts (GITs) contributing to 77% of total MPs accumulation. Fibers were the most common type of MPs, and the dominant size range was 350 μm to 5 mm. The fish S. trutta is of good economic and commercial value. As confirmed by the study, the presence of MPs in them is a drawback and a negative trait for consumption [65].
The research was conducted to assess microplastic ingestion by the freshwater fish Hoplosternum littorale, which is economically and commercially important as it is heavily consumed in the semi-arid regions of South America. A total of 48 fishes were used for the analysis (40 males and eight females). Plastic debris was found inside the guts of 40 individuals, which amounted to 83% of the total sample. A total of 176 plastic particles were retrieved from the fishes, and the number of MPs particles ranged between 1 and 24 particles per fish. About 88.6% of the particles present in the fish gut were <5 mm (156 out of 176 particles). Fibers were the most prevalent type of MP recovered from the gut amounting to 46.6% in total. The study suggests that the gut content of fishes can be used as a qualitative tool to assess microplastic pollution in freshwater [66].
In an investigation carried out in the lower Xingu River Basin in the Amazon, 172 specimens of 16 Serrasalmidae species were collected. In the samples, about 80% of species analyzed, and one-quarter of sample specimens had ingested plastic particles in the size range of 1–15 mm in length [67].
A study was conducted to analyze the level of plastic ingestion by the commercially valuable, planktivorous fish Alburnus tarichi. A total of 3338 pieces of plastic were collected from the GITs of the 101 sampled fishes. The abundance of MPs ranged from 8 to 124 pieces per fish with an average of 34 ± 13 MPs/individual. Fibers constituted the samples majorly – 74%, and blue color MPS was highly prevalent (58%). Out of five samples subjected to FTIR, three were polyethylene and two were polypropylene [68].
| Title of the research | Author | Year | Major objective | Innovative ideas, methods in use, strategies, and proposed solutions | Reference |
|---|---|---|---|---|---|
| The effects of plastic pollution on aquatic wildlife: Current situations and future solutions | Sigler | 2014 | Plastic pollution management measures | 1. Lagrangian drifters to monitor the trajectory of floating marine debris. | [73] |
| Microplastics in the marine environment: Sources, consequences, and solutions | Thompson | 2015 | Photo-oxidative degradation | End of life plastics can be used as raw materials for new production. | [74] |
| Solutions to microplastic pollution – Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies | Talvitie | 2017 | Wastewater effluent treatment | 1. Membrane bioreactor for treatment of primary wastewater effluents | [31] |
| Microplastics pollution and reduction strategies | Wu | 2017 | Microbial biodegradation, and bioremediation of plastic pollutants | 1. Removing microbeads from personal care products | [75] |
| Degradation of plastics and plastic-degrading bacteria in cold marine habitats | Aneta | 2018 | Microorganism on marine water | 1. Biodegradation of plastics using marine bacteria as potential organisms | [76] |
| Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution | Prata | 2019 | Integrated measures for plastic pollution | Life cycle assessment as a tool to improve plastic production | [77] |
| A novel method enabling the accurate quantification of microplastics in the water column of deep ocean | Lie | 2019 | Novel filtration method for sampling microplastics | [78] | |
| Biodegradation and catalytic-chemical degradation strategies to mitigate microplastic pollution | Zhou | 2021 | Catalytic-chemical degradation | Biodegradation or catalytic-chemical degradation for degrading microplastics | [79] |
| Characterization and identification of microplastics using Raman spectroscopy coupled with multivariate analysis | Jin | 2022 | Spectral analysis for characterization and identification of microplastics | 1. Raman spectroscopy coupled with multivariate analysis as a robust analytical method to investigate the spectral profile of microplastics. | [80] |
A study on the Japanese medaka (Oryzias latipes) juveniles based on exposure and depuration tests of fluorescently labeled MPs: Polyethylene (sphere with 20 μm or 200 μm diameter) and polystyrene (sphere with 2 μm or 20 μm diameter) revealed that MPs accumulated majorly in the GITs of the medaka during the exposure study. In the fishes exposed to 20 μm PS- and PE-MPs, fluorescent signals of MPs were observed in the gills and head. The average bioconcentration factor for medaka at various concentrations is as follows: 200 μm PE-MPs – 74.4, 20 μm PE-MPs – 25.7, 20 μm PS-MPs – 16.8, and 2 μm PS-MPs – 139.9. The 2 μm PS-MPs had a higher uptake rate and lower depuration rate [69].
A study was conducted to determine microplastic contamination in the fish Gambusia affinis, which is an excellent freshwater environmental control agent. Microplastics <0.1 mm were prevalent in water samples, gills, and the digestive tracts of the fishes. Black color microplastics were the most dominant in the samples; water (24%), gill (43%), and digestive tract (46%). Fragments were the most abundant microplastic type. The water samples consisted of 4066.67 particles/m3, gill samples contained 1352.78 particles/gram, and 2138.89 particles/gram were present in the digestive tract. Multivariate analysis and post hoc test revealed a significant value below 0.05 (P < 0.05), indicating that the abundance of microplastics varies between organ, water samples, and locations [70].
A study was done to assess the presence of microplastics in commercially sold fishes in Kerala. Both the edible and inedible tissues of pelagic fishes were taken into account. Samples of nine pelagic fishes were collected over a period of 6 months from December 2017 to May 2018. The average abundance of MPs in the edible tissues of fishes was 0.07 ± 0.26 items/fish, and in inedible tissue, it was 0.53 ± 0.77 items/fish. Fragments were the most common type of MPs found both in the edible (57.8%) and inedible (55.6%) tissue samples followed by fibers and sheets. Majority of MPs segregated were white in color. The microplastics were largely in the size range of 200–400 mm, and polyethylene was the predominant polymer type [71].
7. CONCLUSION
More studies on microplastic pollution in freshwater resources are required. Studies on the negative impact of consuming fishes reared in a microplastic polluted environment are the need of the hour. Microplastics are abundant in the environment, and it is high time we realize the complexity of the issue. It is vital to evaluate the ways through which microplastics gain entry into the freshwater systems. In a review by Akdogan and Guven, more than 200 papers published between 2006 and 2018, were analyzed and they concluded stating “whilst marine microplastics have received substantial scientific research, the extent of microplastic pollution in continental environments, such as rivers, lakes, soil and air, and environmental interactions, remains poorly understood [72].” It is also pertinent to analyze the various modes in which aquatic organisms take up microplastics. We are now aware of the fact of microplastics presence in human blood. Studying model organisms and checking ways through which MPs travel along the food chain will help us better understand the current status of microplastic pollution, and come up with sustainable solutions.
8. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
9. FUNDING
The current study received no funding.
10. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
11. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
12. DATA AVAILABILITY
No data has been available for the present review.
13. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Leslie HA, Van Velzen MJ, Brandsma SH, Vethaak D, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int 2022;163:107199. [CrossRef]
2. National Oceanic and Atmospheric Administration (NOAA), U.S. Department of Commerce, What is Aquaculture?Washington, DC:National Oceanic and Atmospheric Administration. Available from:https://www.oceanservice.noaa.gov/facts/aquaculture.html [Last accessed on 2022 Jan 24].
3. Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, et al. A 20-year retrospective review of global aquaculture. Nature 2021;591:551-63. [CrossRef]
4. Ruff EO, Gentry RR, Lester SE. Understanding the role of socioeconomic and governance conditions in country-level marine aquaculture production. Environ Res Lett 2020;15:1040a8. [CrossRef]
5. Krause G, Billing SL, Dennis J, Grant J, Fanning L, Filgueira R, et al. Visualizing the social in aquaculture:How social dimension components illustrate the effects of aquaculture across geographic scales. Mar Policy 2020;118:103985. [CrossRef]
6. Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2018 Meeting the Sustainable Development Goals. Rome:Food and Agriculture Organization;2018.
7. Tezzo X, Bush SR, Oosterveer P, Belton B. Food system perspective on fisheries and aquaculture development in Asia. Agric Hum Values 2021;38:73-90. [CrossRef]
8. Belton B, Bush SR, Little DC. Not just for the wealthy:Rethinking farmed fish consumption in the Global South. Glob Food Secur 2018;16:85-92. [CrossRef]
9. Belton B, Thilsted SH. Fisheries in transition:Food and nutrition security implications for the global South. Glob Food Secur 2014;3:59-66. [CrossRef]
10. Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome:Food and Agriculture Organization;2020.
11. Li D, Liu S. Water quality evaluation. In:Water Quality Monitoring and Management. Ch. 4. Cambridge:Academic Press;2019. 113-59. [CrossRef]
12. Anand PE. The Fish Farming Industry of India. Portsmouth:Global Aquaculture Alliance;2019. Available from:https://www.aquaculturealliance.org/advocate/the-fish-farming-industryofindia [Last accessed on 2021 Jun 03].
13. UN Environment;2018. Available from:https://www.unenvironment.org [Last accessed on 2021 Jun 03].
14. Charles Moore. Available from:https://www.captain-charles-moore.org/about [Last accessed on 2022 Jan 13].
15. United Nations Environment. 2016. Available from:https://www.unenvironment.org/annualreport/2016 [Last accessed on 2021 Jun 03].
16. Newman S, Watkins E, Farmer A, Ten Brink P, Schweitzer JP. The economics of marine litter. In:Marine Anthropogenic Litter. Cham:Springer;2015. 367-94. [CrossRef]
17. Anderson A, Andrady A, Arthur C, Baker J, Bouwman H, Gall S, et al. Sources, fate and Effects of Microplastics in the Marine Environment:A Global Assessment. Vol. 90. GESAMP Report and Studies;2015.
18. Gall SC, Thompson RC. The impact of debris on marine life. Mar Pollut Bull 2015;92:170-9. [CrossRef]
19. Verschoor A, De Poorter L, Roex E, Bellert B. Quick scan and prioritization of microplastic sources and emissions. RIVM Lett Rep 2014;156:1-41.
20. Environmental Protection Agency. National Overview:Facts and Figures on Materials, Wastes and Recycling;2018. Available from:https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures- materials [Last accessed on 2021 Jun 06].
21. The Hindu, Feeding on Microplastics a Scourge Stalks the Sea;2018. Available from:https://www.thehindu.com/sci-tech/energy-and-environment/feeding-on microplastics-a-scourge-stalks-the sea/article 25527355.ece [Last accessed on 2021 Jun 03].
22. Lambert S, Wagner M. Microplastics are contaminants of emerging concern in freshwater environments:An overview. In:Freshwater Microplastics. Berlin:Springer;2018. 1-23. [CrossRef]
23. Peng J, Wang J, Cai L. Current understanding of microplastics in the environment:Occurrence, fate, risks, and what we should do. Integr Environ Assess Manag 2017;13:476-82. [CrossRef]
24. Klein S, Dimzon IK, Eubeler J, Knepper TP. Analysis, occurrence, and degradation of microplastics in the aqueous environment. In:Freshwater Microplastics. Cham:Springer;2018. 51-67. [CrossRef]
25. Magnusson K, Norén F. Screening of Microplastic Particles in and Down-Stream a Waste water Treatment Plant. Stockholm:Swedish Environmental Research Institute;2014.
26. Heinonen M, Talvitie J. Preliminary Study on Synthetic Microfibers and Particles at a Municipal Waste Water Treatment Plant. Helsinki:Baltic Marine Environment Protection Commission;2014.
27. Estahbanati S, Fahrenfeld NL. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016;162:277-84. [CrossRef]
28. Murphy F, Ewins C, Carbonnier F, Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol 2016;50:5800-8. [CrossRef]
29. Dyachenko A, Mitchell J, Arsem N. Extraction and identification of microplastic particles from secondary wastewater treatment plant (WWTP) effluent. Anal Methods 2017;9:1412-8. [CrossRef]
30. Mintenig SM, Int-Veen I, Löder MG, Primpke S, Gerdts G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-fourier-transform infrared imaging. Water Res 2017;108:365-72. [CrossRef]
31. Talvitie J, Mikola A, SetäläO, Heinonen M, Koistinen A. How well is microlitter purified from wastewater?A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res 2017;109:164-72. [CrossRef]
32. Wardrop D, Bott C, Criddle C, Hale R, McDevitt J, Morse, M, et al. Technical Review of Microbeads/Microplastics in the Chesapeake Bay. STAC, Edgewater;2016.
33. Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms:A review. Environ Pollut 2013;178:483-92. [CrossRef]
34. Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, et al. Lost at sea:Where is all the plastic?Science 2004;304:838. [CrossRef]
35. Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, et al. Microplastic ingestion by zooplankton. Environ Sci Technol 2013;47:6646-55. [CrossRef]
36. SetäläO, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 2014;185:77-83. [CrossRef]
37. Mendenhall E. Oceans of plastic:A research agenda to propel policy development. Mar Policy 2018;96:291-8. [CrossRef]
38. Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 2016;178:189-95. [CrossRef]
39. Kwon JH, Chang S, Hong SH, Shim WJ. Microplastics as a vector of hydrophobic contaminants:Importance of hydrophobic additives. Integr Environ Assess Manag 2017;13:494-9. [CrossRef]
40. Kershaw P. Marine Plastic Debris and Microplastics-Global Lessons and Research to Inspire Action and Guide Policy Change. Nairobi:United Nations Environment Programme;2016.
41. Schmidt C, Krauth, T, Wagner S. Export of plastic debris by rivers into the sea. Environ Sci Technol 2017;51:12246-53. [CrossRef]
42. Schwarz AE, Ligthart TN, Boukris E, Van Harmelen T. Sources, transport, and accumulation of different Types of plastic litter in aquatic environments:A review study. Mar Pollut Bull 2019;143:92-100. [CrossRef]
43. Blettler MC, Abrial E, Khan FR, Sivri N, Espinola LA. Freshwater plastic pollution:Recognizing research biases and identifying knowledge gaps. Water Res 2018;143:416-24. [CrossRef]
44. Cable RN, Beletsky D, Beletsky R, Wigginton K, Locke BW, Duhaime MB. Distribution and modeled transport of plastic pollution in the great lakes, the world's largest freshwater resource. Front Environ Sci 2017;5:45. [CrossRef]
45. Sruthy S, Ramasamy EV. Microplastic pollution in Vembanad Lake, Kerala, India:The first report of microplastics in lake and estuarine sediments in India. Environ Pollut 2017;222:315-22. [CrossRef]
46. Boucher J, Faure F, Pompini O, Plummer Z, Wieser O, De Alencastro LF. (Micro) plastic fluxes and stocks in Lake Geneva basin. TrAC Trends Anal Chem 2019;112:66-74. [CrossRef]
47. Dalu T, Banda T, Mutshekwa T, Munyai LF, Cuthbert RN. Effects of urbanisation and a wastewater treatment plant on microplastic densities along a subtropical river system. Environ Sci Pollut Res 2021;28:1-10. [CrossRef]
48. Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y. Accumulation of polystyrene microplastics in juvenile eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol 2018;200:28-36. [CrossRef]
49. Klein K, HeßS, NungeßS, Schulte-Oehlmann U, Oehlmann J. Particle shape does not affect ingestion and egestion of microplastics by the freshwater shrimp Neocaridina palmata. Environ Sci Pollut Res 2021;28:62246-54. [CrossRef]
50. Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod calanus helgolandicus. Environ Sci Technol 2015;49:1130-7. [CrossRef]
51. Lee KW, Shim WJ, Kwon OY, Kang JH. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ Sci Technol 2013;47:11278-83. [CrossRef]
52. Bergami E, Bocci E, Vannuccini ML, Monopoli M, Salvati A, Dawson KA, et al. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotoxicol Environ Saf 2016;123:18-25. [CrossRef]
53. Castro GB, Bernegossi AC, Felipe MC, Corbi JJ. Is the development of Daphnia magna neonates affected by short-term exposure to polyethylene microplastics?J Environ Sci Health A Tox Hazard Subst Eviron Eng 2020;55:935-46. [CrossRef]
54. Hoffschröer N, Grassl N, Steinmetz A, Sziegoleit L, Koch M, Zeis B. Microplastic burden in Daphnia is aggravated by elevated temperatures. Zoology (Jeana) 2021;144:125881. [CrossRef]
55. Pastorino P, Prearo M, Anselmi S, Menconi V, Bertoli M, Dondo A, et al. Use of the zebra mussel Dreissena polymorpha (Mollusca, Bivalvia) as a bioindicator of microplastics pollution in freshwater ecosystems:A case study from Lake Iseo (North Italy). Water 2021;13:434. [CrossRef]
56. Moreschi AC, Callil CT, Christo SW, Junior AL, Nardes C, De Faria É, et al. Filtration, assimilation and elimination of microplastics by freshwater bivalves. Case Stud Chem Environ Eng 2020;2:100053. [CrossRef]
57. Weber A, Jeckel N, Wagner M. Combined effects of polystyrene microplastics and thermal stress on the freshwater mussel Dreissena polymorpha. Sci Total Environ 2020;718:137253. [CrossRef]
58. Kolenda K, Ku?mierek N, Pstrowska K. Microplastic ingestion by tadpoles of pond-breeding amphibians-first results from Central Europe (SW Poland). Environ Sci Pollut Res 2020;27:33380-4. [CrossRef]
59. Zaki MR, Zaid SH, Zainuddin AH, Aris AZ. Microplastic pollution in tropical estuary gastropods:Abundance, distribution and potential sources of Klang River estuary, Malaysia. Mar Pollut Bull 2021;162:111866. [CrossRef]
60. Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, et al. Uptake and accumulation of polystyrene microplastics in Zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 2016;50:4054-60. [CrossRef]
61. Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ 2018;619:1-8. [CrossRef]
62. Guimarães AT, Charlie-Silva I, Malafaia G. Toxic effects of naturally-aged microplastics on zebrafish juveniles:A more realistic approach to plastic pollution in freshwater ecosystems. J Hazard Mat 2021;407:124833. [CrossRef]
63. Qiang L, Lo LS, Gao Y, Cheng J. Parental exposure to polystyrene microplastics at environmentally relevant concentrations has negligible transgenerational effects on Zebrafish (Danio rerio). Ecotoxicol Environ Saf 2020;206:111382. [CrossRef]
64. Sun D, Wang J, Xie S, Tang H, Zhang C, Xu G, et al. Characterization and spatial distribution of microplastics in two wild captured economic freshwater fish from north and west rivers of Guangdong province. Ecotoxicol Environ Saf 2021;207:111555. [CrossRef]
65. O'Connor JD, Murphy S, Lally HT, O'Connor I, Nash R, O'Sullivan J, et al. Microplastics in brown trout (Salmo trutta Linnaeus, 1758) from an Irish riverine system. Environ Pollut 2020;267:115572. [CrossRef]
66. Silva-Cavalcanti JS, Silva JD, de França EJ, De Araújo MC, Gusmao F. Microplastics ingestion by a common tropical freshwater fishing resource. Environ Pollut 2017;221:218-26. [CrossRef]
67. Andrade MC, Winemiller KO, Barbosa PS, Fortunati A, Chelazzi D, Cincinelli A, et al. First account of plastic pollution impacting freshwater fishes in the amazon:Ingestion of plastic debris by piranhas and other serrasalmids with diverse feeding habits. Environ Pollut 2019;244:766-73. [CrossRef]
68. Atici AA, Sepil A, Sen F. High levels of microplastic ingestion by commercial, planktivorous Alburnus tarichi in Lake Van, Turkey. Food Addit Contam Part A Anal Control Expo Risk Asses 2021;38:1767-77. [CrossRef]
69. Liu Y, Qiu X, Xu X, Takai Y, Ogawa H, Shimasaki Y, et al. Uptake and depuration kinetics of microplastics with different polymer types and particle sizes in Japanese medaka (Oryzias latipes). Ecotoxicol Environ Saf 2021;212:112007. [CrossRef]
70. Buwono NR, Risjani Y, Soegianto A. Contamination of microplastics in Brantas river, East Java, Indonesia and its distribution in gills and digestive tracts of fish Gambusia affinis. Emerg Contam 2021;7:172-8. [CrossRef]
71. Daniel DB, Ashraf PM, Thomas SN. Microplastics in the edible and inedible tissues of pelagic fishes sold for human consumption in Kerala, India. Environ Pollut 2020;266:115365. [CrossRef]
72. Akdogan Z, Guven B. Microplastics in the environment:A critical review of current understanding and identification of future research needs. Environ Pollut 2019;254:113011. [CrossRef]
73. Sigler M. The effects of plastic pollution on aquatic wildlife:Current situations and future solutions. Water Air Soil Pollut 2014;225:1-9. [CrossRef]
74. Thompson RC. Microplastics in the marine environment:Sources, Consequences and solutions. In:Marine Anthropogenic Litter. Cham:Springer;2015. 185-200. [CrossRef]
75. Wu WM, Yang J, Criddle CS. Microplastics pollution and reduction strategies. Front Environ Sci Eng 2017;11:1-4. [CrossRef]
76. Urbanek AK, Rymowicz W, Miro?czuk AM. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl Microbiol Biotechnol 2018;102:7669-78. [CrossRef]
77. Prata JC, Silva AL, Da Costa JP, Mouneyrac C, Walker TR, Duarte AC, et al. Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution. Int J Environ Res Public Health 2019;16:2411. [CrossRef]
78. Liu K, Zhang F, Song Z, Zong C, Wei N, Li D. A novel method enabling the accurate quantification of microplastics in the water column of Deep Ocean. Mar Pollut Bull 2019;146:462-5. [CrossRef]
79. Zhou D, Chen J, Wu J, Yang J, Wang H. Biodegradation and catalytic-chemical degradation strategies to mitigate microplastic pollution. Sustain Mat Technol 2021;28:e00251. [CrossRef]
80. Jin N, Song Y, Ma R, Li J, Li G, Zhang D. Characterization and identification of microplastics using Raman spectroscopy coupled with multivariate analysis. Anal Chimica Acta 2022;1197:339519. [CrossRef]