The present study provides plant-derived extracts to characterize the activity and kinetics of protease enzymes of Mentha piperita L and Thymus capitatus L. The results illustrated that these crude extracts have a proteolytic activity within optimum pH (3–9) and optimum temperature (35–50°C). The study of the ionizable groups in or around the active site of these proteases by Dixon-Webb’s plot reveals the occurrences or the presence of aspartic acid with pKa1 at pH 2.9 and pKa2 at pH 3.2, and cysteine amino acids in or around the active site with pKa1 at pH 6.7 and pKa2 at pH 7.2; interestingly, these proteases maybe belonged to the acid proteases and neutral proteases. Enzymatic kinetics studies (km, Vmax, Vmax/km) indicated that egg albumin is a good substrate for proteases of M. piperita L. and T. capitatus L.
Atrooz OM, Alomari FN. Determination of the activity and kinetics parameters of proteases in the crude plant extracts of Mentha piperita L. and Thymus capitatus L. J App Biol Biotech. 2020;8(6):33-37.
In most biological and industrial fields, protease enzymes play a crucial role and responsible for catalyzing the hydrolysis of peptide bonds in proteins and small peptides [1,2]. They participate in metabolism and regulation reactions such as the complement system, blood clotting cascade, apoptosis pathways, and other reactions [3]. There are many classifications for these proteases. They classified as two major groups: Exopeptidases and endopeptidases. In addition, they are classified according to the character of their catalytic active site and action as serine, threonine, cysteine, aspartate, and glutamic acid proteases [4]. Alternatively, proteases may be classified according to the optimal pH as basic proteases, neutral proteases, and acid proteases [5].
Proteases play a role in most physiological processes such as cell maintenance, cell signaling, food digestion, wound healing, and cell differentiation and growth [6]. Deficiency in some proteases may result in many serious disorders and abnormal tissue destruction such as cataracts, cancer, stroke, viral infections, and Alzheimer’s disease. Recently, some proteases inhibitors used for the treatment of many diseases [7].
Proteases are found in animals, fungi, and microorganisms [8,9]. They catalyze the peptide bonds and are characterized as small sized molecules, compact, and spherical structures [2].
Proteases are most industrial enzymes in the world [10]; their proteolytic action makes changes in the physicochemical, biological, and immunological properties of proteins. Modification of protein properties such as solubility, coagulation, emulsification, and foaming is examples on these changes. Improvement of the nutritional characteristics or change of flavors, taste and odors are examples on the hydrolysis of food proteins [11].
In industrial fields, proteases used in food industries, leather, milk clotting, food complements, production of emulsifiers, cheese making, meat processing, detergent, and in the treatment of many human diseases [12]. Proteases can catalyze different pathways as mediators in many cellular events such as blood clotting, apoptosis, inflammation, and other pathways [13].
In addition, to their applications in industry, and biotechnology, they have a very important role in the pharmaceutical industry. Some crude plant extracts with a high content of protease enzymes have been used in traditional medicine. Some plant extracts used in the treatment of cancer, anti-tumors, digestive disorders, and immune modulation problems [14,15].
Proteases occur naturally in all organisms, especially in microbes (bacteria, fungi, and yeast) [16]. Nevertheless, plant proteases participate in some pharmaceutical and industrial applications, and found to play an important role in plant metabolism and in some biochemical mechanisms such as plant germination, breakdown of protein, leaf senescence, and storage [17,18].
The current study was to investigate the protease activity and kinetics in two plants that used widely in folk medicine: Mentha piperita L. and Thymus capitatus L. Mentha piperita L. (Mentha) is a highly aromatic perennial plant, one of the most widely consumed herbs belonging to family Lamiaceae. Mentha has an astringent, antiseptic, antipyretic, antispasmodic, anticatarrhal, rubefacient, stimulant, emmenagogue, antiproliferative [19,20], and antimicrobial [21].
Thymus capitatus L. (Thymus) is a genus containing about 350 species belongs to the family Lamiaceae. Thyme leaves have been used in foods for the flavor, aroma, and preservation of meat, fish, and food products [22]. It possesses biological properties: Antimicrobial activity, antifungal activity, and antiseptic and antioxidant activity, and regulate gene expression [23,24].
In the current study, crude plant extracts of Mentha piperita L. (Mentha) and Thymus capitatus L. (Thymus) used to characterize the activity and kinetic parameters of protease enzymes. A literature review survey indicates that there are no studies or researches about the proteases activity or kinetics in these plant extracts.
Sodium-potassium Tartrate, casein, albumin, and cupric sulfate pentahydrate (Sigma-Aldrich, Germany). Sodium carbonate and egg albumin (BDH .GPR. England). Phenol reagents (Folin–Ciocalteu) (s.d. fine-CHEM Ltd). Sodium phosphate-3-hydrate and sodium acetate-3-hydrate (DAB, PH.EUR, B.P., PH. Franc). Acetic acid glacial (NenTech Ltd., UK). Trichloroacetic acid (TEDIA Company, Inc., USA).
Mentha piperita L. (Mentha) and Thymus capitatus L. (Thymus) freshly collected from local markets (once) in September 2014–2015. The plants’ aerial parts (leaves) thoroughly washed with distilled water and refrigerated at 4oC until used. The research was done in the biochemistry lab at the Department of Biological Sciences, Mutah University.
Forty grams of plant leaves were ground by an electric homogenizer. The suspensions were finely powdered and mixed with phosphate-buffered (0.1 M, pH 7.0). Then, the homogenized mixture was filtered and centrifuged for 10–15 min. The collected supernatant was considered as the crude plant extract (enzyme solution) [1].
Protein concentration determined by the Lowery colorimetric method [25]. The bovine serum albumin was the standard used. The standard curve used to determine the protein amount in the extracts (mg/ml).
The protease activity is determined by Fahmy method [17]. Briefly, to an Eppendorf tube, 50 ml of the crude plant extract (enzyme solution) was added to 650 ml of 50 mM phosphate-buffered (pH 6.5) and 100 ml of egg albumin (as a substrate) at 37°C for 1 h. Then, 200 ul of 10% trichloroacetic acid was added to stop the reaction. After centrifugation, the absorbance of the supernatant measured at wavelength 360 nm using UV-Vis spectrophotometer (Biotech Engineering Management Co. LTD, UK). The activity of protease was defined as the amount of enzyme that hydrolyzes 1 mmol of amino acids (in terms of tyrosine) from egg albumin per minute under the standard assay conditions.
The acetate buffer (50 mM, pH 2.0–5.0) and phosphate-buffered (50 mM, pH 6.0–10.0) were used to determine the effects of pH on the enzyme activity [3]. The initial velocity data obtained at different pH values used to indicate and determine the possible ionizable groups in the active site of the protease enzyme by using Dixon–Webb’s plot [26]. The temperature range 25–60°C was used to perform the effects of temperature on the enzyme activity [3].
Substrate specificity was determined as a relative proteolytic activity of crude plant extracts of proteases by the Patil method using egg albumin as a substrate with minor modifications. Kinetic experiments performed using varying concentrations of substrate (1–3%) and Line weaver–Burk plot used to determine the Km, and the Vmax [27].
The assays were performed in triplicate and expressed as mean ± standard deviation (SD). The statistical analysis was performed by t-test, if P ? 0.05 was statistically significant.
Protease plays a key role in physiological as well as in many pathological processes. Many diseases are linked with deficiency or abnormality of protease enzymes such as Arthritis, cancer, viral infections, and some degenerative diseases. They have found extensive applications in bioremediation processes.
The present study investigated the activity and kinetic parameters of protease enzymes obtained from green leaves of Mentha and Thymus. Egg albumin was used for substrate specificity.
The protein concentrations in crude plant extract estimated by the Lowry method. Table 1 shows the protein concentration in the crude extracts of the selected plants. From the standard curve, the protein concentration in the extracts calculated. The highest protein concentration found to be in the Mentha (58.16 mg/ml) and low concentration in Thymus (5.67 mg/ml), relatively.
Table 1: Protein concentration (μg/ml) in the crude plant extracts of the selected plants. Data presented are Means±SD of n=3 experiments
| Sample | Protein concentration (µg/ml) |
|---|---|
| Mentha piperita L. (Mentha) | 58.16±4.5 μg/ml |
| Thymus capitatus L. (Thymus) | 5.67±1.6 μg/ml |
For Thymus, the profile of pH relative activity ranged between pH 2.0 and 3.5 with maximum relative activity 65% and at pH 6.0–8.0 with maximum relative activity 100% [Figure 1]. Therefore, Thymus has two optimum pH at 3.0 and 6.5. On the other hand, Mentha has two optimum pH at 3.0 and 9.0 with relative activity 100% and 70%, respectively [Figure 1]. In general, the presence of two optimum pH may be due to the presence of isoenzymes for the particular protease.
.png) | Figure 1: The relative activity (%) of the pH profile of the protease enzymes in crude plant extracts of Thymus and Mentha. Data presented are means±SD of n = 3 experiments [Click here to view] |
In the literature, it was reported that the optimum pH for most plants is different and it was ranged from 3.0 to 10.0. For example, Ali et al. [28] reported that Carum copticum has two peaks at pH 3.0 and pH 7.0 while for Allium cepa has one optimum pH at 10.0 (alkaline). In comparison with fungal proteases, the majority of the pH optimum ranged between 8.0 and 12.0, while for bacteria are varied from one strain to another and ranged between 3.0 and 11.0 [29].
Dixon–Webb’s plot used to indicate and determine the ionization groups in the active site of the protease enzymes. According to the Dixon and Webb’s “protein conformation can be maintained within 4-5 pH units but activity different,” it means that the ionizable groups of amino acids in the active site are affected by pH. The analysis of the Dixon–Webb’s plot of the Thymus and Mentha is shown in Figures 2 and 3, respectively, these figures illustrate the presence of aspartic acid and cysteine amino acids in both of the selected plants, which indicate that proteases in the crude plant extracts of these plants may belong to the acidic and neutral proteases.
.png) | Figure 2: Dixon–Webb’s plot for the determination of possible ionizable groups in the active site of proteases of Thymus using egg albumin as a substrate. Data presented are means ± SD of n = 3 experiments [Click here to view] |
.png) | Figure 3: Dixon–Webb’s plot for the determination of possible ionizable groups in the active site of proteases of Mentha using egg albumin as a substrate. Data presented are means ± SD of n = 3 experiments [Click here to view] |
In both Thymus and Mentha, the ionizable groups found to exhibit two types. One type with pKa1 at pH 6.7 and pKa2 at pH 7.2 which suggests the presence of the amino acid cysteine in the active site of the enzyme and therefore maybe belong to the neutral proteases group (Cysteine protease), while the other ionizable groups have pKa1 at pH 3.2, and therefore maybe belong to the acidic proteases group (Aspartic protease) [Figures 2 and 3]. The results of pKas, suggest the presence of aspartic acid and cysteine amino acids in and around the active sites of these protease enzymes [30,31].
Aspartic proteases (EC 3.4.23) are that proteolytic enzymes have two aspartic acids in their active sites. On the other hand, the catalytic mechanisms of the cysteine proteases, it involves the nucleophilic cysteine sulfur group in the catalytic dyad. In general, the cysteine proteases such as Actinidain, Bromelain, Calpains, Caspases, Papain, and others are found in fruits, leaves of different plant families.
The optimum temperature of an enzyme is considered a very important factor especially for those proteases used in the industry. In general, most industrial enzymes require a higher temperature than physiological enzymes.
Figure 4 shows that maximum relative activity (100%) for proteases of Thymus at temperature 40°C. The activity profile of the temperature for Mentha was different. The range of the activity from 25 to 60°C with the lowest relative activity 60% at temperature 45°C and high activity at temperature 35°C ( 100%) and relative activity 90% at temperature 50°C [Figure 4]. This thermal stability of proteases may be due to the presence of some metal ions needed for their biological activities [4]. The activity profile for elevation temperature above 50°C for all plants shows a sharp decrease in the activity. The explanation for these results depends on the protein nature of enzymes that are the elevation of temperature above 60°C leads to protein denaturation.
.png) | Figure 4: The relative activity (%) of the temperature profile of the protease enzymes in crude plant extracts of Thymus and Mentha. Data presented are means±SD of n = 3 experiments [Click here to view] |
In addition to pH and temperature, substrate concentrations considered as one of the main factors for determining the activity of the enzymes. In addition, it can be used for determining the enzyme kinetics (Km and Vmax) to illustrate the enzyme affinity and specificity. In the experiments, different concentrations of egg albumin (0.5–3.0%) were used. It was found that Vmax was reached at a concentration of 3% for the egg albumin substrate.
Line weaver-Burk plots were used to calculate the Km and Vmax of proteases enzymes. Figure 5 illustrates the plots of Km and Vmax for the proteases of the Thymus and Mentha and all the results summarized in Table 2. Michaelis–Menten constant (Km) and maximum reaction velocity (Vmax) of the protease enzymes were determined using the above substrate.
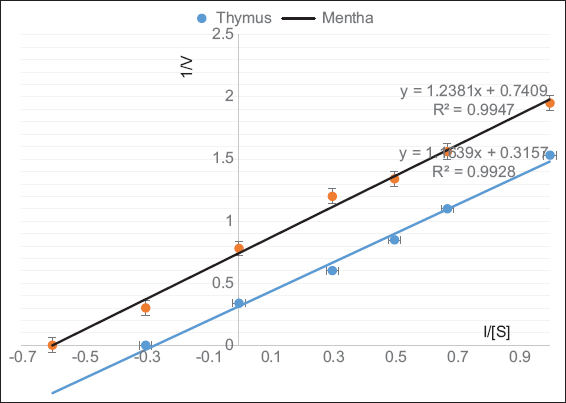 | Figure 5: Determination of Km and Vmax values for protease enzyme in the crude plant extracts of the Thymus and Mentha using egg albumin as a substrate. Data presented are means ± SD of n = 3 experiments [Click here to view] |
Table 2: Comparison between the Vmax, Km, and the ratio of Vmax/ Km for the crude plant extracts (proteases) of the selected plants in means of egg albumin as substrates
| Sample | Egg albumin | ||
|---|---|---|---|
| Vmax (U/mg) | Km (mg/ml) | Vmax/ Km | |
| Mentha piperita L. | 2.94 | 3.33 | 0.883 |
| Thymus capitatus L. | 1.3 | 1.7 | 0.764 |
Studying the kinetics of an enzyme considered a very important value in understanding the affinity and conditions suitable to obtain optimum activity, especially, if these enzymes used for industrial and biochemical applications. Proteases are one of the most groups of enzymes that play a role in many fields of industry.
According to the obtained data from Line weaver-Burk plots, substrate specificity (Vmax/Km) was calculated. Results in Table 2 showed different affinities toward egg albumin as a substrate. Km value is a measure of the affinity of the proteases for the substrate, the smaller the value the higher the affinity of the enzyme for that substrate while Vmax shows the catalytic efficiency, the higher the Vmax the higher the efficiency.
In general, the best substrate for any enzyme depends on the strong binding of the substrate to the enzyme (low Km) and high proteolytic activity (high Vmax). It was found that the egg albumin has more affinity to toward Mentha proteases (Km 1.7 mg/ml) than Thymus proteases (Km 3.33 mg/ml), while Thymus proteases have higher catalytic efficiency (Vmax 2.94 mmol/min) than Mentha proteases (Vmax 1.3 mmol/min). By considering the ratio (Vmax/Km) [Table 2], the observed results indicated that egg albumin was a good substrate for proteases of Mentha (0.764) and Thymus (0.882).
The results showed that the egg albumin is a good substrate for the proteases enzyme in the crude plant extracts of Mentha and Thymus. The activity and kinetics of these proteases have a strong binding to the substrate with high affinity and hydrolytic activity at 35°C and 40°C, and at pH 3.0 and 7.0, respectively. These results encourage the use of these enzymes in various industrial fields, especially food industries. It is the first study to determine the activity and kinetics of the proteases of these plants and the first to indicate that these proteases belong to aspartic and cysteine proteases groups (endopeptidases).
Part of this research based on Fatmah Alomari’s master thesis work. Prof. Omar M. Atrooz was the supervisor, writer, and editor of this paper.
Authors declared that there are no conflicts of interest.
None.
1. Akhtaruzzaman S. Role of thiol proteases in tissue injury. Am J Resp Crit Care Med 2012;150:155-9. [CrossRef]
2. Tran TH, Ton TH, Nguyen PH, Tran TK, Vu TT, Bui LM, et al. Expression and Characterization of a new serine protease inhibitory protein in Escherichia coli. Biomed Res Ther 2020;7:3633-44. [CrossRef]
3. Ibrahim MA, Olatominwa JO, Aliyu AB, Bashir M, Sallau AB. Partial characterization of protease from the leaves of Jatropha curcas. Int J Biol 2012;4:79-85. [CrossRef]
4. Al-Sherri MA. Mostafa S. Products form and properties of protease produced by Bacillus licheniformis. Biol Sci 2004;7:1631-5. [CrossRef]
5. Sandhya C, Sumantha A, Pandey C, Webb CR, Soccol C. Proteases in Enzyme Technology. New Delhi, India: Asiatech Publishers Inc.; 2004. p. 312-25.
6. Vanndita B, Parth K, Prasidhi T, Kameshwar SY. Enzymatic determination of catechol oxidase and protease from fruits (orange, apple) and vegetables (carrot, tomato). IOSR J Pharm Biol Sci 2013;5:29-35. [CrossRef]
7. Rawlings ND, Morton FR, Barrett T. MEROPS: The Peptidase Database. Nucleic Acids J Res 2006;34:D270-2. [CrossRef]
8. Jovanotti GA, Freitas SM, Silva LP. Proteinaceous protease inhibitors: Structural features and multiple functional faces. Curr Enzyme Inhib 2006;2:199-217. [CrossRef]
9. Christeller JT. Evolutionary mechanisms acting on proteinase inhibitor variability. FEBS J 2005;272:5710-22. [CrossRef]
10. Leary D, Vierros M, Hamon G, Arico S, Monagle C. Marine genetic resources: A review of scientific and commercial interest. Marine Pol 2009;33:183-94. [CrossRef]
11. Pardo MF, Lopez MI, Canals F, Zviles FX, Natalucci CL, Caffini N. Purification of balansain I, an endopeptidase from unripe fruits of Bromelia balansae Mez (Bromeliaceae). J Agric Food Chem 2000;48:3795-800. [CrossRef]
12. Cheng K, Lu FP, Li M, Liu LL, Liang XM. Purification and biochemical characterization of a serine alkaline protease TC4from a new isolated Bacillus alcalophilus TCCC11004 in detergent formulations. Afr J Biotechnol 2010;9:4942-53.
13. Ivanova D, Emonet C, Foata F, Affolter M, Delly M, Fisseha M, et al. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase like serine proteases. J Biol Chem 2006;281:17246-52. [CrossRef]
14. Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol 2010;127:760-7. [CrossRef]
15. Padul MV, Tak RD, Kachole MS. Protease inhibitor (PI) mediated defense in leaves and flowers of pigeon pea (protease inhibitor mediated defense in pigeon pea). Plant Physiol Biochem 2012;52:77-82. [CrossRef]
16. Tang J, Li HL, Shen YH, Jin HZ, Yan SK, Liu RH, et al. Antitumor activity of extracts and compounds from the rhizomes of Veratrum dahuricum. Phytother Res 2008;22:1093-96. [CrossRef]
17. Fahmy AS, Ali AA, Mohamed SA. Characterization of a cysteine protease from wheat Triticum aestivum. Bioresour Technol 2004;91:297-304. [CrossRef]
18. Krystyna O, Marlena S. Cellular recycling of proteins in seed dormancy alleviation and Germination. Front Plant Sci 2016;7:1128-36. [CrossRef]
19. Torres-MartÃnez Y, Arredondo-Espinoza E, Puente C, González-Santiago O, Pineda-Aguilar N, et al. Synthesis of silver nanoparticles using a Mentha spicata extract and evaluation of its anticancer and cytotoxic activity. Peer J 2019;7:e8142-55. [CrossRef]
20. Felicia P, Alessandro P, Sara V, Loris B, Marco R, Zorzan M, et al. Anticancer effects of wild mountain Mentha longifolia extract in adrenocortical tumor cell models. J Front Pharmacol 2020;10:1647-58. [CrossRef]
21. Chahrazed B, Amine B, Ryad D, Mahmoud R. The effects of Mentha Ã? piperita essential oil on C. albicans growth, transition, biofilm formation, and the expression of secreted aspartyl proteinases genes. Antibiotics 2019;8:1-15. [CrossRef]
22. Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. 3rd ed. United States: Thomson PDR, Inc.; 2004. p. 815-6.
23. Mostafa K, Pezhman M, Mohammad RD, Saeed DN, Pouran K. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1a content and endurance exercise performance in rats. J Int Soc Sports Nutr 2017;14:11-9. [CrossRef]
24. Khalifa FK, Alkhalaf MI. Effects of black seed and thyme leave dietary supplements against malathion insecticide-induced toxicity in the experimental rat model. J King Saud Univ Sci 2020;32:914-9. [CrossRef]
25. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
26. Patil UK. Studies on Novel Thermostable and Solvent-tolerant Alkaline Protease from Bacteria, Ph.D. Thesis. India: North Maharashtra University, Jalgaon (Maharashtra State); 2010.
27. Weaver HL, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658-66. [CrossRef]
28. Ali S, Qazi A, Khan MR. Protease activity in seeds commonly used as a herbal Medicine. Pak J Med Res 2003;42:2.
29. Hajji M, Kanoun S, Nasri M, Gharshallah N. Purification and characterization of an alkaline serine-protease produced by a new isolated Aspergillus clavatus ES1. Proc Biochem 2007;42:791-7. [CrossRef]
30. Jakubowski H. Biochemistry Online: An Approach Based on Chemical Logic. New York: College of Saint Benedict, Saint John's University. 2013.
31. Alfredo A. Screening of Mentha Cordifolla Opiz buffer crude extract as a putative HIV-1 translational protease inhibitor. BMJ Open 2015;5:A1-53.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Year
Month
Characterization of the crude extract of Portulaca oleracea and the determination of the polyphenol oxidase kinetics in the presence of Cu and Zn
Omar Mohammad Atrooz, Shada Zaher Al-MaitahSolid-state fermentation of groundnut (Arachis hypogaea) shell using Trichoderma sp., tape yeast, and tempeh yeast to produce cellulase
Muhammad Yusuf Abduh, Chalil Rizqullah Ramadhan, Alfanny Putri Fadhlilah, Siti Dhiffah Nabilah Abdul, Khairul Hadi BurhanAntimicrobial effect of nanofluid including Zinc oxide (ZnO) nanoparticles and Mentha pulegium essential oil
Mona Jahanpanahi, Ali Mohamadi SaniApplication of Mentha suaveolens essential oil as an antimicrobial agent in fresh turkey sausages
Abdelaziz Ed-Dra, Fouzia Rhazi Filai, Mohamed Bou-Idra, Badr Zekkori, Aziz Bouymajane, Najia Moukrad, Faouzia Benhallam, Amar BentayebAntimicrobial effects of edible nano-composite based on bean pod shell gum, nano-TiO2, and Mentha pulegium essential oil
Mozhgan Nasiri, Ali Mohamadi Sani, Vahid Hakimzadeh, Mostafa ShahidiAssessment of matK, rbcL, rpoC1, trnA-trnH, ycf5, and ITS for DNA barcoding of a Mentha species from Madinah city, Saudi Arabia
Rashid Ismael Hag Ibrahim