1. INTRODUCTION
Plants have been considered an ultimate source of medicine. Plants offer numerous medicines to the mankind and the essentials of typical traditional schemes of medicines which have been beneficially explored for various years entirely rely on plants. Plant derived drugs have transfigured modern medicinal practice also [1]. Evaluation and analyzing of active plant compounds and plant based drugs could contribute a lot in the advent of pioneering epoch of healthcare scenario and medicaments. Isolation of plant compounds plays a significant role in discovery of different and new therapeutic reliefs. Moreover, demands for plant-derived products are increasing throughout the globe [2]. Besides that, the demands for plant-derived medicines have been increasing throughout the globe. Justicia adhatoda L. belongs to family acanthaceae and is widely acknowledged for its effectiveness in curing respiratory problems. It has been extensively utilized in treating issues such as asthma, chronic bronchitis, and other problems for eras in Ayurveda [3]. J. adhatoda L. embrace countless biologically active compounds and its phytochemical profile could be explored for the improvement of a number of plant based drugs [4]. The fresh leaves of J. adhatoda L. have been known to exhibit stimulant effect on the respiratory tract, it has been used by Yogis and Sadhus to get relief from various ailments [5]. The various biological activities exhibited by J. adhatoda L. could be ascertained as hepatoprotective, antiproliferative, cardioprotective, antimalarial, anti-inflammatory, and oxidative DNA damage protection and all these activities could be ascribed due to the occurrence of efficient bioactive compounds such as primary metabolites, vitamins, secondary metabolites, organic acids, and minerals [6]. Moreover, the environmental condition under which the plants develop holds a significant impact on its various attributes and that ultimately affects its medicinal efficacy [7,8]. Variation in factors such as fluctuation in day length and annual temperature range, solar radiations, and photoperiod affect plant’s morphology and biological mechanisms [9]. Plants alter their performances to adapt to a certain environmental condition [10]. In this perspective, it stands paramount important to highlight variations in biochemicals which occur with the progression of time and different environmental factors.
2. MATERIALS AND METHODS
2.1. Morphological Analysis
The present study was done on J. adhatoda L. for two consecutive years, namely, 2019 and 2020 in Jammu region and in case of morphological analysis, measurements of 5 plants from each sampling site were taken and growth characteristics, namely, plant height, leaf length, and leaf width which were measured by the help of ruler and expressed in centimeters, whereas number of branches were counted. The leaf area was estimated by protocol of Green-armytage [11], In case of crown spread, method described by Blozan [12] was followed.
2.2. Biochemical Composition
Carbohydrates were determined by the method of Thangaraj [13]. Protein content was estimated by following the method of Thangaraj [13]. Amino acids were estimated by following protocol of Thangaraj [13]. Crude fiber was estimated by following the protocol of Chandaka et al. [14]. Crude fat was estimated by Thangaraj’s [13] method. Potassium and sodium were determined in flame photometer by protocol given by Olaniyi et al. [15]. Alkaloids were determined by Harborne’s [16] method. The flavonoids were determined by following Zao et al. [17]. Phenols were determined by following protocol of Sethi and Sharma [18]. Tannin was estimated by the protocol of Saxena et al. [19]. Saponin was determined by protocol of Mir et al. [20].
3. RESULTS AND DISCUSSION
In the present study, significant variation was observed in the carbohydrate content. The varied from 30 ± 4.88 mg/g (1st year) to 25.8 ± 2.9 mg/g (2nd year) [Figure 1]. Carbohydrates act as a source of several metabolic reactions [23]. Previous studies have reported that plants adopt to different environmental conditions by altering several metabolic pathways, for example, carbohydrate synthesis. A significant increase or decrease in carbohydrate content can play an important role in plants, for example, increased carbohydrate content possess a significant impact on osmoregulation, accumulation of sucrose during the time of stress [24]. The protein content varied from 15.9 ± 1.87 mg/g (1st year) to 19.1 ± 2.07 mg/g (2nd year). Proteins play a key role in body development, hormone formation, maintaining fluid balance, enzyme formation, development of immunity, etc. The crude fiber content of J. adhatoda L. leaves varied from 5.6 ± 0.75% (1st year) to 4.9 ± 0.58% (2nd year). Crude fiber plays an essential role in absorbing trace elements in gut [25]. The crude fat varied from 5.6 ± 0.75% (1st year) to 4.9 ± 0.5% (2nd year) [Figure 2]. Fats have also been known to contribute to some medicinal applications [26]. Amino acids varied from 14.3 ± 0.69 mg/g (1st year) to 13.8 ± 0.7 mg/g (2nd year) [Figure 1]. Amino acids play a major role in plant’s metabolism. They act as nitrogen donors in various metabolic reactions [27]. Potassium content was found to be 2.3 ± 0.42 ppm (1st year) and 2.7 ± 0.1 ppm (2nd year). Sodium content varied from 0.26 ± 0.2 ppm (1st year) to 0.14 ± 0.0 ppm (2nd year) [Figure 3]. Potassium and sodium both take part in sustaining ionic balance in cells and also help in production of gastric juice in stomach [28]. Phenols varied from 1.2 ± 0.18 mg/g (1st year) to 1.2 ± 0.03 (2nd year) [Figure 1]. Plants possess ultraviolet (UV) protective systems to cope up with the increasing harmful UV radiations. Phenolic compounds play a key role in defense as well as in other mechanisms of the plant and during stress conditions [29]. Flavonoids varied from 1.7 ± 1.88 mg/g to 2.7 ± 0.67 [Figure 1]. Flavonoids are also considered a major UV protectant compound due to their ability to absorb UV radiations and scavenge UV-induced free radicals [30]. The tannin content varied from 3.68 ± 0.05 mg/g (1st year) to 3.9 ± 0.19 mg/g (2nd year), alkaloids varied from 43.8 ± 2.9% (1st year) to 45.5 ± 0.6% (2nd year), and saponin content varied from 31.9 ± 5.1% (1st year) to 29.1 ± 7.57% (2nd year). However, the variation was not statistically significant [Figure 2]. In morphological analysis, J. adhatoda L. height, the plants varied from 143.6 ± 16.5 cm (1st year) to 156.8 ± 9.88% (2nd year) and crown spread from 198.6 ± 3.2 cm (1st year) to 180.2 ± 27.26 cm (2nd year). Plant’s ability to adapt to the suitable environmental conditions can be related to the morphological or physiological properties displayed by its organs [31-33]. The factors such as competition for nutrition and light lay a strong impact on the growth of plants. In a study, growth of small mountain beech trees was affected due to the competition for light and nutrients [34]. Short stature of plants is also known to possess some benefits for instance, provides protection against stresses like winds, reduced nutrient availability [35]. The number of primary branches in our study were significantly low during 1st year, that is, 95.6 ± 19.26 and high during 2nd year at, that is, 133 ± 23.01 and maximum number of secondary branches were found during 2nd year, that is, 150.2 ± 38.44 and minimum during 1st year at, that is, 127 ± 30.14. No significant change was observed in number of tertiary branches, namely, 47.8 ± 10.96 and 52 ± 18.22 [Figure 4]. With decreasing height, mechanical damage in branches and trunks of the trees were also observed in some studies [36]. The environment in which the leaves are produced has a significant influence on leaf morphology [37,38]. Other abiotic and biotic factors, namely, exposure to strong wind regimes, soil mineralization and nitrification, transpiration rates, rate of photosynthesis, and differences in light intensity could be considered as contributing factors to the variations [39,40]. In the present study, variations in leaf morphology were also insignificant. Leaf length for the 1st year was 12.96 ± 0.76 cm and 14.3 ± 1.4 cm for 2nd year. Leaf width during 1st year was 5.54 ± 0.5 cm and 5.14 ± 0.23 cm for 2nd year [Figure 5]. The leaf area during the 1st year was 47 ± 1.58 cm2 and 45.6 ± 1.14 cm2 for 2nd year.
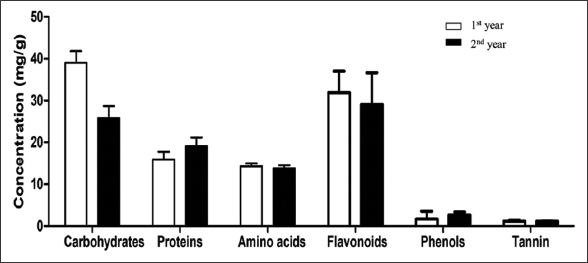 | Figure 1: Concentration of carbohydrates, proteins, amino acids, flavonoids, phenols, and tannin in Justicia adhatoda L. [Click here to view] |
 | Figure 2: Concentration of crude fiber, crude fat, alkaloids, and saponin in Justicia adhatoda L. [Click here to view] |
 | Figure 3: Potassium and Sodium of Justicia adhatoda L. [Click here to view] |
 | Figure 4: Different branches of Justicia adhatoda L. [Click here to view] |
 | Figure 5: Plant height, crown spread, leaf length, leaf width, and leaf area of Justicia adhatoda L. [Click here to view] |
The antibacterial efficacy of J. adhatoda L. leaf extracts was considerable against all the tested strains. All the extracts showed inhibition of microbial growth in comparison to that of standard drug Ampicillin [Figure 6 and Table 1]. J. adhatoda L. leaf extracts were able to inhibit mycelia growth better in Rosellinia in comparison to that of Fusarium [Figure 7 and Table 2]. However, the comparison between antimicrobial activities from leaf extracts of both the years was made and no significant result was observed. Studies have suggested that plant’s antimicrobial activity is mainly due to its phytochemicals, for instance, the antimicrobial effect of phenolic compounds is because they possess a significant impact on the permeability of membrane and ratio of penetration in bacterial cell to promote the damage and inactivation of cellular profile of microbes [41,42]. Yearly variations hold a significant impact on plant biochemical composition, for example, in a study, significant variations in quantity of phytochemicals were recorded in Fragaria ananassa when those plants were harvested at different times of the year. The study revealed that when the plants were harvested earlier in the year, the phytoconstituents were available in low amounts [43]. In another study, changing environmental situation throughout the year and impact of different seasons lead to significant change in quantity of phytochemicals of Rubus chamaemorus. The study lead to a deduction that the extent of phytochemicals and nutritional quality of plants primarily depended upon the time of day as well as the time of season, in which they were harvested [44]. Various bioactive molecules present in plants play their part in antimicrobial action, for example, alkaloids, flavonoids, and terpenes which contain tremendously variable chemical structures with high potential antibacterial activities [45,46]. Plants develop defense strategies to protect themselves from various microbes occurring in the environment. There is an accumulation of defense compounds chiefly, secondary metabolites involving potent antimicrobial activities. It is expected that these bioactive plant derived secondary metabolites play a key role in synthesizing antimicrobial agents with certain pharmacological effect which, in turn, helps if any bacteria or fungi attacks the plants [47].
 | Figure 6: Effect of different concentrations of Justicia adhatoda L. ethanolic leaf extract on Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Klebsiella pneumonia. [Click here to view] |
Table 1: Antibacterial activity of Justicia adhatoda L. ethanolic leaf extracts against selected standard and clinical bacterial isolates. Data are represented as mean±standard deviation.
| S. No. | Bacterial Strains | Year | Antibacterial zone (mm) | ||||
|---|---|---|---|---|---|---|---|
| Control (Ampicillin) | Concentration of leaf extract (mg/ml) | ||||||
| 0.5 | 1 | 1.5 | 2 | ||||
| 1 | Escherichia coli | 1st year | 21.5±0.264 | 12.1±0.529 | 13.1±0.550 | 13.2±1.011 | 13.3±0.404 |
| 2nd year | 21.2±0.655 | 11.4±0.173 | 13.7±0.173 | 13.5±0.493 | 13.3±0.838 | ||
| 2 | Pseudomonas aeruginosa | 1st year | 21.4±0.472 | 11.8±0.173 | 12.7±0.173 | 13±0.152 | 13.7±0.208 |
| 2nd year | 21.2±0.152 | 11.3±0.4 | 12.3±0.435 | 13.2±0.493 | 13.6±0.556 | ||
| 3 | Staphylococcus aureus | 1st year | 21.8±0.55 | 11.2±0.115 | 12.5±0.305 | 12.8±0.404 | 13.1±0.435 |
| 2nd year | 21.7±0.568 | 11.6±0.519 | 12.6±0.115 | 13.3±0.321 | 13.7±0.152 | ||
| 4 | Klebsiella pneumoniae | 1st year | 22.3±0.45 | 12.1±0.602 | 12.3±0.378 | 13.3±0.7 | 13.8±0.1 |
| 2nd year | 22±0.288 | 11.7±0.6 | 12.6±0.2 | 13.2±0.416 | 13.7±0.351 | ||
Table 2: Anti-fungal activity of Justicia adhatoda L. ethanolic leaf extracts against selected fungal pathogens.
| Year | Anti-fungal activity | |||
|---|---|---|---|---|
| Rosellinia necatrix | Fusarium ssp | |||
| (%) Mean Inhibition | Standard deviation | (%) Mean Inhibition | Standard deviation | |
| 1st year | 41.08 | 0.013 | 23.58 | 0.014 |
| 2nd year | 37.20 | 0.023 | 26.82 | 0.024 |
 | Figure 7: Anti-fungal activity of Justicia adhatoda L. [Click here to view] |
4. CONCLUSION
Plant’s ability to adapt to suitable environmental conditions relies on the morphological and physiological properties exhibited by its organs. Daily and seasonal environmental fluxes in temperature, light, precipitation, and humidity play a chief role in regulating plant’s biological mechanisms. The biochemicals of J. adhatoda L. build up its strong nutritional as well as pharmacological profile and have been known to contribute in various important metabolic reactions. The present study justifies its use as an important commercial plant with potent medicinal properties. J. adhatoda L. should be explored for further studies.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors requirements/guidelines.
6. FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All data generated or analyzed during this study are included in this published article.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Dar R, Shahnawaz M, Qazi P. General overview of medicinal plants:A review. J Phytopharmacol 2017;6:349-51. [CrossRef]
2. Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants:Past history and future perspective. J Herb Med Pharmacol 2018;7:1-7. [CrossRef]
3. Hossain TM, Hoq OM. Therapeutic use of Adhatoda vasica. Asian J Med Biol Res 2016;2:156-63. [CrossRef]
4. Jayapriya G, Shoba FG. GC-MS analysis of bio-active compounds in methanolic leaf extracts of Justicia adhatoda (Linn.). J Pharmacogn Phytochem 2015;4:113-7.
5. Gangwar AK, Ghosh, AK. Medicinal uses and pharmacological activity of Adhatoda vasica. Int J Herb Med 2014;2:89-91.
6. Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera:A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016;6:1-14. [CrossRef]
7. Schumann T, Paul S, Melzer M, Dormann P, Jahns P. Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Front Plant Sci 2017;8:1-18. [CrossRef]
8. Wang M, Zhang J, Guo Z, Guan Y, Qu G, Liu J, et al. Morphological variation in Cynodon dactylon (L.) Pers., and its relationship with the environment along a longitudinal gradient. Hereditas 2020;177:104132. [CrossRef]
9. Rapinski M, Liu R, Saleem A, Arnason JT, Cuerrier A. Environmental trends in the variation of biologically active phenolic compounds in Labrador tea, Rhododendron groenlandicum, from Northern Quebec, Canada. Botany 2014;92:783-94. [CrossRef]
10. Read QD, Moorhead LC, Swenson NG, Bailey JK, Sanders NJ. Convergent effects of elevation on functional leaf traits within and among species. Funct Ecol 2014;28:37-45. [CrossRef]
11. Green-Armystage S. Extraordinary leaves. Color Res Appl 2008;31:253-69. [CrossRef]
12. Blozan W. Tree measuring guidelines of the Eastern native tree society. Bull Eastern Native Tree Soc 2006;1:3-10.
13. Thangaraj P. Proximate composition analysis. Prog Drug Res 2016;71:21-31. [CrossRef]
14. Chandaka M, Murali KK, Ramanji KR, Jhansi PL, Eswar Kumar K. Estimation of crude fibre content from natural food stuffs and its laxative activity induced in rats. Int J Pharma Res Health Sci 2017;5:1703-6. [CrossRef]
15. Olaniyi MB, Lawal IO, Olaniyi AA. Proximate, phytochemical screening and mineral analysis of Crescentia cujete L. leaves. J Med Plants Econ Dev 2018;2:1-7. [CrossRef]
16. Harborne JB. Phytochemicals Methods. London:Chapman and Hall Ltd.;1973.
17. Zao Y, Lu Y, Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. In vitro. J Agric Food Chem 2004;52:5032-9. [CrossRef]
18. Sethi A, Sharma RA. Antioxidant activity with total phenolic constituents from Aerva tomentosa Forsk. Int J Pharma Bio Sci 2011;2:596-603.
19. Saxena V, Mishra G, Saxena A, Vishvakarma KK. A comparative study on quantitative estimation of tannins in Terminalia chebula, Terminalia belerica, Terminalia arjuna and Saraca indica using spectrophotometer. Asian J Pharm Clin Res 2013;6:148-9.
20. Mir MA, Sawhney SS, Jassal MM. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J Pharm Pharmacol 2013;2:1-5.
21. Duraipandiyan V, Dhabi-Al N, Balachandran C, Ignacimuthu S, Sankar C, Balakrishna K. Antimicrobial, antioxidant, and cytotoxic properties of vasicine acetate synthesized from vasicine isolated from Adhatoda vasica L. BioMed Res Int 2015;2015:7-14. [CrossRef]
22. Kumar RA, Garampalli KR. In vitro antifungal activity of some plant extracts against Fusarium oxysporum F. sp. lycopersici. Asian J Plant Sci Res 2015;5:22-7.
23. Song X, Peng C, Zhou G, Gu H, Li Q, Zhang C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci Rep 2016;6:1-8. [CrossRef]
24. Korner C. Alpine plant life. Geogr J 1999;31:1-343. [CrossRef]
25. Achi NK, Onyeabo C, Ekeleme-Egedigwe CA, Onyeanula JC. Phytochemical, proximate analysis, vitamin and mineral composition of aqueous extract of Ficus capensis leaves in South Eastern Nigeria. J Appl Pharm Sci 2017;7:117-22.
26. Agbafor KN, Engwa AG, Obiudu IK. Analysis of chemical composition of leaves and roots of Ageratum conyzoides. Int J Curr Res Acad Rev 2015;3:60-5.
27. Ningsih SS, Ariyanto D, Puspitasari D, Jayanegara A, Hamim H, Gunawan H. The amino acid contents in mangrove Rhizophora mucronata leaves in Asahan, North Sumatra, Indonesia. E2S Web Conf 2020;151:1-3. [CrossRef]
28. Pi Z, Stevanato P, Yv LH, Geng G, Guo XL, Yang Y, et al. Effects of potassium deficiency and replacement of potassium by sodium on sugar beet plants. Russ J Plant Physiol 2014;61:224-30. [CrossRef]
29. Bilger W, Rolland M, Nybakken L. UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem Photobiol Sci 2007;6:190-5. [CrossRef]
30. Murai Y, Setoguchi H, Kitajim J, Iwashina T. Altitudinal variation of flavonoid content in the leaves of Fallopia japonica and the needles of Larix kaempferi on Mt. Fuji. Natl Prod Commun 2015;10:407-11. [CrossRef]
31. Corenblit D, Baas A, Balke T, Bouma T, Fromard F, Garofano-Gomez V, et al. Engineer pioneer plants respond to and affect geomorphic constraints similarly along water-terrestrial interfaces world-wide. Glob Ecol Biogeogr 2015;24:1363-76. [CrossRef]
32. Cornelissen JH, Diaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, et al. Corrigendum to:New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 2016;64:715-6. [CrossRef]
33. Heilmeier H. Functional traits explaining plant responses to past and future climate. Flora 2019;254:1-11. [CrossRef]
34. Coomes DA, Allen RB. Effects of size, competition and altitude on tree growth. J Ecol 2007;95:1084-97. [CrossRef]
35. Pellissier L, Fournier B, Guisan A, Vittoz P. Plant traits co-vary with altitude in grasslands and forests in the European Alps. Plant Ecol 2010;211:351-65. [CrossRef]
36. Miyajima Y, Takahashi K. Changes with altitude of the stand structure of temperate forests on Mount Norikura, central Japan. J Forest Res 2007;12:187-92. [CrossRef]
37. Li X, Li Y, Zhang Z, Li X. Influences of environmental factors on leaf morphology of chinese jujubes. PLoS One 2015;10:1-16. [CrossRef]
38. Baumgartner A, Donahoo M, Chitwood DH, Peppe DJ. The influences of environmental change and development on leaf shape in Vitis. Am J Bot 2020;107:676-88. [CrossRef]
39. Gardiner B, Berry P, Moulia B. Review?:Wind impacts on plant growth, mechanics and damage. Plant Sci 2016;245:94-118. [CrossRef]
40. Wang H, Prentice IC, Davis TW, Keenan TF, Wright IJ, Peng C. Photosynthetic responses to altitude?:An explanation based on optimality principles. New Phytol 2016;213:976-82. [CrossRef]
41. Russo M, Suraci F, Postorino S, Serra D, Roccotelli A, Agosteo GE. Essential oil chemical composition and antifungal effects on Sclerotium cepivorum of Thymus capitatus wild populations from Calabria, Southern Italy. Braz J Pharmacogn 2013;23:239-48. [CrossRef]
42. El-Jalel LF, Elkady WM, Gonaid MH, El-Gareeb KA. Difference in chemical composition and antimicrobial activity of Thymus capitatus L. essential oil at different altitudes. Future J Pharm Sci 2018;4:156-60. [CrossRef]
43. Ariza MT, Martinez-Ferri E, Dominguez P, Medina JJ, Miranda L, Soria C. Effects of harvest time on functional compounds and fruit antioxidant capacity in ten strawberry cultivars. J Berry Res 2015;5:71-80. [CrossRef]
44. Hykkerud AL, Uleberg E, Hansen E, Vervoort M, Molmann J, Martinussen I. Seasonal and yearly variation of total polyphenols, total anthocyanins and ellagic acid in different clones of cloudberry (Rubus chamaemorus L.). J Appl Bot Food Qual 2018;91:96-102.
45. Gorniak I, Rafal B, Jaroslaw K. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 2018;18:241-72. [CrossRef]
46. Bahman K, Milad I, Vahid S, Bibi SF. Review on plant antimicrobials:A mechanistic viewpoint. Antimicrob Resist Infect Control 2019;8:1-28. [CrossRef]
47. Reichling J. Plant microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. Ann Plant Rev