1. INTRODUCTION
Thermostable enzymes resist thermal deactivation and are very useful in industrial processes. Thermostability is substance quality to resist irreversible change in its physical or chemical structure, often by resisting polymerization or decomposition, at a high relative temperature. Archaebacterial and thermophilic eubacterial xylanases have a relatively higher half-life at 80°C than those produced from other microbial sources [1]. This thermal resistivity can also be harbor by the insertion of a disulfide bond in xylanase of Thermoanaerobacterium and these modifications protect them from thermal destruction [2,3]. The thermozymes accomplish enzymatic action at very high temperatures. Therefore, the excessive temperature gives rise to high rates of mass transfer and less viscidity aiding the insolubility of reactants and products. It also decreases the chance of contamination of microbes that prefer to grow at moderate temperatures. However, production achieved from thermophilic microbes is considerably less than mesophile microorganisms. Thus, the production can be increased by recombinant DNA technology employing a host-vector system for heterologous expression. If taking into account simply temperature, several xylanases which display optimal activity at elevated temperature were recorded from numerous microbes. These include Bacillus firmus, Streptomyces sp., and Saccharopolyspora sp. which all generate xylanase that displays activity approximately 65°C–90°C. Xylanase (E.C.3.2.1.8) hydrolyzes xylan into D-xylose by breaking β-1, 4 glycosidic linkage, in lignocellulosic, xylose is the 2nd most fermentable sugar. In general, the cell wall of plant cells incorporates the aromatic compound of lignocellulose that has 50% cellulose, 30% xylan, and 25% lignin. Xylan, sustainable hemicelluloses, is the 2nd greatest prevalent heterogeneous polysaccharide complex consisting of homopolymeric residues of 1, 4-linked β-D-xylopyranose and branches with a short-chain composed of residues of α-D-glucuronyl, α-L-arabinofuranosyl, and O-acetyl. It entails 25% timber and the rest lumber and is the main element of the plant cell wall. Hemicelluloses may function as flexible bridges in the matrix and respond as a coat among cellulose fibrils [4]. In different plants, the structural and chemical composition of xylan, as well as its accumulation, is different. Many eukaryotic microbes employ xylan as their primary source of carbon. Endo-xylanase hydrolyzes the xylan and generates several additional enzymes required to break down the replaced xylan [5].
Xylanases are found in a wide range of organisms including marine algae, fungi, yeast, crustaceans, protozoans, snails, seeds, insects, a fungus with filaments, and bacteria but not reported in mammals. Xylanase is mostly produced by the filamentous fungus, which produces more than other microorganisms but the mesophilic fungi may not have the thermal resistivity. Cellulose and xylan trigger xylanase activity, whereas quickly metabolized glycerol or glucose reduces its activity. It is likely to obtain it either by submerged fermentation (SMF) or by solid-state fermentation (SSF) methods. Enzyme production is stronger by SSF than the SMF [5]. The SSF uses typical agricultural waste such as the bran of wheat and another agricultural leftover as a substrate, whereas forced homing of enzyme on the nanoparticles to improve the activity. This has numerous usages in the pulp and paper industries.
Xylanase effectively eliminates the complex of lignin and carbohydrate which are produced throughout the kraft method and functions as a barrier to chemicals of toxic bleaching such as chlorine compounds. The chemical bleaching system creates waste products that are toxic, mutagenic, bioaccumulative, and can trigger biosystem disturbance. The government and environmental activists forbid the consumption of chlorine products for this reason. The best replacement for this may be the employment of xylanase which guarantees hardly any harm to pulp fiber tender and utilize to supply good quality along with the quickly dissolvable pulp. The significant industrial services incorporate chlorine-free dying of wood mash for making of paper. This is required to enhance the nutritive value of silage, wheat flour food additives.
2. IMPOVEMENT IN THERMOSTABILITY OF XYLANASE USING ADVANCED BIOTECHNOLOGICAL TECHNIQUES
Different techniques are applied to enhance the thermostability of xylanase. Some of the approaches are summarized in Figure 1.
 | Figure 1: Different approaches used to improve/enhance the thermostability of xylanase. [Click here to view] |
2.1. Immobilization of Enzyme
Immobilization is a key and widely employed method to enhance the half-life of enzymes and to regain after finishing of reaction as a result, the strategies are cost effective [6]. In addition, immobilization approaches notably enhance the stability of enzymes under harsh environmental conditions such as acidity, an aqueous solution containing organic solvents, high temperature, and alkalinity. Immobilization triggers the enzyme rigidification that hinders the structural destruction mainly in high temperatures. This rigidification may be enhanced by linking the chemical groups that can covalently link supporting matric [7]. Recently, researchers have developed LXy (levan-xylanase) nanohybrid and entrapped them into beads of sodium alginate. This immobilized levan-xylanase nanohybrid sustains near 80% of activity at different pH (3-10) and temperatures (20–90°C) [8]. At the same, xylanase immobilized on calcium alginate beads showed optimal temperature 50–60°C and optimal pH 8-9 [9].
2.2. Enzyme Advancement Using Nanotechnology
The progression in nanotechnology has transformed applied science. Numerous characteristics of industrial enzyme entail xylanase were improved by nanotechnology [8-10,11]. These techniques involve the enzyme adsorption on non-material which may increase the activity and thermal stability of the enzyme [12,13]. Nanoparticles show distinctive properties as supports to immobilize [8]. Grapheme oxide nanosheets were decorated with nanoparticles of superparamagnetic iron oxide (SPGO) which has been developed for xylanase immobilization, covalently. Immobilized xylanase exhibited its pH and thermal stability [14].
2.3. Protein Engineering
The activity and stability of the enzyme can be improved by protein engineering. This can be attained by changing the appropriate amino acid order or linking the particular molecule on sites of target that modified the 3D structure of the enzyme. The thermostability may be achieved by introducing the disulfide bonds, enhancing the intrahydrogen bond, and modification the surface of enzymes [15,16]. Commonly, the role of the enzyme is identified by its amino acid sequence. This is important to know about particular regions and domains accountable for enzyme activities. The knowledge aids to forecast the probable impact of modification in particular amino acids on distinct functions [17]. Commonly, three steps are indulged in the engineering of enzyme: The 1st step encompasses enzyme isolation and its structure identification. The repositories entailing whole functional and sequential details such as FRENDA, AMENDA, and BRENDA have been developed, already [18]. In the 2nd step, recorded details are screened with repositories entailing thermozymes sequence employing tools for multiple sequence alignment to detect a sequence of an amino acid (AA) which requires to be replaced and impact on enzyme function and structure [19]. Molecular modeling is accomplished to detect to require change. Numerous web-based server-like protein data bank repositories, MATARA and FATCAT, have been created to compare the structure and forecast the optimum mutation at the residue of particular amino acids [20]. In addition, tools for docking such as AutoDock, AutoDock Vina, and Glide are being employed to screen the substrate [21]. Finally, the 3rd step entails modification of enzymes employing mutagenesis (site directed) and managed evolution utilizing genetic engineering method in that chimeric gene encrypting for an altered sequence of amino acid is expressed in a host of expression [17,22]. The novel work gene editing is underway to replace the traditional enzyme engineering method for genetic alteration [23]. The important tools, namely, clustered regularly CRISPR-Cas (interspaced short palindrome repeats), TALEN (transcription activator-like effecter nucleases), and ZFNs (zinc-finger nucleases) are utilized to alteration of direct gene sequences in the genome of the host [24,25]. The successful application has been demonstrated in several studies of enzyme engineering to improve the thermostability of xylanase [19,22,26,27]. In recent years, the thermal stability of termite gut endo-β-1, 4-xylanase was improved by cysteine pairs, which created external disulfide bonds. The enzyme produced showed a triple increase in catalytic efficiency at pH 9 at 60°C [28].
2.4. Incorporating the Residues of Glutamic Acid and Proline
MSA (multiple sequence alignment) of XynAS9, as well as 6 xylanases belonging to GH10 with distinct optima temperatures (73°C–90°C) as shown in Figure 2, was achieved. Residues were established which could be linked to their thermophilic properties [29]. A frequent sequence was recognized from the consensus patterns, depending on the outcomes of the MSA [Figure 2]. In comparison to mesophilic equivalents, thermostable xylanases with a maximum temperature of 80°C or greater have a pro (P) amino acid at locations 81 and 185, a greater intensity of glutamic acid at locations 82 and 186, relating to Val (V), Asp (D), Gly, (G) and Ser (S) in XynAS9, respectively [29]. By substituting the respective residual in XynAS9 with glutamic acid and proline, the modeled structure mutant V81P/G82E/D185P/S186E [Figure 3] specified that certain mutation locations are located just at end of a long secondary structure element; that is, genetic changes V81P, as well as G82E, are situated at the inner β-strand 2 of an (α/β) 8 domain and D185P and S186E at the surface of the barrel at the fourth α-helix terminus N [29]. MDS assessment with NAMD demonstrates that such genetic changes reduced XynAS9’s conformational adaptability at a temperature of 400 K with reduced RMSD principles, implying a much more rigid form for the V81P/G82E/D185P/S186E mutant. Thus, MDS findings have confirmed the critical role of proline and glutamic acid replacements in thermostability [29].
 | Figure 2: Utilizing the ClustalW system multiple sequence alignment of xylanases belonging to GH 10 with distinct ideal temperature. TmxB: Thermotoga maritima xylanase; XYL10C, Bispora sp., endo-β-1, 4-xylanase. MEY-1: XylA, Thermobifida alba β-1,4-endoxylanase; XylE: Endo-1,4-xylanase, Penicillium canescens; XynC: Phanerochaete chrysosporium xylanase; and XynAS9, Streptomyces sp. xylanase. S9. Catalytic site with asterisks is stated. The sites for the mutation are dark red and green. [Click here to view] |
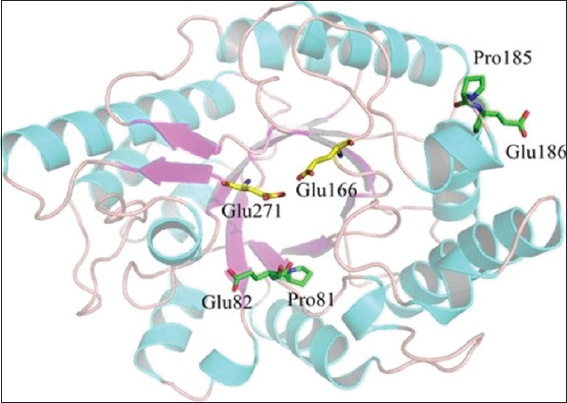 | Figure 3: 3D framework of V81P/G82E/D185P/S186E mutant model. Glu271 and Glu166 are assumed catalytic site. [Click here to view] |
Numerous researches have shown the stabilizing variables in barrel enzymes (α/β) can be ascribed to the core of hydrophobic packaging, cavity filling together with carboxy and aminoterminal loop stabilization regions, and the existence of α-helices prolines at N terminal [30]. GthC5Xyl, however, separated from Geobacillus C5 thermodenitrificans displayed timid thermostability that could be directly linked to its (α/β) catalytic barrel domain [31]. A descriptive study of the protein structure of the thermophiles and mesophile showed greater arginine content on the thermophilic protein surface. In addition, these thermophilic proteins were relatively quite robust at elevated temperatures that could be due to higher arginine content [32]. Mutagenesis technique directed at the site was used by Irfan et al. [30] for the designing of highly thermostable GthC5Xyl mutants. As per previous data, multisequence alignment temperature optima derived from GH10 xylanase varieties given the most widely accessible and direct knowledge about the properties of enzymes. Glutamic acid is seen as a major factor for enzyme thermostability that could be caused by anionic carboxylate and helix creating propensities for the development of salt bridges and bonding with hydrogen [29]. Chen et al., 2015, reported that substitution of Asp (D) with Glu (E) in Aspergillus awamori glucoamylase, elevated the thermostability. In place of Chen et al., 2015, that substitution of Asp (D) with Glu (E) in Aspergillus awamori glucoamylase elevated the thermostability [33,34].
2.5. Disulfide Bond Introduction
Xylanase is an industrial enzyme that is most essential accessible widely in industries. The usage of xylanases is, therefore, reduced by its struggling to manage with different tough circumstances, such as high temperatures and acidic or alkaline extremes [35,36]. The addition of a disulfide bond has been recorded in several analyses of GH family 11 xylanases that boosted aromatic interplay and additional intermolecular forces (IMFs) at the aminoterminus or α-helix, therefore, effectively going to improve the stability of the whole protein [37-40]. In brief, effective aminoterminus and α-helix packaging are important for the xylanases (GH 11 Family) thermostability. Everything is the same, the N-terminus and α-helix relationships to β-sheet central are yet to be elucidated. In research, double disulfide bonds were incorporated by Tang et al. to link the aminoterminus and α-helix to the core of the β-sheet. The disulfide bonds are seen as stabilizing the protein structure by lowering unfolded state entropy. Two disulfide bonds independently link the aminoterminus and α-helix bonds to the core of the β-sheet in the Xyn2 mutant. As a consequence, the Xyn2C14–52 as well as the mutants Xyn2C59–149 half-lives were strengthened at 60°C, suggesting that the aminoterminus and α-helix connection to the core of β-sheet lead to the whole protein stability. In addition, the Xyn2C14–52 and mutants Xyn2C59–149 maintain greater residual activity compared to wild ones type since incubation at 70oC for 10 min. In addition, the mutants demonstrate a significantly reduced residual activity after treatment with 10 mM DDT at 4°C for 12 h, relative to that of the residual activity untreated mutants with the same situation, and wild enzyme type reveals hardly any major difference [41].
2.6. Internal Peptide Replacement Method
XylE holds 53% large amino acid sequence ethnicity with the extremely active and hyperthermophilic XYL10C. Even so, under thermophilic environments that are frequently found in the biorefinery industry, its catalytic function is substantially lower. Once [42] substituted the segment of XylE with its equivalent of XYL10C, they generated seven hybrid enzymes which displayed xylanase activity, whereas three hybrids did not. The above indicated that the replacement of internal peptides could either generate new effective enzymes or create inert enzymes due to inappropriate folding, often with both similar equivalents. Even though the peptides M9, M6, and M3 added greatly to XylE’s catalytic efficiency, replacement with their variations had no additional effects. In addition, the substitution of main peptides or residues on a protein’s surface affects its enzymatic characteristics, such as ideal conditions, catalytic ability, and specific activity [43-45]. Structural analysis revealed that M9, M6, and M3 are all located on XylE’s surface [Figure 4], and substitution of these residues may influence the entire protein structure. Therefore, the improvement in XylE mutants’ catalytic efficiency (kcat/km) can be ascribed to the elevation in Vmax and kcat and lowering in Km. The same findings of proteases and phytases have been recorded [33,46].
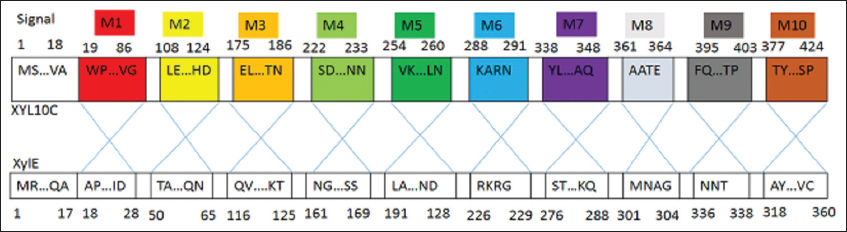 | Figure 4: Schematic description of the portion replacement. Cleavage platforms for every XylE and XYL10C fragment. [Click here to view] |
2.7. Genetic Engineering
Carbohydrate-binding modules (CBMs) are grouped into several families primarily on similarities of sequence, and the online database CAZy (http://www.cazy.org, viewed on October 10, 2021) now has 88 families [47]. Several CBMs alter enzyme thermostability in addition to contributing to substrate binding. CBM22 from Thermotoga maritima’s XynA was merged with Trichoderma reesei’s Xyn2, leading to enhanced thermostability [48]. The thermostability of Bacillus halodurans xylanase was lowered by fusing CBM22 from hyperthermophilic Thermotoga neapolitana [49]. The insertion of CBM3 to the catalytic site of Clostridium thermocellum endoglucanase CelA had no effect on its thermostability [50]. However, eliminating CBM22 from C. thermocellum XynC reduced its thermostability, but deleting CBM36 from Caldicellulosiruptor sp. F32’s Xyn11 enhanced its thermostability [51]. Eighty percent of the CBMs in the present family are somewhere around 50–100 amino acids long [47]. Larger CBMs do not improve the characteristics of chimaeras [52] and even lower the thermostability of wild enzymes [49], according to published studies.
This is because a larger CBM typically has numerous smaller serial submodules, each with its own set of functions [52,53]. Shorter CBMs or submodules have a simpler structure of proteins as well as a clearer impact on the spatial organization of wild enzymes, and they’re more common [49,53]. Meanwhile, fusing the hyperthermophilic CBM9 1–2 module generated from T. maritima GH10 xylanase A to the C-terminus of xylanase from Aspergillus niger GH11 has been shown to lower the heat stability of the Xyn-CBM9 1–2 chimera. The C2 submodule, however, can greatly increase the performance and thermostability of A. niger xylanase by subdividing hyperthermophilic CBM9 1–2 into two smaller submodules [54].
Even though many investigations on the importance of CBM have been published [49,50,54], no studies on the impact of adding CBM to the N-terminus, C-terminus, or both termini on GH11 xylanase (CDBFV), which possess all of the beneficial properties of N. patriciarum, however, lack a CBM in the wild xylanase sequence [19].
Submodule C2 from hyperthermophilic CBM9 1-2 was introduced into the N- and/or C-terminal areas of the CDBFV protein (producing C2-CDBFV, CDBFV-C2, and C2-CDBFV-C2) by genetic engineering to enhance the action and thermostability of the xylanase CDBFV from Neocallimastix patriciarum (GenBank accession no. KP691331) [Figure 5]. In E. coli BL21 (DE3), CDBFV as well as the hybrid proteins were found to be expressed. The C2 submodule had a considerable influence on improving the CDBFV’s thermostability, according to enzymatic property study. The half-lives of the three chimaeras C2-CDBFV, CDBFV-C2, and C2-CDBFV-C2 are 1.5 times (37.5 min), 4.9 times (122.2 min), and 3.8 times (93.1 min) higher than wild-type CDBFV (24.8 min) at the ideal temperature (60.0°C) [55].
 | Figure 5: Chimeras construction. (a) Chimera model diagram construction of C2-CDBFV-C2. The C2L, CDBFV-1, and LC2 genes were amplified using P1/P2, P3/P4, and P5/P6, respectively. Pink represents the C2 gene; yellow represents the linker gene; purple represents the CDBFV gene; the arrow represents the direction of amplification. (b) CDBFV-C2, C2-CDBFV, and CDBFV model diagrams construction. The CDBFV, C2-CDBFV, and CDBFV-C2 genes were amplified using P7/P8, P1/P7, and P6/P8, respectively. [Click here to view] |
2.8. Systems and Synthetic Biology
Soil, the principal habitat for numerous microbes supplying enzymes, like xylanases, is subjected to varied conditions of changing environment. The microorganisms must adjust to conditions that fluctuate, to survive. This is the microbial adjustment that results from several modifications at different levels, like at proteome genome, and transcriptome. In addition, many other control systems, such as regulation of gene expression, allosteric, and post-translational regulations, are also interested in the adjustment of microorganisms under unfavorable circumstances. System biology offers full details as for changes that occur in cells under various situations [56]. Latest events in omics methods, such as proteomics, genomics, metabolomics, and transcriptomics, have allowed complete system biology study. The immense quantity of information was produced and deposited in the freely available directories. Next-generation sequencing (NGS) allows sequencing of entire genomes of many thermophilic microbes around the globe and whole-genome sequences are contained in the datasets of the genome. NGS may also be employed for inspection of adaptive landscapes of enzymes, functional screening of single-nucleotide mutated enzymatic collections, and the essential research of enzyme behavior, under particular conditions [56]. These data can be used for determining the canonical sequences of DNA or the responsible protein sequences for the thermostability of varied enzymes from numerous microorganisms.
In turn, a descriptive gene study and patterns of amino acids which are separated from thermophiles supply useful, information about critical locations, and domains require to be interfered with to increase the stability of a given enzyme. Analyzing data need different computer simulations, such as those focused on constraints modeling and various algorithms based on bioinformatics. Data from system biology thus include preliminary details for the modular design of enzymes for the production of various enzymes which are stress tolerant [57]. In 2008, for example, MSA was used to alter histidine kinase response regulator specificity (HK-RR) of a large number of histidine kinase response regulator specificity pairs already recorded in databases for the detection of the residue of amino acids substituted by histidine kinase [58]. In addition, the approach to synthetic biology often takes an important involvement in the engineering of the enzyme toward advancement assets. Due to genetic synthesis technology’s progress, costs were decreased substantially, which makes de novo synthesis and gene assembly into greater operons [59]. Thus, employing the data preserved in datasets and analysis employing the various strategies which are based on bioinformatics, specific gene coding may be used for thermostable enzymes de novo synthesized [60].Table 1 summarizes some of the different ways to enhance xylanase thermostability.
Table 1: Review of thermostability improvement techniques and their impact on the thermostability of xylanase.
| Xylanase and source | Thermostability improvement techniques | Impact on xylanase thermostability | References |
|---|---|---|---|
| PcXylA and Penicillium canescens | Substitution of a particular amino acid | Half-life time enhanced 2–2.5-fold at 50–60°C. | [1] |
| afxynG1 and Aspergillus fumigatus RT-1 | epPCR used in directed evolution | Half-life time [t1/2] improved 3.5-fold [42 min] at 70°C versus wild form. | [22] |
| Xyl-L and Psychrobacter sp. strain 2–17 | The novel one-step combination method of direct evolution. This includes mutagenesis of saturation and focusing on epPCR | Mutant displayed a 4.3°C rise in its T5015 value of enzyme. | [27] |
| Xyn2 and Trichoderma ressei | Placing two disulfide bonds into Xyn2 To link α-helix and β-sheets to the aminoterminal Of key enzymes. | The half-life of mutants Xyn2C59–149 and Xyn2C14–52 being thermally deactivated, expanded around 1.8- and 2.5-fold at 60°C separately. | [41] |
| XynA and Thermoascus aurantiacus CBMAI 756 | Mutagenesis geared to the site | Thermal stability increased at 70–75°C. | [41] |
| Orpinomyces strain PC-2 and Endo-β-1,4-xylanase | Evolution is driven by epPCR | Half-life time [t1/2] rise to 15.3, 401, 33.2, and 209 min of mutants M4, M3, M2, and M1 mutants, at 60°C, respectively, relative to the wild type [7.92 min] | [41] |
| Thermomyces lanuginosus and Endo-β-1,4-xylanase [xynA] | epPCR | 71% of the residual activity was detected in 2B7-10 [Mutant] at 80°C for sixty min. | [41] |
| Geobacillus stearothermophilus and XT6 | Evolution directed employing epPCR | The maximum temperature went up 77 to 87°C and half-life expanded 52-fold. | [37] |
| GthC5ProXyl from Geobacillus thermodenitrificans C5 | The sequence, good in proline, was combined with xylanase polypeptide C-terminal | At pH 8, an optima higher temperature [70°C] was observed. | [30] |
| T-XynFM from Talaromyces thermophilus F1208 | N-terminal Phe1-Pro16 substituted with Ala1-Gln8 and carboxyterminal amino acid substitution of Phe193 with Ser | A 55% rise in xylanase residual activity at 50°C,12 h. | [122] |
| Metagenomic specimen of termite gut symbiont Xyn12.2 | Cysteine pair was developed, to produce Outer disulfide bonds | A 4.2-fold increase in catalytic efficiency at 50°C. | [28] |
| Bacillus subtilis and XynA | Point mutation | Expanding of specific activity and greater half-life duration, 55°C | [123] |
| Streptomyces sp. 9 and XynAS9 | SDM [Site-directed mutagenesis] | The V81P/G82E double-mutant has improved thermostable properties and heat resistance. | [123] |
| Thermomyces lanuginosus and TLX | Insertion of a Q1C – Q24C disulfide bridge into the N-terminal xylanase region. | The optima temperature was adjusted uphill from 10°C to 75°C at pH 6.5 and thermal inhibition. The temperature rose to about 10°C | [124] |
| Orpinomyces sp. PC-2 | Delete 27 residues of amino acid from the amino-terminal | Elimination of amino terminus amino acids triggers greater thermostability and activity of the enzymes. | [121] |
| XYL7novel metagenomic specimen of termite gut | Directed evolution | Mutants XYL7-TC displayed 4-fold enhancement, Capability to saccharify at 60°C in 4 h | [26] |
3. APPLICATION
Xylanase with the unique feature of the thermoalkaline-resistant property makes it more significant for applications in various industries such as paper and pulp, deinking, use of biomass, and feed and food industries [Figure 6].
 | Figure 6: Industrial applications of xylanase. [Click here to view] |
3.1. Xylanase Used in the Feed and food Sector
3.1.1. Baked goods
Xylanase tries to find utilization in food companies like bakeries. The bread is comprised entirely of wheat which is composed of hemicelluloses like arabinoxylan. Xylanase is capable of solubilizing the WU-AX (water-unextractable arabinoxylan) into WE-AX (water-extractable arabinoxylan) [61]. It leads to consistent dispersal of water and improves the development of gluten networks in the dough. The application of xylanase enhances dough’s rheological qualities such as extensibility, softness, as well as flexibility, together with a specific volume of bread and compactness of bread crumbs [61-63]. In bread, arabinoxylan degradation product, that is, xylooligosaccharides (Xos) has its health advantage [64]. Butt et al., illustrated the function of GH11 endoxylanases from B. subtilis in arabinoxylan solubilization [63]. It enhances viscidity, dough volume, and reduces the agglomeration of gluten and the compactness of the dough leading to the growth of unified, good crumbs. Xylanase of family GH11 (0.12 U/g flour) from Penicillium occitanis Pol6 assisted in improved bread-making processes, such as decreased absorption of water (8%) and increased dough rise (36.8%), thickness (17.8%), specific thickness (34.9%), and cohesiveness. Bread has enhanced sensory and rheological properties (texture, taste, color, softness, and acceptability overall). The bread prepared using xylanase was shown to have low gumminess and springiness [65]. Ghoshal et al. used slightly filtered bacterial xylanase to generate entire wheat bread with improved sensory characteristics (shiny color) [66].
Xylanase inclusion also led to an increase in specific volume and lifespan, with reduced hardness during storing, and decreased staling. Panzea, a fresh generation of xylanase acquired from Bacillus licheniformis, may aid in improving dough characteristics at reduced dosages of enzymes. It allows attaining the perfect texture, shape, volume of loaf, and structure of crumbs [67]. Equally, r-XynBS27 (recombinant xylanase) attained from Pichia pastoris (xynBS27 gene from Streptomyces sp. S27) utilized in making bread as an additive. The recombinant xylanase improved a specific volume and decreased sugar material with a reduction in firmness and rigidity [68].
3.1.2. Clearing up fruit juices
The enzymatic method is commonly used in the processing and clarifying of fruit juices. Organic fruit juices involve polysaccharides such as hemicellulose, cellulose, pectin, lignin, and starch, which reduce juice appearance, for example, cloudy color and elevated viscidity [69]. The enzyme application reduces viscidity and prevents cluster forming by employing centrifugation and filtration methods to remove the suspended and undissolved solids. This improves juice clearness, flavor, and color [69]. Xylanase from Streptomyces sp. was utilized to clarify the juices of pineapple, mosambi, and orange, with 27.9%, 23.6%, and 20.9%, clarity, respectively [70]. Immobilized xylanase derived from Bacillus pumilus VLK-1 was utilized for enrichment with orange (29%) and grape juices (26%) [70]. Xylanase immobilization on 1,3,5-triazine-functional silica-encapsulated magnetic nanoparticles was noticed to clarify the three various kinds of fruit juices at 50°C after 5 h of incubation [71]. Xylanase derived from Streptomyces sp. AOA40 was employed in the fruit juice company to enhance the transparency of orange (18.4%), apple (17.8%), and grape (17.9%) juices [72]. Immobilized xylanase triggered by glutaraldehyde was utilized to clarify tomato juice. Xylanase from P. acidilactici GC25 has been utilized for the treatment of apple, kiwi, orange, peach, grape apricot, and pomegranate, with a rise in reducing sugar and a decline in juice turbidity [73].
3.1.3. Livestock feed
Xylanases perform a significant function in livestock feed by splitting the arabinoxylan feed component and reducing the viscosity of the raw resources. Aspergillus japonicus C03 with excellent endoxylanase and stable cellulase manufacturing capability in the existence of goat ruminal environment has shown applications for ruminant feed [74]. Several kinds of research have noted the accessibility of dried soluble grain (DDGS) distillers to be used in livestock feed and the utilize of exogenous enzyme xylanase in poultry diets to control the high-fiber quality [75].
The extracellular enzymes have effectively increased the nutrient benefit of bioethanol coproducts, as recently mentioned with corn-based DDGS [76]. Xylanases have been engaged in livestock feed for 10 years, as they decrease digesta viscosity in poultry. Xylanase inclusion demonstrates an enhancement in gaining weight and increased feed conversion efficiency due to an enhancement in the digestibility of arabinoxylan in monogastric animal nutrition [77,78]. Passos et al. reported on the use of xylanase as a nutritional additive for the nutrient digestibility and digesta viscosity in young pigs fed corn intestinal morphology diets based on soybean meal [79]. ECONASE XT is a well-known synthetic endo-1,4-β-xylanase that was utilized as food supplements for chicken fattening, piglet weaning, and pig fattening [80].
3.2. Scope of Xylanase in the Pulp and Paper Industry
3.2.1. Bio-bleaching
The procedures of lignin elimination from wood pulp to generate shiny and white completed paper are considered bleaching [81]. Chemical blanching stuff (like chlorine) has traditionally been utilized for bleaching [82]. The usage of ligno-hemicellulolytic bleaching enzymes has obtained momentum worldwide. Xylanases can hydrolyze xylan which is connected to the pulp fiber cellulose and lignin. Thus, interruption of xylan will ultimately contribute to the isolation of such materials, improve fiber wall swelling, and increase the extraction of lignin from the pulp [83,84]. Thus, in a mixture with enzymes for lignin degradation, xylanase assists to boost pulp brightness [85,86]. Cellulose fiber exposures to pulping with enzyme enhance paper bonding strength and improve paper qualities through xylan deterioration and lignin abolition during treatment with enzyme [87]. The enzymatic approach to bio-bleaching has been extremely selective, non-toxic, and environmentally sustainable [88]. The manufacturing of the paper and biomass pulp occurs at differing temperatures and pH. Thermostable xylanases, however, are essential for the bio-bleaching process. An alkaliphilic Bacillus strain developed thermoactive cellulase-free xylanase utilizing elevated temperature 60°C and pH 6–10 active agro-residues and was used for kraft pulp bio-bleaching [89].
3.2.2. Ink dislodgement from waste paper
Ink dislodging from paper waste is needed for its reuse and recycling. Chemical-related processes containing compounds based on chlorine derivatives or chlorine-like ClO−, NaOH, NaCO3, H2O2, and Na2SiO2 were used to extract the ink from the waste paper. This culminated in the production of dangerous effluents and lengthy treatment needed before the environmental disposal [90]. The techniques based on enzymes using laccase and xylanase have been recommended for removing ink from effluents of the pulp and paper industries [91,92].
Virk et al., 2013 [93], investigated the ink removal performance of alkaliphilic bacterial xylanase and laccase along with mechanical deinking such as ultrasound and microwave processes for waste paper recycling [92]. The integration of laccase and xylanase enzymes displayed an expansion in luminosity (21.6%) of various old newspaper pulp (ONP), (4.1%) inkjet printer paper, (3.1%) laser-printed paper, (8.3%) pulp of magazine, and xerox paper pulp of xerox (1.9%) only. Gupta et al. stated that the stimulatory activity of laccase and xylanase enzyme (coculture of B. halodurans FNP135 and Bacillus sp.) culminated in the enhancement of mechanical characteristics such as freeness, burst factor, breaking length, and tear factor by 17.8%, 2.77%, 34.8%, and 2.4% of the old newspaper, respectively [94]. The newspaper’s appearance has also enhanced with an 11.8% reflectivity increase and a 39% whiteness increase. The appropriate treatment of commercial Bacillus halodurans TSEV1 xylanase and cellulase for deinking was evaluated at 1.2 U/mg (each enzyme) [95]. The xylanase and cellulase complex acquired from E. coli SD5 aided the lowering of kappa numbers and hexenuronic acid (Hex A), increased brightness (10%), and recycled paper tear strength [96].
3.3. Xylanase used in the Textile Industry
The processing of textiles is sometimes wide split into scouring, bleaching, and desizing. Desizing includes extracting the sticky substance from plant fiber and scouring for eliminating desized fiber through an inhibitory substance [97].
The traditional approach utilized to desize and scouring includes applying extreme temperatures within the alkaline system, underneath the impact of oxidizing factors. This approach is indeed not chemical intensive and quite unspecific, hampering the valuable cellulosic fraction that compromises the ultimate textile fiber stiffness. Hence, usage of the extremely thermoalkali stabilized xylanolytic cellulase-free enzyme is utilized effectively to desize and scouring [67,97]. Dhiman et al. illustrated the usage of Bacillus stearothermophilus SDX alkalothermophilic xylanase to process the micropyle and cotton fabric. The bioscouring and desizing treatments were conducted at 70°C, pH 9.5, using 5 g/L of xylanase for 90 min. It culminated in losing weight in micropyle for 0.91% and cotton for 0.83% with just an aggregate whiteness index of 11.81% for cotton and micropyle for 52.15% [97]. Compared to regulation, the refined fabric has improved the tensile strength (1.1–1.2%) and tearness value (1.6–2.4%). The collective action of the enzyme pectinase and xylanase was employed to scour the cotton fabrics. Bioscouring was undertaken with 5.0 IU xylanase and 4.0 IU pectinase from Bacillus pumilus strain AJK (MTCC 10414) along with surfactants such as 1.0 mM EDTA and 1% Tween-80 at high pH 8.5 for 1 h at 50°C. It was found the progress in whiteness, clarity, and yellowness by 1.2%, 3.2%, and 4.2%, respectively, which is stronger than chemical-based alkaline scouring [98]. Abd El et al. confirmed an increase in the performance of bio-finishing desizing, and bio-scouring utilizing xylanase adapted from T. longibrachiatum KT693225 hardly any additive requisites [99].
3.4. Xylanase Used in the Chemical and Medicinal Sectors
The indigestible sugars together establish oligomers identified as xylooligosaccharides (XOS) composed of monomer units of xylose [100]. XOS has diverse uses in the biotechnology, food industry [101]. As a prebiotic, XOS plays a critical role because it is not hydrolyzed or ingested in the gastrointestinal system. Thus, XOS preferentially promotes the development of essential gut health controlling gastrointestinal microorganisms [100]. XOS’s capacity as an effective feed substitute is developed by helping to minimize cholesterol, prevent starch retrogradation, and enhance Ca2+ bioavailability, thus enhancing the sensory and nutritional characteristics of foods [35]. Due to its immunomodulatory, anti-cancer, antimicrobial, antioxidant, anti-allergic, anti-inflammatory, and anti-hyperlipidemic activity, XOS has demonstrated application throughout the pharmaceutical industries [25,102-105]. XOS has also demonstrated herbal medicinal and feed uses, such as fisheries and chicken development regulatory activities. The existence of uronic substituents in acidic oligosaccharides may be responsible for these effects. The XOS production method includes the autohydrolysis, water-autoionizing hydronium ions, and in situ organic acids or enzymatic hydrolysis such as xylanase or β-xylosidase of agricultural residue abundant in hemicellulose [104]. A few reports indicated that XOS could be produced enzymatically from various agro-residues such as wood fibers, straws, corn cobs, bran, sugarcane bagasse, and bamboo utilizing bacterial xylanases [106-111]. Bacillus mojavensis A21 alkaline xylanase used corncob xylan to release xylotriose and xylobiose [112]. Bacillus aerophilus KGJ2 xylanase demonstrated efficacy for XOS development, such as xylobiose, xylotriose, and xylose after xylan hydrolysis [113]. Pichia stipitis xylanase hydrolysis generated 2% XOS consists mainly of 14% xylotetraose, 49% xylotriose, and 29% xylobiose [114]. Bhardwaj et al. showed that the slightly purified xylanase extracted from Aspergillus oryzae LC1 resulted in xylotriose, xylobiose, and xylotetraose formation [115].
3.5. Xylanase used in Bio-refineries
Effective transformation of lignocelluloses biomass (LCB) into ethanol (fuel grade) has set off global priority to generate environmentally sustainable renewable energies at a fair price for the transport sector. The method of biotransformation combined with cellulolytic enzyme, the xylanolytic enzyme plays a significant act in the processing of hydrolysis. Many studies recommend that xylanase acquired from many bacteria plays an important function in the saccharification of lignocellulosic biomass for the biorefinery relying on lignocellulose [116]. Fermentation and hydrolysis are critical steps toward the development of bioethanol from biomass of lignocellulose necessitate pretreated biomass hydrolysis to transform complicated LCB carbohydrate polymer to the simplistic monomers that will be quite far transformed by fermentation into ethanol. Hu et al. indicated that xylanase induces fiber swelling through enhancing porosity which helps to improve cellulose accessibility [117]. Concurrent cofermentation and saccharification (CCFS) are implemented to ferment all hexoses (C6) derived from xylan which induces ethanol development, utilizing bacteria cocultivated with xylanase and cellulase producing strain. Centralized processing, as well as concurrent fermentation, saccharification, and delignification, involves the culture of strain for enzyme production in a bioreactor along with ethanol-generating strain. It can be a monoculture or the coculture of various microbes. This would help to reduce the process flow investment incurred for fermenter operation and the manufacturing of different enzymes and ethanol production [118]. Specific engineered microbes are utilized to develop a centralized processing system on monoculture, with lignohemicellulolytic enzymes containing the capability to potentially produce ethanol. For consolidated lignocellulosic biomass processing, Shen et al. developed a thermostable self-splicing bacterial intein-modified xylanase [119].
4. ISSUES AND FUTURE PERFORMANCE IN INDUSTRIAL XYLANASE DEVELOPMENT, PURIFICATION, AND IMPLEMENTATION
Therefore, the investigation for incredible xylanase is on, looking at the new bacterial source with the capacity to generate extremely stable and durable xylanase that occurs all over the globe. Strains originating from different extreme habitats could be useful since they can withstand a variety of stresses such as changes in pH and temperature. Another alternative is to select these temperature and pH-resistant strains and expose them to various optimization techniques for higher xylanase output. The development of biotechnological techniques and tools (recombinant DNA technology) offers an ability to pick the DNA sequences responsible for the production of xylanase, which can be extracted and transmitted effectively to the expression vector. Such expression systems can be controlled with required properties for different industrial purposes for improved development of xylanase. Using various tools of bioinformatics, the accessibility of a massive proportion of genomics, metabolomics, and proteomics data may also be employed to establish various strategies to improve xylanase development. The combination of modern technologies including synthetic biology (DNA oligo-synthesis) and traditional genetic engineering can be employed to achieve the goal of massive yields of xylanase with required commercial property [Figure 7]. Nonetheless, before full-scale implementations, problems correlated with copying the natural process into an artificial model have to be taken care of.
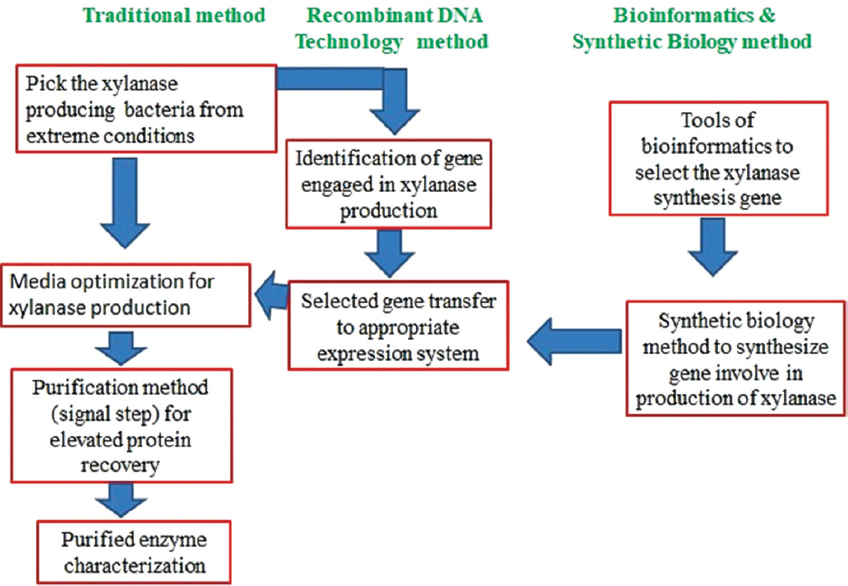 | Figure 7: Future prospective for growth in field of xylanase development, utilizing traditional and modern strategies. [Click here to view] |
5. SYNERGISTIC USAGE OF XYLANASE AND CELLULASE
By facilitating several valuable transforms, enzymes provide significant benefits to the biological base economy in comparison to chemocatalysts. Biocatalysts provide a major environmental benefit over chemical catalysts, as well as many other significant advantages such as specificity, precision, and low-energy consumption. For the past 10 years, cellulase is the third highest enzyme utilized in different industrial processes, also xylanase is one of the commonly utilized enzymes and several commercial applications enable some of these enzymes to function synergistically [120]. These are applications [Table 2]that involve the production of bioethanol, waste paper deinking, the processing of animal feed, the removal of fine synthetic fibers [stoning], and medical products.
Table 2: Synergistic effect and applications of xylanases.
| Source of enzyme | Enzyme synergy | Application | References |
|---|---|---|---|
| Trichoderma orientalis EU7-22 | Glucosidase + Xylanase + Cellulase+β-cellobiohydrolase | Saccharification of biomass | [125] |
| Gloeophyllum trabeum | Fungal xylanase + Commercial cellulase + polysaccharide monooxygenase | Production of bioethanol | [126] |
| Yarrowia lipolytica | Xylanase + Cellulase + Lytic polysaccharide | Saccharification of biomass | [127] |
| Microbulbifer strain CMC-5 | Xylanase + agarase + Cellulase + carrageenase + Alginate lyase | Saccharification of biomass | [128] |
| B. sonorensis, Bacillus amyloliquefaciens | Xylanase and cellulase | Agro-waste; saccharification of biomass | [129] |
| Escherichia coli SD5 | Xylanase + cellulase | Waste paper deinking | [97] |
| B. velezensis 157 | Pectinase + xylanase + cellulase + amylase | Agro-waste treatment [SSF] | [130] |
| Aspergillus oryzae SBS50 | Xylanase + phytase amylase + cellulase | Saccharification of biomass and production of biofuel | [131] |
| Pseudotheobromae C1136 | Cellulase + xylanase + Laccase | Saccharification of biomass | [132] |
| Exogenous fibrolytic enzymes [EFE] | Xylanase + Cellulase | Improved animal feed consistency, high milk production in cows | [133] |
6. FUTURE CHALLENGES
New methods for obtaining novel xylanase genes from metagenomics libraries have newly been implemented. Essential structural motifs of the xylanase protein have been stabilized using protein engineering strategies. The use of a combination of system biology experiments, proteomics, and genomics methodologies to produce xylanases that perform well at high temperatures and pH for industrial usage indicates a possible pathway for the development of xylanases that function well at extreme temperatures and pH [121]. This would also get the industries excited to transform to enzyme systems. In addition to the above advantages, the generation of many tons of industrial waste is preventable. Governments and environmental conservation body groups around the globe are making relentless attempts in reduces pollution rates and save money. Certainly, that approach will lead to this aim. Over the following two decades, these strategies appear to put the industry on the frontier position and it may be the center for industrial research and innovation, attracting researchers and research scientists from all over the world.
7. CONCLUSION
Among the industrially useful enzymes, thermostable xylanase is a major player. Thermostable xylanases have been derived from bacteria, Actinobacteria, yeast, and fungi. Many industries, such as biofuels, pulp and paper, food and feed, and animal feed, need thermostable xylanase. Since thermostability is a requirement for xylanases in industrial applications, many attempts have been devoted to identifying or creating novel thermostable enzymes. Several molecular methods and protein engineering strategies are discussed in this study to improve the operational stability of xylanases so that they can be used in more commercial processes.
8. ACKNOWLEDGMENT
Mr. Manoj Verma, Director, MRD LifeSciences Pvt. Ltd., Lucknow, deserves my heartfelt gratitude. I am grateful to Dr. Pallavi Sharma (Research Scientist MRDLS, Lucknow) for her invaluable assistance during the review process, and I am also grateful to the almighty, without whose blessings nothing would have been possible.
9. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
10. FUNDING
No funding is required to complete this manuscript. The authors are thankful to Shri Ramswaroop Memorial University, Lucknow Deva Road Barabanki, and MRD LifeSciences, Lucknow for providing all technical support.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
The authors have adhered to the accepted ethical standards of a genuine research study.
13. DATA AVAILABILITY
All the data have been taken from reputed journals only.
14. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Dahlberg L, Holst O, Kristjansson JK. Thermostable xylanolytic enzymes from
2. Selvarajan E, Veena R. Recent advances and future perspectives of thermostable xylanase. Biosci Biotechnol Res Asia 2017;14:421-38. [CrossRef]
3. Shao W, Obi S, Puls J, Wiegel J. Purification and characterization of the (alpha)-glucuronidase from
4. Saha BC. Hemicellulose bioconversion. J Ind Microbiol Biotechnol 2003;30:279-91. [CrossRef]
5. Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Župan?i?S. Production of fungal xylanases. Bioresour Technol 1996;58:137-61. [CrossRef]
6. Moehlenbrock MJ, Minteer SD. Introduction to the field of enzyme immobilization and stabilization. In:Enzyme Stabilization and Immobilization. Berlin:Springer;2017. 1-7. [CrossRef]
7. Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 2007;40:1451-63. [CrossRef]
8. Jampala P, Preethi M, Ramanujam S, Harish BS, Uppuluri KB, Anbazhagan V. Immobilization of levan-xylanase nanohybrid on an alginate bead improves xylanase stability at wide pH and temperature. Int J Biol Macromol 2017;95:843-9. [CrossRef]
9. Kumar L, Nagar S, Mittal A, Garg N, Gupta VK. Immobilization of xylanase purified from
10. Chen M, Zeng G, Xu P, Lai C, Tang L. How do enzymes “meet“nanoparticles and nanomaterials?Trends Biochem Sci 2017;42:914-30. [CrossRef]
11. Wang J, Liu Z, Zhou Z. Improving pullulanase catalysis via reversible immobilization on modified Fe3O4@polydopamine nanoparticles. Appl Biochem Biotechnol 2017;182:1467-77. [CrossRef]
12. Ansari SA, Husain Q. Potential applications of enzymes immobilized on/in nano materials:A review. Biotechnol Adv 2012;30:512-23. [CrossRef]
13. Li C, Jiang S, Zhao X, Liang H. Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogelnan ofibers for improving stability and recycling. Molecules 2017;22:179. [CrossRef]
14. Singh S, Madlala AM, Prior BA. Thermomyces lanuginosus:Properties of strains and their hemicellulases. FEMS Microbiol Rev 2003;27:3-16. [CrossRef]
15. Basheer SM, Chellappan S. Enzyme engineering. In:Bioresources and Bioprocess in Biotechnology. Berlin, Germany:Springer;2017. 151-68. [CrossRef]
16. Pucci F, Rooman M. Physical and molecular bases of protein thermal stability and cold adaptation. Curr Opin Struct Biol 2017;42:117-28. [CrossRef]
17. Kahrani ZF, Emamzadeh R, Nazari M, Rasa SM. Molecular basis of thermostability enhancement of Renilla luciferase at higher temperatures by insertion of a disulfide bridge into the structure. Biochim Biophys Acta Proteins Proteom 2017;1865:252-9. [CrossRef]
18. Chang A, Scheer M, Grote A, Schomburg I, Schomburg D. BRENDA, Amenda and FRENDA the enzyme information system:New content and tools in 2009. Nucleic Acids Res 2009;37:D588-92. [CrossRef]
19. Han N, Miao H, Ding J, Li J, Mu Y, Zhou J,
20. Ebert MC, Pelletier JN. Computational tools for enzyme improvement:Why everyone can-and should-use them. Curr Opin Chem Biol 2017;37:89-96. [CrossRef]
21. Wijma HJ, Floor RJ, Janssen DB. Structure-and sequence-analysis inspired engineering of proteins for enhanced thermostability. Curr Opin Struct Biol 2013;23:588-94. [CrossRef]
22. Bin Abdul Wahab MK, Bin Jonet MA, Illias RM. Thermostability enhancement of xylanase
23. Donohoue PD, Barrangou R, May AP. Advances in industrial biotechnology using CRISPR-Cas systems. Trends Biotechnol 2018;36:134-46. [CrossRef]
24. Yadav R, Kumar V, Baweja M, Shukla P. Gene editing and genetic engineering approaches for advanced probiotics:A review. Crit Rev Food Sci Nutr 2018;58:1735-46. [CrossRef]
25. Gupta PK, Agrawal P, Hedge P, Akhtar MS. Xylooligosaccharides and their anticancer potential:An update. In:Anticancer Plants:Natural Products and Biotechnological Implements. Berlin, Germany:Springer;2018. 255-71. [CrossRef]
26. Qian C, Liu N, Yan X, Wang Q, Zhou Z, Wang Q. Engineering a high-performance, metagenomic-derived novel xylanase with improved soluble protein yield and thermostability. Enzyme Microb Technol 2015;70:35-41. [CrossRef]
27. Acevedo JP, Reetz MT, Asenjo JA, Parra LP. One-step combined focused epPCR and saturation mutagenesis for thermostability evolution of a new cold-active xylanase. Enzyme Microb Technol 2017;100:60-70. [CrossRef]
28. Boonyapakron K, Jaruwat A, Liwnaree B, Nimchua T, Champreda V, Chitnumsub P. Structure-based protein engineering for thermostable and alkaliphilic enhancement of endo-β-1, 4-xylanase for applications in pulp bleaching. J Biotechnol 2017;259:95-102. [CrossRef]
29. Wang K, Luo H, Tian J, Turunen O, Huang H, Shi P,
30. Irfan M, Guler HI, Ozer A, Sapmaz MT, Belduz AO, Hasan F,
31. Irfan M, Guler HI, Shah AA, Sal FA, Inan K, Belduz AO. Cloning, purification and characterization of halotolerant xylanase from
32. Sriprang R, Asano K, Gobsuk J, Tanapongpipat S, Champreda V, Eurwilaichitr L. Improvement of thermostability of fungal xylanase by using site-directed mutagenesis. J Biotechnol 2006;126:454-62. [CrossRef]
33. Chen W, Ye L, Guo F, Lv Y, Yu H. Enhanced activity of an alkaline phytase from
34. Sun DP, Sauer U, Nicholson H, Matthews BW. Contributions of engineered surface salt bridges to the stability of T4 lysozyme determined by directed mutagenesis. Biochemistry 1991;30:7142-53. [CrossRef]
35. Motta FL, Andrade CC, Santana MH. A review of xylanase production by the fermentation of xylan:Classification, characterization and applications. In:Sustainable Degradation of lignocellulosic Biomass-Techniques, Applications And Commercialization. London:IntechOpen;2013.
36. Paës G, Berrin JG, Beaugrand J. GH11 xylanases:Structure/function/properties relationships and applications. Biotechnol Adv 2012;30:564-92. [CrossRef]
37. Zhang ZG, Yi ZL, Pei XQ, Wu ZL. Improving the thermostability of
38. Joo JC, Pack SP, Kim YH, Yoo YJ. Thermostabilization of
39. Yang HM, Yao B, Meng K, Wang YR, Bai YG, Wu NF. Introduction of a disulfide bridge enhances the thermostability of a
40. Ayadi DZ, Sayari AH, Ben HH, Ben MS, Mezghani M, Bejar S. Improvement of
41. Tang F, Chen D, Yu B, Luo Y, Zheng P, Mao X,
42. You S, Xie C, Ma R, Huang H, Herman RA, Su X,
43. Wang Y, Feng S, Zhan T, Huang Z, Wu G, Liu Z. Improving catalytic efficiency of endo-β-1, 4-xylanase from
44. Jia H, Li Y, Liu Y, Yan Q, Yang S, Jiang Z. Engineering a thermostable β-1, 3-1, 4-glucanase from
45. Trollope KM, Görgens JF, Volschenk H. Semirational directed evolution of loop regions in
46. Yang JH, Park JY, Kim SH, Yoo YJ. Shifting pH optimum of
47. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active enzymes database (CAZy):An expert resource for glycogenomics. Nucleic Acids Res 2009;37:D233-8. [CrossRef]
48. Jun H, Bing Y, Keying Z, Xuemei D, Daiwen C. Thermostable carbohydrate binding module increases the thermostability and substrate-binding capacity of
49. Mamo G, Hatti-Kaul R, Mattiasson B. Fusion of carbohydrate binding modules from
50. Sajjad M, Khan MI, Zafar R, Ahmad S, Niazi UH, Akhtar MW. Influence of positioning of carbohydrate binding module on the activity of endoglucanaseCelA of
51. Sajjad M, Khan MI, Akbar NS, Ahmad S, Ali I, Akhtar MW. Enhanced expression and activity yields of
52. Liu L, Zeng L, Wang S, Cheng J, Li X, Song A,
53. Sunna A, Gibbs MD, Bergquist PL. The thermostabilizing domain, XynA, of
54. Liu L, Cheng J, Chen H, Li X, Wang S, Song A,
55. Miao H, Ma Y, Zhe Y, Tang X, Wu Q, Huang Z,
56. Wrenbeck EE. Deep Sequencing Driven Protein Engineering:New Methods and Applications in Studying the Constraints of Functional Enzyme Evolution. Michigan:Michigan State University;2017. [CrossRef]
57. Santero E, Floriano B, Govantes F. Harnessing the power of microbial metabolism. Curr Opinion Microbiol 2016;31:63-9. [CrossRef]
58. Kohanski MA, Collins JJ. Rewiring bacteria, two components at a time. Cell 2008;133:947-8. [CrossRef]
59. Tian J, Ma K, Saaem I. Advancing high-throughput gene synthesis technology. Mol Biosyst 2009;5:714-22. [CrossRef]
60. Esvelt KM, Wang HH. Genome-scale engineering for systems and synthetic biology. Mol Syst Biol 2013;9:641. [CrossRef]
61. Courtin CM, Delcour J. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci 2002;35:225-43. [CrossRef]
62. Camacho NA, Aguilar G. Production, purification, and characterization of a low-molecular-mass xylanase from
63. Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT. Xylanases and their applications in baking industry. Food Technol Biotechnol 2008;46:22-31.
64. Polizeli M, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi:Properties and industrial applications. Appl Microbiol Biotechnol 2005;67:577-91. [CrossRef]
65. Driss D, Bhiri F, Siela M, Bessess S, Chaabouni S, Ghorbel R. Improvement of breadmaking quality by xylanase GH 11 from
66. Ghoshal G, Shivhare US, Banerjee UC. Effect of xylanase on quality attributes of whole-wheat bread. J Food Qual 2013;36:172-80. [CrossRef]
67. Bajpai P. Sources, production, and classification of xylanases. In:Xylanolytic Enzymes. Tokyo:Academic Press, Elsevier;2014. 43-52. [CrossRef]
68. Cunha CC, Gama AR, Cintra LC, Bataus LA, Ulhoa CJ. Improvement of bread making quality by supplementation with a recombinant xylanase produced by
69. Danalache F, Mata P, Alves VD, Moldão-Martins M. Enzyme-assisted extraction of fruit juices. In:Fruit Juices. Amsterdam, Netherlands:Elsevier;2018. 183-200. [CrossRef]
70. Rosmine E, Sainjan NC, Silvester R, Alikkunju A, Varghese SA. Statistical optimisation of xylanase production by estuarine
71. Shahrestani H, Taheri-Kafrani A, Soozanipour A, Tavakoli O. Enzymatic clarification of fruit juices using xylanase immobilized on 1, 3, 5-triazine-functionalized silica-encapsulated magnetic nanoparticles. Biochem Eng J 2016;109:51-8. [CrossRef]
72. Adigüzel AO, Tunçer M. Production, characterization and application of a xylanase from
73. Adiguzel G, Faiz O, Sisecioglu M, Sari B, Baltaci O, Akbulut S,
74. Facchini FD, Vici AC, Reis VR, Jorge JA, Terenzi HF, Reis RA,
75. Pirgozliev V, Whiting I, Rose SP, Ivanova SG, Staykova G, Amerah AM. Variability between wheat dry distillers grains with solubles samples influence the effectiveness of exogenous enzymes when fed to broiler chickens. Vet Med Anim Stud 2016;6:61-9.
76. Liu N, Ru YJ, Tang DF, Xu TS, Partridge GG. Effects of corn distillers dried grains with solubles and xylanase on growth performance and digestibility of diet components in broilers. Anim Feed Sci Technol 2011;163:260-6. [CrossRef]
77. Paloheimo M, MäntyläA, Kallio J, Puranen T, Suominen P. Increased production of xylanase by expression of a truncated version of the xyn11A gene from nonomuraeaflexuosa in
78. Van Dorn R, Shanahan D, Ciofalo V. Safety evaluation of
79. Passos AA, Park I, Ferket P, Von Heimendahl E, Kim SW. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim Nutr 2015;1:19-23. [CrossRef]
80. Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos M De, Bories G,
81. Beg Q, Kapoor M, Mahajan L, Hoondal GS. Microbial xylanases and their industrial applications:A review. Appl Microbiol Biotechnol 2001;56:326-38. [CrossRef]
82. Subramaniyan S, Prema P. Biotechnology of microbial xylanases:Enzymology, molecular biology, and application. Crit Rev Biotechnol 2002;22:33-64. [CrossRef]
83. Thomas L, Sindhu R, Binod P, Pandey A. Production of an alkaline xylanase from recombinant
84. Sharma D, Chaudhary R, Kaur J, Arya SK. Greener approach for pulp and paper industry by xylanase and laccase. Biocatal Agric Biotechnol 2020;25:101604. [CrossRef]
85. Viikari L, Kantelinen A, Sundquist J, Linko M. Xylanases in bleaching:From an idea to the industry. FEMS Microbiol Rev 1994;13:335-50. [CrossRef]
86. Pérez J, Munoz-Dorado J, De la Rubia T, Martinez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin:An overview. Int Microbiol 2002;5:53-63. [CrossRef]
87. Lin X, Wu Z, Zhang C, Liu S, Nie S. Enzymatic pulping of lignocellulosic biomass. Ind Crops Prod 2018;120:16-24. [CrossRef]
88. Bajpai P. Biotechnology for Pulp and Paper Processing. Berlin, Germany:Springer;2012. [CrossRef]
89. Azeri C, Tamer UA, Oskay M. Thermoactivecellulase-free
90. Maity C, Ghosh K, Halder SK, Jana A, Adak A, Mohapatra PK,
91. Chandra R, Singh R. Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem Eng J 2012;61:49-58. [CrossRef]
92. Dhiman SS, Garg G, Sharma J, Kalia VC, Kang YC, Lee JK. Reduction in acute ecotoxicity of paper mill effluent by sequential application of xylanase and laccase. PLoS One 2014;9:e102581. [CrossRef]
93. Virk AP, Puri M, Gupta V, Capalash N, Sharma P. Combined enzymatic and physical deinking methodology for efficient eco-friendly recycling of old newsprint. PLoS One 2013;8:e72346. [CrossRef]
94. Gupta V, Garg S, Capalash N, Gupta N, Sharma P. Production of thermo-alkali-stable laccase and xylanase by co-culturing of
95. Kumar V, Satyanarayana T. Production of endoxylanase with enhanced thermostability by a novel polyextremophilic
96. Kumar NV, Rani ME, Gunaseeli R, Kannan ND. Paper pulp modification and deinking efficiency of cellulase-xylanase complex from
97. Dhiman SS, Sharma J, Battan B. Pretreatment processing of fabrics by alkalothermophilic xylanase from
98. Singh A, Kaur A, Patra AK, Mahajan R. A sustainable and green process for scouring of cotton fabrics using xylano-pectinolytic synergism:Switching from noxious chemicals to eco-friendly catalysts. 3 Biotech 2018;8:184. [CrossRef]
99. Abd El Aty AA, Saleh SA, Eid BM, Ibrahim NA, Mostafa FA. Thermodynamics characterization and potential textile applications of
100. Vazquez MJ, Alonso JL, Dom?nguez H, Parajo JC. Xylooligosaccharides:Manufacture and applications. Trends Food Sci Technol 2000;11:387-93. [CrossRef]
101. Chang S, Chu J, Guo Y, Li H, Wu B, He B. An efficient production of high-pure xylooligosaccharides from corncob with affinity adsorption-enzymatic reaction integrated approach. Bioresour Technol 2017;241:1043-9. [CrossRef]
102. Chen HH, Chen YK, Chang HC, Lin SY. Immunomodulatory effects of xylooligosaccharides. Food Sci Technol Res 2012;18:195-9. [CrossRef]
103. Kallel F, Driss D, Bouaziz F, Neifer M, Ghorbel R, Chaabouni SE. Production of xylooligosaccharides from garlic straw xylan by purified xylanase from
104. Aachary AA, Prapulla SG. Xylooligosaccharides (XOS) as an emerging prebiotic:Microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr Rev Food Sci Food Saf 2011;10:2-16. [CrossRef]
105. Li T, Li S, Du L, Wang N, Guo M, Zhang J,
106. Huang C, Jeuck B, Du J, Yong Q, Chang H, Jameel H,
107. Moniz P, Ho AL, Duarte LC, Kolida S, Rastall RA, Pereira H,
108. Gowdhaman D, Ponnusami V. Production and optimization of xylooligosaccharides from corncob by
109. Otieno DO, Ahring BK. A thermochemical pretreatment process to produce xylooligosaccharides (XOS), arabinooligosaccharides (AOS) and mannooligosaccharides (MOS) from lignocellulosic biomasses. Bioresour Technol 2012;112:285-92. [CrossRef]
110. Jayapal N, Samanta AK, Kolte AP, Senani S, Sridhar M, Suresh KP,
111. Xiao X, Bian J, Peng XP, Xu H, Xiao B, Sun RC. Autohydrolysis of bamboo (
112. Haddar A, Driss D, Frikha F, Ellouz-Chaabouni S, Nasri M. Alkaline xylanases from
113. Gowdhaman D, Manaswini VS, Jayanthi V, Dhanasri M, Jeyalakshmi G, Gunasekar V,
114. Ding C, Li M, Hu Y. High-activity production of xylanase by
115. Bhardwaj N, Kumar B, Agarwal K, Chaturvedi V, Verma P. Purification and characterization of a thermo-acid/alkali stable xylanases from
116. Basit A, Liu J, Miao T, Zheng F, Rahim K, Lou H,
117. Hu J, Arantes V, Saddler JN. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase:Is it an additive or synergistic effect?Biotechnol Biofuels 2011;4:1-14. [CrossRef]
118. Chadha BS, Kanwar SS, Garcha HS. Simultaneous saccharification and fermentation of rice straw into ethanol Acta Microbiol Immunol Hung 1995;42:71-5.
119. Shen B, Sun X, Zuo X, Shilling T, Apgar J, Ross M,
120. Bajaj P, Mahajan R. Cellulase and xylanase synergism in industrial biotechnology. Appl Microbiol Biotechnol 2019;103:∇-24. [CrossRef]
121. Ventorim RZ, Mendes TA, Trevizano LM, Camargos AM, Guimarães VM. Impact of the removal of N-terminal non-structured amino acids on activity and stability of xylanases from
122. Li Q, Sun B, Xiong K, Teng C, Xu Y, Li L,
123. Alponti JS, Maldonado RF, Ward RJ. Thermostabilization of
124. Wang Y, Fu Z, Huang H, Zhang H, Yao B, Xiong H,
125. Long C, Cheng Y, Cui J, Liu J, Gan L, Zeng B,
126. Sanhueza C, Carvajal G, Soto-Aguilar J, Lienqueo ME, Salazar O. The effect of a lytic polysaccharide monooxygenase and a xylanase from
127. Guo Z, Duquesne S, Bozonnet S, Nicaud JM, Marty A, O'Donohue MJ. Expressing accessory proteins in cellulolytic
128. Jonnadula R, Imran M, Poduval PB, Ghadi SC. Effect of polysaccharide admixtures on expression of multiple polysaccharide-degrading enzymes in
129. Berikashvili V, Sokhadze K, Kachlishvili E, Elisashvili V, Chikindas ML.
130. Chen L, Gu W, Xu H, Yang GL, Shan XF, Chen G,
131. Singh B. Engineering fungal morphology for enhanced production of hydrolytic enzymes by
132. Zhang W, Wu S, Cai L, Liu X, Wu H, Xin F,
133. Arriola KG, Oliveira AS, Ma ZX, Lean IJ, Giurcanu MC, Adesogan AT. A meta-analysis on the effect of dietary application of exogenous fibrolytic enzymes on the performance of dairy cows. J Dairy Sci 2017;100:4513-27. [CrossRef]