1. INTRODUCTION
Safou or African plum is a non-timber forest product from tropical forest bordering the Gulf of Guinea and is highly appreciated as a seasonal delicacy. The fruit is a fleshy elliptic drupe (4–15 cm long and 3–6 cm diameter [1]) that is produced annually or biannually by the safou tree, Dacryodes edulis (G. Don) H. J. Lam, of the Burseraceae family. Overall, safou has a high nutritional value. Its mesocarp or pulp can be eaten dry, raw, roasted, or boiled in hot water [2]. Safou contains 40-70% of lipids (on dry matter) [3] and its oil is widely used in pharmaceutical, cosmetics, and food industries [4]. Safou pulp powder could be used directly as a partial animal fat (butter) substitute in biscuit making [5]. As a percentage of dry matter, the pulp also contains about 25.9% proteins and 17.9% fiber [6], as well as calcium, potassium, phosphorus, magnesium, iron, and manganese [2]. Despite its good nutritional quality, <6% of the safou produced in Cameroon is exported because the ripe fruit is naturally highly perishable, lasting only 2–3 days at ambient temperature [7]. Little is currently known about the fruit ripening physiological features that could explain the poor fresh fruit harvesting, handling, and storage. Efforts have been made, with limited success, to improve the shelf life of safou to <2 weeks by treating the fruit with 1-methylcyclopropene [7]. The best long-term storage practice currently involves drying the fruits before packaging and processing [8]. There is a need to improve knowledge on safou development to overcome postharvest loss and better control the fruit marketability [9] by mastering both the physical and physiological maturation processes.
Moreover, there is great variability in agronomic performance and fruit quality in D. edulis, due to a high rate of heterozygosity within the populations. This heterozygosity is due to the high pollen dispersal potential and dominant cross-pollination, which has, in turn, leads to significant genetic flow between populations [10]. The breeding pattern has also introduced wide ecological plasticity which helps D. edulis tree to adapt and grow in a varied lowland and upland range of climatic conditions. This climatic spatial heterogeneity, in turn, leads to great heterogeneity in the dates of occurrence of safou maturation and ripening stages, also known as a plastic response of phenological traits or stages [11]. In horticulture, the identification and understanding of the temporal and spatial progression of phenological traits are a prerequisite for any cultural intervention depending on a precise stage, but also for any prediction of the phenological stage allowing an optimal crop harvest as well as their effective management [12]. Sound knowledge of the different fruit developmental and ripening stages and their respective durations are required to be potentially used as a standard biological keynote for physiological and biotechnological studies in D. edulis fruit maturation. Furthermore, as far as our knowledge, there is no work on prediction of the period of maturation and ripening in D. edulis. In this study, the morphological changes that occurred during safou’s maturation and ripening have been assessed in relation to climatic factors (average temperature, cumulative temperature, and rainfall) over two consecutive production seasons in four major safou production localities situated in three of the five main agro-ecological zones (AEZ) of Cameroon.
2. MATERIALS AND METHODS
2.1. Study Area
This study was conducted in four localities in Cameroon, corresponding to three AEZ of the country [13]. These localities are (i) Foumban (AEZ III, in the Western Highlands at an elevation of 1,100 to 2,000 m above sea level (ASL) and a monomodal rainfall distribution); (ii) Njombe (AEZ IV, in South-western Cameroon, at an elevation of 0–800 m ASL and a monomodal rainfall regime); (iii) Makenene; and (iv) Yaounde (AEZ V, a Humid Forest Zone in Southern Cameroon at an elevation of <800 m ASL with bimodal rainfall pattern) [13].
Climate data (rainfall, temperature, and relative humidity) for the different localities in 2011(Y0), 2012 (Y1), and 2013 (Y2) were obtained from the Cameroon Ministry of Transport Climate Service.
2.2. Experimental Design and Sampling
Four safou trees, separated by at least 50 m intervals, were selected per site. These safou trees included a landrace and domesticated trees that were selected on the basis of farmer interviews (about tree age, production frequency, fruit quality, flowering density, fruit fall, and accessibility). On the experimental farms, cultivated trees were integrated into cropping systems among food crops in Yaounde (Tree 13, Tree 14, Tree 15, and Tree 16) or in cocoa or plantain based agroforests in Foumban (Tree 1, Tree 2, Tree 3, and Tree 4), Makenene (Tree 5, Tree 6, Tree 7, and Tree 8), and Njombe (Tree 9, Tree 10, Tree 11, and Tree 12).
All accessible flowers on trees were first labeled at the budding stage. Floral buds were monitored weekly and flowers were subsequently labeled at the anthesis stage. The third labeling was done at the fruit set stage. At 2 weeks interval and from fruit set to ripening, 10 labeled fruits were randomly selected and collected on each tree for morphological parameter measurement. The experiment was conducted over 2 consecutive production years, that is, 2012 (Y1) and 2013 (Y2). Due to fruit fall, data could not be collected on some preselected trees.
2.3. Measurements of Morphological Characteristics
The length and diameter of each fruit were measured using digital calipers (Vernier Caliper, 200 mm, Mitutoyo, Paris, France). The fruit length was measured on the polar axis of the fruit, that is, from apex to bottom using a measuring tape. The fruit diameter was considered as the maximum fruit width, as measured perpendicular to the polar axis. The fruit volume was determined using the displacement volume measurement method [14] by placing the fruit in a measuring cylinder and recording the rise in the water level. Fruits were weighed individually on an accurate electronic balance (RADWAG, Clarkson Laboratory and Supply Inc., USA).
The daily fruit growth rate (DFGR) was calculated with the following formula:
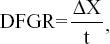 |
A panel of five plant scientists scored the fruit color, shape, and epidermis texture.
2.4. Determination of Fruit Development and Ripening Stages
The different fruit development and ripening phases, that is, early putative cell division, putative elongation, pre-ripening, and ripening, were determined based on changes in the observed and measured parameters. The putative cell division period was considered as the time from fruit set to the onset of increased fruit length, diameter, and volume. The putative elongation period was defined as the period during which there was a continuous increase in the measured morphological parameters. The pre-ripening phase was defined as the time from no change in fruit growth to the fruit color change (start of darkening). The ripening period was considered as a time during which the fruit color changed (darkening). The fruit set-to-ripening time was also recorded.
2.5. Determination of Reproductive Phenophases
For each safou tree studied, the chronology of changes from floral bud emergence to fruit ripening over the 2 consecutive years of study was recorded. The correlation between the phenophase chronology and climatic parameters was established. The climatic parameters considered were the average temperature, cumulative temperature, cumulative rainfall, and the average relative humidity.
2.6. Statistical Analysis
The variance homogeneity was tested using the Levene test. The data (length, diameter, volume, and fresh weight) were subjected to an analysis of variance using IBM SPSS 20.0 software (SPSS Inc., Chicago, IL). Means were compared using Student–Newman–Keuls and Duncan tests and differences were considered significant at the 5% probability level. Bilateral correlations between the chronology of the appearance of phenophases, the morphological and climatic parameters were done using both Spearman and Pearson.
3. RESULTS AND DISCUSSION
3.1. Results
3.1.1. Climatic conditions
The climatic records for the study sites [Figure 1] revealed that at Foumban and Makenene the average annual rainfall in 2011 (Y0: 1 year before the study) were significantly higher than the levels in Y1 (P ≤ 0.001) and Y2 (P ≤ 0.01), while at Njombe, the average annual rainfall in Y0 was significantly lower than in Y1 and Y2 (P ≤ 0.01). With regard to temperature, the annual values in Y0 and Y1 at Makenene were significantly lower than that of Y2 (P ≤ 0.001), while at Yaounde, the temperature in Y2 was significantly lower than in Y0, which was also significantly lower than in Y1 (P ≤ 0.001). The relative humidity at Makenene in Y0 was significantly lower than in Y2 and Y1 (P ≤ 0.05). The relative humidity at Njombe in Y1 was significantly higher than in Y0 and Y2 (P ≤ 0.05). Then, the relative humidity at Yaounde in Y2 was significantly lower than in Y0 and Y1 (P ≤ 0.001).
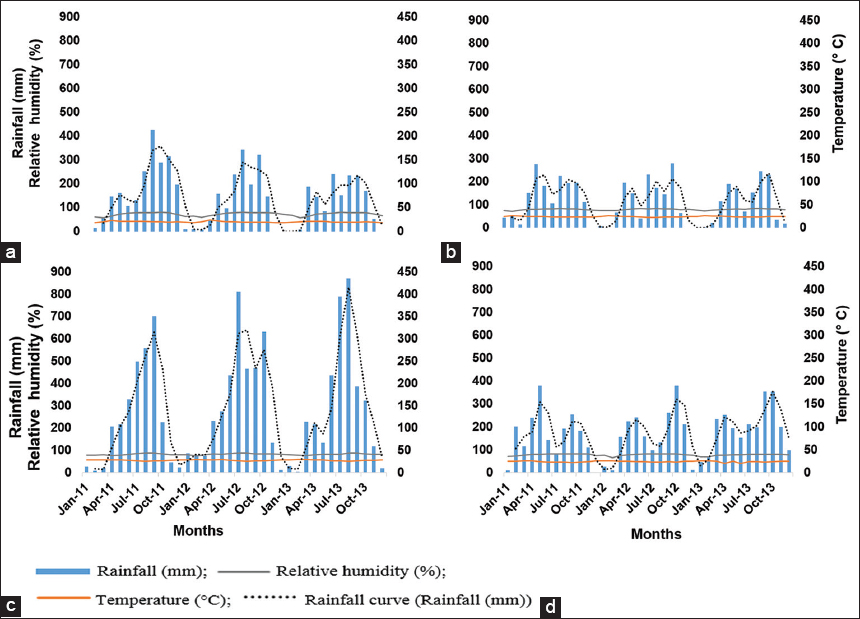 | Figure 1: Meteorological data for the different study sites from 2011 to 2013. (a) Foumban (AEZ III), (b) Makenene (AEZ V), (c) Njombe (AEZ IV), and (d) Yaounde (AEZ V) [Click here to view] |
3.1.2. Characteristics of ripe D. edulis fruits
The age of the trees used in this study, according to the farmers, ranged from 7 to 40 years old in (Y1) and 8 and 41 years old in Y2. At ripening, the color of the fruits from these trees [Figure 2] was either blue (Trees 5, 6, 8, 14, and 16) or dark blue (Trees 1, 2, 3, 4, 9, 10, 13, and 15). The fruit shape was spheroidal (Trees 1, 3, 6, and 8), elongate (Trees 2, 5, and 16), obovate (Trees 4, 9, 10, 14, and 15), or ovate (Tree 3), while the epidermis was either smooth or rough [Table 1].
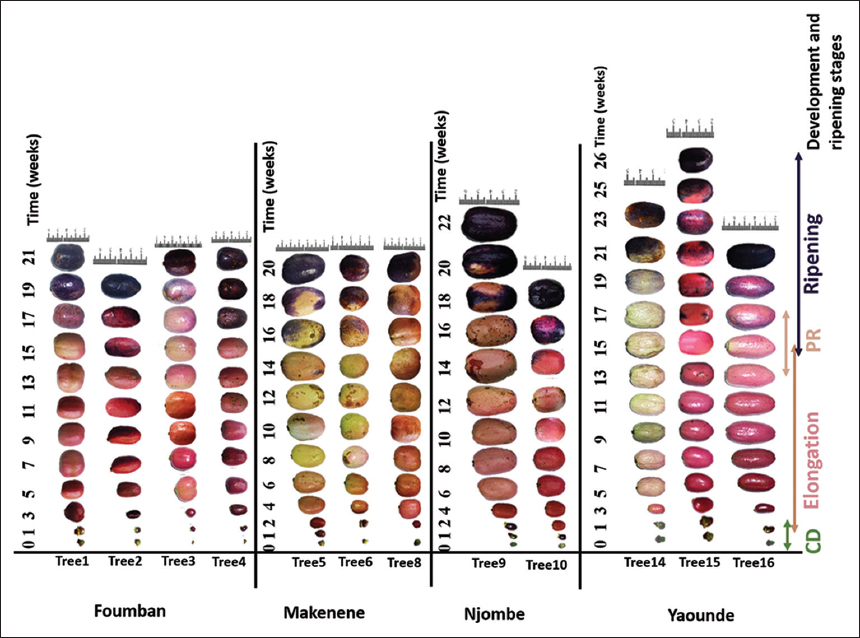 | Figure 2: Developmental and ripening stages of Dacryodes edulis fruits from different agro-ecological zones [Click here to view] |
Table 1: Morphological characteristics of mature average-sized Dacryodes edulis fruits from the four study sites in 2012 (Y1) and 2013 (Y2).
| Year | Localities (AEZ) | Tree number | Length | Diameter | Volume | Fresh weight | Color | Form | Epidermis | Tree age |
|---|---|---|---|---|---|---|---|---|---|---|
| mm | cm3 | g | Year | |||||||
| Y1 | Foumban (III) | 1 | 65.5±1.4c | 35.8±1.2c,d | 49.7±0.8b,c | 37.9±0.7a,b | Dark blue | Sp | Smooth | 10 |
| 2 | 70.0±1.7d,e | 28.0±0.8a | 44.8±2.9b | 35.9±1.1a | Dark blue | El | Smooth | 40 | ||
| 3 | 56.2±2.1b | 36.3±2.0d | 47.8±1.8b,c | 35.6±1.0a | Dark blue | Sp | Smooth | 10 | ||
| 4 | 64.6±2.0c | 33.0±1.4b | 49.0±1.7b,c | 39.0±2.3a,b | Dark blue | Ob | Smooth | 10 | ||
| Makenene (V) | 5 | 85.8±3.7h,i | 43.7±2.8h,i | 71.0±3.2f,g,h | 54.5±2.4c,d | Blue | El | Smooth | 8 | |
| 6 | 65.0±1.9c | 41.9±1.4g,,h | 48.0±6.3b,c | 61.0±9.8d,e,f | Blue | Sp | Rough | 20 | ||
| 7 | 81.6±2.8f,g,h | 41.6±1.3g,h | 68.3±4.9e,f,g | 70.4±11.1f,g | Blue | El | Smooth | 8 | ||
| 8 | 81.5±1.8f,g,h | 52.1±1.9m | 92.5±6.9j,k | 86.8±4.0h | Blue | Sp | Rough | 8 | ||
| Njombe (IV) | 9 | 73±3.4e | 45.0±1.8i,j,k | 78.7±6.5f,g,h,i | 62.3±2.4d,e,f | Dark blue | Ob | Smooth | 15 | |
| 10 | 66.6±3.8c,d | 44.5±2.8I,j | 60.5±2.3d,e | 65.8±8.5e,f,g | Dark blue | Ob | Smooth | 15 | ||
| 11 | nd | nd | nd | nd | nd | nd | nd | |||
| 12 | nd | nd | nd | nd | nd | nd | nd | |||
| Yaounde (V) | 13 | 82.9±4.2g,h | 39.4±1.9f,g | 79.7±12g,h,i | 61.7±4.9d,e,f | Dark blue | Ov | Smooth | Unknown | |
| 14 | 80.4±2.3f,g | 48.6±1.8l | 85.0±10i,j,k | 83.1±2.7h | Blue | Ob | Rough | >20 | ||
| 15 | 76.6±2.3f | 40.1±1.4f,g | 56.7± 3.7c,d | 64.1±5.9ef,g | Dark blue | Ob | Smooth | 7 | ||
| 16 | 89±4.7i | 36.8±3.3d | 67.5±8.2e,f | 57.5±5.3d,e | Blue | El | Smooth | >20 | ||
| Y2 | Foumban (III) | 1 | 48.4±4.6a | 32.8±3.0b | 31.8±4.5a | 31.9±2.7a | Dark blue | Sp | Smooth | 11 |
| 2 | 77.4±5.9f | 33.6±0.7b,c | 69.7±4.9e,f,g | 46.4±4.7b,c | Dark blue | Sp | Smooth | 41 | ||
| 3 | 59.3±2.5b | 33.6±1.1b,c | 50.8±1.7b,c,d | 40.3±3.7a,b | Dark blue | Sp | Smooth | 11 | ||
| 4 | 69.5±3.5d,e | 37.4±1.8d,e | 55.8±3.7c,d | 52.0±3.8c,d | Dark blue | Ob | Smooth | 11 | ||
| Makenene (V) | 5 | 78.0±9.1f,g | 45.8±4.8i,j,k | 71.8±14.8f,g,h | 70.6±18.0f,g | Blue | El | Smooth | 9 | |
| 6 | 71.5±2.7e | 46.8±1.4j,k,l | 71.7±6.5f,g,h | 70.0±2.9f,g | Blue | Sp | Rough | 21 | ||
| 7 | 77.0±3.7f | 39.9±1.3e,f,g | 60.5±2.1d,e | 54.1±5.1c,d | Blue | El | Smooth | 9 | ||
| 8 | 71.6±5.5e | 47.3±3.3k,l | 89.9±3.8j,k | 84.7±2.5h | Blue | Sp | Rough | 9 | ||
| Yaounde (V) | 13 | 81.2±2.3f,g,h | 38±1.2d,e,f | 81.7±6.8h,i,j | 58.45±2.8d,e | Dark blue | Ov | Smooth | Unknown | |
| 14 | 81.3±2.1f,g,h | 45±1.4i,j,k | 78.7±5g,h,i | 73±5.0g | Blue | Ob | Rough | >20 | ||
| 15 | nd | nd | nd | nd | nd | nd | nd | |||
| 16 | nd | nd | nd | nd | nd | nd | nd |
nd: Not determined, Sp: Spheroidal, El: Ellipsoidal, Ob: Obovate, Ov: Ovate, For each parameter measured, mean±standard deviations (based on measurements of 10 fruits) followed by the same letter are not significantly different at the 5% probability level
The value of morphological parameters did not necessarily decrease with the age of the tree. The lengths and diameter of ripe fruits from Tree 2 in Y2 were significantly higher than those in Y1. The same parameters on Tree 4 and 6 in Y2 were significantly higher (P < 0.01) than those from the same trees in Y1 [Table 1].
3.1.3. Fruit development and ripening
The fruit set-to-ripening period [Figure 2] ranged from 19 to 25 weeks in Y1 and was 21 weeks in Y2 on the same trees at Foumban. That time ranged from 18 to 21 weeks in Y1 and from 19 to 21 weeks in Y2 at Makenene. However, the African plum trees needed 18–22 weeks in Y1 at Njombe, while at Yaounde, the trees needed 19–26 weeks in Y1 and 19–23 weeks in Y2 between fruit set and ripening. Nevertheless, the average time between fruit set and ripening in Y1 (21.36 ± 2.56 weeks) was not significantly different (P ≥ 0.05) to that in Y2 (20.3 ± 1.11 weeks).
In all the sites, the fruit length, diameter, volume, and fresh weight kinetics were sigmoidal [Figure 3]. Fruits reached the maximum value of these morphological parameters, respectively, listed, between 8.25 ± 2.5 and 15 ± 1.63; 9.75 ± 0.96 and 13.5 ± 1; 10.75 ± 1.26 and 14.5 ± 1.91; 12.75 ± 4.92; and 17 ± 4.32 weeks [Table S1].
 | Figure 3: Morphological characteristics of Dacryodes edulis fruits collected at Foumban (AEZ III), Makenene (AEZ: V), Njombe (AEZ IV), and Yaounde (AEZ V) in 2012 and 2013 [Click here to view] |
Table S1: Reference periods for morphological changes in some parameters during safou development studied in Cameroon, 2012 (Y1) and 2013 (Y2)
| Years and locality | Development | Length | Diameter | Volume | Fresh weight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time PECD (weeks) | Time PE (weeks) | Time PR (weeks) | Time R (weeks) | S-HL (weeks) | S-HLGR (weeks) | S-HD (weeks) | S-HDGR (weeks) | S-HV (weeks) | S-HVGR (weeks) | S-HW (weeks) | S-HWGR (weeks) | ||
| Y1 | Foumban | >1 | 14.25±1.71b | 3.5±4.12a | 3.5±1a | 15±1.63b | 5.5±1a | 13.5±1a | 5.5±1b | 14.5±1.91a | 8±2b,c | 17±4.32a,b | 8±1.15a |
| Makenene | >1 | 12±0a,b | 3.5±0a | 3±0a | 11.75±1.71a | 4.25±0.5a | 11.25±0.96a | 4.25±0.5a,b | 12.75±1.5a | 5.75±1.26a,b | 14.25±1.71a,b | 7.25±5.25a | |
| Njombe | <1 | 12±1.41a,b | 2±2.83a | 5±1.41a | 12±2.83a | 5±1.41a | 11±4.24a | 5±1.41a,b | 13±1.41a | 7±1.41a,b,c | 20±2.83b | 8±2.83a | |
| Yaounde | >1 | 9.5±1a | 6.5±1.91a | 5.25±2.5a | 9.75±0..96a | 3.75±0.96a | 9.75±0.96a | 3.25±1.26a | 10.75±1.26a | 5.75±0.96a,b | 14.75±1.26a,b | 5.25±0.5a | |
| Y2 | Foumban | <1 | 12±0a,b | 4±1.63a | 4 ±0a | 11±1.63a | 4±1.15a | 11.5±3a | 3±1.63a | 12.5±1.91a | 9±2.31c | 16.5±1.91a,b | 7±1.63a |
| Makenene | >1 | 11±2.71a,b | 3±2.58a | 4±1.63a | 8.25±2.5a | 3.75±1.5a | 10.75±1.26a | 3.75±1.5a,b | 12.25±2.22a | 4.75±1.26a | 12.75±4.92a | 5.25±1.71a | |
| Njombe | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Yaounde | <1 | 12±0a,b | 5±1.41a | 3±1.41a | 11±0a | 4±1.41a | 13± 0a | 3 ±0a | 13±0a | 11± 0c,d | 15±0a,b | 5±0a | |
PECD: Putative early cell div, PE: Putative elongation, PR: Pre-ripening, R: Ripening, S: Fruit set, HL: Highest length value, HLGR: Highest length growth rate, HD: Highest diameter value, HDGR: Highest diameter growth rate, HV: Highest volume value, HVGR: Highest volume growth rate, HW: Highest fresh weight value, HWGR: Highest fresh weight growth rate, Nd: Not determined
In contrast, the curves showing the evolution of growth rates of these morphological parameters were sinusoidal and linear shapes [Figure 4]. Maximum fruit growth rates were reached between 3.75 ± 1.5 and 5.5 ± 1; 3 ± 1.63 and 5.5 ± 1; 4.75 ± 1.26 and 11 ± 0; and 5.25 ± 1.71 and 8 ± 2.83 weeks for length, diameter, volume, and fresh weight, respectively [Table S1].
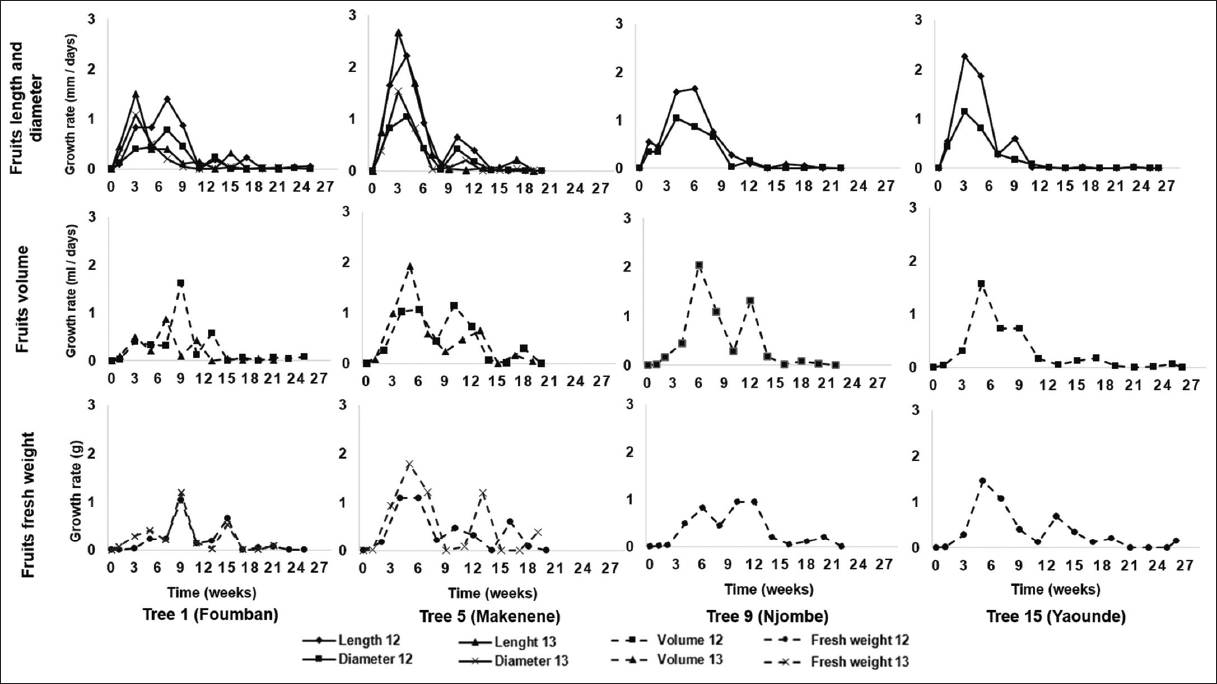 | Figure 4: Growth rate of measured parameters in Dacryodes edulis fruits collected at Foumban (AEZ III), Makenene (AEZ: V), Njombe (AEZ IV), and Yaounde (AEZ V) in 2012 and 2013 [Click here to view] |
The two sets of these curves showed four main safou development and ripening phases. There were successively the early putative cell division phase, putative elongation phase, pre-ripening phase, and a ripening phase. In all fruits, the putative cell division phase started from the fruit set and ended during the putative elongation phase. The duration of fruit putative elongation phase was between 9.5 ± 1 and 14.25 ± 1.71 weeks. That of pre-ripening phase was between 2 ± 2.83 and 6.5 ± 1.91 weeks. The average duration of the fruit ripening process was between 3 ± 0 and 5.25 ± 2.5 weeks [Table S1].
3.1.4. Identification of development and ripening periods as well as reproductive phenophases
In the studied population, safou flower buds emerged late in Y2 as compared to Y1. In Y1, flower buds emerged on 92.86% of the trees in January and 7.14% in February; in Y2, flower buds emerged on 80% of the trees in February, with the rest emerging in January (10%) and March (10%)) [Table S2].
Table S2: Reference dates for the development of reproductive and developmental phenological stages for each Dacryodes edulis tree studied in Cameroon, 2012 and 2013
| Trees | Date floral buds | Date anthesis | Date fruit set | Date putative elongation | Date ripening onset | Date end of ripening |
| Tree 1 | 11/01/2012 | 01/02/2012 | 08/02/2012 | 06/06/2012 | 18/07/2012 | 01/08/2012 |
| 06/03/2013 | 20/03/2013 | 27/03/2013 | 12/06/2013 | 24/07/2013 | 21/08/2013 | |
| Tree 2 | 18/01/2012 | 01/02/2012 | 08/02/2012 | 23/05/2012 | 23/05/2012 | 20/06/2012 |
| 06/02/2013 | 20/02/2013 | 27/02/2013 | 12/06/2013 | 26/06/2013 | 24/07/2013 | |
| Tree 3 | 20/01/2012 | 03/02/2012 | 10/02/2012 | 08/06/2012 | 08/06/2012 | 06/07/2012 |
| 30/01/2013 | 20/02/2013 | 27/02/2013 | 29/05/2013 | 26/06/2013 | 24/07/2013 | |
| Tree 4 | 20/01/2012 | 03/02/2012 | 10/02/2012 | 11/05/2012 | 06/07/2012 | 03/08/2012 |
| 06/02/2013 | 20/02/2013 | 27/02/2013 | 29/05/2013 | 26/06/2013 | 24/07/2013 | |
| Tree 5 | 09/01/2012 | 30/01/2012 | 06/02/2012 | 29/04/2012 | 14/05/2012 | 25/06/2012 |
| 04/02/2013 | 25/02/2013 | 04/03/2013 | 06/05/2013 | 17/06/2013 | 15/07/2013 | |
| Tree 6 | 30/01/2012 | 13/02/2012 | 20/02/2012 | 07/05/2012 | 11/06/2012 | 25/06/2012 |
| 11/02/2013 | 25/02/2013 | 04/03/2013 | 10/06/2013 | 10/06/2013 | 22/07/2013 | |
| Tree 7 | 02/01/2012 | 23/01/2012 | 30/01/2012 | 14/05/2012 | 11/06/2012 | 25/06/2012 |
| 04/02/2013 | 25/02/2013 | 04/03/2013 | 03/06/2013 | 01/07/2013 | 15/07/2013 | |
| Tree 8 | 16/01/2012 | 30/01/2012 | 06/02/2012 | 14/05/2012 | 11/06/2012 | 25/06/2012 |
| 11/02/2013 | 25/02/2013 | 04/03/2013 | 03/06/2013 | 17/06/2013 | 15/07/2013 | |
| Tree 9 | 22/01/2012 | 05/02/2012 | 12/02/2012 | 07/05/2012 | 03/06/2012 | 15/07/2012 |
| nd | nd | nd | nd | nd | nd | |
| Tree 10 | 22/01/2012 | 05/02/2012 | 12/02/2012 | 28/05/2012 | 20/05/2012 | 17/06/2012 |
| nd | nd | nd | nd | nd | nd | |
| Tree 11 | nd | nd | nd | nd | nd | nd |
| nd | nd | nd | nd | nd | nd | |
| Tree 12 | nd | nd | nd | nd | nd | nd |
| nd | nd | nd | nd | nd | nd | |
| Tree 13 | 07/02/2012 | 28/02/2012 | 06/03/2012 | 29/05/2012 | 26/06/2012 | 24/07/2012 |
| 24/02/2013 | 17/03/2013 | 24/03/2013 | 09/06/2013 | 21/07/2013 | 04/08/2013 | |
| Tree 14 | 22/01/2012 | 12/02/2012 | 19/02/2012 | 06/05/2012 | 01/07/2012 | 29/07/2012 |
| 03/02/2013 | 17/02/2013 | 24/02/2013 | 02/06/2013 | 07/07/2013 | 04/08/2013 | |
| Tree 15 | 29/01/2012 | 19/02/2012 | 26/02/2012 | 13/05/2012 | 24/06/2012 | 26/08/2012 |
| nd | nd | nd | nd | nd | nd | |
| Tree 16 | 29/01/2012 | 19/02/2012 | 26/02/2012 | 29/04/2012 | 24/06/2012 | 22/07/2012 |
| nd | nd | nd | nd | nd | Nd |
nd: not determined
A significant difference was observed between Y1 and Y2 for the climatic conditions that occurred during some phenological and developmental phases [Figure 5]: Average temperature and cumulative temperature between floral buds and ripening onset (P < 0.05); average temperature between floral bud emergence and the end of fruit ripening (P < 0.05); average temperature and cumulative temperature between floral bud emergence and the end of elongation (P < 0.05); and cumulative temperature between floral bud emergence and the end of ripening (P < 0.01). Significant differences were also observed between Y1 and Y2 for: time to highest fruit length growth rate (P < 0.05); time to highest fruit diameter growth rate (P < 0.01); time to highest fruit volume growth rate (P < 0.05); and time to highest length value (P < 0.05) [Table 2].
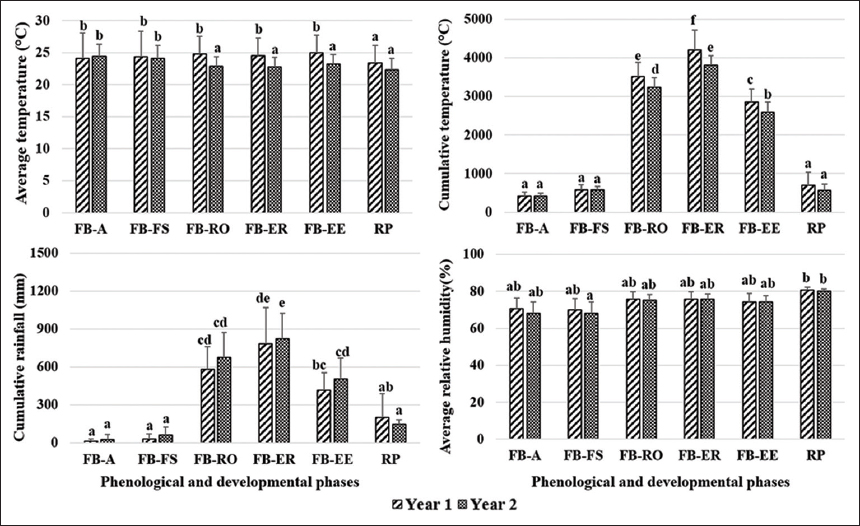 | Figure 5: Comparison of climatic parameters recorded during the different phenological and developmental phases in two consecutive Dacryodes edulis fruits production seasons. FB-A: Floral bud emergence and anthesis, FB-FS: Floral bud emergence and fruit set, FB-RO: Floral bud emergence and ripening onset, FB-ER: Floral bud emergence and end of ripening, FB-EE: Floral bud emergence and end of elongation, RP: Ripening period. The bars bearing the same letter are not significantly different at 5% probability level [Click here to view] |
Table 2: Comparison of time recorded during the different phenological and developmental phases in the two consecutive Dacryodes edulis fruits production seasons
| Parameters | Y1 | Y2 |
|---|---|---|
| Time of vegetative growth between 2 consecutive production seasons (days) | nd | 198.25±24.05 |
| Time between floral bud emergence and anthesis (days) | 17.06±3.59a | 16.92±3.09a |
| Time between floral bud emergence and fruit - set (days) | 24.06±3.59a | 23.92±3.09a |
| Time between floral bud emergence and ripening onset (days) | 143.94±20.6a | 141.17±9.16a |
| Time between floral bud emergence and end of ripening (days) | 172.38±19.44a | 166.83±7.6a |
| Time between floral bud emergence and end of elongation (days) | 115.5±15.12a | 112.00±8.85a |
| Ripening time (days) | 29.31±13.09a | 25.67±6.92a |
| Time to highest fruit length growth rate (days) | 84.88±17.31b | 70.58±12.58a |
| Time to highest fruit diameter growth rate (days) | 32.38±7.61b | 22.75±7.29a |
| Time to highest fruit volume growth rate (days) | 46.38±11.10a | 58.92±17.42b |
| Time to highest fresh fruit weight growth rate (days) | 49.88±20.11a | 40.25±11.75a |
| Time to highest fruit length value (days) | 84.88±17.31b | 70.58±12.38a |
| Time to highest fruit diameter value (days) | 79.63±15.52a | 82.25±11.75a |
| Time to highest fruit volume value (days) | 89.25±13.4a | 88.08±9.38a |
| Time to highest fruit fresh weight value (days) | 115.5±23.43a | 103.25±19.15a |
| Time between fruit set and end of ripening (days) | 148.31±17.75a | 140.58±5.97a |
| Time between fruit set and ripening onset (days) | 119.00±17.71a | 114.92±6.5a |
nd: Not determined, for each parameter measured, mean±standard deviations followed by the same letter are not significantly different at the 5% probability level
Meanwhile, the delay in the date of flower bud emergence led to an overall delay in harvest date (end of ripening) from June to July (for Tree 2, Tree 5, Tree 6, Tree 7, Tree 8) and from early July to late July (Tree 3) or from late July to early August (for Tree 13 and Tree 14) [Tables S2].
3.1.5. Relationships between fruit development and ripening phases, phenophases, and measured parameters
Pearson and Spearman correlation coefficients calculation [Table 3] revealed a positive correlation between (A) the time from floral bud emergence to anthesis and (B) the time from floral bud emergence to fruit set (P < 0.01 in Y1 and Y2); between (N) the cumulative temperature during the floral bud emergence to fruit set period and A (P < 0.01 in Y1 and Y2), B (P < 0.01 in Y1 and Y2) and (M) the cumulative temperature during the floral bud emergence to anthesis period (P < 0.01 in Y1 and Y2); between (O) the cumulative temperature during the floral bud emergence to fruit ripening onset period and (C) the time from the floral bud emergence to fruit ripening onset (P < 0.01 in Y1 and Y2); between (P) the cumulative temperature during the floral bud emergence to late fruit ripening period and (H) the average temperature during the floral bud emergence to fruit set period (P < 0.01 in Y1 and Y2); between (Q) the cumulative temperature during the floral bud emergence to late fruit elongation period and (E) the time from floral bud emergence to late fruit elongation (P < 0.01 in Y1 and Y2); between (R) the cumulative temperature for the ripening period and (G) the average temperature during the floral bud emergence to anthesis period (P < 0.01 in Y1 and P < 0.05 in Y2); between (X) the cumulative rainfall for the ripening period and (F) the time during the ripening period (P < 0.01 in Y1 and Y2); and between (AN) the time from fruit set to fruit ripening onset and (D) the time from the floral bud emergence to late ripening period (P < 0.01 in Y1 and P < 0.05 in Y2) [Table 3].
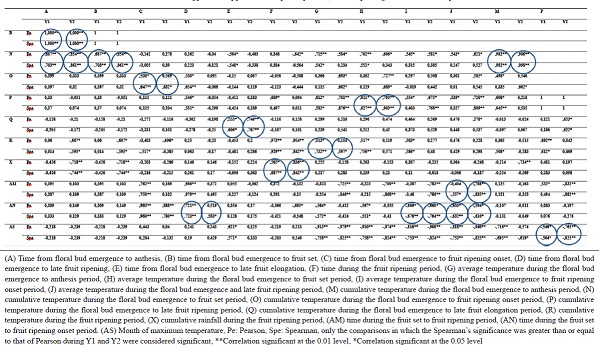 | Table 3: Bilateral correlation between the chronology of the appearance of phenophases, the morphological and climatic parameters in Y1 and Y2 [Click here to view] |
Predicting the values of (P) and (R) allows to predict the ripening dates. (P) being the thermal time, the regression line of (P) versus (H) postulates that knowledge of the values of (H) predicts those of (P). It is, therefore, a forecasting model estimated by the relation (P) = a(H) + b, that is, (P) = 101.4(H) +1583 (correlation coefficient, r = 0.97), a highly significant relation. Similarly, the relation (R) = c(G) − d, that is, (R) = 44.714 (G) − 429.04 (correlation coefficient, r = 0.85) obtained from the regression line of (R) versus (G) postulates that knowledge of the values of (G) allows to predict those of (R).
The Pearson and Spearman correlation coefficients revealed a negative correlation between (AM) the time during the fruit set to end of ripening period and (J) average temperature during the floral bud emergence to end of ripening period (P < 0.05 in Y1 and P < 0.01 in Y2); between AN and (I) the average temperature during the floral bud emergence to ripening onset period (P < 0.01 in Y1 and Y2) and J (P < 0.01 in Y1 and P < 0.05 in Y2).
4. Discussion
D. edulis fruit maturation and ripening indexes are not clearly defined and the role of climate in the prediction of ripening time has not been assessed. At the target sites, it was found that the shortest time between fruit set and fruit ripening was 18 weeks (Makenene and Njombe in Y1), but this period was sometimes as long as even 26 weeks (Yaounde Y1). This period, therefore, did not depend on the study site or the fruit size and could vary between years. During this period, safou fruit development and ripening could be divided into four major phases, respectively, putative cell division, putative elongation, pre-ripening (phase defined as the time from no change in fruit growth to fruit color change or start of darkening), and ripening (time of fruit darkening) phases. The putative cell division phase began at fruit set and continued during the elongation phase. The putative fruit elongation phase began 1–2 weeks after the onset of cell division and the duration was between 9.5 and 14.25 weeks in Y1 and 11–12 in Y2. The maximum growth rate of all of the parameters studied was reached during this elongation phase and fruits from Makenene and Yaounde had the highest maximum growth rates, which were reached earlier after fruit set compared to fruits from Foumban and Njombe. That of pre-ripening phase was between 2–6.5 and 3–5 weeks (respectively in Y1 and Y2). However, the duration of the fruit ripening process was on average 3–5.25 weeks in Y1 and 3–4 weeks in Y2. The time of each phase, therefore, sometimes varied between the studied trees and between years in the same tree, suggesting that there was high variability between D. edulis fruits, including those from the same AEZ. These findings were in accordance with those of previous studies showing that apparently homogeneous groups may exhibit considerable variations [2].
The fruit set-to-preripening period ranged from 14 weeks (Makenene (AEZ V) in Y1 and Y2, Njombe (AEZ IV) in Y1) to 23 weeks (Foumban (AEZ III) in Y1), which was higher than the period reported in a previous study by Kengue [15] in Yaounde (AEZ V). Before the ripening phase, immature fruits changed from green to red and then to pink. In this study, some immature fruits turned white before starting to take the color of the ripe fruit (14.3% in Y1 and 70% in Y2), while others did not. As anthocyanins are the pigments responsible for the red color in plant tissues [16], we suggest that these compounds degenerate and enzymes responsible of their synthesis are down-regulated before the synthesis of black or blue pigments. Some previous authors showed that immature pink safou gradually whitened before taking on the characteristic color of ripe fruits [7,17].
During D. edulis fruits development and ripening, there was no significant difference between time from floral bud emergence and end of ripening between Y1 and Y2, although the average temperature during this period and the thermal time were significantly higher in the 1st year. This is due to the fact that average temperature during floral bud emergence to anthesis remained non-significantly different between Y1 and Y2. Previous studies showed that the high temperatures after flowering accelerated the rate of their phenology, reduced the development time of fruits, accelerated their growth, and thus advancing the harvest date [18,19].
Our results also showed that, within a given AEZ, the morphological parameters of safou fruits from the same tree varied significantly between years. The values of these parameters did not necessarily decrease with the age of the tree. This could have been due to the availability of minerals (soil-borne potassium, calcium, nitrogen, and phosphorus), which are known for their beneficial effects on fruit growth [20,21] and/or their uptake by trees [22].
Correlations between the different parameters (dates of flowering, fruit set, fruit elongation, ripening onset, end of fruit ripening, values of temperature, rainfall, and average relative humidity) showed that it was possible to predict the chronology of certain reproductive phenophases and consequently the succession of development stages (anthesis, fruit set, end of elongation, ripening onset, and end of ripening). Gordo and Sanz [23], Grab and Craparo [24], and Wang et al. [25] already showed that temperature was one of the most important factors with regard to plant phenology. These correlations confirmed that the cumulative temperature to complete safou fruit ripening (thermal time) could be predicted on the basis of the value of the average temperature between floral buds and fruit set. Similarly, knowing the average temperature between floral bud emergence and anthesis can help to predict the temperature that the fruit will need to cumulate during the ripening phase. These results can thus be exploited for various purposes such as controlling the effects of climate change on safou fruit development and ripening or developing predictive models of the succession of these phenophases. This could facilitate the prediction of harvest dates to avoid marketing highly perishable safou fruit and to determine the ideal agronomic intervention period.
5. CONCLUSION
Under conditions of this study, based on the targeted morphological parameters (i.e., fruit color, diameter, length and volume), there was high morphological variability in D. edulis fruits depending on the year of investigation. Four phases of fruit development and ripening were determined during the fruit set-to-ripening period, despite the high species heterogeneity. Knowing the average temperature during the floral bud emergence to fruit set period can help predicting the cumulative temperature necessary from flower bud emergence to complete safou ripening. Similarly, the average ambient temperature during the floral bud emergence to anthesis period can help to determine the cumulated temperature required by the fruit during the ripening phase. These results will help farmers to determine accurately the best periods for fruit harvest and sale to enhance the safou marketability while knowing that predictions of plant phenology may also depend on water availability that was not considered in this study.
6. ACKNOWLEDGMENT
The authors would like to thank Mrs. Clovis Gamaleu, Justin Lowe, and Aliyou Nsangou for their technical assistance while also providing us with the safou trees that were used in this study.
7. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
10. ETHICAL APPROVALS
This study does not involve the use of animals or human subjects.
REFERENCES
1. Todou G. Distribution, Adaptation Environnementale et DiversitéGénétique de
2. Kadji BR, Kone FM, Sika AE, Dabonne S. Physico-chemical properties of safou (
3. Ondo-Azi AS, Ella Missang C, Nsikabaka S, Silou T, Chalchat JC. Variation in physicochemical and morphological characteristics of safou (
4. Mbofung CM, Silou T, Mouaragadja I. Chemical characterisation of safou (
5. Ndindeng SA, Ambang Z, Woin N, Ngome AF, Njebu AS, Mapiemfu-LamaréD,
6. Iloamaeke IM, Onuigbo CC, Umedum LN, Umeobika CU, Oforah PU. Production and characterization of biodiesel from the seed of
7. Ndindeng SA, Talle, Bigoga J, Kengue J, Boffa JM. Predictors of organoleptic quality of boiled and dried pulp of safou (
8. Oluwatooyin FO, Kudirat TS, Olubukola VO. Potential of African pear (
9. Youmbi E, Mbeuyo M, Tchinda ND, Amougou A. Physico-chemical characterisation and classification of fruits of
10. Tchinda ND, Wanjala BW, Muchugi A, Fotso F, Nzweundjl G, Ndoumou DO,
11. Berteaux D, Réale D, McAdam AG, Boutin S. Keeping pace with fast climate change:Can arctic life count on evolution?Integr Comp Biol 2004;44:140-51. [CrossRef]
12. Park I, Jones A, Mazer SJ. PhenoForecaster:A software package for the prediction of flowering phenology. Appl Plant Sci 2019;7:e01230. [CrossRef]
13. Kana JR, Gnonlonfin BG, Harvey J, Wainaina J, Wanjuki I, Skilton RA,
14. Hughes SW. Archimedes revisited:A faster, better, cheaper method of accurately measuring the volume of small objects. Phys Educ 2005;40:468-74. [CrossRef]
15. Kengue J. Le Safoutier(
16. Sugiura T, Ogawa H, Fukuda N, Moriguchi T. Changes in the taste and textural attributes of apples in response to climate change. Sci Rep 2013;3:2418. [CrossRef]
17. Anegbeh PO, Ukafor V, Usoro C, Tchoundjeu Z, Leakey RR, Schreckenberg K. Domestication of
18. Day K, Lopez G, DeJong TM. Using growing degree hours accumulated thirty days after bloom to predict peach and nectarine harvest date. Acta Hortic 2008;803:163-7. [CrossRef]
19. Impa SM, Vennapusa AR, Bheemanahalli R, Sabela D, Boyle D, Walia H,
20. Husain A, Muhammad S, Sikandar H, Rizwan U, Muhammad A, Abbas J,
21. Brunetto G, De Melo GW, Toselli M, Quartieri M, Tagliavini M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev Bras Frutic 2015;37:1089-104. [CrossRef]
22. Theron K. Size matters:Factors influencing fruit size in pear. Acta Hortic 2011;909:545-55. [CrossRef]
23. Gordo O, Sanz JJ. Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Change Biol 2010;16:1082-106. [CrossRef]
24. Grab S, Craparo A. Advance of apple and pear tree full bloom dates in response to climate change in the Southwestern Cape, South Africa:1973-2009. Agric For Meteorol 2011;151:406-13. [CrossRef]
25. Wang HJ, Ge QS, Rutishauser T, Dai YX, Dai JH. Parameterization of temperature sensitivity of spring phenology and its application in explaining diverse phenological responses to temperature change. Sci Rep 2015;5:8833. [CrossRef]