1. INTRODUCTION
Pleurotus florida, a saprophytic fungus, belonging to the family Pleurotaceae, is farmed globally due to its palatable basidiocarp and modest cultivation methods [1]. The oyster mushroom is particularly recognized for its rapid development and capacity to generate fruiting bodies on various substrates [2]. This is the second most economically significant mushroom genus, noted for its flavour, valuable nutritional value, rapid growth rate, and ability to decompose nearly all forms of lignocellulosic biomass [3]. Wheat straw, among the other lignocellulosic materials, is typically discarded via open-field burning, resulting in significant environmental contamination issues [4]. Wheat straw may facilitate the cultivation of oyster mushrooms, thereby offering a solution for converting lignocellulosic waste into valuable edible protein biomass and providing an economical substrate for mushroom cultivators [5]. However, the production of mushrooms is not up to the mark with the increasing global demand for mushrooms. In this regard, researchers and farmers both have turned their attention toward optimizing the substrate composition and nutrient levels, particularly available forms of C and N. In the way of substrate optimization, supplementation of substrates with selected oilseed cakes (OSC) rich in organic C and N is one of the best choices that can improve the nutrient level of the substrate, which can improve the mushroom yield and quality.
The complete extraction of oil from seeds is referred to as OSC, including groundnut seed cake (GSC), neem seed cake (NSC), among others. The OSC are regarded as a valuable source of carbon-to-nitrogen (C/N) content, which aids in mushroom production. The utilization of de-oiled cakes is a crucial method for enhancing both the quality and quantity of mushrooms. A variety of nitrogen supplements have been investigated in the process of efficacious mushroom farming, including mustard, sunflower, cotton, groundnut, and NSCs. The OSC are abundant in proteins, amino acids, and other complexes that enhance mushroom growth and decrease the mycelial run time in mushroom cropping [6]. Combinations of the substrates with de-fatted cakes have been shown to affect both the production and nutraceutical potential of mushrooms [6,7]. Supplemental substrates provide organic nitrogen, crucial for mycelial growth, yet may impede mushroom productivity and biological efficiency (BE) [8]. The elevated levels of nutrient supplements impede the growth of mushroom mycelium, likely attributable to the higher amounts of nitrogen-rich substrates resulting in a diminished C/N ratio [9]. Nonetheless, a reduced quantity of supplements has proven useful in mushroom development. Naraian et al. [7] elucidated that the characteristics of lignocellulosic materials, specifically the C/N ratio, are pivotal for mycelial growth in mushrooms. Li et al. [10] posited that the physical characteristics and C/N ratio of the medium enhance its biodeterioration and facilitate the growing of mushrooms. Substrates with an optimal C/N ratio may generate more mushroom production [11]. Therefore, including cakes in the substrate is anticipated to enrich the C/N ratio, bioconversion efficiency, and production efficiency of the oyster mushrooms.
The versatile mycelia of oyster mushrooms secrete a variety of lignolytic enzymes, including manganese peroxidase (MnP) and laccase [12]. Thus, cultivated edible mushrooms can decompose lignocellulosic materials by generating a variety of enzymes [13]. They can subsequently utilize these components as nutrients for their favourable expansion. Produced enzymes decompose substrates into simpler constituents able to be absorbed through the hyphal surface to facilitate the growth and productivity of mushrooms [14]. The proliferation of palatable mushrooms on substrates and the subsequent emergence of fruitings are profoundly influenced by the quantity of ligninolytic enzymes produced by the mushrooms to degrade the complex substrate components of the substrates [15]. The incorporation of OSC into the substrate augments the enzymatic activity of mushrooms, hence markedly enhancing the bio-degradation rate of biomass [6]. This study exhibits its novelty because it compares and improves oyster mushroom productivity and quality by using groundnut and NSCs as organic supplements.
The current research article was focused on the supplementation of two different OSC (groundnut and NSC) in wheat straw substrate to observe their impact on the growth, yield, BE, and lignolytic enzyme profiling of oyster mushroom (P. florida).
2. MATERIALS AND METHODS
2.1. Mushroom Culture and Maintenance
The purified mycelia of the mushroom (P. florida) used were gained from the Mushroom Training and Research Centre, V.B.S. Purvanchal University, Jaunpur. The freshly purified culture of the mushroom was continuously maintained in slants. A potato dextrose agar medium was used to prepare the pure culture. The inoculated Petri plates were stocked in a dark cultivation room for 6–8 days to promote mycelium growth.
2.2. Preparation of Spawn
Spawn was derived from wheat grains drenched in water and cooked to exterminate the seed embryo; then, these were air-dried and combined with 4% (w/w) calcium carbonate and 2% (w/w) gypsum or calcium sulfate. Thermo-resistant polypropylene bags containing 0.5 kg of broiled grains were decontaminated through autoclaving for 45 min at 121°C. After cooling, autoclaved grains were introduced with P. florida inoculum (2 mycelia discs) and cultured at 25 ± 1°C for 10 days in the dark.
2.3. Supplementation and Spawning
The chopped wheat straw substrate was subjected to a treatment with a solution containing 75 ppm bavistin and 500 ppm formalin overnight. Surplus water was removed in a shaded environment to maintain 65–70% moisture with a pH of 6.5. The substrate and grain spawn were meticulously combined by hand, placed into polythene bags, and securely sealed with threads. Before spawning, cropping bags were meticulously disinfected using a 2% (v/v) formalin solution to prevent any surface contamination. Two distinct oilseed cakes, namely GSC (Arachis hypogaea) and NSC (Azadirachta indica) (GSC and NSC), were utilized as supplements and incorporated into the substrate before spawning. Six combinations of oilseed cakes with wheat straw substrate (1, 2, 3, 5, 8, and 10%; w/w) and unsupplemented wheat straw (0%) were subjected to three replications (n = 3).
2.4. Spawn Run, Cropping, and Harvesting
Poly bags measuring 18 × 22 inches with a thickness of 100 gauge were utilized for the filling of the sterilized damp wheat straw substrate. Spawning was conducted at a rate of 5% (w/w) of spawn. For a proper spawn run, filled bags were incubated in a cropping room at 28°C in a dark environment with 85% relative humidity [Figure 1].
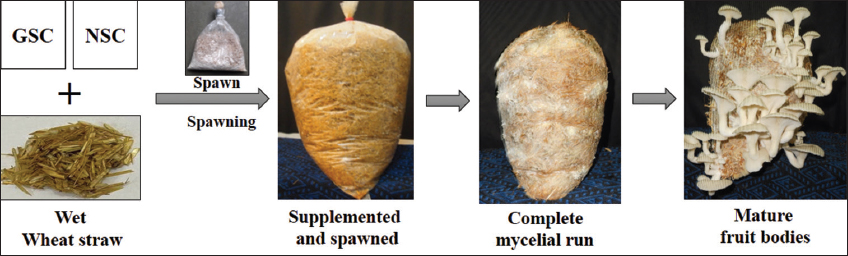 | Figure 1: Oil seed cake supplemented polybag cultivation process yielding fruitbodies (basidiocarps) of oyster mushroom (Pleurotus florida). [Click here to view] |
The appropriately sized basidiocarps were picked by winding to uproot them from their substrate base. Fruitings were collected thrice in separate flushes from the mushroom bed. Following the initial harvest, the packets were scraped, immersed in a bucket for 5 min, and subsequently positioned in the cultivation house, where water was regularly sprayed while awaiting pinhead initiation. The water spraying persisted until the mushrooms were ready for harvest, and further continued.
2.5. Evaluation of Yield and BE
The yield of mushrooms was evaluated by the total weight of all fruitings collected across the three distinct harvestings, utilizing the formula provided below.
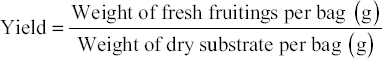 |
Mushrooms were analysed to assess the BE of the oyster mushroom. The BE of mushroom crops was determined using the formula shown below.
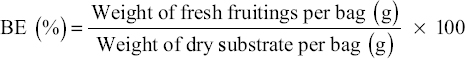 |
2.6. Extraction of Crude Enzyme Extract
Samples of oyster mushroom fruit bodies were collected and pulverized in a mortar and pestle with 50 mL of sodium acetate buffer (50 mM) (pH 5.4), then filtered employing muslin cloth. The obtained filtrate was centrifuged at 4000 × g for 15 min using a centrifuge machine (Remi C-24BL, India). The resultant supernatant served as the enzyme extract for subsequent investigations.
2.7. MnP Assay
The enzymatic activity of MnP (EC 1.11.1.13) was quantified through the prescribed protocol via oxidizing phenol red [16]. One millilitre of the reaction mixture was incubated for 5 min at 20 ± 1°C with the occurrence of 0.1 mM H2O2 to facilitate the enzymatic reaction. The reaction was concluded by mixing 50 µL of NaOH (4 M), and the optical density was recorded at 610 nm. However, a single unit of the MnP activity was presented as the quantity of enzyme necessary to oxidize 1 µmol of the phenol red per minute.
2.8. Laccase Assay
For the assessment of laccase (EC 1.10.3.2.) activity oxidation of 2, 2’-azinobis [3-ethylbenzothiazolone-6-sulfonic acid] (ABTS) was estimated. An aliquot (10 µL) of crude enzyme extract was combined with 90 µL of sodium acetate (50 mM) buffer (pH 4.5) holding 1 mM ABTS and allowed to react at ambient temperature. The 100 µL aliquot of trichloroacetic acid, 20% (w/v) was mixed to pause the reaction. The optical density was observed using a spectrophotometer (RS Cube, India) (420 nm). However, the individual unit of the laccase activity was presented as the quantity of enzyme used to oxidize 1 µmol/min of ABTS under the specified test conditions.
2.9. Statistical Analysis
All experiments of this paper were conducted in triplicate (n = 3), and the mean data together with standard deviations are presented.
3. RESULTS AND DISCUSSION
3.1. Effect of GSC on MnP and Laccase
The addition of GSC at various concentrations (1, 2, 3, 5, 8, and 10%) differentially affected the MnP and laccase activities of P. florida. The GSC demonstrated superior enzyme activity compared to the NSC in terms of oilseed cake quality. The maximum MnP activity (88 IU/mL) and laccase activity (30 IU/mL) were achieved with 5% (w/w) GSC, whereas 10% (w/w) GSC supplementation resulted in the lowest activities of 21 IU/mL and 10 IU/mL, respectively. A lower concentration of GSC facilitated enzyme secretion, but concentrations beyond 5% led to a steady decline in enzyme secretion [Figure 2]. Enzymes such as MnP and laccase play a critical role in degrading lignin and other complex polymers present in lignocellulosic substrates, making them easily available for mushroom development, fructification, and yield of mushrooms. In an earlier investigation, Sharma and Kumar [17] found that the combination of substrate with nitrogen-rich cakes increased the release of degradation enzymes (peroxidases and laccases), which ultimately led to the acceleration of the degradation of complex lignocellulosic materials. Ample evidence indicates that the cakes contain a significant quantity of protein and its derivative amino acids, namely arginine, thiamine, biotin, cystine, phenylalanine, and isoleucine [7,18]. The research indicated that cottonseed cake, mustardseed cake, and groundnut cake exhibit a respectable amount of organic proteins. Organic nitrogen supplies are essential for laccase formation and cannot be replaced by inorganic components [19]. The efficacy of the augmentation was significantly contingent upon the comparative levels of the cakes. Related findings were similarly reported by Krishnan and Chandra [20] in their investigation of submerged culture for the synthesis of β-amylase. However, the reason for suppression of enzyme activity with the supplementation of a higher concentration of OSC might be due tothe thermogenesis in the supplemented substrate.
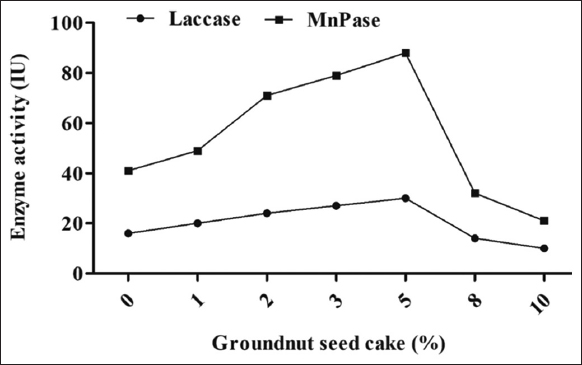 | Figure 2: Effect of groundnut seed cake supplementation on the laccase and manganese peroxidase activity of oyster mushroom Pleurotus florida. [Click here to view] |
3.2. Effect of NSC on MnP and Laccase
The different amounts of 1, 2, 3, 5, 8, and 10% GSC supplementation significantly affected the enzyme activity (MnP, and laccase) of the oyster mushroom P. florida. The maximum activity of MnP (56 IU/mL) and laccase (29 IU/mL) was observed with 1% NSC supplementation, whereas the minimum activity of MnP (9 IU/mL) and laccase (1 IU/mL) occurred with 10% supplementation [Figure 3]. The NSC was determined to be inferior to GSC for the enhancement of enzyme activity in oilseed cake. All measured concentrations of NSC evidently hindered the colonization of fungal mycelia and subsequently inhibited enzyme function. It is considered that the NSC provides a substantial level of protein (45–50%), phosphate, iron, and calcium [21]. In contrast to the GSC, it has shown a negative impact on enzyme synthesis (laccase and MnP). This may be associated with the existence of specific hazardous elements that possess antibacterial characteristics. Bawa et al. [22] indicated that NSC contains several triterpenoids, such as nimbin, salaninand azadirone. Azadirachtin, a chemical derived from neem, obstructs microbial enzymes such as ATPase and DNA gyrase, hence impeding DNA replication and protein synthesis. Salannin and other neem derivatives obstruct fungal enzymes that participate in several metabolic processes, including those essential for cell wall synthesis. These phenomena might have influenced the synthesis of fungal MnP and laccase enzymes. The occurrence of phosphorus might be a subsequent aspect contributing to adverse effects due to the addition of NSC, which has occurred in the present study.
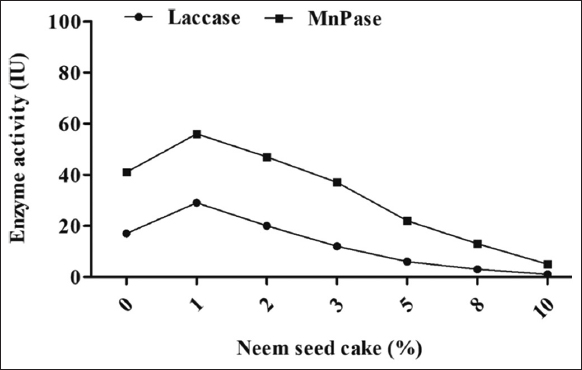 | Figure 3: Effect of neem seed cake supplementation on the laccase and manganese peroxidase activity of oyster mushroom Pleurotus florida. [Click here to view] |
3.3. Effect of GSC and NSC on Fruit Body Yield
The incorporation of GSC and NSC at varying concentrations (1, 2, 3, 5, 8, and 10%; w/w) distinctly influenced the yield of the P. florida. The GSC was identified as the preeminent oilseed cake in terms of fruit body output. The highest fruit body production of 2583 g was attained with 5% (w/w) GSC, whereas 10% (w/w) GSC supplementation produced the lowest yield of 467 g. Consequently, it was clear that a reduced GSC level promoted mushroom production, whereas levels beyond 5% led to a consistent decrease in output. Conversely, the supplementation of NSC resulted in the highest fruit body yield of 1432 g, whilst the lowest yield was 103 g. The inclusion of NSC did not adequately enhance mushroom yield, despite its minimal concentration. Utilization of 1% had a favourable impact; however, levels over 1% adversely affected fruit body yield output. Overall, the comparison revealed that the inclusion of GSC had a beneficial impact, but NSC led to a detrimental effect on fruit body production [Figure 4]. The use of de-oiled cakes is critical for improving the value and fruit body yield. The majority of cakes are acknowledged for their richness in amino acids, which promote mushroom development and reduce mycelial run time in a mushroom production setup [6]. De-oiled seed cake supplementation has been demonstrated to encourage the productivity and nutraceutical potency of mushrooms [6]. NSC, as a supplement in mushroom growth, can influence mushroom yield both positively and adversely, contingent upon the concentration and species of mushroom. Khade et al. [23] demonstrated that treatment with lower quantities than 2% neem cake (NC) improved yields of the mushroom (Hypsizygus pulmarius), supporting our findings. Nevertheless, similar to the findings of the current investigation, NSC supplementation adversely affected the growth of mushroom fruiting bodies [6]. The negative effects of NSC supplementation can be ascribed to the presence of several growth-inhibitory macromolecules, which may have impacted both mycelial growth and mushroom yield. Bawa et al. [22] indicated that NSC comprehends triterpenoids, salanin, nimbin, and azadirone, which demonstrate inhibitory effects on the growth of mushroom mycelium. Literature reveals that the presence of nimbin, salannin, and azadirone in neem, demonstrates antibacterial properties against fungal mycelia through multiple mechanisms. This encompasses the disruption of fungal cell membrane integrity, the inhibition of ergosterol manufacture, and the alteration of fungal enzyme activity, ultimately impeding fungal growth and development. Nimbin and nimbidin are recognized for their ability to compromise the lipid bilayer of microbial cell membranes, resulting in the release of cellular contents and subsequent cell death. The antifungal efficacy of nimbin, salannin, and azadirone is ascribed to their capacity to disrupt fungal cellular architecture, impede critical metabolic functions, and inhibit the synthesis of deleterious byproducts, thereby resulting in diminished fungal proliferation and enhanced mycelial development in specific cases. The inhibitory incidences might have occurred after NSC addition, which has negatively influenced. Furthermore, studies suggest that the incorporation of NC may negatively impact mushroom yield, potentially due to increased substrate temperature. The presence of an antimicrobial nature of NSC may be another cause to reduce the fruit body yield.
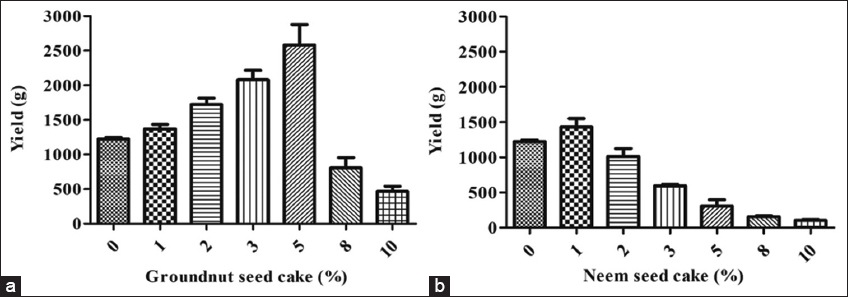 | Figure 4: Fruit body yield of oyster mushroom influenced by the supplementation of oil seed cake (a) Groundnut seed cake and (b) Neem seed cake. [Click here to view] |
3.4. Effect of GSC and NSC on BE
The introduction of GSC and NSC in different quantities markedly affected the BE of oyster mushrooms. Lower concentrations of GSC markedly improved BE; however, concentrations over 5% (w/w) led to a significant decrease. The highest biological efficacy (258.3%) was observed with 5% GSC supplementation. The addition of NSC produced no advantageous impact on the BE of P. florida. The maximum BE (143.2%) was seen with 1% NSC; however, it was simultaneously reduced in groups supplemented with NSC at levels beyond 1%. Consequently, it was apparent that the GSC was superior to the NSC in terms of BE [Figure 5a and b]. Renganathan et al. [24] similarly documented the comparative impact of groundnut oil cake (4%) combined with paddy straw on the BE of various Pleurotus mushroom species: Pleurotus citrinopileatus and Pleurotus eous (132.09%), P. eous and Pleurotus ostreatus (134.03%), and P. citrinopileatus and P. florida (130.03%). The biological efficiencies were considerably lower than those in our investigation. Nevertheless, an increase in the content of NC resulted in minimal biological yield [6], which contradicts our findings. The declining biological yield trend is attributed to elevated nitrogen levels, which disrupt the C/N and impede mushroom growth [25]. The adverse impact of NSC can also be ascribed to the presence of several antimicrobial metabolites inside it. Our findings indicate that various authors have similarly demonstrated that lower concentrations of oilseed cakes, such as sunflower seed cake, cottonseed cake, mustard seed cake, GSC, and mahua seed cake, positively affect the BE of oyster mushrooms [26,27]. On the other hand, in alignment with our findings, Singh and Mahendra [28] found that the NC-supplemented substrate yielded biological efficiencies of 59.60% and 61.20% in oyster mushroom Pleurotus sajor-caju, which are much lower than the BE attained in our work.
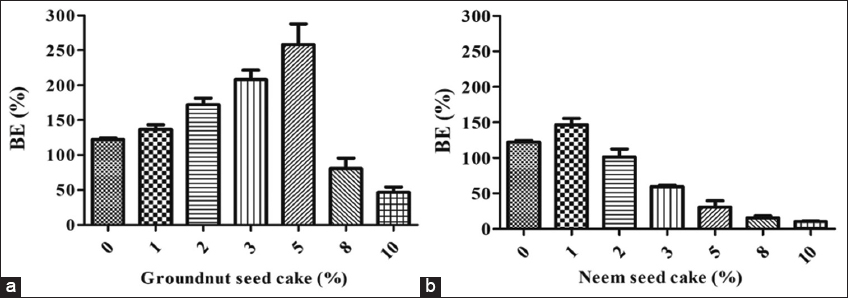 | Figure 5: Biological efficiency of oyster mushroom (Pleurotus florida) influenced by supplementation of oil seed cakes (a) Groundnut seed cake (b) Neem seed cake. [Click here to view] |
The findings of all aspects of the current study demonstrated that the mushrooms produce lignocellulolytic enzymes that facilitate the breakdown of lignocellulosic substrates. Mushroom mycelia generate enzymes, including cellulase, xylanase, and laccase [29], to decompose the substrate’s intricate polymers into simpler molecules. The enzymes generated by mushroom mycelia decompose the substrate, liberating nutrients that facilitate mushroom growth. Therefore, enzyme synthesis is crucial to promote mushroom growth and development. Enzymatic activity directly influences substrate breakdown and nutrient accessibility. Effective enzyme synthesis and substrate decomposition are essential for maximizing mushroom yield and quality.
The mushrooms subsequently employ these enzymes in the creation of their fruitings. The effective biodegradation of the substrate is crucial for mushroom growth and productivity. The decomposition of the substrate liberates nutrients vital so that mushrooms can continue to grow and develop. The degree of biodegradation and nutrient accessibility directly influences mushroom production and quality. In light of this, the growing of mushrooms can be regarded as a significant biotechnological procedure for the reduction and monetization of waste from agricultural and industrial processes.
4. CONCLUSION
OSC are rich in proteins, amino acids, and many other organic complexes able to supply the proper amount of nitrogen and carbon to mushroom mycelia. Based on the examinations of distinct parameters of the current study, GSC was determined to be a more effective supplement than NSC for the production of oyster mushrooms. The ideal concentrations of GSC and NSC for enhancing mushroom productivity, BE, and enzyme activity were determined to be 5% and 1%, respectively. Nonetheless, elevated quantities of both GSC and NSC were determined to be detrimental to mushroom cultivation, leading to diminished yields and BE. GSC was seen to enhance the activity of lignolytic enzymes, such as MnP and laccase, at reduced doses. The study recommends utilizing a lower dose of GSC (5%) as a supplement to wheat straw for optimal growing of oyster mushrooms. The findings offer significant insights into the utilization of OSC as supplements for oyster mushroom culture and underscore the potential of GSC as an exceptional supplement for enhancing mushroom production and enzyme activity. Having a surplus amount of nitrogen, proteins, soluble amino acids, and carbon sources, OSC can be explored in future studies of mushroom research and application in commercial oyster mushroom cultivations.
5. ACKNOWLEDGMENT
The financial grant (project No.: F. No.41-513/2012/(SR) from the UGC, New Delhi, is highly recognized. The authors also thank V.B.S.P. University, Jaunpur, for providing infrastructure and facilities.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided on request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Lakhe AA, Zinjade AS, Mane SB. Growth and yield performance of Pleurotus florida (oyster mushroom) on different agriculture substrates. Int J Sci Res Sci Technol. 2024;11(5):272-9.[CrossRef]
2. Fernandes A, Barros L, Martins A, Herbert P, Ferreira IC. Nutritional characterisation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. Produced using paper scraps as substrate. Food Chem. 2015;169:396-400.[CrossRef]
3. Tolera KD, Abera S. Nutritional quality of oyster mushroom (Pleurotus ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci Nutr. 2017;5(5):989-96.[CrossRef]
4. Bangar SP, Kajla P, Ghosh T. Valorization of wheat straw in food packaging:A source of cellulose. Int J Biol Macromol. 2023;227:762-76.[CrossRef]
5. Yang W, Guo F, Wan Z. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J Biol Sci. 2013;20:333-8.[CrossRef]
6. Singh G, Tiwari A, Rathore H, Prasad S, Hariprasad P, Sharma S. Valorization of paddy straw using de-oiled cakes for P. ostreatus cultivation and utilization of spent mushroom substrate for biopesticide development. Waste Biomass Valor. 2021;12:333-46.[CrossRef]
7. Naraian R, Sahu RK, Kumar S, Garg SK, Singh CS, Kanaujia RS. Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. Environmentalist. 2009;29:1-7.[CrossRef]
8. Khan NA, Yasin O, Aslam HM, Ikram A, Maqbool R, Akhtar M, et al. Yield improvement of oyster mushroom (Pleurotus ostreatus) production using cotton seed cake with combination of wheat straw amended with rice bran cellulosic waste materials. Int J Biosci. 2019;14(2):340-9.
9. Economou CN, Diamantopoulou PA, Philippoussis AN. Valorization of spent oyster mushroom substrate and laccase recovery through successive solid state cultivation of Pleurotus, Ganoderma, and Lentinula strains. Appl Microbiol Biotechnol. 2017;101(12):5213-22.[CrossRef]
10. Li H, Zhang Z, Li M, Li X, Sun Z. Yield, size, nutritional value, and antioxidant activity of oyster mushrooms grown on perilla stalks. Saudi J Biol Sci. 2017;24:347-54.[CrossRef]
11. Jin Z, Li Y, Rena J, Qin N. Yield, nutritional content, and antioxidant activity of Pleurotus ostreatus on corncobs supplemented with herb residues. Mycobiology. 2018;46(1):24-32.[CrossRef]
12. Zapasnik A, Bryla M, Sokolowska B, Waskiewicz A. Pleurotus spp.-an effective way in degradation mycotoxins? A comprehensive review. Mycotoxin Res. 2025;41(1):1-13.[CrossRef]
13. Kumla J, Suwannarach N, Sujarit K, Penkhrue W, Kakumyan P, Jatuwong K, et al. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules. 2020;25(12):2811.[CrossRef]
14. Ghorai S. Edible mushroom:A potent producer of industrial enzymes. J Sci Res. 2022;66(1):148-66.[CrossRef]
15. Gomathi V, Esakkiammal M, Thilagavathi SS, Ramalakshmi A. Lignocellulosic enzyme production by Termitomyces spp from termite garden. Univ J Agric Res. 2019;7(2):100-111.[CrossRef]
16. Glenn JK, Gold MH. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985;242:329-41. [CrossRef]
17. Sharma JP, Kumar S. Effect of supplementation of substrate with brans and oil cakes on yield of oyster mushroom (Pleurotus spp.). Indian Phytopathol. 2009;62(3):341-4.
18. Stevenson DG, Doorenbos RK, Jane J, Inglett GE. Structures and functional properties of starch from seeds of three soybean (Glycine max (L.) Merr.) Varieties. Starch. 2006;58:509-19.[CrossRef]
19. Revankar SR, Lele SS. Increased production of extracellular laccase by the white rot fungus Coriolus versicolor MTCC 138. World J Microbiol Biotechnol. 2006;22:921-6.[CrossRef]
20. Krishnan T, Chandra AK. Effect of oilseed cakes on alpha-amylase production by Bacillus licheniformis CUMC305. Appl Environ Microbiol. 1982;44:270-4.[CrossRef]
21. Rao PU. Chemical composition and biological evaluation of debitterized and defatted neem (Azadirachta indica) seed kernel cake. J Am Oil Chem Soc. 1987;64:1348-51. [CrossRef]
22. Bawa GS, Orunmuyi M, Agbaji AS, Ladan Z, Okekeif UO. Effect of different methods of processing neem (Azadirachta indica) seeds on performance of young rabbits. Pak J Nutr. 2007;6(3):212-6.[CrossRef]
23. Khade RS, Jadhav AC, Dhavale MC, Gaikwad AP. Evaluation of different supplementations on growth and yield of elm oyster (Hypsizygus ulmarius) mushroom. Int J Curr Microbiol Appl Sci. 2019;8(11):1084-95.[CrossRef]
24. Renganathan P, Kannan R, Raj TS, Murugavel K. Effect of various organic amendments on yield performance of multispore isolates of Pleurotus spp. Plant Arch. 2019;19(2):2899-903.
25. Hoa HT, Wang CL, Wang CH. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015;43(4):423-34. [CrossRef]
26. Mamiro DP, Mamiro PS. Yield and mushroom size of Pleurotus ostreatus grown on rice straw basal substrate mixed and supplemented with various crop residues. J Anim Plant Sci. 2011;10(1):1211-8.
27. Naraian R, Arora NK, Garg SK. Improved submerged fermentation of corn cob with mechanically broken oil seed cakes and decolorisation of textile dyes by enzyme extract of Pleurotus florida PF05. Res Environ Life Sci. 2009;2(2):83-90.
28. Singh UP. Evaluation of different supplementation effect to capsulated on the yield of oyster mushroom (P. Sajor caju.) J Pharmacogn Phytochem. 2020;9(1):1-3.
29. Maniyam MN, Gunalan P, Azman HH, Abdullah H, Yaacob NS. Decolorization of selected industrial synthetic dyes using laccase from an indigenous isolate strain SK1. J Appl Biol Biotechnol. 2025;13(2):113-21.[CrossRef]