1. INTRODUCTION
The goal to end hunger and food insecurity by the year 2030 is currently threatened by the changing precipitation pattern and the more intense weather [1] that results from climate change. Regional increases in temperature, aridity, and drought have led to significant crop failures and in some cases species extinction as a consequence of climate change [2]. Water scarcity is among the most critical abiotic stress elements affecting crop growth and productivity globally and could cause severe yield losses [3,4] by impairing photosynthesis [5] and other physiological processes [6-8]. An estimated 163 million people across the globe currently experience unprecedented dry spells compared to 50 years ago [2]. As the effect of climate change continues to increase, water scarcity and drought stress are becoming more frequent and severe, posing a substantial threat to sustainable agriculture and food security. It is projected that an estimated 3 billion people will experience water scarcity in 2025 [9] as irrigation water is increasingly becoming a scarce resource across the globe.
Mungbean (Vigna radiata L. Wilczek) is an important warm-season pulse cultivated in several regions of the world including Asia, America, and Africa. It prides as the most nutritious pulse known [10] with high levels of protein, amino acids (phenylalanine, leucine, isoleucine, lysine, and arginine) [11], vitamins and minerals, and considerable amounts of fiber and resistant starch which aids digestion, in addition to antioxidants that decrease the risk of chronic diseases [12]. Despite its numerous economic benefits, its production is often limited by abiotic stress factors [13]. Breeding high-yielding mungbean genotypes for stress tolerance can help to cushion the undesirable effect of climate change on crop productivity.
Several studies on mungbean tolerance to WS have been largely a one-off withholding of water [14-17] at specific stages of the crop development. These studies focused on mungbean response to WS either at seedling, vegetative, or reproductive stages employing various approaches including agronomic, physiological, and biochemical analysis [18,19]. The objective of this study was to evaluate the response of mungbean accessions to intermittent water deficit stress to identify high-yielding and water deficit resilient mungbean accession(s) on the premise that sufficient yield variability could exist in contrasting genotypes. Successful evaluation of these genotypes could lead to the identification of mungbean genotypes that could withstand prolonged periods of water scarcity which is a critical component of sustainable mungbean production. This study tested the hypothesis that mungbean genotypes could vary in their tolerance to intermittent water deficit stress. This study quantified WS in terms of agronomic and physiological traits to be able to ascertain how different mungbean accessions responded to intermittent water deficit stress.
2. MATERIALS AND METHODS
2.1. Site Description
Two experiments were carried out in a screen house at the Teaching and Research Farm of the Department of Crop Science, University of Nigeria, Nsukka between December 2019 to February 2020 and March to June 2020. Nsukka is located on latitude 06° 52’ N, longitude 07° 24’ E, and altitude 447, with mean annual rainfall, solar irradiance, and temperature of 1276 ± 706 mm, 1452 ± 269 w m-2, and 32°C, respectively [20]. Relative humidity varies across the seasons (rainy and dry seasons) with lower limits (39–41%) occurring between December and January (dry spell), while the upper limit (about 89%) occurs between July and August which corresponds to the peak of raining season [21].
2.2. Treatments and Experimental Design
The treatments comprised ten mungbean accessions (Tvr18, Tvr19, Tvr24, Tvr28 Tvr32, Tvr34, Tvr49, Tvr65, Tvr79, and Tvr83) sourced from the “Genetic Resource Center, International Institute of Tropical Agriculture (IITA), Ibadan” and three irrigation intervals: No-stress (I1), 3-day intermittent water-stress (I3), and 7-day intermittent water-stress (I7). ETC (3–5 mm) corresponding to 100% of mungbean daily evapotranspiration rate [17] was administered at the different water-stress levels. The experiment design was a split plot design replicated 3 times, with genotype as the main plot and WS as the sub-plot treatments. 4 kg of topsoil was filled in polyethylene bags with four perforations at the bottom to facilitate drainage. Poultry manure was applied at the rate of 10 g/pot [22]. Seeds were primed for 12 h before seeding to stimulate germination, and at 2 weeks after planting, the number of seedlings per pot was reduced to one.
2.3. Data Collection and Measurement
Data were recorded for agronomic and physiological traits following the standard procedures used by Ihejiofor et al. [22,23] and Ukwu et al. [24]. “The number of days (NOD) to seed emergence was determined by recording the days from the day the seeds were sowed until the day of seed emergence. NOD to flowering was obtained by recording the days from the date of seeding to when a flower opens. NOD to pod formation was recorded by calculating the days from seeding to when at least the first pod is formed. The number of leaves (NOL) per plant was obtained by tallying the number of fully expanded leaves per plant. Plant height was recorded as the distance between the plant’s base and the peak of the terminal leaf buds using a measuring tape. Leaf area (LA) was determined following the procedure of Chukwu et al. [25] as Y = 0.1686 + 1.017 LW with measurements taken on the 4th fully expanded leaf from the apex. Stem diameter (SD) was determined with a measuring Vernier caliper at 5 cm above ground level of each plant. Relative water content (RWC) was determined following the procedure of Bangar et al. [26]. 0.1 g of fully expanded leaf sample was weighed, and fresh weight (FW) was recorded. The leaf sample was then placed in a Petri dish containing distilled water for 4 h at room temperature, and the turgid weight (TW) of the sample was recorded. Samples were oven dried at 70°C for 24 h, and dry weight (DW) was taken. RWC% was then computed as ([FW–DW]/[TW–DW] × 100). The number of seeds (NOS) per pod was determined by taking the average NOS from five pods per plant. Grain yield per plant was recorded as the total weight of dry seeds per plant, and stress tolerance index (STI) was computed as STI = ([Vc × Vs]/Vc2); where Vc is the value of no-water-stress (control) and Vs is the value of water-stressed plants.”
2.4. Statistical Analysis
To detect the significant differences among treatments, “analysis of variance (ANOVA) was carried out for all data using GenStat 18th edition, and post-ANOVA mean separation was achieved using the least significant difference at P < 0.05. Cluster analysis was done using IBM SPSS version 23 using the average linkage method.” Graphs were constructed using GraphPad Prism 6.
3. RESULTS
3.1. Weather Conditions at the Experimental Site
The meteorological data sourced from the University of Nigeria Meteorological Station provides insights into the weather conditions at the experimental site. The highest maximum temperatures, reaching 34.9°C, 35.5°C, and 35.7°C, were registered during December 2020, January, and February 2021, coinciding with the months that saw the least amount of rainfall (0%, 0%, and 6.5%, respectively) and the highest number of sunny days (38%, 61%, and 32%, respectively), as illustrated in Figure 1. In contrast, the month of June 2021 exhibited the highest percentage of rainy days (52.1%) and relative humidity (82%). Interestingly, this period also experienced the lowest percentage of sunny days at 6.5%.
 | Figure 1: Weather report of the experimental site from December 2020 to June 2021. [Click here to view] |
3.2. Effects of Accession and Water Stress (WS) on Phenological Attributes of Mungbean
The results presented herein represent the average of two plantings that exhibited statistically similar outcomes. This study investigated the influence of genotype and intermittent WS on the growth characteristics of mungbean. The mungbean accessions had a significant impact on both phenology and growth traits (P < 0.05). With the exception of Tvr83, which showed a delayed emergence at 4.5 days after planting (DAP), all other genotypes emerged within 2 days [Figure 2]. The response of mungbean accessions to intermittent WS displayed significant variation in terms of phenology (P < 0.05). Specifically, Tvr83 exhibited delayed seed emergence (8 DAP) under the imposed I7 water-stressed condition, while the remaining genotypes emerged within 2 days, regardless of the presence of WS.
 | Figure 2: Effect of accession and water stress on days to emergence of mungbean. I1: No stress; I3: 3 days without water intermittently; and I7: 7 days without water intermittently. [Click here to view] |
Variability in the flowering pattern was also evident, with Tvr18, Tvr24, and Tvr65 exhibiting a longer flowering duration (46–48 days after planting, DAP) compared to the other accessions, which initiated flowering at 40–42 DAP. This divergence in flowering timing was similarly observed in the NOD required for pod formation. Notably, Tvr32, Tvr49, and Tvr83 began pod development at 43 DAP, earlier than the remaining accessions, while Tvr65 had the latest NOD to pod formation, occurring at 50.89 DAP [Figure 3].
 | Figure 3: Effect of water stress and accession on days to flowering and podding of mungbean. I1: No stress; I3: 3 days without water intermittently; I7: 7 days without water intermittently; DTF: Days to flowering; and DTPF: Days to pod formation. [Click here to view] |
WS also had a significant impact on the phenological attributes of mungbean (P < 0.05). Plants subjected to I1 and I3 conditions exhibited an earlier emergence, occurring at 2 DAP, in contrast to those under I7 conditions, which had a slightly delayed emergence at 2.67 DAP. In addition, the imposition of I7 WS led to a delay in flowering by 3 to 5 days and in pod formation by 2 days [Figure 3].
Unstressed plants were comparable to I3 water-stressed plants (P > 0.05) in NOD to pod formation [Figure 3]. With the exception of Tvr28, which exhibited earlier flowering and pod development (38.00 and 40.67 days) under I3 water-stressed conditions compared to both the unstressed (42.00 and 44.33 days) and I7 water-stressed (46.00 and 47.50 days) conditions, all other accessions followed a consistent pattern. They displayed a trend of late of earlier flowering and pod formation as the level of WS increased [Figure 3]. The days to flowering (DTF) ranged from 38.00–45.67 in I1 plants, 38.00–47.33 in I3 plants, and 41.33–51.00 in I7 plants. Similarly, the days to podding ranged from 40.03 49.00, 40.67–52.00, and 43.50–53.33 in I1, I3, and I7 plants, respectively.
3.3. Effects of Accession and WS on Mungbean Growth Attributes
The impact of accession on mungbean growth attributes is depicted in Figure 4. Notably, Tvr28 and Tvr32 displayed significantly taller plants with broader leaves compared to other accessions. However, SD remained statistically similar across all accessions (P > 0.05), except for Tvr34 and Tvr83, which stood out as distinct (P < 0.05). While Tvr34 exhibited significantly fewer leaves, Tvr83 had the shortest plants, smallest leaf sizes, thinner stems, and the highest number of leaves (NOL) compared to the other accessions, which displayed relatively similar growth traits (P > 0.05) in most measurements [Figure 4]. Plant height ranged from 10.32 cm (Tvr83) to 29.98 cm (Tvr28), leaf number ranged from 7.25 (Tvr34) to 37.90 (Tvr83), LA spanned from 38.70 cm² (Tvr83) to 101.10 cm² (Tvr28), and SD varied from 0.90 cm (Tvr83) to 1.67 cm (Tvr24).
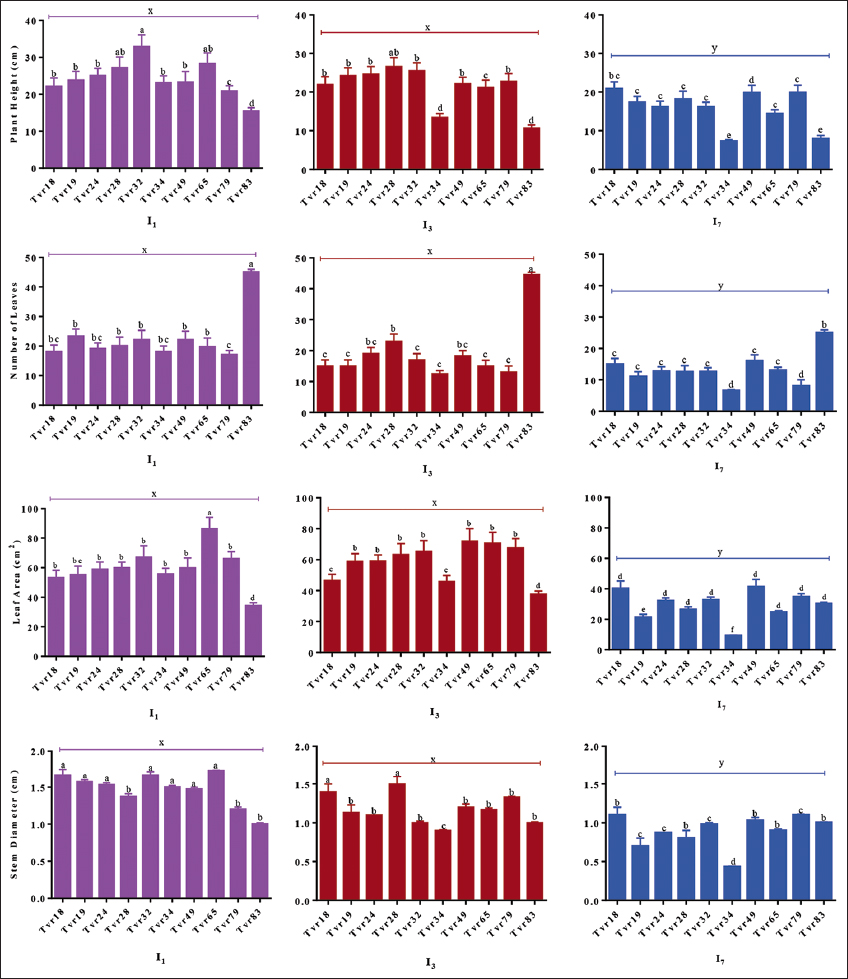 | Figure 4: Effect of accession and water stress on growth traits of mungbean. I1: No stress; I3: 3 days without water intermittently; and I7: 7 days without water intermittently. Columns show mean values of three replicates and bars show standard errors; Bars with contrasting alphabets are significantly different at P < 0.05. [Click here to view] |
WS exerted a significant impact on mungbean growth attributes (P < 0.05). Plants subjected to I7 WS experienced a substantial reduction in plant height (30%), leaf number (47%), LA (42%), and SD (32%) compared to those under I1 conditions, which recorded taller plants, a higher leaf count, larger LA, and thicker stems.
The response of mungbean accessions to WS varied significantly, particularly under I7 conditions, where all measured traits showed reduced values. The accessions exhibited a similar response pattern (P > 0.05) to I1 and I3 WS conditions in terms of plant height, NOL, LA, and SD throughout the study duration, and they outperformed their counterparts under I7 WS (P < 0.05).
Notably, the tallest plants (Tvr32 at 32.77 cm), the highest leaf count (Tvr83 at 44.96), the largest LA (Tvr65 at 86.10 cm²), and the thickest stems (Tvr65 at 1.73 cm) were all recorded in unstressed plants, contrasting starkly with the 7-day water-stressed plants, which displayed the shortest plants (Tvr83 at 7.90 cm), the fewest leaves (Tvr34 at 6.56), the smallest LA (Tvr34 at 9.25 cm²), and the thinnest stems (Tvr34 at 0.43 cm) [Figure 4].
3.4. Effect of accession and WS on RWC and STI in mungbean
Across the ten accessions, there were no significant differences (P > 0.05) observed in their response to WS in terms of RWC. Nonetheless, three accessions – Tvr28, Tvr32, and Tvr83 – exhibited a greater propensity to retain more water in conditions of water deficit [Figure 5]. These accessions recorded the least percentage reduction in RWC under both I3 and I7 water-stressed conditions, with reductions of 2.37% and 19.48%, 1.98% and 21.90%, and 4.26% and 28.30%, respectively. RWC exhibited a consistent decline with increased levels of WS across all accessions (P < 0.05), ranging from 82.61–86.55% in I1, 79.50–84.45% in I3, and 59.10–69.65% in I7 water-stressed plants [Figure 5].
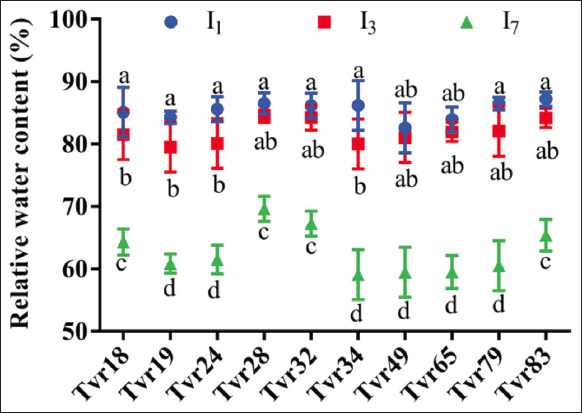 | Figure 5: Effect of water stress and accession on the relative water content of mungbean. I1: No stress; I3: 3 days without water intermittently; and I7: 7 days without water intermittently. Columns show mean values of three replicates and bars show standard errors; Bars with contrasting alphabets are significantly distinct at P < 0.05. [Click here to view] |
The STI for grain yield revealed that four accessions – Tvr24, Tvr28, Tvr32, and Tvr83 – attained tolerance scores exceeding 70 under I3 WS conditions. However, only two accessions – Tvr18 and Tvr83 – achieved tolerance scores exceeding 30 under the more severe I7 WS condition [Figure 6].
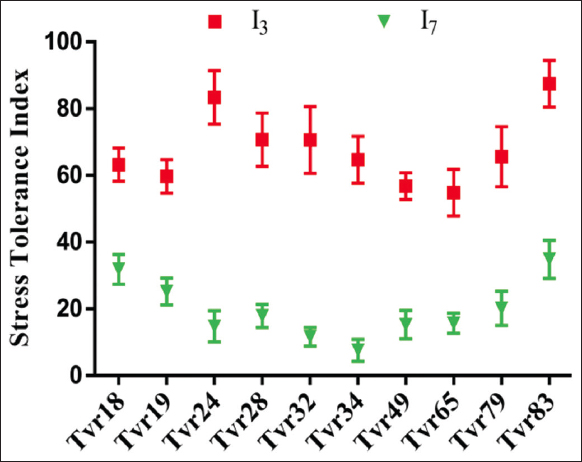 | Figure 6: Effect of water stress and accession on stress tolerance index of mungbean. I1: No stress; I3: 3 days without water intermittently; and I7: 7 days without water intermittently. [Click here to view] |
3.5. Effects of Accession and WS on Yield Traits of Mungbean
The yield characteristics of mungbean exhibited a significant influence of genotype at P ≤ 0.05. Notably, there was variation in the number of pods (NOP) among the different accessions.
The accessions Tvr83 (5.83), Tvr28 (4.11), and Tvr18 displayed statistical similarity (P > 0.05) in NOP per plant, surpassing (P < 0.05) Tvr49 (1.89), Tvr65 (2.00), and Tvr79 (2.11), which had comparatively lower pod counts. The number of seeds per pod (NOS per pod) varied significantly (P < 0.05) among the accessions, with Tvr18 (7.22) and Tvr32 (7.00) yielding a higher NOS compared to the others, especially Tvr79 (4.89), Tvr34 (4.56), and Tvr83 (3.50), which produced relatively fewer seeds [Figure 7].
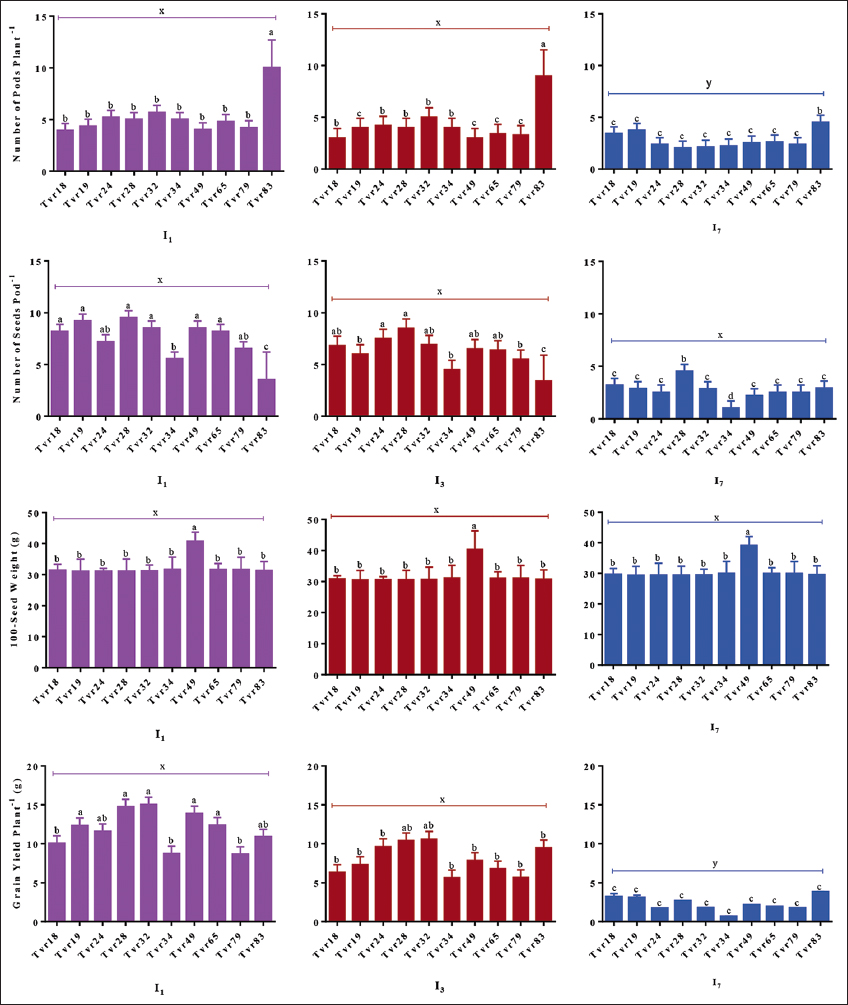 | Figure 7: Effect of water stress on yield and yield traits of ten mungbean accessions. I1: Watering once daily; I3: Watering once in 3 days; and I7: Watering once in 7 days. Columns show mean values of three replicates and bars show standard errors; Bars with contrasting alphabets are significantly distinct at P < 0.05. [Click here to view] |
Grain yield was significantly different (P < 0.05) among the accessions, with Tvr32, Tvr28, and Tvr18 producing higher yields (7.23–7.93 g) compared to Tvr65 and Tvr79, which yielded the least (3.93–3.99 g). Notably, Tvr49 recorded a significantly higher 100-seed weight at 40.60 g, in contrast to the other accessions, which exhibited statistical similarity with weights slightly above 30 g [Figure 7].
The imposition of WS had a significant effect (P < 0.05) on the yield attributes of mungbean. The number of pods per plant (NOP), NOS per pod, and NOS per plant were all significantly reduced (P < 0.05) under both I3 and I7 stressed conditions, declining by 27%, 24%, and 27% in I3, and by 51%, 45%, and 58% in I7, respectively. While pod length and width remained unaffected by I3 WS (P > 0.05), they were significantly affected by I7 WS (P < 0.05), resulting in a reduction of 30% and 31% in pod length and width, respectively. Grain yield per plant and the weight of 100 seeds varied with WS. Mean grain yield was similar in I1 (5.58 g) and I3 (4.82 g) plants but significantly decreased (P < 0.05) in I7 (2.05 g) plants. Similarly, the weight of 100 seeds was comparable in I1 (41.25 g) and I3 (39.41 g) plants but significantly decreased (P < 0.05) in I7 (37.12 g) plants [Figure 7].
The accessions responded in a similar pattern to the levels of imposed WS in yield and yield components. Higher yield values were recorded for unstressed plants than the water-stressed plants, and these values decreased with the severity of the WS. I1 plants generally produced more pods and seeds than I7 plants. NOP per plant ranged from 3.92–5.67, 3.00–5.00, and 2.10–3.71 in I1, I3, and I7 plants, respectively. NOS per pod ranged from 3.50–9.50, 3.00–8.50, and 1.00–4.50 in I1, I3, and I7 plants, respectively. NOS per plant ranged from 17.50–47.50, 15.00–34.50, and 2.20–10.78 in I1, I3, and I7 plants, respectively [Figure 7].
Grain yield varied (P < 0.05) across water-stressed levels, with unstressed plants recording superior grain yield per plant (5.46–14.99 g) than I3 (4.62–10.59 g) and I7 water-stressed (0.66–3.19 g) plants. Genotypic responses across the WS levels in terms of 100 seed weight, were similar, and ranged from 30.98–40.70 g, 30.58–40.30 g, and 29.28–39.00 g in I1, I3, and I7 plants, respectively.
3.6. Cluster Analysis of Growth and Yield Components of Ten Mungbean Accessions
Cluster analysis was able to delineate the ten accessions into four sub-sub clusters, three sub-clusters, and two super-clusters at an Euclidean distance of 5, 10, and 15, respectively [Figure 8]. At Euclidean distance of 5, Tvr18, Tvr19, Tvr28, and Tvr32 were grouped as sub-sub cluster A1, Tvr24, Tvr49, Tvr65, and Tvr79 were grouped as sub-sub cluster A2 while Tvr34 and Tvr83 were outliers in A3 and A4, respectively. At an Euclidean distance of 10, sub-sub clusters A1 and A2 were collapsed together to form a new sub-cluster B1 with Tvr34 and Tvr83 occupying B2 and B3, respectively. At a higher Euclidean distance of 15, however, all nine accessions excluding Tvr83 were collapsed into a super-cluster C1 while Tvr83 stood out in C2.
 | Figure 8: Cluster analysis of growth and yield components of ten mungbean accessions using the average linkage method. A1-A4 are sub-sub clusters, B1-B3 are sub-clusters, C1 and C2 are super-clusters. [Click here to view] |
4. DISCUSSION
Significant differences were observed among the accessions in terms of agronomic characteristics (P < 0.05). Variability was evident in phenological traits such as the NOD to emergence, NOD to flowering, and NOD to pod formation, as well as in plant height, NOL, LA, pod, and seed traits. These differences suggest variations in their genetic makeup. Accessions Tvr32, Tvr28, and Tvr18 were closely clustered together in sub-sub cluster A1 [Figure 8] and displayed superior characteristics in terms of plant height, LA, NOS per pod, NOS per plant, and grain yield per plant. This clustering suggests a common ancestry among these accessions, implying that crosses between them may not yield superior F1 progenies due to potential inbreeding depression [27].
In contrast, Tvr83 stood out from the other accessions as it exhibited the smallest LA, higher NOL, higher NOP per plant (P < 0.05), and comparable grain yield per plant (P > 0.05) compared to Tvr32, Tvr28, and Tvr18, which were considered the best performers. The smaller leaf size of Tvr83 may be an adaptation to water-stressed conditions, as smaller leaves are known to reduce water loss through transpiration [28]. The distinct nature of Tvr83, as indicated by the dendrogram [C2 in Figure 8], suggests that it is a distant relative of the other accessions and could be a valuable candidate for mungbean hybridization and introgression programs, especially in traits where it demonstrated superiority [29].
The success of crop improvement relies on the presence of genetic diversity [30]. Clustering of genotypes aids in organizing a diverse set of genotypes into more homogeneous groups, which is essential for identifying distant relatives of a species, a prerequisite for crop improvement through hybridization. Accessions that are closely related tended to cluster together in sub-sub clusters A1, A2, A3, and A4; sub-clusters B1, B2, and B3; and super-clusters C1 and C2 at Euclidian distances of 5, 10, and 15, respectively [Figure 8], aligning with previous studies by Gayacharan et al. [31] and Mwangi et al. [28].
Intermittent water-stressed condition (I7) significantly reduced growth traits such as plant height, NOL, and LA, as well as yield traits such as NOP per plant, NOS per pod, NOS per plant, 100-seed weight, and grain yield per plant compared to unstressed plants. In contrast, I3 plants showed non-significant decreases (P > 0.05) in growth and yield component traits relative to the control. The non-significant reduction in these traits in I3 plants may be attributed to the complex physiological mechanisms the plants employ to cope with stress, such as reducing leaf size and closing stomata to minimize water loss [32]. This finding aligns with the previous studies by Tawfik [33] and Khan et al. [34] reporting significant reductions in plant height, LA, NOP, NOS, seed weight, and yield under drought-stressed conditions.
The reduction in RWC values under stressed conditions was significant across all measured traits, with I7 stressed plants experiencing greater decrease (31.63%) in leaf water potential compared to I3 (7.80%) stressed plants [Figure 5]. Decreased RWC in leaves often triggers stomata closure, limiting CO2 uptake, and ultimately reducing photosynthesis [35]. Reduction in growth traits under WS may result from altered physiological processes such as reduced CO2 assimilation, impaired cell division and elongation, loss of turgor pressure, reduced stomatal conductance, and photochemistry, as plants commonly respond to drought stress by closing stomata and reducing transpiration [36-42].
Significant reductions in grain yield (63.3%) and 100-seed weight (10.01%) were observed in I7 stressed plants compared to unstressed plants, possibly due to reduced assimilate transport from leaves to seeds, and it is consistent with the findings by Zare et al. [42] and Zhou et al. [43], who reported yield reductions in mungbean under drought stress.
The RWC and STI are crucial physiological parameters for distinguishing drought-tolerant genotypes from susceptible ones. Based on these parameters, four accessions – Tvr83, Tvr19, Tvr28, and Tvr18 – were identified as the most drought-tolerant accessions under I7 conditions. In contrast, under less severe I3 conditions, four accessions – Tvr28, Tvr32, Tvr24, and Tvr83 – demonstrated higher tolerance (STI > 70) than others, indicating their ability to withstand short intermittent water deficits.
The variability among the accessions in response to WS, characterized by delayed seed emergence, reduced plant height, LA, NOL, seed weight, and RWC in I7 plants across all accessions, reflects differences in their tolerance or susceptibility to prolonged intermittent WS. Tvr34 and Tvr65 were the most affected accessions and are considered susceptible to WS. Conversely, Tvr28, Tvr18, Tvr19, and Tvr83 showed the least percentage decrease in RWC and STI, indicating their higher tolerance to WS. This aligns with findings by Chowdhury et al. [44], who reported greater decreases in RWC in drought-susceptible soybean genotypes compared to drought-tolerant types. Pod and seed traits were relatively higher in I1 and I3 conditions than in I7 plants, which are consistent with the previous studies by Zhou et al. [43] and Khan et al. [34]. The comparable performance of I1 and I3 plants in terms of plant height, NOL, LA, NOP, pod length, and 100-seed weight suggests that most mungbean accessions can tolerate short intermittent water deficit stress, corroborating previous report by Ahmad et al. [45].
5. CONCLUSION
This study has revealed promising grain-yield outcomes in four mungbean accessions – Tvr28, Tvr18, Tvr19, and Tvr83 – under severe I7 water-stressed conditions, although with slightly lower mean values compared to the control. Except for Tvr49 and Tvr65, which exhibited significantly reduced (P < 0.05) grain yield under moderate I3 WS relative to the control, the remaining eight accessions demonstrated comparable grain yield (P > 0.05) to unstressed plants. The study underscores the pressing need to develop and adopt climate-resilient mungbean cultivars. As water scarcity is expected to intensify due to climate change, the identified accessions, particularly Tvr28, Tvr18, Tvr19, and Tvr83, that demonstrated appreciable grain yield under severe WS (I7 conditions) could serve as valuable genetic resources for breeding programs aimed at developing mungbean varieties capable of withstanding intermittent rainfall patterns, consequently contributing to food security by providing a reliable source of nutrition even in water-scarce regions. The accession Tvr83, identified as a distant relative of other accessions, presents an opportunity to expand genetic diversity in mungbean breeding programs. This genetic diversity can be harnessed for further crop improvement efforts, especially in traits related to leaf number, pod number, and seed production, ensuring the preservation and utilization of valuable genetic resources.
6. ACKNOWLEDGMENTS
The authors acknowledge the Genetic Resource Center, International Institute of Tropical Agriculture (IITA) for making the seeds available.
7. AUTHORS’ CONTRIBUTIONS
All authors significantly contributed to the conception and design of the study, acquisition, and analysis of data, participated in drafting or critically revising the manuscript for significant intellectual content, provided their consent for submission to the present journal, granted final approval for the published version and agreed to take responsibility for all aspect of the research.
8. FUNDING
This research did not receive any external funding from public or private organizations.
9. CONFLICTS OF INTEREST
The authors declared that there are no conflicts of interest.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All data generated in this study are included in the article.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. UN. The Sustainable Development Goals Report. New York, USA;2020. 66.
2. Pörtner HO, Roberts DC, Adams H, Adelekan I, Adler C, Adrian R, et al. Technical summary. In:Poloczanska ES, Mintenbeck K, Tignor M, Alegría A, Craig M, Langsdorf S, et al., editors. Climate Change 2022:Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK, New York, USA:Cambridge University Press;2022. 37-118. [CrossRef]
3. Neenu S, Biswas AK, Rao AS. Impact of climate factors on crop production. A review. Agric Rev 2013;34:97-106.
4. UN. United Nations Climate Change Annual Report 2021. p. 1-74. Available from:https://unfccc.int/sites/default/files/resource/unfccc_annual_report_2020.pdf [Last accessed on 2021 Jul 21].
5. Torres-Ruiz JM, Diaz-Espejo A, Perez-Martin A, Hernandez-Santana V. Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiol 2015;35:415-24. [CrossRef]
6. Raza MA, Saleem MF, Haider KI. Combined application of glycinebetaine and potassium on the nutrient uptake performance of wheat under drought stress. Pak J Agric Sci 2015;52:19-26.
7. Zhang D, Jiao X, Du Q, Song X, Li J. Reducing the excessive evaporative demand improved photosynthesis capacity at low costs of irrigation via regulating water driving force and moderating plant water stress of two tomato cultivars. Agric Water Manag 2018;199:22-33. [CrossRef]
8. Liang G, Liu J, Zhang J, Guo J. Effects of drought stress on photosynthetic and physiological parameters of tomato. J Am Soc Hortic Sci 2020;145:12-7. [CrossRef]
9. Hanjra MA, Qureshi ME. Global water crisis and future security in an era of climate change. Food Policy 2010;35:365-77. [CrossRef]
10. Anjum NA, Umar S, Iqbal M, Khan NA. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russ J Plant Physiol 2011;58:92-9. [CrossRef]
11. Arumugam R, Rajasekaran S, Nagarajan SM. Response of arbuscular mycorrhizal fungi and Rhizobium inoculation on growth and chlorophyll content of Vigna unguiculata (L) walp var. Pusa 151. J Appl Sci Environ Manage 2010;14:113-5. [CrossRef]
12. Tang D, Dong Y, Ren H, Li L, He C. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem Cent J 2014;8:4. [CrossRef]
13. Saleem MF, Raza MA, Ahmad S, Khan IH, Shahid AM. Understanding and mitigating the impacts of drought stress in cotton-a review. Pak J Agric Sci 2016;53:609-23. [CrossRef]
14. Sadeghipour O. Effect of withholding irrigation at different growth stages on yield and component of mungbean (Vigna radiate L. Wilczek) varieties. Am Eurasian J Agric Environ Sci 2008;4:590-4.
15. Ranawake AL, Dahanayaka N, Amarasingha UG, Rodrigo WD, Rodrigo UT. Effect of water stress on growth and yield of mung bean (Vigna radiata L). Trop Agric Res Ext 2012;14:1-4. [CrossRef]
16. Ambachew S, Alamirew T, Melese A. Performance of mungbean under deficit irrigation application in the semi-arid highlands of Ethiopia. Agric Water Manag 2014;136:68-74. [CrossRef]
17. Hussen A, Worku W, Zewdie M. Effects of deficit irrigation and phosphorus levels on growth, yield, yield components and water use efficiency of mung bean (Vigna radiata (l.) Wilczek) at Alage, Central Rift valley of Ethiopia. Agric Res Technol Open Access J 2019;21:556167. [CrossRef]
18. Islam S, Akter MB, Paul NK, Khan MA, Amin R, Hakim MA. Physiological and biochemical responses of mungbean genotypes under water stress conditions. J Crop Sci Biotechnol 2018;21:431-9.
19. Sharma R, Gupta SK, Batra P. Identification of differentially expressed genes and regulatory networks in contrasting drought-tolerant mungbean genotypes under water stress. Plant Mol Biol Rep 2021;39:161-77.
20. Okoro EC, Ugwu EB, Onah IG, Omeje LC. Rainfall and solar irradiance monitoring in Nsukka zone, Nigeria. Eur J Stat Probab 2021;9:1-10.
21. Atlas. Nsukka Weather Atlas;2021. Available from:https://www.weather-atlas.com/en/nigeria/nsukka-climate#humidity_relative [Last accessed on 2022 Jul 24].
22. Ihejiofor PN, Ukwu UN, Adeoye G. Determination of kolgrace bio-fertilizer rate for optimum greengram (Vigna radiata L. Wilczek) production in Ibadan, Southwest Nigeria. Agro Sci 2022;21:82-7. [CrossRef]
23. Ihejiofor PN, Ukwu UN, Adeoye GO. Comparative effects of different levels of kolgrace organic fertilizer on the growth and yield attributes of greengram (Vigna radiata (L) Wilczek) in the screenhouse. Asian J Res Agric For 2020;6:1-7. [CrossRef]
24. Ukwu UN, Agbirionwu AC, Dauda N, Adewuyi SO, Osadebe VO, Anozie CC. Response of mungbean (Vigna radiata L Wilczek) genotypes to. different spacing types in derived savannah agroecology of Southeast Nigeria. Niger J Biotechnol 2023;40:77-85. [CrossRef]
25. Chukwu C, Ogoke IJ, Onyia VN. A Quick and Non-destructive Method of Determining Leaf Area in Mungbean (Vigna radiata L). In:Proceedings of the Crop Science Society of Nigeria, FUT-Owerri;2020. p. 220-5.
26. Bangar P, Chaudhury A, Tiwari B, Kumar S, Kumari R, Bhat KV. Morphophysiological and biochemical response of mungbean [Vigna radiata (L) Wilczek] varieties at different developmental stages under drought stress. Turk J Biol 2019;48:58-69. [CrossRef]
27. Brown J, Caligari PO, Campos HA. Plant Breeding. 2nd ed. New Jersey:Wiley Black Publishing Ltd.;2014. 40-64.
28. Mwangi JW, Okoth OR, Kariuki MP, Piero NM. Genetic and phenotypic diversity of selected Kenyan mung bean (Vigna radiata L. Wilczek) genotypes. J Genet Eng Biotechnol 2021;19:142-56. [CrossRef]
29. Ukwu NU, Olasanmi B. Crossability among five Cassava (Manihot esculenta Crantz) varieties. Mod Concepts Dev Agron 2018;2:1-6. [CrossRef]
30. Ebert AW, Engels JM. Plant biodiversity and genetic resources matter!Plants 2020;9:1706. [CrossRef]
31. Gayacharan C, Tripathi K, Meena SK, Panwar BS, Lal H, Rana JC, et al. Understanding genetic variability in the mungbean (Vigna radiata L.) gene pool. Ann Appl Biol 2020;177:346-57. [CrossRef]
32. Lambers H, Chapin II, Pons TL. Photosynthesis, respiration and long distance transport. In:Plant Physiological Ecology. 2nd ed. Germany:Springer;2008. 11. [CrossRef]
33. Tawfik KM. Effect of water stress in addition to potassiomag application on mungbean. Aust J Basic Appl Sci 2008;2:42-52.
34. Khan MB, Hussain M, Raza A, Farooq S, Jabran K. Seed priming with CaCl2 and ridge planting for improved drought resistance in maize. Turk J Agric For 2015;39:193-203. [CrossRef]
35. Baroowa B, Gogoi N. Morpho-physiological and yield responses of black gram (Vigna mungo L.) and green gram (Vigna radiata L.) genotypes under drought at different growth stages. Res J Recent Sci 2016;5:43-50.
36. Hussain M, Malik MA, Farooq M, Ashraf MY, Cheema MA. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J Agron Crop Sci 2008;194:193-9. [CrossRef]
37. Taiz L, Zeiger E. Plant Physiology. 5th ed. Sunderland, MA, USA:Sinauer Associates;2010. 464.
38. Iqbal A, Fahad S, Iqbal M, Alamzeb M, Ahmad A, Anwar S, et al. Special Adaptive Features of Plant Species in Response to Drought. Cham:Springer;2020. 77-118. [CrossRef]
39. Martin-StPaul N, Delyon S, Cochard H. Plant resistance to drought depends on timely stomatal closure. Ecol Lett 2017;20:1437-47. [CrossRef]
40. Olsovska K, Kovar M, Brestic M, Zivcak M, Slamka P, Shao HB. Genotypically identifying wheat mesophyll conductance regulation under progressive drought stress. Front Plant Sci 2016;7:1111. [CrossRef]
41. Luo DD, Wang CK, Jin Y. Plant water-regulation strategies:Isohydric versus anisohydric behavior. Chin J Plant Ecol 2017;41:1020-32. [CrossRef]
42. Zare M, Dehghani B, Alizadeh O, Azarpanah A. The evaluation of various agronomic traits of mungbean (Vigna radiata L.) genotypes under drought stress and non-stress conditions. Int J Farming Allied Sci 2013;2:764-70.
43. Zhou R, Kong L, Wu Z, Rosenqvist E, Wang Y, Zhao L, et al. Physiological response of tomatoes at drought, heat and their combination followed by recovery. Physiol Plant 2019;165:144-54. [CrossRef]
44. Chowdhury JA, Karim MA, Khaliq QA, Ahmed AU, Mondol AM. Effect of drought stress on water relation traits of four soybean genotypes. SAARC J Agric 2017;15:163-75. [CrossRef]
45. Ahmad A, Selim MM, Alderfasi AA, Afzal M. Effect of drought stress on mungbean (Vigna radiata L.) under arid climatic conditions of Saudi Arabia. In:Miralles I Garcia JL, Brebbia CA, editors. Ecosystem and Sustainable Development. Vol. 192. Southampton, UK:WIT Press;2015. 185-93. [CrossRef]