1. INTRODUCTION
Electroplating wastewater contains a variety of contaminants, including heavy metals, cyanide complexes, and a complex mixture of effluents. It is typically characterized by high levels of biochemical oxygen demand, chemical oxygen demand, dissolved solids, suspended solids, total solids, and turbidity. Approximately 29% of the waste generated in the electroplating industry is considered toxic and hazardous [1]. The electroplating process enhances the surface properties of objects, making them corrosion-resistant and abrasion free. However, it also releases toxic solvents and vapors from hot plates during the process. Research has primarily focused on heavy metals, which are some of the most toxic environmental pollutants. These pollutants pose significant risks to soil, surface water, and groundwater resources [2].
Heavy metals such as Cr (VI), Pb (II), and Ni persist in the environment for extended periods, leading to long-term risks to human health and ecosystems. Improper disposal of untreated lead, chromium, and nickel can cause serious health issues, including neurological damage, kidney impairment, and gastrointestinal disorders in humans. This highlights the urgent need for effective treatment methods to reduce heavy metal contamination in water and wastewater. Various technologies are available for the removal of lead, chromium, and nickel from water, including chemical precipitation, membrane filtration, and adsorption [3,4].
Modified plant-based biosorbents are regarded as one of the most promising, sustainable, and effective treatment methods among the existing technologies. In India, agricultural and plant biomass waste is abundant, making it a valuable resource for efficient heavy metal adsorption. Biomass materials are known for their high porosity and the presence of functional groups that bind heavy metals tightly, enabling effective removal from water and wastewater [5,6]. Although biomass materials are easily accessible for heavy metal removal, they require specific modifications to enhance their removal efficiency. Modifications are typically carried out through physical or chemical treatments. In physical modification, the biomass is crushed or ground into a fine powder to increase its surface area or processed through gasification, where gases such as carbon dioxide are passed through to enhance porosity and adsorption capacity. Chemical modification involves washing the biosorbent with acids such as nitric acid, hydrochloric acid, or potassium chloride [7].
Biosorption, an eco-friendly method, plays a crucial role in addressing heavy metal pollution in water. This technique uses biological materials such as bacteria, fungi, algae, or plant residues to adsorb and remove heavy metal ions from aqueous solutions [8]. The process is driven by mechanisms such as ion exchange, complexation, chelation, and physical adsorption. Biological materials, such as carboxyl, hydroxyl, amino, and sulfhydryl groups on their surfaces, serve as active sites for binding heavy metal ions [9].
One of the main advantages of biosorption is its cost-effectiveness and sustainability. Many biosorbents come from abundant, low-cost sources such as agricultural residues, fruit peels, and microorganisms, aligning with the principles of a circular economy by repurposing waste materials for environmental remediation [6]. Agricultural residues, such as crop residues, fruit peels, and sawdust, have shown remarkable biosorption capabilities due to the presence of functional groups that bind heavy metal ions. For instance, cellulose and lignin in crop residues offer plentiful sites for metal ion binding, and the porous nature of these materials enhances their adsorption capacity [10].
The primary aim of this study is to review innovative biosorbents for removing heavy metals from electroplating wastewater, providing valuable insights for future research in this area. The biosorbent used in this study is the fruit waste of Ficus benghalensis (Banyan tree). The banyan tree, India’s national tree, thrives in rainforests and monsoon regions [11]. This fast-growing, evergreen species can reach up to 30 m in height and is characterized by its wide-spreading growth, supported by accessory trunks and aerial roots. The tree’s fruit is inedible to humans, contains tiny flowers and seeds, and is small, orange-red, and sour in taste. It typically appears from March to May. The fruit waste is collected from nearby agricultural areas, powdered, modified, and used as a biosorbent [12].
Effective biosorbents should possess a high surface area, abundant functional groups, and excellent adsorption capacity. Researchers have explored various parts of the F. benghalensis tree for developing cost-effective biosorbents for pollutant removal. Gul et al. [13] demonstrated that F. benghalensis leaves had an adsorption capacity of 19.5 mg/g for brilliant green dye under optimal conditions. Yousafa et al. [14] studied the biosorption potential of F. benghalensis leaves for Ni (II) and Cd (II) ions in a synthetic binary metal ion mixture. Kumar et al. [15] investigated the adsorption of As (V) using F. benghalensis stem powder as a low-cost natural biosorbent and found significant removal efficiency across a wide pH range (3.0–11.0). Kiruthiga et al. [16] evaluated phosphoric acid-activated carbon derived from the aerial roots of F. benghalensis for Cr (VI) and Cu (II) removal, confirming its effectiveness as an inexpensive adsorbent. George and Tembhurkar [17] reported that biosorbents derived from F. benghalensis leaves achieved a maximum fluoride removal efficiency of 92.2%. Hymavathi and Prabhakar [18] utilized F. benghalensis leaf powder for Co (II) adsorption, reporting a maximum sorption capacity (qmax) of 5.65 mg/g and a removal efficiency of 98.73%. These studies highlight the versatility and efficacy of F. benghalensis-based biosorbents in removing various contaminants from aqueous solutions.
The novelty of this study lies in exploring F. benghalensis fruit as a biosorbent, which has not been widely studied compared to other parts of the tree. Unlike previous research that focused on the leaves, stems, and roots, this study examines the unique structural, chemical, and environmental advantages of the fruit. The fruit contains functional groups such as hydroxyl (-OH), carboxyl (-COOH), and carbonyl (-C=O), which enhance its interaction with pollutants, particularly heavy metals and organic dyes. Using F. benghalensis fruit waste (FBFW) as a biosorbent offers a cost-effective, sustainable, and innovative approach to pollutant removal.
2. MATERIALS AND METHODS
2.1 Materials
Potassium Hydroxide (Merck, Supelco, Pellets EMPLURA, ACS Reagent Grade) was used for adsorbent modification. Standard Stock Solutions of Cr (VI), Ni (II), and Pb (II) (Inorganic Ventures, USA, 1000 μg/mL each) were used to prepare stock solutions for the adsorption experiments by dissolving the appropriate amounts of metal salts in deionized water (Milli-Q). Hydrochloric acid (Merck, Supelco, fuming 37%, ACS Reagent), nitric acid (Merck, Supelco, 65% EMPLURA, ACS Reagent), and sodium hydroxide (Merck, Supelco, Pellets EMPLURA, ACS Reagent Grade) were used for solution preparation in pH experiments.
2.2. Preparation of Modified FBFW Powder (FBFWP) Adsorbent
The FBFW was collected from nearby urban and agricultural fields in Navi Mumbai. The collected fruits were washed with deionized water (18 MΩ resistivity, obtained from a Milli-Q DI water system) to remove dust particles and impurities. The fruits were then oven-dried at 80°C for 12 h, crushed using an electric grinder to make powder, and sieved through a mechanical sieve to obtain particle fractions ranging from 75 to 150 μm.
In the first step, the obtained FBFW biosorbent was modified using potassium hydroxide (KOH) [19]. An appropriate amount of KOH (to achieve a 0.5% KOH concentration in the final absorbent, with an excess of 10%) was dissolved in water, corresponding to the FBFW biosorbent. The required amount of FBFW biosorbent (20 g) was treated with this solution and stirred for 5 h. Afterward, the resulting powder was rinsed with deionized water and oven-dried at 100°C for 5 h. In the second step, the oven-dried material was allowed to cool to room temperature and then stored for further experiments.
Potassium hydroxide (KOH) modification plays a crucial role in enhancing the adsorption capacity of biomass materials and other porous adsorbents. This chemical activation process facilitates the development of a highly porous structure by reacting with carbonaceous materials to form potassium compounds. These compounds etch the material, creating new micro- and mesopores, thereby increasing the surface area and pore volume, which provides more adsorption sites. In addition, KOH modification introduces oxygen-containing functional groups (e.g., hydroxyl -OH, carboxyl -COOH, and carbonyl -C=O) onto the adsorbent surface. These functional groups enhance chemical interactions such as hydrogen bonding and electrostatic attraction, improving the adsorption of polar molecules, including heavy metal ions, dyes, and organic pollutants. The incorporation of oxygen-containing groups often increases the negative surface charge, further enhancing the material’s ability to attract and adsorb positively charged species, such as heavy metal cations. By simultaneously increasing surface area, pore volume, functional groups, and electrostatic interactions, KOH modification significantly improves adsorption efficiency, making it an effective method for pollutant removal [20].
2.3. Adsorbent Characterization
To characterize the properties of FBFWP, X-ray diffraction (XRD) played a key role. In this study, a benchtop XRD (Japan), which utilizes Nickel-filtered Cu Kα radiation (λ = 1.54 Å) at 15 mA and 30 kV, was employed for the analysis of FBFWP. The XRD pattern of the powdered adsorbent was recorded over a 2θ range of 0°–80° with a step size of 0.02°/s. The biosorbent was analysed using Fourier Transform Infrared (FTIR) spectroscopy (FTIR: AGILENT CARY 630 WITH ATR & DIAL PATH). The analysis was conducted over a wave range of 4000 to 400 /cm at a resolution of 4 /cm. Microstructural analysis was performed using a field emission scanning electron microscope (SEM) (Zeiss Ultra 55) operating at an accelerating voltage of 5 kV. The specific surface area of the FBFWP biosorbent was determined through nitrogen adsorption isotherms using a Micropore Analyzer (ASAP 2020, Micromeritics, USA).
2.4. Cr, Ni and Pb Adsorption Experiments
The synthesized FBFWP-modified biosorbent was used to study the uptake of Cr, Ni, and Pb ions in water. To investigate the effects of various parameters, such as adsorbent size, adsorbent dosage, ion concentrations of Cr, Ni, and Pb, pH, contact time, and competing anions, batch experiments were conducted using FBFWP. Inductively coupled plasma mass spectrometry (ICP-MS) was employed to detect Cr, Ni, and Pb ions in aqueous solutions by calibrating high-purity standards.
In these experiments, predefined dosages of FBFWP was added to a 250 mL polypropylene flask containing 100 mL of synthetic Cr (VI), Ni (II), and Pb (II) ion solutions, depending on the specific experiment. The flasks were shaken in a rotary shaker (Remi, India) at 150 (±5) rpm at room temperature (30°C ± 2°C). Samples of the Cr, Ni, and Pb ion solutions were withdrawn at predetermined time intervals and analyzed for residual ion concentrations.
The effect of adsorbent size was studied to select the final adsorbent for detailed experiments. Dose optimization studies were performed to determine the optimal adsorbent dosage required for efficient removal of Cr, Ni, and Pb ions from aqueous solutions. Adsorption kinetics were conducted to evaluate the effects of contact time and the initial concentrations of Cr, Ni, and Pb ions. Experiments were carried out with an optimized adsorbent dose and varying concentrations of Cr, Ni, and Pb (5 mg/L, 10 mg/L, 20 mg/L, and 30 mg/L) and adsorbent dosages (2 g/L, 5 g/L, 10 g/L, and 20 g/L). Batch kinetic study samples were withdrawn at time intervals ranging from 20 to 180 min.
The effect of solution pH on Cr, Ni, and Pb removal was also evaluated by adjusting the pH of the solution between 2 and 11 using 0.1 M HNO3 and 0.1 M NaOH solutions. Blank experiments (without the addition of adsorbent) were conducted to assess the initial concentrations of Cr, Ni, and Pb ions in each experiment. In addition, regeneration experiments were carried out as batch adsorption-desorption cycles using mild acidic solutions (e.g., HCl). After regeneration, the adsorption experiment was repeated up to three cycles to assess the reusability of the adsorbent. The samples collected during the experiments were filtered using 0.22 μm disposable membrane filters, and the filtered samples were analyzed using ICP-MS (Agilent Technologies, 7800 ICP-MS).
3. RESULTS AND DISCUSSION
3.1. Characterization of the FBFWP Biosorbent
The modified FBFWP was further analyzed to investigate its structural properties and the characteristics of the modified material. The XRD pattern of the FBFWP biosorbent [Figure 1a] reveals a typical amorphous structure, with distinct sharp peaks at 15°, 21°, and 25°, suggesting the presence of silica-like material, such as quartz. These crystalline silica phases not only serve as active adsorption sites, enhancing chemisorption and electrostatic interactions with pollutants, but also facilitate complexation with heavy metals and organic contaminants through surface functional groups. In addition, the silica domains contribute to the material’s catalytic properties, potentially supporting advanced pollutant degradation mechanisms.
 | Figure 1: (a) Powder X-ray diffraction and (b) Fourier-transform infrared of modified Ficus benghalensis fruit waste powder biosorbent. [Click here to view] |
Figure 1b presents the Fourier-transform infrared (FTIR) spectrum of the modified FBFWP biosorbent. The peaks observed at 3316 and 3254 cm−1 indicate the presence of –OH groups, while the band at 1729 cm−1 suggests the presence of a C = O group. The peak at 1431 cm−1 corresponds to –CH2– stretching vibrations, and the band at 763 cm−1 signifies the existence of R–CH groups. These IR peaks correspond to functional groups derived from limonoids, flavonoids, and carotenoids present in the FBFWP biosorbent [21]. These findings are consistent with previous literature identifying alcohol, phenolic, or water molecules [22]. The presence of these functional groups confirms the existence of active adsorption sites, such as –OH and –COOH, which play a key role in pollutant removal through mechanisms like hydrogen bonding, ion exchange, and surface complexation. Furthermore, the FTIR data provides insights into chemical modifications in the biosorbent following treatments, such as acid activation or metal adsorption. Shifts or attenuation of these peaks may indicate structural changes or interactions with contaminants. This molecular-level characterization highlights the biosorbent’s inherent reactivity, supporting its potential for use in environmental remediation applications.
Figure 2a and b present the SEM micrographs of the modified FBFWP biosorbent, offering high-resolution insights into its surface morphology and porosity. The images reveal distinct morphological features: Figure 2a shows heterogeneous structures, including cubical, diamond, triangular, and hexagonal shapes, while the higher-resolution Figure 2b captures finer details, such as flower-like or petal-like architectures. These structural changes are likely due to the incorporation of KOH during the activation process, which induces pore formation and alters the surface topography. The development of such porous, hierarchical morphologies - enhanced by KOH activation - creates a larger surface area and exposes more active sites, crucial for pollutant adsorption.
 | Figure 2: (a and b) Scanning electron microscope of modified Ficus benghalensis fruit waste powder biosorbent. [Click here to view] |
In addition, comparing these micrographs with pre-activation or post-adsorption SEM data (if available) could offer further insight into the adsorption mechanism, as changes in pore structure or surface roughness may indicate interactions with contaminants. This morphological characterization highlights how chemical activation optimizes the biosorbent’s physical structure, working in synergy with its chemical functional groups (e.g., –OH, –COOH) to improve remediation efficiency. These changes can be attributed to the effect of KOH incorporation into the FBFWP biosorbent.
The BET surface area analysis quantifies the specific surface area, pore volume, and pore size distribution of the FBFWP biosorbent, all of which are critical parameters for determining its adsorption capacity. For the pristine (unmodified) biosorbent, the BET surface area was measured at 1.11 m2/g, while the KOH-modified sorbent showed a dramatic increase to 67 m2/g. This 60-fold increase in surface area, along with the well-developed porosity, directly correlates with improved adsorption efficiency. The modified biosorbent offers more exposed active sites and enhanced pore accessibility for pollutant interaction.
The stark difference in surface area confirms the significant impact of chemical activation - specifically KOH incorporation - on the restructuring of the material’s porosity, likely through etching and pore-widening mechanisms observed in the SEM [Figure 2b]. The increased surface area and optimized pore network work in tandem with the functional groups identified via FTIR (e.g., –OH, –COOH) and the crystalline-amorphous domains revealed by XRD, collectively enhancing the biosorbent’s potential for environmental remediation. These structural and textural modifications emphasize the importance of activation treatments in optimizing biomass-derived materials for high-performance environmental applications.
3.2. Impact of Size of the Biosorbent
Figure 3 presents the impact of adsorbent particle size on the removal of various heavy metals, aiming to identify the optimal particle size for biosorbent in further studies. The experiments were conducted with an initial concentration of 10 mg/L, at room temperature (30°C), with 150 rpm stirring, an adsorbent dose of 5 g/L, and a reaction time of 90 min for Cr, Pb, and Ni. The concentrations of the respective heavy metal ions were measured using ICP-MS. The graph in Figure 3 illustrates the removal efficiency for different particle sizes (75, 90, and 150 μm) for Cr, Pb, and Ni ions.
 | Figure 3: Impact of particle size of modified Ficus benghalensis fruit waste powder biosorbent on Cr, Pb and Ni removal (%). [Click here to view] |
The 75 μm particle size achieved approximately 98.5% removal of Cr ions and 99.7% removal of Pb ions, while for Ni ions, it demonstrated a removal efficiency of over 48.2%. No significant differences in removal efficiency were observed for Cr, Pb, and Ni ions with changes in the adsorbent particle size [23]. Previous studies have shown that adsorption increases as the particle size of the adsorbent decreases. Smaller particles offer a larger surface area and more active sites on the biosorbent’s surface, enhancing the likelihood of collisions between the adsorbate and adsorbent. In addition, smaller particles move more quickly in solution and reach equilibrium faster than larger particles, resulting in more interactions between the surface and the solution. In contrast, with larger particle sizes, there is a greater possibility of intraparticle diffusion from the outer surface, and mass transfer is influenced by contact time. Some parts of the particles may become blocked, preventing absorption and reducing the equilibrium and adsorption capacity. The increased adsorption observed with smaller adsorbent particles is likely due to their larger surface area. Similar findings were reported by Evilyani et al. [24], Holliday et al. [25], and Mane et al. [26]. The FBFWP biosorbent with a 75 μm particle size was selected for further experiments due to its smaller particle size and high removal capacity.
3.3. Effect of Dose and Concentration on the Removal Efficiency of Heavy Metals
The impact of varying doses of modified FBFWP biosorbent on the removal of selected heavy metal ions (Cr, Pb, and Ni) was evaluated to determine the optimal dose for subsequent batch adsorption experiments. Using simulated wastewater allowed precise control over key variables such as metal ion concentration, pH, temperature, and contact time, enabling a systematic assessment of biosorption efficiency. This controlled setting helps optimize biosorption conditions before applying the process to real wastewater, ensuring maximum effectiveness. It also facilitates the evaluation of the biosorbent’s capacity to remove specific heavy metals under different conditions. Moreover, critical adsorption parameters - such as isotherms, kinetics, and thermodynamics - can be thoroughly investigated, offering valuable insights into the adsorption mechanism and overall biosorbent performance.
Experiments were conducted with initial concentrations of Cr, Pb, and Ni (5 mg/L, 10 mg/L, 20 mg/L, and 30 mg/L) and varying biosorbent doses (2 g/L, 5 g/L, 10 g/L, and 20 g/L) over a reaction time of 90 min. Figure 4a illustrates the effect of a 2 g/L biosorbent dose on the removal of Cr, Pb, and Ni at concentrations of 5 mg/L, 10 mg/L, 20 mg/L, and 30 mg/L. The results show that a 2 g/L dose reduced the initial Cr concentration from 5 mg/L to approximately 92%, Pb to about 88%, and Ni to roughly 55%. As the initial concentration of metal ions increased, the removal efficiency decreased [27]. Figure 4b presents the results for a 5 g/L FBFWP biosorbent dose, which efficiently reduced Cr and Pb concentrations (5 mg/L, 10 mg/L, 20 mg/L, and 30 mg/L) by about 85%, while Ni removal showed around 60% efficiency at an initial concentration of 5 mg/L. Figure 4c and d show results for biosorbent doses of 10 g/L and 20 g/L, respectively, where Cr and Pb ions achieved approximately 90% removal efficiency, and Ni ions showed around 50% efficiency at concentrations of 5 mg/L, 10 mg/L, 20 mg/L, and 30 mg/L. As the biosorbent dose increases, the number of available adsorption sites increases, thereby enhancing removal efficiency. Similar findings were reported by Surisetty et al. [28] in their study on Pb removal from aqueous solutions using F. benghalensis leaves.
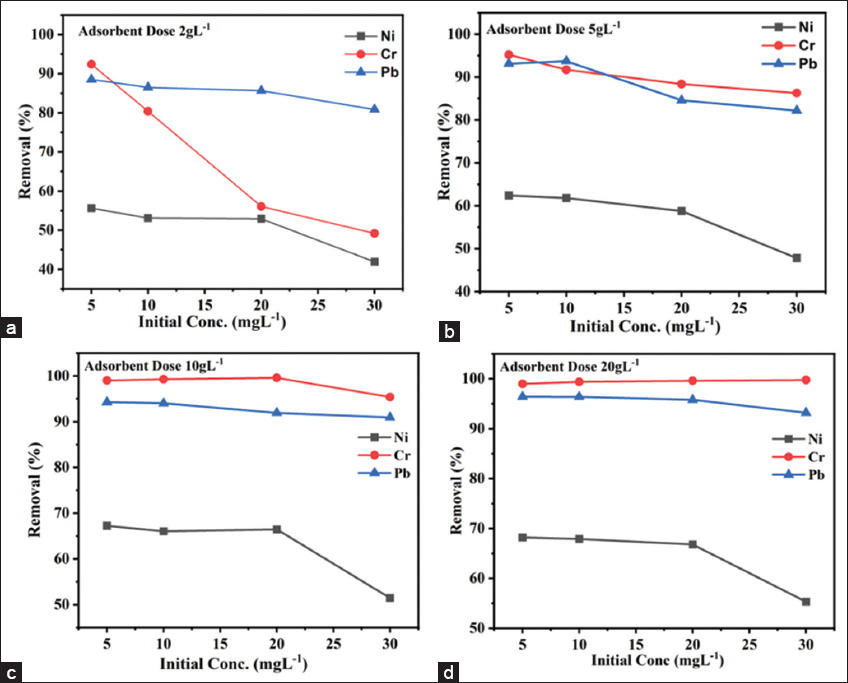 | Figure 4: (a-d) Effect of dose on modified Ficus benghalensis fruit waste powder biosorbent for Cr, Pb and Ni removal (%). [Click here to view] |
3.4. Effect of pH for Heavy Metal Adsorption
The pH of the aqueous solution plays a crucial role in the adsorption of both cations and anions at the liquid-solid interface, with the interaction of metal ions with surface functional groups showing significant pH dependence. Figure 5 presents the results of the pH adsorption study for the FBFWP biosorbent in the uptake of Cr, Pb, and Ni from water. Since pH adsorption experiments are essential for understanding the practical applications of any adsorbent, a series of experiments was conducted to examine the behavior of FBFWP biosorbent under varying acidic and alkaline conditions [29]. The experiments were performed with an initial concentration of 10 mg/L of the selected heavy metals, an adsorbent dose of 5 g/L, at room temperature (30°C), and a reaction time of 120 min. Acidic (0.1M HCl) and alkaline (0.1M NaOH) solutions were used to adjust the pH. Figure 5 shows that the maximum removal efficiencies were observed at pH 5 and pH 7, with Cr removal efficiencies of 98.9% and 91.7%, and Pb removal efficiencies of 96.9% and 96.6%, respectively. For Ni ions, the maximum removal efficiency was observed to be 62.6% at pH 5 and 50.2% at pH 7. Figure 5 also reveals that the removal efficiency for Cr, Pb, and Ni decreased as the pH increased from 5 to 9. FBFWP biosorbent exhibited the highest removal efficiency for Cr, followed by Pb and Ni ions. Surisetty et al. [28] reported that FBFWP contains ionizable functional groups, such as carboxyl groups, which can affect adsorption under varying pH conditions. According to Sa? et al. [30] increasing the pH generally enhances metal ion removal efficiency. The surface complexation theory suggests that this improvement occurs because higher pH reduces the competition between protons and metal species for surface sites, alongside a decrease in surface charge. In addition, Surisetty et al. [28] explained that the carboxylate groups on the surface of F. benghalensis result in a high negative charge density, which facilitates interaction with positively charged metal ions, thereby promoting the biosorption process. The present study confirmed that pH 5 was the most efficient for the biosorption process compared to other pH levels. pH is a critical factor in biosorption as it influences the ionization of functional groups on the biosorbent surface and the speciation of metal ions in solution. At pH 5, functional groups such as carboxyl (-COOH) and hydroxyl (-OH) on biosorbents (e.g., biomass, algae, and bacteria) are deprotonated, generating negatively charged binding sites that enhance the attraction of positively charged metal ions. At lower pH (<5), an excess of H+ ions compete with metal cations for these binding sites, reducing biosorption efficiency. Conversely, at higher pH (>5), metal ions may precipitate as hydroxides rather than binding to the biosorbent. Therefore, pH 5 provides an optimal balance, where metal ions remain soluble and available for biosorption, while the biosorbent functional groups are sufficiently deprotonated for effective binding.
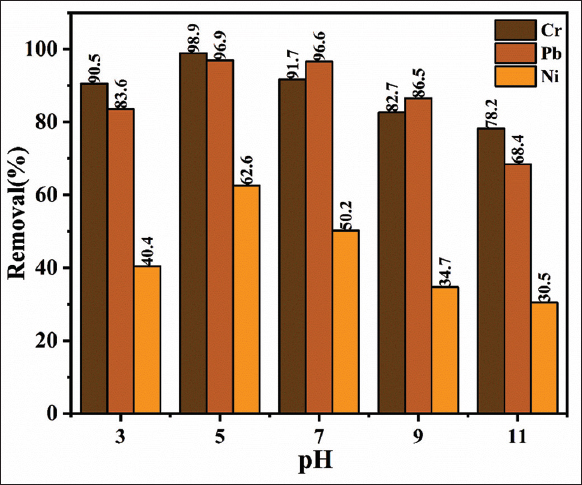 | Figure 5: pH-adsorption study of Ficus benghalensis fruit waste powder biosorbent for Cr, Pb and Ni uptake from water (at 10 mg/L concentration, 5 g/L adsorbent dose and reaction time of 120 min). [Click here to view] |
3.5. Adsorption Isotherm and Kinetics
Batch adsorption experiments were conducted to determine the Qmax adsorption capacity and the characteristics of the FBFWP biosorbent. Figure 6a and b display the results of the Langmuir and Freundlich isotherms for the FBFWP biosorbent’s uptake of Cr, Pb, and Ni ions from aqueous solutions. The linear form of the Langmuir adsorption isotherm is represented as [7].
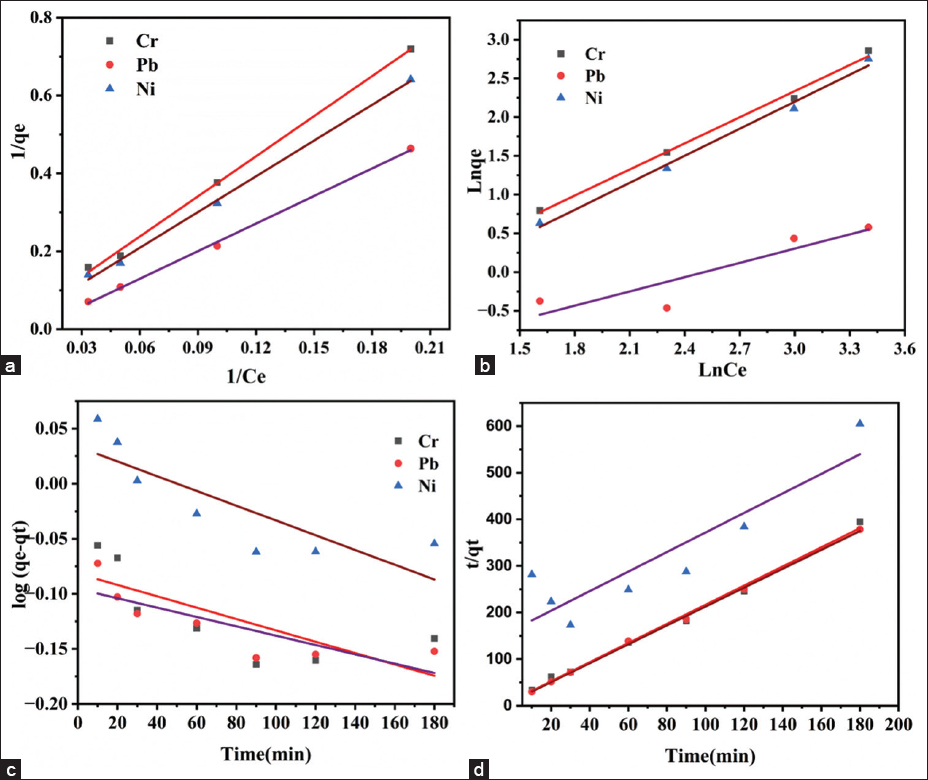 | Figure 6: Langmuir adsorption isotherm (a), Freundlich adsorption isotherm (b) (Fitted by linear regression) and Pseudo first-order kinetic model (c) Pseudo second-order kinetic model (d) of Cr, Pb and Ni uptake on the surface of Ficus benghalensis fruit waste powder biosorbent. [Click here to view] |
Where, qe represents the amount of Cr, Pb, and Ni metal ions adsorbed per unit mass of the FBFWP biosorbent, while Ce denotes the concentration of metal ions remaining in the solution (mg/L) at equilibrium. qm is the maximum adsorption capacity of the adsorbent, and KL (mg/L) is the Langmuir equilibrium constant.
The Freundlich adsorption model is represented as [8]:
Ln (qe) = ln (kf) + 1/n ln (Ce) (2)
where, qe represents the amount of adsorbate adsorbed per unit weight of adsorbent at equilibrium (mg/g), qm denotes the maximum adsorption capacity (mg/g), and Ce refers to the equilibrium concentration of adsorbate in the solution (mg/L).
The regressed parameters are presented in Table 1. The maximum adsorption capacities (Qmax) derived from the Langmuir model were 81.2 mg/g, 83.3 mg/g, and 40 mg/g for Cr, Pb, and Ni ions, respectively. The correlation coefficients (R2 ~ 0.99) suggest that the Langmuir adsorption model effectively describes the adsorption equilibrium for FBFWP concerning Cr, Pb, and Ni. The Langmuir adsorption isotherm indicates that the adsorption behavior of FBFWP follows a monolayer adsorption pattern [31]. According to the Langmuir model, monolayer adsorption occurs on a surface with a finite number of homogeneous binding sites, where each site binds to only one metal ion, with no interactions between adsorbed molecules. The study’s results confirm that FBFWP adheres to this monolayer adsorption pattern, meaning metal ions bind to specific sites on the biosorbent until saturation is achieved. This supports the assumption of a homogeneous surface in the Langmuir model and underscores the biosorbent’s effectiveness in removing metal ions under equilibrium conditions.
Table 1: Characteristics of Langmuir and Freundlich adsorption Isotherms of FBFWP biosorbent to the uptake of Cr, Pb and Ni from aqueous solutions.
| Langmuir Adsorption Isotherm | Freundlich Adsorption Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Cr | Pb | Ni | Parameter | Cr | Pb | Ni |
| KL | 0.009 | 0.0051 | 0.0089 | n | 1.123 | 0.614 | 1.163 |
| 1/Qm | 0.032 | 0.012 | 0.025 | lnK | 1.035 | 1.54 | 1.28 |
| Qm | 81.25 | 83.33 | 40 | KF | 2.815 | 4.665 | 3.597 |
| R2 | 0.994 | 0.998 | 0.998 | R2 | 0.994 | 0.810 | 0.992 |
Figure 6d shows the results of the pseudo-second-order kinetic model, plotted as time (t) versus t/Qt, used to determine the rate constant and Qe values. This model is expressed by Eq. (3) [32], as follows:
 | Figure 7: Regeneration study. [Click here to view] |
Where, K2 is the rate constant for pseudo-second-order adsorption, and the intercept and slope of the t versus t/Qt plot provide values for K2 and Qe, respectively [28]. The pseudo-second-order fitting parameters for the FBFWP biosorbent are listed in Table 2. The kinetic data for the FBFWP biosorbent fit well with the pseudo-second-order model, showing correlation coefficients close to unity for Cr and Pb, while for Ni, the coefficient was 0.81. This suggests that the adsorption mechanism follows chemisorption, where the adsorption rate is dependent on the adsorption capacity rather than the adsorbate concentration. The regression parameters align with the literature, indicating that the pseudo-second-order kinetic model is suitable for the biosorbent data. The lower R2 value (0.81) for Ni indicates that its adsorption mechanism is not solely governed by chemisorption but likely involves a combination of physisorption and chemisorption. This behavior can be attributed to the unique hydration energy and ionic radius of Ni (II) compared to Cr and Pb, which affect its interaction with the biosorbent. As a result, Ni exhibits a lower affinity for the adsorption sites, leading to weaker attachment and making it more susceptible to desorption or competitive displacement by other ions [33].
Table 2: Characteristic kinetics of Pseudo 1st order and Pseudo 2nd order models to of FBFWP adsorbent to the uptake of Cr, Pb and Ni from aqueous solutions.
| Pseudo 1st order Isotherm | Pseudo 2nd order Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Cr | Pb | Ni | Parameter | Cr | Pb | Ni |
| R2 | 0.68 | 0.55 | 0.69 | R2 | 0.993 | 0.998 | 0.81 |
| qe | 250 | 20 | 142.8 | qe | 0.50 | 0.49 | 0.48 |
| K1 | 0.0042 | 0.0052 | 0.019 | K2 | 0.21 | 0.18 | 0.013 |
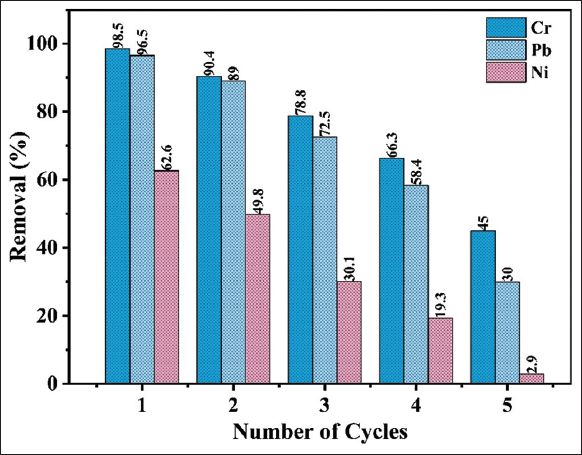
3.6. Regeneration Study
The regeneration study of the biosorbent provides an added advantage for bioremediation, particularly in wastewater treatment. In this study, 0.1M HCl was used as an eluent to recover Cr, Pb, and Ni from the loaded FBFWP biosorbent. It was observed that the biosorbent’s adsorption capacity decreased with repeated use. Despite this, the FBFWP biosorbent remained effective up to the second cycle for reducing Cr and Pb, but its efficiency significantly dropped after the third cycle. For Ni, the reusability of the FBFWP biosorbent proved inefficient after the first cycle, with further declines in performance, making regeneration impractical and not recommended. The weak retention of Ni suggests it may undergo leaching or a different desorption mechanism, reducing its adsorption efficiency over multiple cycles. In contrast, Cr and Pb form stronger chemical bonds with the biosorbent, ensuring stable adsorption and enabling efficient reusability over repeated cycles [34,35].
4. CONCLUSION
In this study, the FBFWP biosorbent was used to remove heavy metals, including Cr, Pb, and Ni. The IR peaks confirmed the presence of functional groups associated with limonoids, flavonoids, and carotenoids in the FBFWP biosorbent, which aligned with literature reports identifying alcohols, phenols, and water molecules. A biosorbent dose of 2 g/L reduced the initial Cr concentration of 5 mg/L by approximately 92%, Pb by 88%, and Ni by 55%. However, removal efficiency decreased with higher initial metal concentrations. Increasing the biosorbent dose improved removal efficiency due to the greater availability of adsorption sites. The optimal pH conditions were found to be pH 5 and pH 7, achieving removal efficiencies of 98.9% and 91.7% for Cr and 96.9% and 96.6% for Pb, respectively. For Ni, the maximum efficiencies were 62.6% and 50.2%. pH 5 proved to be most effective due to the negatively charged binding sites that facilitated biosorption.
The Langmuir adsorption model effectively described the equilibrium for Cr, Pb, and Ni, indicating a monolayer adsorption mechanism. Kinetic data followed the pseudo-second-order model, with correlation coefficients close to unity for Cr and Pb, suggesting chemisorption driven by adsorption capacity. For Ni, the model was less fitting (R2 = 0.81). The biosorbent remained effective for two cycles with Cr and Pb but showed very low reusability for Ni after the first cycle, making regeneration impractical.
Despite its effectiveness, commercial adsorbents such as activated carbon often require expensive regeneration processes. In terms of cost-effectiveness, FBFWP is likely to be more economical than commercial adsorbents, as it is derived from natural materials, reducing production costs. The FBFWP biosorbent demonstrated reusability for at least two cycles with Cr and Pb, making it a viable low-cost alternative. However, its low reusability for Ni could limit its application for multi-cycle adsorption in Ni-contaminated wastewater. Given these findings, FBFWP presents a sustainable, cost-effective, and efficient alternative to commercial adsorbents, particularly for Cr and Pb removal, making it a promising option for heavy metal remediation in wastewater treatment.
5. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
6. FUNDING
I confirmed that this research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
7. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
8. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
9. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Rajoria S, Vashishtha M, Sangal VK. Treatment of electroplating industry wastewater:A review on the various techniques. Environ Sci Pollut Res Int 2022;29:72196-246. [CrossRef]
2. Somyanonthanakun W, Ahmed R, Kron Tong V, Thongmee S. Studies on the adsorption of Pb (II) from aqueous solutions using sugarcane bagasse-based modified activated carbon with nitric acid:Kinetic, isotherm and desorption. Chem Phys Impact 2023;6:100181. [CrossRef]
3. Mitra S, Chakraborty AK, Tareq AM, Emran TB, Nainu F, Khusro A, et al. Impact of heavy metals on the environment and human health:Novel therapeutic insights to counter the toxicity. J King Saud Univ Sci 2022;34:101865. [CrossRef]
4. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl 2012;101:133-64. [CrossRef]
5. Syeda HI, Ibrahim Sultan I, Razavi KS, Pow-Seng Y. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes:A review. J Water Process Eng 2022;46:102446. [CrossRef]
6. Chong ZT, Soh LS, Yong WF. Valorization of agriculture wastes as biosorbents for adsorption of emerging pollutants:Modification, remediation and industry application. Results Eng 2023;17:100960. [CrossRef]
7. Soffian MS, Halim FZ, Aziz F, Mukhlis A. Rahman MA, Amin MA, Awang Chee DN. Carbon-based material derived from biomass waste for wastewater treatment. Environ Adv 2022;9:100259. [CrossRef]
8. Karnwal A. Unveiling the promise of biosorption for heavy metal removal from water sources. Desalin Water Treat 2024;319:100523. [CrossRef]
9. Singh V, Ahmed G, Vedika S, Kumar P, Chaturvedi SK, Rai SN, et al. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods:Isotherms, thermodynamics and kinetics study. Sci Rep 2024;14:7595. [CrossRef]
10. Natasa K, Maia AS, Teodorovi´CA, Atanasova N, Langergraberf G, Crini G, et al. Bio-waste valorisation:Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem Eng J Adv 2022;9:100239. [CrossRef]
11. Ritika N. Development of value-added products from banyan tree (Ficus benghalensis) fruit powder. Int J Basic Appl Sci 2022;11:141-7.
12. Radhakrishnan AJ, Venkatachalam S. A holistic approach for microwave assisted solvent extraction of phenolic compounds from Ficus benghalensis fruits and its phytochemical profiling. J Food Process Eng 2020;43:e13536. [CrossRef]
13. Gul S, Gul A, Gul H, Khattak R, Ismail M, Khan SU, et al. Removal of brilliant green dye from water using Ficus benghalensis tree leaves as an efficient biosorbent. Materials (Basel) 2023;16:521. [CrossRef]
14. Yousafa A, Salmanb M, Saleema F, Razzaqa M, Hayata A. Mobilization and scavenging of Ni+2 and Cd+2 from binary metal ions mixture to study the influence of co-cation on biosorption. Green Chem Lett Rev 2023;16:2233988. [CrossRef]
15. Kumar A, Pandey JP, Kumar S. Biosorptive removal of arsenate by Ficus benghalensis stem powder from aqueous medium. Indian J Adv Chem Sci 2020;8(4):206-215.
16. Kiruthiga M, Ramesh K, Rajappa A, Kamalakannan K. Optimization of rhodamine-b dye sorption potential from aqueous solution using activated carbon developed from the aerial root of Ficus benghalensis L. JAdv Sci Res 2021;12:205-16. [CrossRef]
17. George A, Tembhurkar A. Biosorptive removal of fluoride from aqueous solution onto newly developed biosorbent from Ficus benghalensis leaf:Evaluation of equilibrium, kinetics, and thermodynamics. Sustain Chem Pharm 2018;10:125-33. [CrossRef]
18. Hymavathi D, Prabhakar G. Studies on the removal of cobalt (II) from aqueous solutions by adsorption with Ficus benghalensis leaf powder through response surface methodology. Chem Eng Commun 2017;204:1401-11. [CrossRef]
19. Ofomaja AE, Naidoo EB, Modise SJ. Biosorption of copper (II) and lead (II) onto potassium hydroxide treated pine cone powder. J Environ Manage 2010;91:1674-1685. [CrossRef]
20. Chen H, Yang X, Liu Y, Lin X, Wang J, Zhang Z, et al. KOH modification effectively enhances the Cd and Pb adsorption performance of N-enriched biochar derived from waste chicken feathers. Waste Manag 2021;130:82-92. [CrossRef]
21. Irshad MA, Abdullah, Latif M, Nasim I, Nawaz R, Zahoor AF, et al. Efficient chromium removal from leather industrial wastewater in batch experimental study:Green synthesis and characterization of zinc oxide nanoparticles using Ficus benghalensis extracts. Ecotox Environ Safe 2024;281:116616. [CrossRef]
22. Kumar B, Smita K, Cumbal L, Debut A. Green approach for fabrication and applications of zinc oxide nanoparticles. Bioinorg Chem Appl 2014;2014:523869. [CrossRef]
23. Mathews AP, Zayas I. Particle size and shape effects on adsorption rate parameters. J Environ Eng ASCE 1989;115:41-55. [CrossRef]
24. Evilyani V, Kurniawati D, Putra A, Oktaviya B. Impact of particle size and contact time biosorption of rhodamine b by lengkeng seeds (Euphoria longan Lour) Biosorbent. Int J Sci Res Eng Dev 2021;4:2581-7175.
25. Holliday MC, Parsons DR, Zein SH. Agricultural pea waste as a low-cost pollutant biosorbent for methylene blue removal:Adsorption kinetics, isotherm and thermodynamic studies. Biomass Convers Biorefinery 2024;14:6671-85. [CrossRef]
26. Mane PV, Rego RM, Yap PL, Losic D, Kurkuri MD. Unveiling cutting-edge advances in high surface area porous materials for the efficient removal of toxic metal ions from water. Prog Mater Sci 2024;146:101314. [CrossRef]
27. Eftekhari E, Hasani H, Fashandi H. Removal of heavy metal ions (Pb2+and Ni2+) from aqueous solution using nonwovens produced from lignocellulosic milkweed fibres. J Ind Text 2021;51:695-713. [CrossRef]
28. Surisetty VR, Kozinski J, Rao Nageswara L. Biosorption of lead ions from aqueous solution using Ficus benghalensis L. J Eng 2013;2013:1-8. [CrossRef]
29. El-Sadaawy M, Abdelwahab O. Adsorptive removal of nickel from aqueous solutions by activated carbons from doum seed (Hyphaene thebaica) coat. Alex Eng J 2014;53;399-408. [CrossRef]
30. Sa?Y, Kaya A, Kutsal T. The simultaneous biosorption of Cu (II) and Zn on Rhizopus arrhizus:Application of the adsorption models. Hydrometallurgy 1998;50:297-314. [CrossRef]
31. Ehiomogue P, Ahuchaogu II, Ahaneku IE. Review of Adsorption Isotherms Models;2021. Avaialable from:https://www.researchgate.net/publication/358271705 [Last accessed on 2025 May 12].
32. Ho YS, Mckay G. Pseudo-second order model for sorption process. Process Biochem 1999;34:451-65. [CrossRef]
33. Kostoglou M, Karapantsios TD. Article why is the linearized form of pseudo-second order adsorption kinetic model so successful in fitting batch adsorption experimental data?Colloids Interfaces 2022;6:55. [CrossRef]
34. Akinhanmi TF, Ofudje EA, Adeogun AI, Aina P, Joseph IM. Orange peel as lowcost adsorbent in the elimination of Cd (II) ion:Kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour Bioprocess 2020;7:2-16. [CrossRef]
35. Gupta VK, Nayak A, Agarwal S. Bioadsorbents for remediation of heavy metals:Current status and their future prospects. Environ Eng Res 2015;20:1-18. [CrossRef]