1. INTRODUCTION
Climate change (CC) is a serious threat which affects human health, food security, water availability, and general socioeconomic advancement in many nations. Irregular monsoons, droughts, and floods due to CC create an impact on farmers’ income and agricultural production. One-degree increase in average worldwide temperature causes 3–7% decline in the productivity of agricultural crops [1]. The estimated reduction of yield due to CC was 4.5–9% during the period 2020–2039 and in annual gross domestic product, it declined from 0.7% to 1.35% [2]. Flood affects the productivity of agricultural crops at 15,966.345 ha and horticultural crops at 2,328.73 ha in Thanjavur District, Tamil Nadu, India [3]. Flooding poses a significant threat to both food production and economic prosperity on a global scale. Annually, it impacts approximately 17 million km2 of land surface, resulting in substantial damage to agricultural crop yields [4]. Waterlogging affects nearly 10% of the world’s land area and in between 2006 and 2016, floods were responsible for two-thirds of the total global crop loss and damage [5].
Flooding leads to reduction in soil redox potential, alteration of soil chemical composition, increased ionic elements, and increased toxicity. Flooding increases the soil pH and sodium adsorption ratio and affects the physiochemical properties, and it not only affects the crop yield but also makes the soil unproductive and unfit for cultivation due to depletion of nutrients [6]. Excess irrigation suppresses the growth and development of plant due to reduced gaseous exchange in the root zone which elevates soil salinity. The intense rainfall-induced fertile topsoil erosion affects productivity. Plants require the optimum level of water throughout their life cycle; the increased water content affects cell integrity and leads to plant mortality. Waterlogged soil lacks adequate aeration, hindering normal plant growth and function, thus making plants more prone to diseases, reducing resistance mechanisms, and causing excessive soil nutrient leaching. Flooded fruit crops exhibit symptoms such as chlorosis, wilting, leaf scorching, shedding of leaves, fruit drop, stem and limb dieback, and tree mortality [7].
The objective of this review is to describe how waterlogging impedes the growth and development of fruit crops amidst global warming and the increased likelihood of heavy rainfall, which can significantly. We focus on the changes in cell decisions on integrity and their symptoms in fruit species to aid in future thrust in research and improve fruit production under the altered changing environmental conditions. This is one aspect which has not received adequate attention. The review focuses on taking it as a major chapter beginning with the cell wall and then various parts of the cell.
2. IMPACTS OF FLOODING IN PLANTS
2.1. Limited Oxygen Availability (Hypoxia)
Waterlogging replaces air from the soil pores which hinders the gas exchange between soil and atmosphere and it affects root respiration and root activity resulting in an energy deficit. Adenosine triphosphate (ATP) is the primary energy source for the cell and its metabolism, but in flooded plants, ATP production was restricted. Flooding-induced O2 deficiency affects the photosynthetic activity and it leads to decreased chlorophyll levels, reduced stomatal aperture, premature leaf aging, and a decline in leaf area, it also affects the source–sink activity of photosynthetic products. Insufficient O2 affects the electron transport chain components dependent metabolic reactions due to the alteration of redox balance in cells. Hence, maintaining NADH/NAD+ ratio is important for normal cell functioning under flood stress. Electron transport components coupled with a reduced intracellular environment promote reactive oxygen species (ROS) generation which limits the energy supply leading to enzyme inactivation, lipid peroxidation, and DNA damage [8]. Under hypoxia conditions, metabolic activity and ATP production are disrupted, which hinders root and vegetative growth. Stagnant water creates more stress than moving water, and banana trees under non-circulating flooding show tolerance up to 24–48 h but the circulating water showed tolerance up to 72 h toward flood [9]. Figure 1 explains the impact of flooding on plants. An area worth research is the level of anoxia that the plant is subjected to and how to overcome this by oxygenation and also ensuring the availability of oxygen.
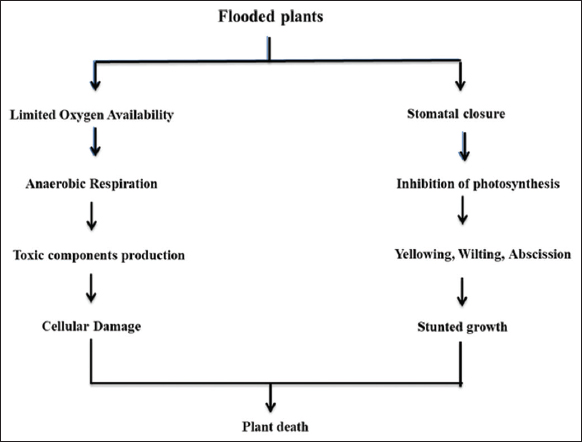 | Figure 1: Flooding affects plant metabolism and cell integrity [Click here to view] |
2.2. Anerobic Respiration
Flooding leads to an energy crisis, and low O2 availability halts ATP production which disrupts the electron transport chain and mitochondrial respiration. To maintain the energy level, plants shift from erobic respiration to anerobic. Normally, the tricarboxylic acid (TCA) cycle produces 38 ATP molecules from 1 molecule of glucose but the glycolysis and ethanol fermentation pathway produce 2 ATP molecules. Anerobic respiration leads to energy wastage and lifts the fermentation level and cytoplasmic acidosis and also increases the toxicity due to acetaldehyde, ethanol, and lactic acid accumulation [10]. In anerobic fermentation, two pathways involved in energy production are lactate dehydrogenase (LDH), which enhance the production of lactic acid and alcohol dehydrogenase (ADH), in which pyruvate is converted into acetaldehyde [11]. Extended flooding induces the formation of ROS, lactic acid, hydrogen peroxide (H2O2), aldehydes, and ethanol which leads to cellular death and complete senescence of plants. These conditions not only affect the vegetative growth but also affect the reproductive phase by yield reduction [12]. Depending upon the adaptation level of species, flood stress causes cell death within an hour or can extend to days. The degree of tolerance/susceptibility to waterlogging of fruit crops is an area worth to be probed.
2.3. Effect on Photosynthesis
Flooded plants prevent water loss by reducing stomatal conductance, which unintendedly affects the photosynthetic activity and also some factors such as low water potential, irregular photo assimilates transportation, reduced chlorophyll content, and reduced photosynthetic enzymes activity, which affects the net photosynthetic rate [13]. Flood affects leaf gaseous exchange and CO2 net assimilation resulting in leaf yellowing and senescence due to reduced photosynthetic activity and degradation of chlorophyll [14]. Increased photosynthetic rate coincides with morpho-anatomical adaptations such as hypertrophied lenticels and adventitious roots (ARs) formation [15]. Other factors that limit photosynthesis were reduced and increased activity of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) [15], and soluble carbohydrates accumulation [16], resulting in reduction of leaf area, suppression of leaf formation, formation of leaf lesions, and abscission.
3. EFFECTS OF FLOOD STRESS AT CELLULAR LEVEL
Flood stress-induced oxidative stress generates ROS such as superoxide, hydrogen peroxide, and hydroxyl radicals, and also, the anerobic respiration by-products such as lactic acid and ethanol can disrupt cell function and lead to cell death. The adequate level of ROS synthesis showed beneficial impacts such as promotion of plant growth and development, stimulation of cell division, and enlargement but the abounded synthesis of ROS can induce oxidative stress resulting in tissue death [17]. Excess ethanol accumulation affects membrane fluidity, functions of membrane proteins, and induces cell depolarization causing cell death. The promotion of protein denaturation and cell turgor alteration leads to cellular dehydration [18]. Lactic acid synthesis induces cellular acidification and disturbs the pH equilibrium that changes the enzyme function and cell integrity. Decreased pH alters the distribution and concentration of crucial ions such as K+, Ca+, and Mg++ which affects signal transmission, membrane-based transport, nutrient absorption, enzyme functions, and other cellular operations [19]. Figure 2 explicates the impact of flood stress on cellular cell.
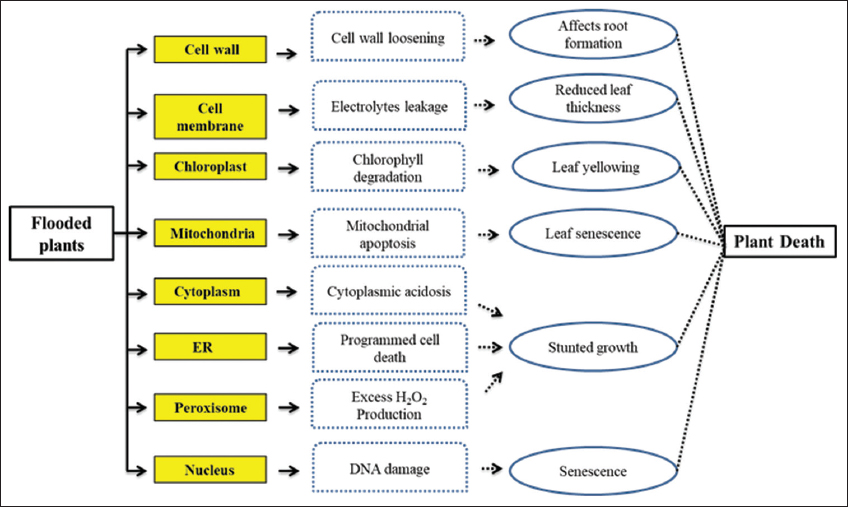 | Figure 2: Flooding effects in cell integrity and plant response [Click here to view] |
3.1. Cell Wall
Flooding affects cell wall expansion, bringing cell wall loosening, increased lignification, changes in cell wall porosity, and hindering cell wall degradation. Flooding activates enzymes such as expansins, xyloglucan endotransglucosylases/hydrolases, and pectin methylesterases, which cause changes in cell wall expansion, elasticity, and flexibility [20]. Flood-induced alteration in pH levels and ion concentrations causes cell wall loosening [21]. Flooding-induced down-regulation of proteins such as β-galactosidase, β-glucanase, and β-glucosidase inhibits the cell wall elongation and impedes plant growth [22]. Waterlogging leads to down-regulation of genes responsible for cell wall degradation and structure alteration, which hinders the root growth and formation of lateral roots [23].
3.2. Cell Membrane
Flooding disrupts ionic and nutrient equilibrium leading to electrolyte leakage such as potassium, calcium, and magnesium from the cytoplasm; which elevates membrane permeability and membrane protein damage [24]. Accumulation of harmful ions such as Na+ and Cl- by roots during waterlogging affects the cell integrity by disrupting the ionic balance [25]. Flood stress reduces turgor pressure, which leads to loosen arrangement of cells and widening of cell gaps observed as reduced thickness of leaf [26]. Elaborate studies on loss of cell integrity and osmolyte leakage as a consequence of flooding are observations that need to be addressed.
3.3. Cytoplasm
Cytoplasmic acidosis triggered by the acid leakage from vacuole affects the root tips and hypoxia tolerance capacity which leads to cell death. Due to the inhibition of energy generation, cytoplasmic acidosis leads to loss of viability of hypoxia tissue by low pH levels. Anerobic respiration induces lactic acid production, but cytoplasmic acidosis triggers ethanol production which suppresses the lactic acid production during hypoxia causing cell death [27].
3.4. Chloroplast
Prolonged waterlogging affects photosynthetic enzyme activities due to reduced synthesis of chlorophyll observed as leaf yellowing, senescence, and shedding. New leaf production is also suppressed finally resulting in death due to reduction of photosynthetic rate [28]. Flood-induced stomatal closure also declines the leaf ability to capture light for photosynthesis [29]. Floods also cause reduction in photosynthetic pigments, carotenoids, rubisco activity, and sucrose transportation from source to sink [30]. Flood stress affects the chloroplast arrangement and chloroplast abundance within mesophyll cells [31] and alters the structure of chloroplast such as initiation of lamella structure loosening, increased osmophilic particle count, and swelling and rounding of chloroplast [32]. Structurally, it causes damage to chloroplast outer membranes, disruption of grana-lamellae arrangements, alterations in shape, and plasmolysis [33].
3.5. Mitochondria
Mitochondria plays a crucial role in cellular respiration and energy production, besides cell differentiation, apoptosis and transmission of cellular information [34]. Altered mitochondrial structure due to rupture and degradation of outer membranes by flooding can lead to mitochondrial fusion [33]. Waterlogging induces swelling and eventual disintegration of mitochondria [35]; Vitamin C within mitochondria triggers programmed cell death (PCD) which triggers cell necrosis; their mechanism adapts flood stress. Flooding affects the integrity of mitochondria making it unable to provide sufficient energy to transport the photosynthetic products to potential sinks within chloroplasts. This delayed transport of excess starch damages the structure of thylakoids, impacting the photosynthetic efficiency and activity of chloroplast [36].
3.6. Endoplasmic Reticulum (ER)
Depleted ATP level and O2 starvation by flood stress affect the protein synthesis in ER, unfolded or misfolded protein accumulation induced by the unfolded protein response (UPR) pathway [37], which triggers PCD pathway. UPR prompts ER stress by upregulation of chaperone proteins which limits the synthesis of new proteins and augments protein degradation pathways, either ending in cellular dysfunction or apoptosis [38]. Waterlogging affects the ribosomal proteins resulting in reduced protein abundance, protein synthesis, and glycosylation and also delays protein translocation. Calnexin, an ER-localized molecular chaperone is involved in protein folding got reduced during waterlogging [39].
3.7. Peroxisome
During unfavorable conditions, peroxisomes act as a safeguard toward ROS due to the overabundance of glutathione peroxidase (GPX), ascorbate peroxidase, glutathione reductase (GR), catalase (CAT), peroxiredoxins, dehydro-, monodehydro-ascorbate reductase, and superoxide dismutase (SOD) [40]. H2O2 production in peroxisomes induces oxidative stress, but the enzyme monodehydroascorbate reductase present in both peroxisomes and mitochondria catalyzes the decomposition of hydrogen peroxide. Glutathione is a non-protein thiol that plays an important role against oxidative damage by ROS within peroxisomes [41].
3.8. Nucleus
Flood alters the protein level and nuclear function by various transcription factors such as SNORKEL1, SNORKEL2, and SUBMERGENCE1 which provides tolerance to submergence. In addition, mRNA levels of proteins are also reduced due to flooding [42]. Extreme stress causes DNA fragmentation, and these fragmentations induce replication stress during mitosis that leads to chromosomal abnormalities and mutations [43]. Waterlogging affects the cellular signaling pathways and ROS-induced DNA damage leads to cell death [44]. Table 1 gives the flood-induced changes at cellular level and the plant response.
Table 1: Flood-induced changes in cell organelle and plant response.
| Cell organelle | Flood stress-induced changes | Plant response | References |
|---|---|---|---|
| Cell wall | Cell wall loosening and degradation | Suppression of lateral root formation | [113] |
| Cell membrane | Leakage of electrolytes such as potassium, calcium, and magnesium from the cytoplasm | Reduced leaf thickness | [137] |
| Cytoplasm | Cytoplasmic acidosis | Wilting, stunted growth, and yellowing of leaves | [91] |
| Chloroplast | Chlorophyll degradation, disruption of grana lamellae, and thylakoid arrangements | Yellowing and shedding of leaves | [103] |
| Mitochondria | Swelling and disintegration of mitochondria; Mitochondrial apoptosis, Programmed cell death, | Leaf senescence and Stunted growth | [132] |
| Endoplasmic reticulum | Unfolded or misfolded protein accumulation leads to PCD | Stunted growth | [82] |
| Peroxisome | Excess production of H2O2 | Hinder plant growth and development | [101] |
| Nucleus | DNA fragmentation and ROS-induced DNA damage causing cell death | Senescence | [121] |
PCD: Programmed cell death, ROS: Reactive oxygen species
4. MECHANISMS AND ADAPTATIONS BY PLANTS
Plants undergo various mechanisms for survival and adaptation in the face of different challenging environmental stresses, which encompass alterations in their physical structure as well as physiological, biochemical, and molecular reactions.
4.1. Antioxidant Production
Hydrogen peroxide (H2O2), superoxide radicals (O2•–), and hydroxyl radicals (HO?) trigger lipid oxidation, breakdown of leaf membrane, oxidative damage to DNA, protein, and severe damage to cell organelles [45]. H2O2 controls the aerenchyma formation [46], but the lowest concentration acts as signaling molecule to mitigate stress [47]. To maintain the equilibrium of ROS, plants are dependent on active antioxidants and systems of antioxidant enzymes such as CAT, POD, and SOD, thereby mitigating oxidative damage [48]. Elevated H2O2 content in leaves impairs photosynthetic organs causing premature senescence. CAT regulates H2O2 levels by converting it into O2 [49]. Exogenous application of γ-aminobutyric acid enhances chlorophyll content and photosynthetic rate by stimulating antioxidant enzyme activity such as CAT, GR, SOD, ascorbate peroxidase (APX), and POD and also suppresses malondialdehyde (MDA) and H2O2 levels, thereby enhancing tolerance to waterlogging [50].
Increased levels of antioxidant enzymes such as CAT, SOD, GR, APX, MDA, GPX, and proline were involved in ROS scavenging mechanisms in bananas under waterlogging [51]. CAT and APX mitigate stress-induced damage [52]; GPX acts as an oxygen radical scavenger [53]. Production of very few leaves with reduction in biomass production, root length, and plant height was observed in apple trees under hypoxia stress due to an abundance of superoxide radicals and hydrogen peroxide [54]. Increased carotenoids, proline, and soluble sugars were observed in the flooded purple passion plant (Passiflora edulis f. edulis) [55]. Castro-Duque et al. [56] found out that the foliar application of glycine betaine and hydrogen peroxide improves the ability of leaf water potential, stomatal conductance, relative water content, photochemical efficiency, chlorophyll content, and net photosynthesis in waterlogged Cape gooseberry (Physalis peruviana). All these reveal the pivotal role of ROS and the regulatory role of antioxidants that are critical in flood tolerance. Hence, this area deserves maximum attention as it is the basic physiological mechanism underlying flood tolerance.
4.2. Regulations through Gene Expression and Transcriptional Factor
H2O2 regulates ethylene (ET) signal transduction and influences the transcription of downstream hypoxia-responsive genes, such as ADH1 and ERF73/HRE1, in Arabidopsis [57]. ET signaling enhances ethanol fermentation which temporarily alleviates energy deficiencies and enhances plant adaptability to waterlogging and the expression of Plant Cysteine Oxidase and ET Response Factor ERFVIIs which were crucial for oxygen sensing in flooded conditions specifically, MaERFVII-3, MaERFVII-2, MaERFVII-1, and ADH1 genes are crucial for flooding tolerance in banana [51]. ET biosynthesis enzymes such as 1-aminocyclopropane-1-carboxylic acid oxidase and S-adenosyl-L-methionine upregulated in flooded roots, because ET is the key regulator for the flooding response [58]. ERFVII transcriptive factor promotes ATP production through glycolysis and fermentation during hypoxia [59].
Downregulated NCED1 gene and upregulated ABA degradation gene (ABA 8’ -hydroxylase gene) were the genes associated with ABA pathway regulating flood stress [60]. Overexpressed AtACO5 in Arabidopsis enhances the production of ET and expansion of cells, boosting tolerance toward flood stress [61], while overexpressed CsARN6.1 facilitates AR formation [62]. Constitutive expression of TaERFVII.1 improves immunity against waterlogging stress, leading to increased rate of survival and content of chlorophyll [63]. Similarly, overexpression of HvERF2.11 enhances the biosynthesis genes of antioxidants (AtPOD1, AtSOD1) and biosynthesis gene of ET (AtACO1) and provides resistance toward flooding [64]. Expression of PDC and ADH-related genes enhances ethanol fermentation to provide energy support for the waterlogged plants [65]. GmADH2 gene enhanced seed germination under waterlogging [66] and PDC1 gene showed increased tolerance toward flood stress [67].
4.3. Hormonal Regulation
Plant hormones or phytohormones are the signaling molecules that regulate the normal functioning of plants and enhance tolerance toward abiotic stress [Table 2].
Table 2: Effects of plant hormones on various cellular functions during flooding stress.
| Hormones | Fruit crop | Functions | References |
|---|---|---|---|
| SA | Peach | Increases the photosynthesis and root activity | [124] |
| Melatonin | Apple | Inhibits ROS and MDA-induced chlorosis | [138] |
| Peaches | Improves the antioxidant enzymatic activities | [28] | |
| Ethylene | Guava | Adventitious root formation | [51] |
| Mango | Formation of adventitious roots | [98] | |
| Pond apple | Formation of hypertrophied stem lenticels | [72] | |
| Mango | Development of hypertrophied lenticels to excrete the toxic compounds | [57] | |
| Kiwifruit | High antioxidant activity | [76] |
ROS: Reactive oxygen species, MDA: Malondialdehyde
4.3.1. ET
Flooding affects the gas exchange leading to rapid ET accumulation within plant cells prompting adaptive mechanisms such as the formation of aerenchyma, enhancement of gas diffusion through ARs, implementation of survival strategies like shoot elongation, and promotion of hyponastic growth to elevate leaves above water [68]. It is also involved in post-hypoxia recovery by restoring substrates for the TCA cycle [69]. Maintaining water equilibrium and gas exchange during low oxygen conditions is the role of aquaporin. ET acts on aquaporin through the phosphorylation of AtPIP2;1 [70]. ET triggers rapid cell expansion of petiole under flooding [61] and interacts with gibberellic acid (GA) and brassinosteroid, stimulating hypocotyl elongation [71]. Initiation of AR, high scavenging activity of ROS, improved level of photosynthetic pigmentation, and high root surface area were noted by the action of exogenous ET application [8].
4.3.2. Abscisic acid (ABA)
ABA regulates the opening and closing of stomata by controlling guard cell. Decreased level of ABA leads to ET accumulation which stimulates the development of aerenchyma and ARs in flooded plants [60]. ABA-stimulated GA accumulation promotes shoot elongation [72]. Overexpressed RAP2.6L (AP2/ERF family gene) promotes ABA biosynthesis which initiates the antioxidant defense activation and stomata closure, ultimately reducing oxidative degradation, delaying senescence, and enhancing flood tolerance [32].
4.3.3. Gibberellin
GA restores internode elongation, allowing the plants to extend above the water surface and re-establish gas exchange during flooding [73]. SD1 gene-induced GA synthesis under waterlogging promotes rapid leaf stalk internodal growth [74]. Exogenous GA treatment effectively reduces MDA content under waterlogged conditions [75].
4.3.4. Other plant growth regulators (PGR)
Other PGRs act mostly through regulation of ET and hence are dealt together.
The influence of auxin is by promoting ET production which facilitates IAA transport to flooded plant regions initiating cell division and AR development [76].
Increased cytokinin in plants shows increased expression of genes that are involved in photosynthesis, photochemical quenching, photochemical efficiency, chlorophyll levels, electron transport rates, and CO2 assimilation. Cytokinins crosstalk with other hormones such as ABA and jasmonates to mitigate the plant stress [77].
Exogenous salicylic acid (SA) spray on waterlogged peach trees significantly increases ethanol dehydrogenase activity, protective enzyme activity, and proline content. SA protects cell organelles, balancing photosynthesis and root activity during waterlogging [78]. Excess synthesis of SA inside the cell induces PCD that stimulates lipid oxidation, development of aerenchyma, and formation of AR primordia through which it transfers oxygen to the root tissues, mitigating flood stress [79].
Methyl jasmonate application elevates ET level which enhances the formation of aerenchyma cells mitigating the flood stress [80].
Exogenous 2,4-epi-brassinolide (EBR) enhances the transportation of carbohydrates under insufficient availability of oxygen, triggers root glycolytic enzyme activity, enhances antioxidant enzyme activity, reduces ROS production, and improves the stress tolerance [81]. EBR promotes ET production which leads to increased enzymatic activity for degradation of cell wall, enhancing hypocotyl expansion, AR formation, oxygen supply, and enhancing tolerance toward insufficient availability of oxygen [82].
Melatonin (MT) application to waterlogged peach trees showed enhanced antioxidant activities, inhibited lipid peroxidation and hydrogen peroxide accumulation, facilitated aerenchyma development for improved anaerobic respiration, and also involved in the upregulation of genes related to calcium signaling [83]. MT application restores biochemical and physiological parameters, up regulates the biosynthesis genes of polyamines such as ADC, SPMS, and SPDS but exhibits reduced expression of ET biosynthesis and its signaling genes [84]. MT application reduces the flood-induced chlorosis and wilting. It maintains aerobic respiration, preserves photosynthesis, and reduces oxidative damage with recovered antioxidant enzymatic activity in waterlogged apple tree [85].
Proline is reported to improve photosynthesis, activity of antioxidants, and water content of the leaves by reducing oxidative damage and membrane injury [86].
The above chapter opens out another vital area where the direct and indirect effects of one growth regulator acting individually or through a regulation of the other which in most cases is ET need to be probed at length. Hormonal crosstalk should be another thrust area of research.
5. MORPHOLOGICAL ADAPTATIONS
To mitigate flood stress, plants develop morphological adaptations to maintain gas exchange, improving water and nutrient absorption, minimizing water loss, and averting physical harm.
5.1. ARs Formation
Initiation of ARs due to endogenous auxin acts as the substitute for the decayed root system which has lost the ability to absorb the water and nutrients. It facilitates gas exchange, water, and nutrient uptake and improves nutrient utilization efficiency thereby enhancing plant survival during flood [87]. Flooded mango trees develop ARs above the water line, to absorb and translocate O2 to the submerged roots [88]. AR occurs on younger trees under extended flooding period which is not observed in oxygenated conditions [89]. Kongsri et al. [90] observed the ARs formation in 5 weeks of flooded guava trees.
5.2. Aerenchyma Formation
Aerenchyma formation induces the formation of interconnected intercellular spaces, which facilitates gas exchange to mitigate the low oxygen conditions. This is stimulated by ET in the mature sections of the root. It diffuses the photosynthetic and atmospheric oxygen from aerated parts to submerged roots, thereby sustaining aerobic respiration in the roots [91].
5.3. Hypertrophic Lenticels
The formation of large cracks on the surfaces of stems and roots is called hypertrophic lenticels positioned just above the water surface which act as entry points for oxygen to mitigate the flood stress [92]. Hypertrophic lenticels in flooded mango trees excrete toxic components which are the byproducts of anerobic respiration that also enhances O2 diffusion to the roots [89].
5.4. Leaf Epinasty
Downward curvature of leaves due to the faster growth of the upper part of petioles (adaxial) compared to the lower part (abaxial), which was influenced by ET and high concentration of auxin [93]. During flooding, ET stimulates epinasty of the petiole along with partial closure of stomata thus maintaining the water equilibrium through reduced interception of light and reduced foliar transpiration [94].
5.5. Internodal or Shoot Elongation
During submerged conditions, internodes elongate rapidly in response to rising water levels, ensuring that leaves remain above the water surface to prevent anoxia [95]. The specialized aerial roots observed in submerged plants to elevate the oxygen supply are called pneumatophores, whose basic function is to breathe [96].
6. SYMPTOMS AND IMPACTS OF FLOOD STRESS IN FRUIT CROPS
Flooding inhibited the shoot growth by hindering leaf formation and leaf expansion, besides promoting abscission and premature leaf senescence [97]. Reduced root growth in flooded plants due to limited O2 and phytotoxins produced during anerobic respiration which cause root decay and declined nitrogen content [98]. Flooding leads to nitrogen deficiency due to denitrification in which the soil microbes convert nitrate into nitrogen gas [99]. Leaching causes 10–40% reduction of nitrogen [100]. Waterlogging alters the nutrient uptake and carbohydrate translocation which leads to leaf chlorosis [101]. Manetas [102] reported that the flood-induced leaf reddening is due to the excess production of anthocyanin for scavenging free radicals. Trees under continuous flooding lead to inhibited root and shoot growth and if it extends complete death will occur [94]. Waterlogging stops root growth and causes damage to root apices as it is the first plant organ affected by the flooding [7].
Root, shoot, and stem growth were affected by the action of reduced carbohydrate generation which was influenced by the reduced photosynthetic rate, declined chlorophyll content affects the papaya yield and quality [103]; reduced stomatal conductance affects the leaf elongation rate [87]. Prolonged waterlogging causes complete cessation of water and nutrient uptake and transport by the roots, which leads to leaf yellowing, necrosis, and abscission [104]. Flooding also affects the fruit crops in the reproductive phase such as flower bud initiation, flowering, fruit set, fruit growth, and premature fruit drop due to an imbalance of plant hormones and photosynthetic arrest [105]. Waterlogged peach trees (cv. Red Globe) produced small-sized fruits with rapid softening and reduced shelf life with low flesh firmness, also reduced chlorophyll content with low branch growth was observed [106]. Reduced soil redox potential, root conductivity, potential of leaf water, and conductance of stomata with removal of fibrous roots and cessation of shoot growth have been observed in flooded sour orange (Citrus aurantium L.) and rough lemon (Citrus jambhiri Lush.) seedlings [107]. Leaf wilting and defoliation observed in waterlogged seedling of Prunus betulaefolia (Birchleaf pear) and Prunus communis (European pear) are due to root anaerobiosis which leads to alteration in root osmotic potential, leaf water potential, and a decline in root hydraulic conductivity [108]. A climacteric fruit harvested from the flooded trees excretes more ET and ripens earlier [109]. Fruit softening was observed due to the action of hydrolytic enzymes in the cell wall [110]. In carambola (Averrhoa carambola) trees, short-term flooding increases the availability of micronutrients in soil and stimulates flowering and fruit production [111]. Short-term flooding affects the soil redox potential and improves its micronutrient content by reducing Fe3+ to Fe2+ which is metabolized by the plants [94]. In commercial Annona species, short-term flooding affects the net CO2 assimilation and vegetative growth which leads to defoliation, reduced flowering, and fruit set [112]. Short-term flooding paradoxically improves the micronutrient availability by reducing the pH which is very important in the case of calcareous soils releasing the bound micronutrients such as Fe, Mn, and Mg in soluble form and making them available to crops which have been elaborately reviewed by Schaffer et al. [94]. Fe3+ is converted to Fe2+ cation that is easily taken up by the crops. Another pathway is by having a situation of hypoxic condition permitting stomatal closure and a major shift in the resource allocatory pattern that promotes flowering and fruit development.
In mango trees, 3 days of flooding leads to reduced assimilation of net CO2, conductance of stomata, and transpiration. Similarly, reduced stem radial growth and root dry weight were observed in 14 days flooded mango trees. Increased net CO2 assimilation with improved Fe and Mn uptake in 10–20 days of flooding and complete mortality of trees was observed in 110 days of flooding [113], limitation of available photo assimilates due to increased root respiration and reduced net photosynthesis by flooding in mango trees [94]. Flooding causes deprivation of O2 in the rhizosphere leading to reduction in diameter of root collar, length of taproot, and dry weight of roots in Lulo fruit (Solanum quitoense var. septentrionale) [114]. Ojeda et al. [115] concluded that the reduced chlorophyll index, plant growth, net CO2 assimilation, and increased root electrolyte leakage were observed in 4-week flooded Soursop (Annona muricata) tree. In Pitanga cherry (Eugenia uniflora) seedlings, flooding showed detrimental effects in plant dry weight, leaf gas exchange, and plant growth due to limited photosynthesis [116]. Waterlogged Cape gooseberry (P. peruviana L.) shows the symptoms of leaf chlorosis, necrosis, abscission, and epinasty due to chlorophyll content reduction [117], whereas flooded apple trees show yield reduction with suppressed tree vigor [118].
Flood induces senescence of young leaves by the transportation of mobile elements such as N, P, and K from the older leaves to younger [119]. Reduced leaf count with low content of chlorophyll, leaf N, and P content was observed in waterlogged purple passion plant (P. edulis) [55]. Less than 7 days of flooded yellow Passion fruit trees show increased diameter of root, thickness of epidermis, thickness of cortex, thickness of endodermis, and aerenchyma generation [120]. Flood stress symptoms vary in different fruit crops. Symptoms in guava were chlorosis, wilting, dropping of leaves, stem bark broken, and AR formation [90], in avocado (Persea americana) leaf abscission [121], and Papaya (Carica papaya) shows that leaf senescence was observed [122].
7. FLOOD STRESS-INDUCED BIOTIC STRESSES
The stagnant water acts as the ideal medium for microbial growth, particularly Phytophthora cinnamomi leads to Phytophthora root rot, which affects the feeder roots and induces root tissue decay, disturbs water and nutrient transportation, and causes branch-dieback and eventual tree death [123]. Accumulation of reduced ions and by-products of anerobic respiration controls aerobic microbes and favors the generation of anerobic fungus such as Phytophthora, Pythium, and Fusarium spp. [124]. Waterlogging-induced Phytophthora root rot has been reported in Avocado [125] and Papaya [126]. High incidence of P. cinnamomi-induced Phytophthora root rot and 25–30% of root necrosis due to photosynthesis inhibition was observed in waterlogged avocado trees [127]. Flood-induced Fusarium oxysporum infection leads to banana wilt [9] and Cape gooseberry root rot [128]. Water-soaked lesions at the base region of stem turned into black necrotic area due to Pythium aphanidermatum observed in papaya trees under waterlogging [126].
An integrated management strategy to cope with flood stress is the need of the hour as a single approach may not yield desired results. The most important of these could be having adequate drainage provisions [129], accumulation versus availability of nutrients [94], and rootstock selection [85,130,131]. Mycorrhizal application and their capacity to withstand flooding and applications of CaO2 and MgO2 has to be need based and follow up based on site-specific recommendations. Application of MT for flood tolerance in Malus baccata has also been reported to increase the endurance of apple seedlings to waterlogged soils.
7.1. Critical Analysis of Classification of Fruit Crops Based on Flood Stress
According to Crane et al. [7], class 1 type shows tolerance toward flood stress, withstand under high water conditions up to a week and also the growth and production were affected. Some examples are guava, sapodilla, Abiu fruit, and citrus. The class 2 type shows moderate tolerance as several days toward flooding; some of the examples are lychee, longan, canistel, mango, carambola, and banana. The class 3 type was sensitive trees which cannot survive in flooded soil conditions and their examples are papaya, sugar apple, jackfruit, atemoya, avocado, passion fruit, and mamey sapote.
8. ROOTSTOCK MANIPULATION IN MANAGING FLOOD STRESS
Fruit trees grafted with flood-tolerant rootstocks show reduced hypoxia stress. For citrus, Citrus macrophylla rootstock [132]; in avocado, “Dusa™” rootstock [125] and in annona, Annona glabra (pond apple) rootstock [112] show greater tolerance toward flood stress. Rootstock P. japonica for peach seedlings provides tolerant to waterlogging by reduced accumulation of ET in the stems and roots [133]. Adaptation to calcareous soil and tolerance to the Phytophthora root rot by the rootstock selection of distinct group of West Indian and Guatemalan varieties in avocado [134]. Seedling-propagated guava plants were more tolerant toward flood stress than the shoot layered [90]. Flooded “Newport” plum shows high tolerance, minimal defoliation, and high survival rate, also maintaining the net photosynthetic rate [135]. Flooding affects photosynthesis through which it reduces sugar content and root vigor of the grafted mango seedlings, damage starts from scion due to the restricted supply of carbohydrates to the roots, which induce the rootstock death [136].
It is an established fact that for plums and apricots, myrobalan (Prunus cerasifera) is a good rootstock. Mariana (Prunus mariana) and prunus hybrids also show good tolerance to waterlogging. Prunus insititia and Prunus domestica, mariana, and myrobalan can tolerate 10–60 days of root asphyxiation depending on cultivar and species in summer and up to 120–145 days of water immersion in winter compared to 70–75 days for apricot and 80–85 days for peach [137,138]. This has been attributed to a genetic entity endowed with character reflected in terms of buffering effect on soil pH [139] and nutrient uptake efficiency [140]. Another similar report available is on MP-29 rootstock that performed better than Flordaguard (both prunus) for peaches under hypoxia conditions due to enhanced antioxidant activity, osmolyte content, and nutrient absorption [141]. The use of Pyrus betulifolia for pear, trifoliate oranges in citrus, and A. glabra for annona have also been reported to tolerate flood stress [130].
Heavy precipitation leads to water saturation of soil pores resulting in hypoxia. Rootstock variation in tolerance to stresses in general has been attributed to differences in photosynthetic activity assimilate partitioning and antioxidant enzyme activity [135,141-145]. Another major aspect that merits consideration is the flooding and co-incidence of Phytophthora root rot which is often noticed in avocados. The answer to this field problem is by choice of Phytophthota cinnamomi-resistant P. americana rootstock [134].
A major point for consideration here would be the critical level to which rootstock adapts to varying intensity of flood. However, this is a vital area for exploitation and a point of investigation yet to be initiated in the international network for rootstock research. Having understood the feasibility, there is also an imperative need to prioritize rootstock breeding and also take up gene editing studies as long-term strategies to ensure durable results.
9. RECOVERY PRACTICES
Pruning the tree branches when the waterlogging ends, maintains the shoot/root ratio; Sanclemente et al. [121] concluded that the pruned avocado trees under waterlogging recovered more quickly than those not pruned. Application of solid fertilizers improves the redox potential of flooded soil; the application of magnesium peroxide (MgO2) and calcium peroxide (CaO2) improves total area of leaf along with dry weight of the waterlogged papaya tree cv. Red Lady, high recovery, and survival rate were also observed [146]. Mycorrhizal colonization improves the nutritional conditions, root health, and nutrient uptake and also promotes growth and biomass of flooded plants. Waterlogged purple passion fruit plants (P. edulis) inoculated with mycorrhizae mixture retain high number of leaves with high leaf chlorophyll and proline content which also maintains leaf N and P content [55]. Crops under flood stress are affected in two ways: (a) hypoxia – deprived of adequate oxygen and (b) Anoxia – absence or deficiency of oxygen – severe hypoxia. It is already mentioned in the next two lines (By adding solid oxygen fertilizers to mitigate the stress). One possible method is adding air into the root zone which is difficult under plant stress, particularly under water stress. A second method is by application of solid oxygen fertilizer. The oxygen fertilizers commonly available are CaO2 and MgO2 [147]. Thani et al. [146] in their studies on Red Lady variety of papaya showed that application of CaO2 followed by flood irrigation minimized the effect of flood as revealed by the minimal reductions in stem, leaf, root, and plant dry matter.
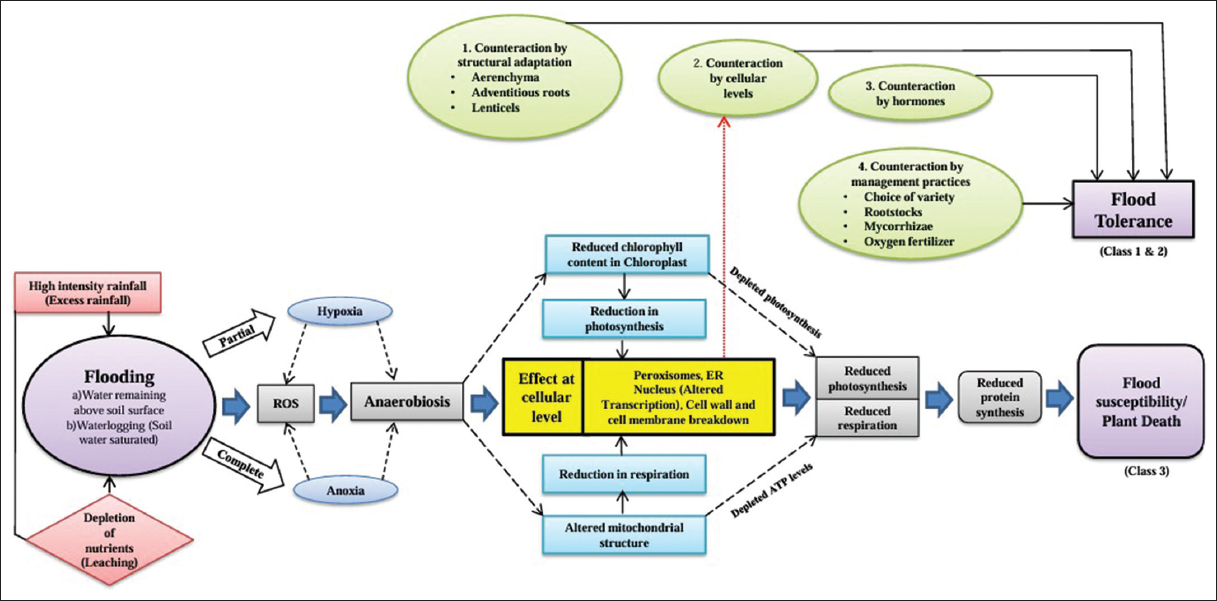 | Figure 3: Model showing effect of flood stress and tolerance by fruit crops [Click here to view] |
10. CONCLUSION
Flood stress is increasingly common due to CC – an accepted fact. This issue is expected to worsen as severe weather events are becoming more frequent and intense. CC will disrupt precipitation patterns, potentially triggering unexpected heavy rains continued with dry spells and intensifying flood stress. Prolonged submersion leads to oxygen deficit and nutrient deficiencies that result in substantial yield reduction of crops with severe financial loss. Fertile topsoil loss by soil erosion was induced by flooding disrupts the root system and complete survival of plant. Plants face different challenges during flooding, depending on the depth and duration of water. Flooding initiates oxidative stress and triggers anerobic respiration with the release of toxic components which can damage the cell organelles and leads to plant death. Over accumulation of ROS is the prime reason for the destruction of cellular function, which leads to oxidative damage. On the other side, flood stress-induced stomatal closure affects photosynthesis which shows the symptoms of yellowing, wilting, and abscission of leaves. This photosynthetic inhibition affects energy production and supply, which leads to stunted growth and complete plant death. Plants undergo adaptive strategies for survival which includes the generation of aerenchyma and AR for better O2 supply and leaf position above the water table for the energy production. Flood stress poses a severe threat to agricultural productivity potentially resulting in decreased crop yields and food shortage. Efforts should focus on developing crop varieties with flood tolerant, sustained land usable practices, restoring ecosystems, and enacting policies to reduce the effects of CC. By incorporating this kind of efforts, we can reduce the detrimental impacts of flooding on ecosystems and agriculture. Based on the present level of knowledge, the whole process of flood stress and its effects are condensed and compressed in the form of a model presented below [Figure 3].
Mitigation aspects need to be focused and follow a definite management plan that systematically reduces the negative impacts. As already stated, the first and foremost aspect is having adequate drainage provisions as already stated above or adapting cultivated terrain for the purpose, choice of flood-tolerant varieties and rootstocks, and adoption of solid oxygen fertilizers and EDTA or Na2 EDTA. However, more research is warranted on solid oxygen fertilizer as initial results are promising and will certainly be a future line of research.
11. ACKNOWLEDGMENT
The first authors acknowledge the KITS for the notable scholarship during the period of study.
12. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
13. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
14. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
15. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
16. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
17. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Dhanya P, Ramachandran R. Farmers'perceptions of climate change and the proposed agriculture adaptation strategies in a semi-arid region of south India. J Integr Environ Sci. 2016;13(1):1-18. [CrossRef]
2. Kamdi PJ, Swain DK, Wani SP. Developing climate change agro-adaptation strategies through field experiments and simulation analyses for sustainable Sorghum production in semi-arid tropics of India. Agric Water Manag. 2023;286:108399. [CrossRef]
3. Thilagavathi G, Tamilenthi S, Ramu C, Baskaran R. Application of GIS in flood hazard zonation studies in Papanasam Taluk, Thanjavur District, Tamil Nadu. Adv Appl Sci Res. 2011;2(3):574-85.
4. FAO, UN. The Future of Food and Agriculture: Trends and Challenges. Rome, Italy:FAO ;2017. 180.
5. Fukao T, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM. Submergence and waterlogging stress in plants:A review highlighting research opportunities and understudied aspects. Front Plant Sci. 2019;10:340. [CrossRef]
6. Chohan M, Panhwar RN, Soomro AF, Kaloi GM, Mari AH, Gadehi MA. Evaluation of soil physico-chemical properties in pre and post flood conditions at Thatta Sindh Pakistan. JOARPS. 2023;4(2):594-600. [CrossRef]
7. Crane JH, Balerdi CF, Schaffer B. Managing Your Tropical Fruit Grove Under Changing Water Table Levels. Doc. HS957. Gainesville, FL:Horticultural Sciences Department, UF/IFAS Extension;2020. [CrossRef]
8. Manghwar H, Hussain A, Alam I, Khoso MA, Ali Q, Liu F. Waterlogging stress in plants:Unraveling the mechanisms and impacts on growth, development, and productivity. Environ Exp Bot. 2024;2024:105824. [CrossRef]
9. Bolanos E. Flooding effect on banana plantations in the Caribbean of Costa Rica. Corbana. 2019;45(65):131-40.
10. Xie LJ, Zhou Y, Chen QF, Xiao S. New insights into the role of lipids in plant hypoxia responses. Prog Lipid Res. 2021;81:101072. [CrossRef]
11. Borella J, Becker R, Lima MC, de Oliveria DD, Braga EJ, de Oliveria AC, et al. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci Agric. 2019;76:51-62. [CrossRef]
12. Zhou W, Chen F, Meng Y, Chandrasekaran U, Luo X, Yang W. Plant waterlogging/flooding stress responses:From seed germination to maturation. Plant Physiol Biochem. 2020;148:228-236. [CrossRef]
13. Taiz L, Zeiger E. Plant Physiology. 5th ed. Sunderland, MA:Sinauer Associates;2010.
14. Pan DL, Wang G, Wang T, Jia ZH, Guo ZR, Zhang JY. AdRAP2.3, a novel ethylene response factor VII from Actinidia deliciosa, enhances waterlogging resistance in transgenic tobacco through improving expression levels of PDC and ADH genes. Int J Mol Sci. 2019;20:1189. [CrossRef]
15. Herrera A. Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front Plant Sci. 2013;4:106. [CrossRef]
16. Ferner E, Rennenberg H, Kreuzwieser J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012;32(2):135-45. [CrossRef]
17. Guo W, Xing Y, Luo X, Li F, Ren M, Liang Y. Reactive oxygen species:A crosslink between plant and human eukaryotic cell systems. Int J Mol Sci. 2023;24(17):13052. [CrossRef]
18. Wang Z, Bianco V, Pirone D, Memmolo P, Villone MM, Maffettone PL, et al. Dehydration of plant cells shoves nuclei rotation allowing for 3D phase-contrast tomography. Light Sci Appl. 2021;10(1):187. [CrossRef]
19. Jaffar NS, Jawan R, Chong KP. The potential of lactic acid bacteria in mediating the control of plant diseases and plant growth stimulation in crop production - A mini review. Front Plant Sci. 2023;13:1047945. [CrossRef]
20. Tenhaken R. Cell wall remodelling under abiotic stress. Front Plant Sci. 2015;5:118089. [CrossRef]
21. Gall HL, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C. Cell wall metabolism in response to abiotic stress. Plants. 2015;4(1):112 66. [CrossRef]
22. Kong FJ, Oyanagi A, Komatsu S. Cell wall proteome of wheat roots under flooding stress using gel-based and LC MS/MS-based proteomics approaches. Biochim Biophys Acta Biomembr. 2010;1804:124-36. [CrossRef]
23. Nanjo Y, Maruyama K, Yasue H, Yamaguchi-Shinozaki K, Shinozaki K, Komatsu S. Transcriptional responses to flooding stress in roots including hypocotyl of soybean seedlings. Plant Mol Biol. 2011;77:129-44. [CrossRef]
24. Lei S, Zeng B, Yuan Z, Su X. Changes in carbohydrate content and membrane stability of two ecotypes of Calamagrostis arundinacea growing at different elevations in the drawdown zone of the Three Gorges Reservoir. PLoS One. 2014;9(3):e91394. [CrossRef]
25. Tamang BG, Fukao T. Plant adaptation to multiple stresses during submergence and following Desubmergence. Int J Mol Sci. 2015;16(12):30164-80. [CrossRef]
26. Zhang RD, Zhou YF, Yue ZX, Chen XF, Cao X, Ai X, Jiang B, Xing Y. The leaf-air temperature difference reflects the variation in water status and photosynthesis of sorghum under waterlogged conditions. PLoS One. 2019a;14:e0219209. [CrossRef]
27. Roberts JK, Callis J, Jardetzky O, Walbot V, Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci. 1984;81(19):6029-33. [CrossRef]
28. Wu YS, Yang CY. Physiological responses and expression profile of NADPH oxidase in Rice (Oryza sativa) seedlings under different levels of submergence. Rice. 2016;9:2. [CrossRef]
29. Yan K, Zhao S, Cui M, Han G, Wen P. Vulnerability of photosynthesis and photosystem I in Jerusalem artichoke (Helianthus tuberosus L.) exposed to waterlogging. Plant Physiol Biochem. 2018;125:239-46. [CrossRef]
30. Anee TI, Nahar K, Rahman A, Mahmud JA, Bhuiyan TF., Alam MU, et al. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants. 2019;8:196. [CrossRef]
31. Ren BZ, Zhang JW, Dong ST, Liu P, Zhou B. Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of Summer Maize. PLoS One. 2016;11:e0161424. [CrossRef]
32. Liu GQ, Zhu HJ, Zhou BB, Sheng JY. Zang X. Effects of drought and flooding stress on photosynthetic characteristics of pecan Carya illinoinensis (Wangenh.) and ultrastructure of its chloroplast. Jiangsu J Agric Sci. 2012;28:1429-33. [CrossRef]
33. Shi F, Pan Z, Dai P, Shen Y, Lu Y, Han B. Effect of waterlogging stress on leaf anatomical structure and ultrastructure of Phoebe sheareri seedlings. Forests. 2023;14(7):1294. [CrossRef]
34. Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am Physiol Soc J. 2007;292:670-86. [CrossRef]
35. Yang WC, Lin KH, Wu CW, Chang YJ, Chang YS. Effects of waterlogging with different water resources on plant growth and tolerance capacity of four herbaceous flowers in a bioretention basin. Water. 2020;12:1619. [CrossRef]
36. Sharma S, Bhatt U, Sharma J, Kalaji HM, Mojski J, Soni V. Ultrastructure, adaptability and alleviation mechanisms of photosynthetic apparatus in plants under waterlogging:A review. Photosynthetica. 2022;60:430-44. [CrossRef]
37. Pollard TD, Earnshaw WC, Lippincott-Schwartz J, Johnson G. Cell Biology E-Book. Netherlands:Elsevier Health Sciences;2022.
38. Coe H, Michalak M. ERp57, a multifunctional endoplasmic reticulum resident oxidoreductase. Int J Biochem Cell Biol. 2010;42(6):796-9. [CrossRef]
39. Komatsu S, Kuji R, Nanjo Y, Hiraga S, Furukawa K. Comprehensive analysis of endoplasmic reticulum-enriched fraction in root tips of soybean under flooding stress using proteomics techniques. J Proteom. 2012;77:531-60. [CrossRef]
40. Nyathi Y, Baker A. Plant peroxisomes as a source of signalling molecules. Biochim Biophys Acta. 2006;1763:1478-95. [CrossRef]
41. Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:217037. [CrossRef]
42. Komatsu S, Hiraga S, Nouri MZ. Analysis of flooding-responsive proteins localized in the nucleus of soybean root tips. Mol Biol Rep. 2014;41:1127-39. [CrossRef]
43. Ubogoeva EV, Zemlyanskaya EV, Xu J, Mironova V. Mechanisms of stress response in the root stem cell niche. Exp Bot. 2021;72(19):6746-54. [CrossRef]
44. Renziehausen T, Frings S, Schmidt-Schippers R. 'Against all floods':Plant adaptation to flooding stress and combined abiotic stresses. TPJ. 2024;117(6):1836-55. [CrossRef]
45. Shriti S, Bhar A, Roy A. Unveiling the role of epigenetic mechanisms and redox signaling in alleviating multiple abiotic stress in plants. Front Plant Sci. 2024;15:1456414. [CrossRef]
46. Steffens B, Geske T, Sauter M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011;190:369-78. [CrossRef]
47. Sahu PK, Jayalakshmi K, Tilgam J, Gupta A, Nagaraju Y, Kumar A, et al. ROS generated from biotic stress:Effects on plants and alleviation by endophytic microbes. Front Plant Sci. 2022;13:1042936. [CrossRef]
48. Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress:Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. [CrossRef]
49. Lee YH, Kim KS, Jang YS, Hwang JH, Lee DH, Choi IH. Global gene expression responses to waterlogging in leaves of rape seedlings. Plant Cell Rep. 2014;33:289-99. [CrossRef]
50. Salah A, Zhan M, Cao C, Han Y, Ling L, Liu Z, et al. g-aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity and growth of waterlogged maize seedlings. Sci Rep. 2019;9:484. [CrossRef]
51. Teoh EY, Teo CH, Baharum NA, Pua TL, Tan BC. Waterlogging stress induces antioxidant defense responses, aerenchyma formation and alters metabolisms of banana plants. Plants. 2022;11(15):2052. [CrossRef]
52. Mahmood U, Hussain S, Hussain S, Ali B, Ashraf U, Zamir S, et al. Morpho-physio-biochemical and molecular responses of maize hybrids to salinity and waterlogging during stress and recovery phase. Plants. 2021;10:1345. [CrossRef]
53. Li H, Wang H, Wen WJ, Yang GW. The antioxidant system in Suaeda salsaunder salt stress. Plant Signal Behav. 2020;15:7. [CrossRef]
54. Bai T, Li C, Ma F, Feng F, Shu H. Responses of growth and antioxidant system to root-zone hypoxia stress in two Malus species. Plant Soil. 2010;327(1):95-105. [CrossRef]
55. Chebet D, Kariuki W, Wamocho L, Rimberia F. Effect of arbuscular mycorrhizal inoculation on growth, biochemical characteristics and nutrient uptake of passion fruit seedlings under flooding stress. Int J Agric Res. 2020;16(4):24-31.
56. Castro-Duque NE, Chávez-Arias CC, Restrepo-Díaz H. Foliar glycine betaine or hydrogen peroxide sprays ameliorate waterlogging stress in Cape gooseberry. Plants. 2020;9(5):644. [CrossRef]
57. Yang CY. Hydrogen peroxide controls transcriptional responses of ERF73/HRE1 and ADH1 via modulation of ethylene signalling during hypoxic stress. Planta. 2014;239:877-85. [CrossRef]
58. Tamang BG, Li S, Rajasundaram D, Lamichhane S, Fukao T. Overlapping and stress-specific transcriptomic and hormonal responses to flooding and drought in soybean. Plant J. 2021;107:100-17. [CrossRef]
59. Tsuchiya Y, Nakamura T, Izumi Y, Okazaki K, Shinano T, Kubo Y, et al. Physiological role of aerobic fermentation constitutively expressed in an aluminum-tolerant cell line of tobacco (Nicotiana tabacum). Plant Cell Physiol. 2021;62:1460-77. [CrossRef]
60. Dawood T, Yang X, Visser EJ, te Beek TA, Kensche PR, Cristescu SM, et al. A co-opted hormonal cascade activates dormant adventitious root Primordia upon flooding in Solanum dulcamara. Plant Physiol. 2016;170(4):2351-2364. [CrossRef]
61. Rauf M, Arif M, Fisahn J, Xue GP, Balazadeh S, Mueller-Roeber B. NAC transcription factor speedy hyponastic growth regulates flooding induced leaf movement in Arabidopsis. Plant Cell. 2013;25:4941-55. [CrossRef]
62. Xu X, Ji J, Xu Q, Qi X, Weng Y, Chen X. The major-effect quantitative trait locus CsARN6.1 encodes an AAA ATPase domain-containing protein that is associated with waterlogging stress tolerance by promoting adventitious root formation. Plant J. 2018;93:917-30. [CrossRef]
63. Wei X, Xu H, Rong W, Ye X, Zhang Z. Constitutive expression of a stabilized transcription factor group VII ethylene response factor enhances waterlogging tolerance in wheat without penalizing grain yield. Plant Cell Environ. 2019;42:1471-85. [CrossRef]
64. Luan H, Guo B, Shen H, Pan Y, Hong Y, Lv C, et al. Overexpression of Barley transcription factor HvERF2. 11 in Arabidopsis enhances plant waterlogging tolerance. Int J Mol Sci. 2020;21:1982. [CrossRef]
65. Zhang P, Lyu D, Jia L, He J, Qin S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genomics. 2017;18:649. [CrossRef]
66. Tougou M, Hashiguchi A, Yukawa K, Nanjo Y, Hiraga S, Nakamura T, et al. Responses to flooding stress in soybean seedlings with the alcohol dehydrogenase transgene. Plant Biotechnol J. 2012;29:301-5. [CrossRef]
67. Zhang JY, Huang SN, Wang G, Xuan JP, Guo ZR. Overexpression of Actinidia deliciosa pyruvate decarboxylase 1 gene enhances waterlogging stress in transgenic Arabidopsis thaliana. Plant Physiol Biochem. 2016;106:244-52. [CrossRef]
68. Sasidharan R, Voesenek LA. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015;169:3-12. [CrossRef]
69. Tsai KJ, Lin CY, Ting CY, Shih MC. Ethylene-regulated glutamate dehydrogenase fine-tunes metabolism during anoxia reoxygenation. Plant Physiol. 2016;172:1548-62. [CrossRef]
70. Qing D, Yang Z, Li M, Wong WS, Guo G, Liu S, et al. Quantitative and functional phosphorproteomic analysis reveals that ethylene regulates water transport via the C-terminal phosphorylation of aquaporin PIP2;1 in Arabidopsis. Mol Plant. 2016;9:158-74. [CrossRef]
71. Kohnen MV, Schmid-Siegert E, Trevisan M, Petrolati LA, Sénéchal F, Müller-MouléP, et al. Neighbor detection induces organ-specific transcriptomes, revealing patterns underlying hypocotyl-specific growth. TPC. 2016;28(12):2889-904. [CrossRef]
72. Khan MI, Trivellini A, Chhillar H, Chopra P, Ferrante A, Khan NA, et al. The significance and functions of ethylene in flooding stress tolerance in plants. Environ Exp Bot 2020;179:13. [CrossRef]
73. Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, et al. The ethylene response factors SNORKEL 1 and SNORKEL 2 allow rice to adapt to deep water. Nature. 2009;460:1026-30. [CrossRef]
74. Kuroha T, Nagai K, Gamuyao R, Wang DR, Furuta T, Nakamori M, et al. Ethylene-gibberellin signalling underlies adaptation of rice to periodic flooding. Science. 2018;361:181-6. [CrossRef]
75. Wang C, Zhu J, Dai S. Effects of chemical control and nutrient control on waterlogging of rapeseed in flower and fruit stage. JAAS. 2016;44:136-8. [CrossRef]
76. Alaguero-Cordovilla A, Sánchez-García AB, Ibáñez S, Albacete A, Cano A, Acosta M, et al. An auxin-mediated regulatory framework for wound-induced adventitious root formation in tomato shoot explants. Plant Cell Environ. 2012;44:1642-62. [CrossRef]
77. Pavlu J, Novák J, KoukalováV, LuklováM, BrzobohatýB, ?ernýM. Cytokinin at the crossroads of abiotic stress signalling pathways. Int J Mol Sci. 2018;19(8):2450. [CrossRef]
78. Wang G, Fan W, Peng F. Physiological responses of the young peach tree to waterlogging and spraying SA at different timing. Int J Fruit Sci. 2015;32:872-8.
79. Kim YH, Hwang SJ, Waqas M, Khan AL, Lee JH, Lee JD, et al. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front Plant Sci. 2015;6:714. [CrossRef]
80. Hudgins J, Franceschi VR. Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol. 2004;135:2134-49. [CrossRef]
81. Kang YY, Guo SR, Li J, Duan JJ. Effect of root applied 24- epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Regul. 2009;57:259-69. [CrossRef]
82. Ma Y, Guo S. 24-epibrassinolide improves cucumber photosynthesis under hypoxia by increasing CO2 assimilation and photosystem II efficiency. Photosynthetica. 2014;52:96-104. [CrossRef]
83. Gu X, Xue L, Lu L, Xiao J, Song G, Xie M, et al. Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J Plant Growth Regul. 2020;40:2178-90. [CrossRef]
84. Zhang Q, Liu X, Zhang Z, Liu N, Li D, Hu L. Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front Plant Sci 2019b;10:44. [CrossRef]
85. Zheng X, Zhou J, Tan DX, Wang N, Wang L, Shan D, et al. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. Seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front Plant Sci. 2017;8:483. [CrossRef]
86. Ghosh UK, Islam MN, Siddiqui MN, Cao X, Khan MA. Proline, a multifaceted signalling molecule in plant responses to abiotic stress:Understanding the physiological mechanisms. Plant Biol. 2022;24(2):227-39. [CrossRef]
87. Lambers H, Oliveira RS. Biotic influences:Interactions among plants. In:Plant Physiological Ecology. Germany:Springer;2019. 615-48. [CrossRef]
88. Schaffer B, Andersen PC. Subtropical and tropical crops. In:Handbook of Environmental Physiology of Fruit Crops. Vol. 2. United States:CRC Press;1994.
89. Whiley AW, Schaffer B. Stress physiology. In:Litz RE, editor. The Mango:Botany, Production and Uses. Wallingford, UK:CAB International Press;1997. 147-73.
90. Kongsri S, Nartvaranant P, Boonprako U. A comparison of flooding tolerance of guava tree propagated from shoot layering and seedling. Int Symp Bot Gard Landsc. 2020;1298:625-32. [CrossRef]
91. Rankenberg T, Geldhof B, Veen H, Holsteens K, Poel B, Sasidharan R. Age-dependent abiotic stress resilience in plants. Trends Plant Sci. 2021;26(7):692-705. [CrossRef]
92. Jackson MB, Ishizawa K, Osamu I. Evolution and mechanisms of plant tolerance to flooding stress. Ann Bot. 2009;103:137-42. [CrossRef]
93. Bradford KJ, Dilley DR. Effects of root anaerobiosis on ethylene production, epinasty, and growth of tomato plants. Plant Physiol. 1978;61(4):506-9. [CrossRef]
94. Schaffer B, Davies F, Crane JH. Responses to tropical and subtropical fruit trees to flooding in calcareous soil. Hort Sci. 2006;41(3):549-55. [CrossRef]
95. Nagai K, Hattori Y, Ashikari M. Stunt or elongate?Two opposite strategies by which rice adapts to floods. J Plant Res. 2010;123:303-9. [CrossRef]
96. Taiz L, Zeiger E, Moller IM, Murphy A. Plant Physiology and Development. 6th ed. Porto Alegre:Artmed Publishing;2017. 858.
97. Kozlowski TT. Responses of woody plants to flooding and salinity. Tree Physiol 1997;17(7):490. [CrossRef]
98. Pezeshki SR. Wetland plant responses to soil flooding. Environ Exp Bot. 2001;46(3):299-312. [CrossRef]
99. Alaoui-Sosse B, Gerard B, Binet P, Toussaint ML, Badot PM. Influence of flooding on growth, nitrogen availability in soil, and nitrate reduction of young oak seedlings (Quercus robur L.). Ann For Sci. 2005;62(6):593-600. [CrossRef]
100. O'Legg J, Meisinger J. Soil nitrogen budgets. In:Stevenson FJ, editor. Nitrogen in agricultural soils Agronomy Monograph 22. Madison, Wisconsin:American Society of Agronomy;1982. pp. 503-66. [CrossRef]
101. Drew MC, Sisworo EJ. Early effects of flooding on nitrogen deficiency and leaf Chlorosis in barley. New Phytol. 1977;79(3):567-71. [CrossRef]
102. Manetas Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red?Flora Morphol Distrib Funct Ecol Plants 2006;201(3):163-77. [CrossRef]
103. Khondaker NA, Ozawa K. Papaya plant growth as affected by soil air oxygen deficiency. Int Symp Papaya. 2007;740:225-32. [CrossRef]
104. Fischer G, Ramírez F, Casierra-Posada F. Ecophysiological aspects of fruit crops in the era of climate change - A review. Agron Colomb. 2016;34(2):190-9. [CrossRef]
105. Kozlowski TT, Pallardy SG. Physiology of Woody Plants. Netherlands:Elsevier;1996.
106. Insausti P, Gorjon S. Floods affect physiological and growth variables of peach trees (Prunus persica L.) Batsch, as well as the postharvest behaviour of fruits. Sci Hortic. 2013;152:56-60. [CrossRef]
107. Syvertsen JP, Zablotowicz RM, Smith ML. Soil temperature and flooding effects on two species of citrus:I. Plant growth and hydraulic conductivity. Plant Soil. 1983;72:3-12. [CrossRef]
108. Andersen PC, Lombard PB, Westwood MN. Effect of root anaerobiosis on the water relations of several Pyrus species. Physiol Plant. 1984;62(2):245-52. [CrossRef]
109. Kader AA. Postharvest Technology of Horticultural Crops. Vol. 3311. California:University of California Agriculture and Natural Resources;2002.
110. Sozzi GO. Strategies for the regulation of postharvest fruit softening by changing cell wall enzyme activity. Production Practices and Quality Assessment of Food Crops:Postharvest Treatment and Technology. Vol. 4. Dordrecht, Netherlands:Springer;2004, 135-72. [CrossRef]
111. Joyner ME. Effects of Flooding on Gas Exchange and Growth of Carambola Trees (Averrhoa carambola L.). MS thesis. Gainesville:University of Florida;1989.
112. Nunez-Elisea R, Schaffer B, Fisher JB, Colls AM, Crane JH. Influence of flooding on net CO2 assimilation, growth and stem anatomy of Annona species. Anne Bot. 1999;84(6):771-80. [CrossRef]
113. Larson KD, Schaffer B, Davies FS. Mango responses to flooding in limestone soil. Proc Flor State Hortic Soc. 1991;104:33-8.
114. Cardona WA, Bautista-Montealegre lG, Flórez-Velasco N, Fischer G. Biomass and root development response of lulo (Solanum quitoensevar. septentrionale) plants to shading and waterlogging. Rev Colomb Cienc Hortic. 2016;10(1):53-65. [CrossRef]
115. Ojeda M, Schaffer B, Davies FS. Flooding, root temperature, physiology and growth of two Annona species. Tree Physiol. 2004;24(9):1019-25. [CrossRef]
116. Mielke MS, Schaffer B. Photosynthetic and growth responses of Eugenia uniflora L. seedlings to soil flooding and light intensity. Environ Exp Bot 2010;68(2):113-21. [CrossRef]
117. Aldana F, García PN, Fischer G. Effect of waterlogging stress on the growth, development and symptomatology of Cape gooseberry (Physalis peruviana L.) plants Rev Acad Colomb Cienc Exactas Fis Nat 2014;38(149):393-400. [CrossRef]
118. Qu QG, Li LX, Fei ZF, Xia WH, Rui SH. Effect of water stress on microstructure of apple leaves and Newborn roots. Acta Hort Sin. 1999;26(3):147-51.
119. Klaring HP, Zude, M. Sensing of tomato plant response to hypoxia in the root. Sci Hortic. 2009;122(1):17-25. [CrossRef]
120. Govea KP, Neto AR, Resck NM, Moreira LL, Júnior VV, Pereira FL, et al. Morpho-anatomical and physiological aspects of Passiflora edulis Sims (passion fruit) subjected to flooded conditions during early developmental stages. Biotemas. 2018;31(3):15-23. [CrossRef]
121. Sanclemente MA., Schaffer B, Gil PM, Vargas AI, Davies FS. Pruning after flooding hastens recovery of flood-stressed avocado (Persea americana Mill.) trees. Sci Hortic. 2014;169:27-35. [CrossRef]
122. Rodriguez G, Schaffer B, Basso C, Vargas A. Effect of flooding duration and portion of the roots submerged on physiology, growth, and survival of papaya (Carica papaya L.). J Faculty Agron 2014;40(3):10.
123. Hardham AR. Phytophthora cinnamomi. Mol Plant Pathol. 2005;6(6):589-604. [CrossRef]
124. Fischer G, Orduz-Rodríguez JO. Ecophysiology in fruit trees. In:Manual for the Cultivation of Fruit Trees in the Tropics. Produmedios;2012. 54-72.
125. Reeksting BJ, Taylor NJ, Van den Berg N. Flooding and Phytophthora cinnamomi:Effects on photosynthesis and chlorophyll fluorescence in shoots of non-grafted Persea americana (Mill.) rootstocks differing in tolerance to Phytophthora root rot. S Afr J Bot. 2014;95:40-53. [CrossRef]
126. Koul B, Pudhuvai B, Sharma C, Kumar A, Sharma V, Yadav D, et al. Carica papaya L.:A tropical fruit with benefits beyond the tropics. Diversity. 2022;14(8):683. [CrossRef]
127. Ploetz RC, Schaffer B. Effects of flooding and Phytophthora root rot on net gas exchange and growth of avocado. Phytopathology. 1989;79(2):204-8. [CrossRef]
128. Villarreal-Navarrete A, Fischer G, Melgarejo LM, Correa G, Hoyos-Carvajal L. Growth response of the cape gooseberry (Physalis peruviana L.) to waterlogging stress and Fusarium oxysporum infection. In:XXIX International Horticultural Congress on Horticulture:Sustaining Lives, Livelihoods and Landscapes (IHC2014). Vol. 1178. 2014. 161-8. [CrossRef]
129. Kaur G, Singh G, Motavalli PP, Nelson KA, Orlowski JM, Golden BR. Impacts and management strategies for crop production in waterlogged or flooded soils:A review. Agron J. 2020;112(3):1475-501. [CrossRef]
130. Nunez-Elisea R, Schaffer B, Crane JH, Colls AM. Effects of flooding on growth and leaf gas exchange of Annona species. In:Proceedings of Congreso Internacional de Annonaceaes. Texcoco, Mexico:Chapingo Autonomous University;1997. 124-32.
131. Korkmaz N, A?kin MA, Altunlu H, Polat M, Okatan V, Kahramano?lu ?. The effects of melatonin application on the drought stress of different citrus rootstocks. Turk J Agric. 2022;46(4):585-600. [CrossRef]
132. Perez-Jimenez M, Perez-Tornero O. Short-term waterlogging in citrus rootstocks. Plants 2021;10(12):2772. [CrossRef]
133. Mizutani F, Yamada M, Tomana T. Differential water tolerance and ethanol accumulation in Prunus species under flooded conditions. JJSHS. 1982;51(1):29-34. [CrossRef]
134. Ploetz R, Schnell RJ, Haynes J. Variable response of open-pollinated seedling progeny of avocado to Phytophthora root rot. Phytoparasitica. 2002;30:262-8. [CrossRef]
135. Ranney TG. Differential tolerance of eleven Prunus taxa to root zone flooding. J Environ Hortic. 1994;12(3):138-41. [CrossRef]
136. Saeki A, Iwasaki N. The submergence of the graft union causes the death of grafted mango trees (Mangifera indica L.) under flooding. Agronomy. 2020;10(8):1121. [CrossRef]
137. Crossa-Raynaud P, Audergon JM, Apricot rootstocks. In:Rom CR, Carlson RF, editors. Rootstocks for Fruits Crops. New York:John Wiley and Sons;1987. 295-320.
138. Vachun Z. Rootstocks for apricots –the current situation and main problems. Acta Hortic (ISHS). 1995;384:459-66. [CrossRef]
139. Paunovic SA. The effect of rootstocks on yield and quality of Prunes CVS Pozegaca and Stanley. Symp Plum Genet Breed Pomol. 1978;74:175-84. [CrossRef]
140. Bojic M, Paunovic AS. The effect of inter-rootstock on the content and seasonal changes of major elements, N, P, K, Ca, and Mg in the leaves of apricot cv. Hungraian Best. Acta Hortic. 1988;209:131-40. [CrossRef]
141. Richmond-Cosie L, Schaffer B, Shahid MA, Chaparro JX, Sarkhosh A. Responses of 'Flordaguard'and 'MP-29'Prunus spp. rootstocks to hypoxia and high root zone temperature. PEI. 2024;5(5):70007. [CrossRef]
142. Pimentel P, Almada RD, Salvatierra A, Toro G, Arismendi MJ, Pino MT, et al. Physiological and mor phological responses of Prunus species with different degrees of tolerance to long-term root hypoxia. Sci Hortic. 2014;180:14-23. [CrossRef]
143. Teskey R, Wertin T, Bauweraerts I, Ameye M, McGuire M, Steppe K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2014;38(9):1699-712. [CrossRef]
144. Bhusal N, Kim HS, Han SG, Yoon TM. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long- term waterlogging conditions. Environ Exp Bot. 2020;176(104):111. [CrossRef]
145. Wu J, Wang J, Hui W, Zhao F, Wang P, Su C, et al. Physiology of plant responses to water stress and related genes:A review. Forests. 2022;13(2):324. [CrossRef]
146. Thani QA, Vargas AI, Schaffer B, Liu G, Crane JH. Responses of papaya plants in a potting medium in containers to flooding and solid oxygen fertilization. Proc Florida State Horticult Soc. 2016;129:27-34.
147. Liu G, Porterfield DM. Oxygen enrichment with magnesium peroxide for minimizing hypoxic stress of flooded corn. JPNSS. 2014;177(5):733-40. [CrossRef]