1. INTRODUCTION
Lignocellulose derived from grasses, wood, forestry residues, agricultural, and municipal wastes is the major component of plant biomass and the most abundant, renewable raw material for producing high value-added bioproducts. Lignocellulose comprises three polymers: cellulose, hemicelluloses, and lignin. Cellulose is the major constituent of plants and the most abundant homopolysaccharide on the Earth. It makes up approximately 45% of the dry weight of wood [1].
It is a linear, crystalline, and unbranched polymer of D-glucopyranose units joined by 1,4-β-D-glycosidic linkages. Hemicelluloses are heteropolysaccharides making up about 25%–30% of the total dry weight, which includes D-xylose, D-mannose, D-galactose, D-glucose, L-arabinose, 4-O-methyl-glucuronic, D-galacturonic, and D-glucuronic [1]. Xylan is the main hemicellulosic, complex carbohydrate and it requires groups of hydrolyzing enzymes to break the β-1,4-xylan subunits into xylose.
The xylanolytic system involves several enzymes, such as α-arabinofuranosidase (E.C.3.2.1.55), α-glucuronidase (E.C.3.2.1.139), acetylxylan esterase (E.C.3.1.1.72), β-xylosidase (E.C.3.2.1.37), and endo-1,4-β-xylanase (E.C.3.2.1.8) [2,3]. Among these, endo-1,4-β-xylanases directly take part in the degradation of the glycosidic bond and produce xylooligosaccharides. Xylanases are inducible enzymes genetically having single-chain glycoproteins ranging from 6 to 80 kDa and are active at pH 4.5–6.5 [4]. Xylanases are produced by various microorganisms, including crustaceans, insects, bacteria, filamentous fungi, and yeasts. Among these, filamentous fungi are the good producers of xylanases when compared to other microbes due to their maximum production of the enzyme and fungi are easy to cultivate [5].
The cost of the carbon source also plays important role in the enzyme production. To reduce the cost of production of xylanases, inexpensive and easily available lignocellulosic sources should be preferred over expensive and pure xylan under solid state fermentation (SSF) and liquid state fermentation (LSF). It has been observed that the cost of maintenance and risk of contamination are low and the enzyme yield is high in LSF when compared to SSF [6].
Xylanases have enormous potential applications in many industrial processes. In the paper industry, it is used in the pre-bleaching process and in the feed industry it improves the digestibility of the ruminant feed [7]. Other applications of xylanases are in the food industry, in fruit juices and wine clarification [8], in production of biofuels, such as bioethanol, extraction of coffee and plant oils, etc. [9].
Therefore, considering the applications and importance of xylanases in industries, this study aims to produce and optimize the production of xylanase using wheat bran and corn as carbon sources by the fungal strain Aspergilllus flavus isolated from the decaying litter collected from the Orchha Wildlife Sanctuary.
2. MATERIALS AND METHODS
2.1. Sample collection
Degrading litter samples were collected from different regions of the Orchha Wildlife Sanctuary, Madhya Pradesh, situated at 25° 21′ 6.91″ N and 78° 38′ 25.19″ 78° 38′ 25.19″E (Figure 1). Samples were collected in sterile polythene bags with the help of a spatula and brought to the laboratory for isolating fungal strains.
2.1.1. Isolation of fungi
Isolation of fungal strains from decaying litter was carried out by using serial dilution agar plating [10] and direct inoculation method [11]. For isolation, a suspension of litter was prepared in double-distilled water which was then serially diluted up to 103 times. About 1.0 ml of this suspension was streaked over the potato dextrose agar medium, supplemented with a pinch of antibiotic, ampicillin, to avoid bacterial contamination. The petri plates were incubated at 30°C for fungal growth.
2.2. Identification of fungi
The isolated fungi were identified and characterized based on their morphological characters and microscopic analysis using taxonomic guides and tools. The morphological characteristics included growth of colony (length and width), presence and absence of aerial mycelium, color of the colony, and spore pigmentation. Standard texts and photographs were used for identification [12–14].
2.3. Examination of xylanolytic activity
The isolated fungi were screened for xylanolytic activity using the xylan agar diffusion method. Fungal isolates were allowed to grow on the malt extract agar (MEA) medium (g/L): oat spelt xylan, 10.0; peptone, 5.0; yeast extract, 5.0; K2HPO4, 0.2; and agar, 20.0 [15]. The inoculated plates were incubated at 30°C for growth. On appearance of growth, the plates were flooded with 0.1% congo red solution for 10 minutes and then washed with 1 M NaCl solution. Strains with clear zones on the plates were proved positive for the xylanolytic activity.
 | Figure 1: Location of the orchha wildlife sanctuary. [Click here to view] |
2.4. Enzyme production under LSF
LSF for xylanase production was carried out in a modified Mandel’s medium [16] containing (g/L): KH2%PO2%, 2.0; MgSO2%.7H2%O, 0.3; CaCl2%, 2.0; FeSO2%.7H2%O, 0.5; ZnSO2%.7H2%O, 1.4; MnSO2%.H2%O, 1.6; peptone 0.3; yeast extract, 0.75; distilled water, 1l; and pH, 5.5. About 0.5 g of the substrate (corn cobs, wheat bran) was suspended in 25 ml of culture medium and then the flasks were sterilized at 121°C for 20 minutes. On cooling, 1 ml of fungal spore suspension was inoculated into the flasks under sterile conditions. Then, the flasks were incubated at 30°C.
2.5. Optimization of enzyme production and assay
To obtain maximum xylanase production, several cultural conditions were optimized with respect to incubation period, temperature, pH, carbon source, and nitrogen sources like peptone and yeast extract. The enzyme assay was also optimized for incubation time, temperature, pH, and substrate (xylan) concentration.
2.5.1. Incubation period
The period of inoculation of inoculum was optimized in terms of incubation period to find out the time period for maximum xylanase production.
2.5.2. Temperature
The effect of temperature on xylanase production was determined to incubate the flasks at different temperature ranges: 20°C, 25°C, 30°C, 35°C, and 40°C.
2.5.3. pH
To find the optimum pH for maximum xylanase production, pH of the culture medium varied at 3.0, 4.0, 5.0, 5.5, 6.0, 6.5, 7.0, and 8.0. pH was maintained by 0.1N NaOH and 0.1N HCl with the help of an electronic pH meter.
2.5.4. Carbon source
Agriculture byproducts, i.e., corn cobs and wheat bran, were used as the carbon source for enzyme production. To find out the maximum xylanase production, substrate was obtained at different concentrations (g/L): 2.0, 4.0, 8.0, 12.0, 16.0, 18.0, 20.0, 22.0, 24.0, 26.0, 32.0, and 40.0.
2.5.5. Nitrogen sources
Both peptone and yeast extract are good sources of nitrogen. Different concentrations were obtained in the production medium: peptone (g/l): 0.1, 0.2, 0.3, 0.4, 0.5, 0.8, 1.0, and 1.2; and yeast (g/l): 0.6, 0.65, 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, and 1.2.
Certain parameters, such as incubation time, temperature, pH, and substrate concentration, were also optimized for the enzyme assay.
2.6. Optimization of xylanase activity
After the standardization of parameters for enzyme production, some parameters, such as incubation period, temperature, pH, and substrate concentrations, were optimized to find out maximum enzyme activity. For incubation period, the enzyme reaction mixture was incubated at different time intervals, such as 5, 10, 15, 30, 45, and 60 minutes. Similarly, optimum temperature for enzyme activity was assayed at various temperatures: 40°C, 45°C, 50°C, 55°C, 60°C, and 65°C. The pH of the reaction mixture was assayed at different pH: 4.0, 4.5, 5.0, 5.5, and 6.0. For optimization of substrate concentration, oat spelt xylan (Himedia) was obtained at different concentrations (mg/ml): 1.0, 2.0, 5.0, 10.0, 15.0, and 20.0.
2.7. Harvesting of the enzyme
The biomass of each flask was filtered through Whatman filter paper grade 1 and the filtrate was centrifuged at 10,000 rpm for 10 minutes. The supernatant was collected in sterilized glass bottles and stored at 4°C in the deep freezer (REMI) for assay purpose.
2.8. Enzyme assay
Enzyme assay was carried out by using 3, 5-dinitrosalicyclic acid method [17]. For the assay purpose, the reaction mixture contained 1.0 ml of substrate solution (1% oat spelt xylan prepared in buffer solution), 1.0 ml of 0.05M sodium citrate buffer at pH 5.3, and 1.0 ml of harvested crude enzyme. The reaction mixture was stopped by adding 3.0 ml of 3,5-dinitrosalicyclic acid (DNS). The reaction mixture was incubated at 55°C for 10 minutes. Absorbance was measured at wavelength 540 nm using spectrophotometer (Systronics 104) [18]. The enzyme activity was expressed as International units (IU)/ml.
3. RESULTS AND DISCUSSION
Saprophytic fungi play a very important role in ecosystems as decomposers, which break down the complex organic material into simple inorganic forms. They are the major component of soil microbiota ecosystems, which utilize the organic substrates present in the litter as energy and nutrient source by the process of decomposition [19]. During this study, the collection of litter samples was carried out in the month of January and October. Totally, 26 fungal strains were isolated from decaying litter soil samples. All the strains were identified with cultural characteristics and microscopic examinations of each strain with the help of standard texts, out of which A. flavus was selected for further studies (Figure 2). It was characterized to have yellow conidial heads at a young stage and become greyish green at an older stage, conidiophores develop from the substratum, conidia globose to sub-globose, echinulate, yellowish green, sometimes elliptical when young [12].
Xylanolytic activity of A. flavus was examined by a well-diffusion method on the MEA plates, supplemented with 0.1% oat spelt xylan as the sole carbon source. After 72 hours of inoculation, growth around the wells appeared and spread around, forming a clear zone. To visualize a clear zone, petri plates were flooded with 0.1% congo red solution followed by 1M NaCl (Figure 3). The appearance of a clear zone is indicative of the ability of fungus to metabolize xylan in the culture medium. After that strain was studied for xylanase production with cheaper substrates, such as wheat bran and corn cobs, and various growth parameters, such as incubation period (days), temperature, pH, carbon, and nitrogen sources, it was optimized for maximum enzyme production under LSF.
 | Figure 2: A. flavus (a) Slant culture (b) Microscopic view. [Click here to view] |
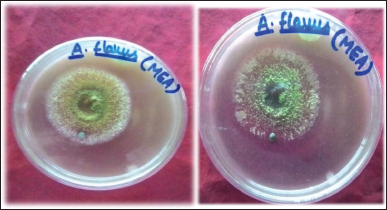 | Figure 3: Showing hydrolyzed zone (a) before congo red treatment and (b) after congo red treatment. [Click here to view] |
3.1. Optimization of incubation period on enzyme production
During this study, maximum xylanase production in terms of released sugar was high in wheat bran after 8 days of incubation, but it required a longer incubation period when compared to corn cobs powder, i.e., 6 days of incubation (Fig. 4 and Table 1). Subsequently, xylanase production was decreased due to scarcity of nutrients and accumulation of toxic compounds in the medium [20].
3.2. Optimization of temperature on enzyme production
Temperature is one of the essential parameters to find out the maximum xylanase production under LSF. To determine the optimum temperature for maximum xylanase production, A. flavus was cultured at different temperatures, ranging from 20°C to 40°C. At 30°C, maximum xylanase production was observed for both substrates. It was found that at 30°C, A. flavus produced xylanase on wheat bran 0.75 IU/ml and for corn cobs it was 1.28 IU/ml. Several studies have reported similar results for species Aspergilllus [21,22]. Above the optimum temperature, it leads to denaturing the enzymes and thus decreasing the production of enzymes under the fermentation process. The effect of A. flavus on xylanase production at various temperatures is shown in (Figure 5 and Table 2).
 | Figure 4: Effect of incubation period on xylanase production by A. flavus. [Click here to view] |
 | Table 1: Effect of incubation period on xylanase production by A. flavus. [Click here to view] |
3.3. Optimization of pH on enzyme production
The pH of the culture medium also affects the enzyme production. Most of the fungi are reported for producing xylanase in acidic medium [23,24]. They are able to grow at pH ranges of 5.0–8.0 [25]. This study observed that pH 6.0 and 7.0 were the optimum pH for corn cobs and wheat bran, respectively, for producing maximum xylanase by A. flavus, shown in (Figure 6 and Table 3). The enzyme production decreased while increasing the pH from the optimum pH. Low enzyme production was observed when pH of the culture medium was adjusted at 8.0 [26]. According to Ho and Hood [27], at pH 6.5, maximum xylanase activity was found in Aspergillus brasiliensis after 72 hours of incubation.
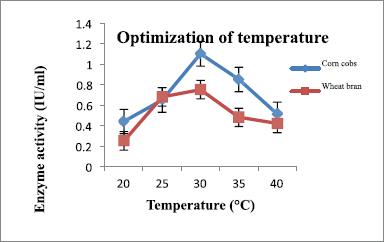 | Figure 5: Effect of temperature on xylanase production by A. flavus. [Click here to view] |
 | Table 2: Effect of temperature on xylanase production by A. flavus. [Click here to view] |
 | Figure 6: Effect of pH on xylanase production by A. flavus. [Click here to view] |
3.4. Optimization of substrate concentration on enzyme production
Another parameter is substrate concentration, i.e., the amount of substrate which has to be hydrolyzed by the fungal strain and during which hydrolyzing enzymes are released in the growth medium. The results are shown in (Figure 7 and Table 4), where the highest production was 1.22 IU/ml with 18 mg/ml concentration for corn cobs and 1.28 IU/ml with 16 mg/ml concentration for wheat bran. It can be explained that initially the substrate concentration was directly proportional to enzyme production, i.e., the increase in substrate amount favors the fungal growth, thus enzyme production was also increased. Above the optimum concentration, the fungi showed a declining trend in xylanase production because high substrate concentration led to medium viscosity and blocked oxygen supply in the medium [20].
 | Table 3: Effect of pH on xylanase production by A. flavus. [Click here to view] |
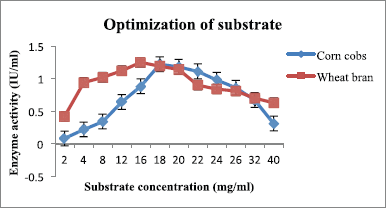 | Figure 7: Effect of substrates (corn cobs and wheat bran) on xylanase production by A. flavus. [Click here to view] |
 | Table 4: Effect of substrate concentration on xylanase production by A. flavus. [Click here to view] |
3.5. Optimization of nitrogen sources on enzyme production
Peptone and yeast extract were supplemented to the medium and suggested by many researchers as nitrogen sources [22]. The optimum concentration of peptone and yeast extract for maximum enzyme production was 0.5 mg/ml (Fig. 8 and Table 5) and 0.75 mg/ml (Fig. 9 and Table 6), respectively, for both substrates.
3.6. Characterization of enzyme
After the optimization of physical parameters for enzyme production, the enzyme was assayed for its maximum activity in IU/mL. During investigation, it was found that enzyme was highly active at 15 and 30 minutes of incubation (Fig. 10 and Table 7), 60° and 55°C (Fig. 11 and Table 8), pH 5.5 of buffer solution (Fig. 12 and Table 9), and 20.0 and 15.0 mg/ml of the xylan substrate (Fig. 13 and Table 10) for corn cobs and wheat bran, respectively.
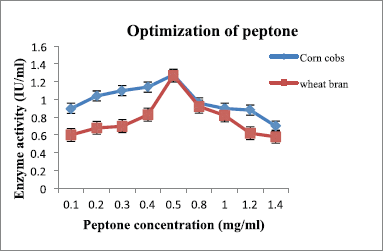 | Figure 8: Effect of peptone on xylanase production by A. flavus. [Click here to view] |
 | Table 5: Effect of peptone concentration on xylanase production by A. flavus. [Click here to view] |
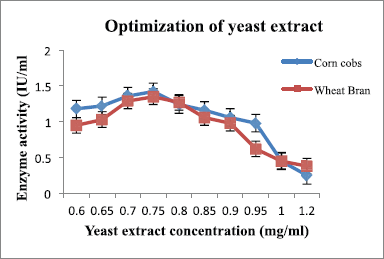 | Figure 9: Effect of yeast extract on xylanase production by A. flavus. [Click here to view] |
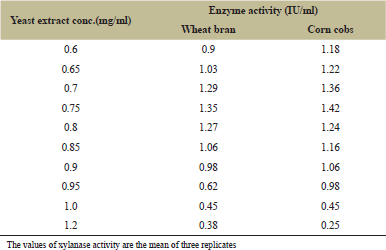 | Table 6: Effect of yeast extract concentration on xylanase production by A. flavus. [Click here to view] |
 | Figure 10: Effect of the incubation period on xylanase assay by A. flavus. [Click here to view] |
 | Table 7: Effect of incubation time (minute) on xylanase assay by A. flavus. [Click here to view] |
 | Figure 11: Effect of temperature on xylanase assay by A. flavus. [Click here to view] |
 | Table 8: Effect of temperature on xylanase assay by A. flavus. [Click here to view] |
 | Figure 12: Effect of pH on xylanase production by A. flavus. [Click here to view] |
 | Table 9: Effect of pH on xylanase assay by A. flavus. [Click here to view] |
 | Figure 13: Effect of xylan concentration on xylanase production by A. flavus. [Click here to view] |
 | Table 10: Effect of xylan concentration on xylanase assay by A. flavus. [Click here to view] |
4. CONCLUSION
The xylanase enzyme produced in this study is quite important with regard to the fact that it is produced by using cheaper agricultural residues, such as wheat bran and corn cobs, as substrates. The enzyme produced by A. flavus has become important for being cellulase-free, and thus can be recommended for industrial applications.
ACKNOWLEDGEMENT
The authors are thankful to the Principal of Bipin Bihari College, Jhansi (UP), for providing necessary facilities for the present work.
CONFLICT OF INTEREST
Authors declared that they do not have any conflicts of interest.
REFERENCES
1. Pérez J, Muñoz-Dorado J, de la Rubia T, Martinez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin. Int Microbiol 2002;5:53–63. CrossRef
2. Rahman AS, Sugitani N, Hatsu M, Takamizawa K. A role of xylanase, α-L-arabinofuranosidase and xylosidase in xylan degradation. Can J Microbiol 2003;49:58–64. CrossRef
3. Selvarajan E, Veena R. Recent advances and future perspectives of thermostable xylanase. Biomed Pharmacol J 2017;10:261–79. CrossRef
4. Ahmad Z. Production and characterization of xylanase for utilization in baking industry. University of Agriculture, Faisalabad, Pakistan, 2009.
5. Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupancic S. Production of fungal xylanases. Biores Technol 1996;58:137–61. CrossRef
6. Ho HL, Lau LY. Bioprecessing of agricultural wastes as optimized carbon source and optimization of growth conditions for xylanase production by Aspergillus brasiliensis in agitated solid state fermentation (SSF). J Biodiv Bioprocess Dev 2014;1:125.
7. Bedford MR, Classen HL. Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. J Nutr 1992;122:560–9. CrossRef
8. Biely P. Microbial xylanolytic systems. Trends Biotechnol 1985;3:286–90. CrossRef
9. Kuhad RC, Singh A. Lignocellulose biotechnology: current and future prospects. Crit Rev Biotech 1993;13:151–72. CrossRef
10. Apinis AE. Occurence of thermophilous micro fungi in certain alluvial soils near Nottingham. Nova Hedwigia, Zeitschr, Kryptogamenk 1963;5:57–78.
11. Warcup JH. The soil plate method for isolation of fungi from soil. Nature 1950;166:117. CrossRef
12. Nagamani A, Kunwar IK, Manoharachary C. Handbook of soil fungi. I.K. International Pvt. Ltd., New Delhi, India, 2006.
13. Gilman JC. A manual of soil fungi. 2nd edition, The Lowa State College Press, Ames, IA, 1957. CrossRef
14. Barnett HL, Hunter BB. Illustrate genera of imperfect fungi. 3rd edition, Burgess Publishing Company, Minneapolis, MN, 1972.
15. Nakamura S, Wakabayashi K, Nakai R, Aono R, Horikoshi K. Purification and some properties of an alkaline xylanase from alkaliphilic Bacillus sp. Strain 41M-1. Appl Environ Microbiol 1993;59(7):2311–6. CrossRef
16. Mandels M, Sternburg D. Recent advances in cellular technology. J Ferment Technol 1976;54:267–86.
17. Bailey M, Bailey J, Poutanen K. Interlaboratory testing of methods for assay of xylanase acivity. J Biotechnol 1992;23(3):257–70. CrossRef
18. Miller GL. Use of 3, 5-dinitrosalicylic reagent for the determination of reducing sugar. Anal Chem 1959;31:426–8. CrossRef
19. Jatav BK, Sharma TK, Dassani S. Succession of microfungi on leaf litter of Acacia catechu in Datia, Madhya Pradesh, India. J Pure Appl Micrbiol 2020;14(1):581–90. CrossRef
20. Bharti AK, Kumar A, Kumar A, Dutt D. Wheat bran fermentation for the production of cellulase and xylanase by Aspergillus niger NFCCI 4113. Res J Biotechnol 2018;13(5):11–18.
21. Kumar AB, Kumar A, Kumar A, Dharam B. Wheat bran fermentation for the production of cellulase and xylanase by Aspergillus niger 4113. Res J Biotechnol 2018;13(5):11–18.
22. Kanimozhi K, Nagalakshmi PK. Xylanase production from Aspergillus niger by solid state fermentation using agricultural waste as substrate. Int J Curr Microbiol App Sci 2014;3(3):437–46.
23. Subramaniyan S, Prema P. Biotechnology of microbial xylanases: enzymology, molecular biology and application. Crit Rev Biotechnol 2002;22(1):33–46. CrossRef
24. Shah AR, Madamwar D. Xylanase production by newly isolated Aspergillus foetidus strain and its characterization. Proc Biochem 2005;40:1763–71. CrossRef
25. Paredes LO, Maldonalo SH, Solis A. Solid substrate fermentation: a biotechnological approach to bioconversion of wastes In: Martin AM (ed.). Bioconversion of waste material to industrial products. 2nd edition, Kluwer, Dordrecht, Netherlands, pp 103–53, 1998. CrossRef
26. Tallapragada P. Venkatesh K. Isolation, identification and optimization of xylanase enzyme produced by Aspergillus niger under submerged fermentation. J Microbiol Biotech Res 2011;1(4):137–47.
27. Ho HLH, Hood JS. Optimization of medium formulation and growth conditions for xylanase production by Aspergillus brasiliensis in submerged fermentation (SmF). J Biodivers Bioprospect Dev 2014;1(1):1–13.