1. INTRODUCTION
Batik industry is one of the rapidly growing industries in the East coast states of Malaysia, especially Kelantan and Terengganu. This industry is generating an enormous contribution to Malaysia’s economic growth due to the high demand both locally and abroad [1]. Batik products are mostly manufactured by small-scale industries or small medium enterprises and are mostly produced in the backyards of homes or near the river [2]. There is concern about the large volume of the effluent discharge from batik processing that includes a large amount of water that contribute to strong color, abundance of suspended solid, high chemical oxygen demand (COD), high biochemical oxygen demand (BOD), and chemicals during their wet processing. According to Yaacob et al. [3], this water pollution issue has gained attention by the community in Kelantan, over concerns of water quality. Through various processes and the use of products containing certain chemicals, the wastewater from batik manufacturing carries a number of pollutants such as grease, wax, softening agent, surfactants, dye, and many other additives. These recalcitrant chemical substances are difficult to remove and non-biodegradable, potentially remaining in the environment for an extended period of time [4,5]. It is found that batik industry in Kelantan has the lowest compliance (5%) and does not meet the standard B permit limit based on the department’s law and regulations [2].
Currently, there are many technologies available to treat batik effluents such as physical, chemical, and biological methods [4-6]. However, chemical and physical treatment usually present drawbacks in terms of cost-effectiveness, complexity of operation, waste generation (sludge), and high energy consumption [5]. Biological treatment is found to have more advantages compared to physical and chemical treatment, which is cheaper and easier to operate [7]. In recent studies, there is an increased focus on dye degradation and decolorization of the wastewater generated from the textile industry. According to Siti-Zuraida et al. [5], there is a biological method that uses microorganisms such as bacteria, fungi, and algae able to remove dyes with high efficiency at low costs from large volumes of wastewater.
Wastewater treatment using microalgae offers more benefits over conventional wastewater treatment. For instance, this approach is relatively cheap, requires low energy, reduce sludge formation and can reduce greenhouse gas emission through sequestering carbon dioxide [8]. These microorganisms have the ability to grow in autotrophic and heterotrophic conditions, and the biomass produced from the wastewater treatment process can be used as feedstock for biofuel such as biodiesel and bioethanol. Microalgae have been showed to have the capability to remove color from various dyes through biosorption, bioconversion, and bioagulation mechanisms [9]. The biosorption capability of microalgae can be attributed to the high surface area and high binding affinity during the treatment. Furthermore, the microalgae cell surface has a wide range of functional groups such as hydroxyl carboxylate and amino phosphate that are responsible for the accumulation of ion or dye on the surface of the microalgae cell biopolymer.
Different types of microalgae have been tested for decolorization and nutrient removal such as Chlorella sp., Desmodesmus sp., Spirogyra sp., Chlamydomonas sp., Haematococcus sp, and Scenedesmus obliquus [1,9-11]. It is reported that microalgae can decolorize and remove nutrients from wastewater with up to 98% effectiveness within 6 days of cultivation before discharge into the environment. However, one of the limitations for the development of microalgae wastewater treatment is harvesting and biomass recovery process. To overcome this problem, immobilization of microalgae process has been proposed as an alternative approach to bypass the harvesting and recovery limitation.
Wastewater treatment using immobilized microalgae has been reported to have many advantages over algae free cell. For instance, it requires less space, and solves problems during the cell harvesting process. Immobilized microalgae are also more stable and resistant to harsh environments that contain high salinity, metal toxicity, pH value and recovering the concentrated microalgal cells for subsequent process in a less destructive way can enhance the cost-effectiveness by reusing the regenerated process. Previous studies reported that utilization of immobilized microalgae is beneficial for nutrient removal as it is effective for removal of phosphate, nitrogen and various metals [12,13]. Immobilized Desmodesmus isolated from Jordan, placed in calcium alginate were found capable of decolorizing dye up to 98% within 6 days of cultivation [14]. In another study, immobilized Chlorella sp. has been reported to be useful to remove 48% color from textile wastewater [10].
Based on literature review, there are many information on wastewater treatment using algae, but inadequate information on batik effluent treatment using immobilized microalgae. The color removal and biodegradation of textile wastewater has been prominantly reported internationally. However, locally, there are limited reports on batik effluent and the decolorization and nutrient removal from its wastewater. Therefore, the purpose of this study is to determine the efficiency of immobilized microalgae for batik effluent treatment. The optimization of decolorization and total nitrogen (TN) removal is evaluated using a statistical approach, Box-Behnken Design (BBD). At the end of the study, the BOD, COD, decolorization, and TN removal are evaluated. The information generated from this study is important to identify an eco-friendly and low cost approach to remediate the batik effluent and protect the environment.
2. MATERIALS AND METHODS
The batik effluent was collected from batik processing factory at Rumah Gahara Galore, Penambang. Kelantan. The effluent is stored in the holding tank before discharged into the river stream. The chemical parameters such as pH, COD, BOD, and TN of each batch of the effluent collected were characterized using the method described by APHA (1998).
2.1 Microalgae and Cultivation Condition
A microalgal species, Chlorella sp. was used throughout in this study. Modified algae growth (MLA) medium that consists with 0.49 g/L magnesium sulfate (MgSO4.7H2O), 1.7 g/L sodium nitrate (NaNO3), 0.14 g/L di-potassium phosphate (K2HPO4), and 0.03 g/L calcium chloride (CaCl2.2H2O) was used for the cultivation and seed preparation. The medium was sterilized using 0.22 μm millipore filter before cultivation process. For seed culture preparation, the culture was incubated under light with a photon intensity of 1000 lux. The cultivation temperature was 30.0°C ± 2.0°C. The microalgae cultures growth were monitored and harvested during the late logarithmic growth phase. Each harvested sample was centrifuged at 4500 rpm for 15 min. The pellet was then rinsed twice and was standardized to optical density of 680 (OD680) of 1.0 before be inoculated for subsequent experiments.
2.2. Preparation on Immobilized Chlorella sp.
Three types of immobilize carrier, namely alginate, starch, and carboxylmethyl cellulose (CMC) were study in this screening. Preparation for each polymer beads is describe below.
2.2.1. Preparation of alginate- immobilized microalgae
An active Chlorella sp. seed culture with optical density at 688 nm (OD688) of 1.0 was harvested by centrifugation at low speed (4500 rpm) for 10 min. Pellet with rich algal biomass was then washed with deionized water and re-suspended in deionized water to form a concentrated algal suspension. The algal suspension was then mixed with 4% sodium alginate solution in 1:1 ratio that is 4 mL of concentrated algal with 4 mL sodium alginate solution. Mixture was stir gently until algae evenly distributed. The mixture was dropped into 6% calcium chloride using a syringe to form uniform algal beads. The beads were left in crosslink solution (CaCl2) about 12 h for hardening. After hardening step done, separated beads from solution using sterile strainer/filter, and beads were rinsed several times with deionized water to remove remaining calcium chloride. Blank alginate beads were prepared with the same way as the algal beads except used deionized water instead of cell suspension.
2.2.2. Preparation of starch-immobilized microalgae
Preparation of algae starch beads was almost same with algae alginate beads. The active Chlorella sp. culture was harvested by centrifugation at low speed (4500 rpm) for 10 min. Cell residue then was washed with deionized water and re-suspended in deionized water to form a concentrated algal suspension. The algal suspension was mixed with 2% of starch solution. The mixture was then homogeneously mixed until algae evenly distributed. The mixture was dropped into 6% calcium chloride using a syringe to form uniform algal beads. The beads were left in crosslink solution (CaCl2) in 12 h for hardening. Strong beads then were separated from solution using sterile strainer/ filter, and beads were rinsed several times with deionized water to remove remaining calcium chloride. Blank alginate beads were prepared.
2.2.3. Preparation of CMC–immobilized microalgae
The active Chlorella sp. culture with optical density at 688nm (OD688) of 1.0 was harvested by centrifugation at low speed (4500 rpm) for 10 min. Cell residue then was washed with deionized water and re-suspended in deionized water to form a concentrated algal suspension. CMC solution was prepared by dissolved 2mL of distilled water with 0.25 g of CMC. Same volume of concentrated algal solution was then homogeneously mixed with CMC mixture. Mixture was stir gently until algae evenly distributed. The mixture was dropped into 6% calcium chloride using a syringe to form uniform algal beads. The beads were left in crosslink solution (CaCl2) about 12 h for hardening. After hardening step done, separated beads from solution using sterile strainer/filter, and beads were rinsed several times with deionized water to remove remaining calcium chloride. Blank alginate beads were prepared in the same way as the algal beads except deionized water instead of cell suspension were used.
2.3. Determination of Optimum Conditions
BBD was used to optimize and investigate the interactive effect of cultivation condition such as pH, light intensity, and algal bead concentration on the decolorization and TN removal from batik effluent. A multi-level with three parameters matrix was employed to determine the synergistic effect between the three parameters. A total of 17 experimental as proposed by the design were carried out and the responses for this study were decolorization and TN removal percentage. The optimal point value was analyzed by fitting second-order polynomial model. The equation for the predicting the response was expressed as follow:
Where Y is response (decolorization and TN removal, percentage), β0 and βi are the model coefficients, and Xi and Xj are the coded for independent variables. The fit of the model was expressed by the coefficient of determination (R2). The statistical significance of each parameter was determined through P values, where smaller p-values indicate the effective parameters. The contour plots were used to show the individual and cumulative effect of the variables on the decolorization and TN removal. These graphical representations for models are plotted as a function of two variables, while keeping other variables at the central level.
2.4. Determination of Decolorization of Batik Effluent
The flasks containing samples were kept for 96 h in a controlled condition at temperature surrounding of 25°C with 12 h light and 12 h dark with presence of fluorescent light. The samples were withdrawn at regular intervals and analyzed for decolorization for every 24 h. The decolorization effectiveness was carried out through absorbance reading at maximum wavelength (Æ›max) of 290 nm using UV-vis spectrophotometer (Hitachi U-1990). The efficiency of color removal was expressed as percentage ratio of the decolorized dye absorbance to that of initial one based on the following Equation 2.
Where Dye(i) = initial dye absorbance, Dye(f) = final dye absorbance.
2.5. Determine of Nitrogen and COD Removal
The percentage of color removal in the samples was measured by equation that described by (Siti-Zuraida et al., 2013). COD reduction was measure by high range kit purchased by Hach Company. BOD5 analysis was determined using standard method for examination of water and wastewater that is 5210 B (5 day BOD test).
TN removal efficiency (%):
2.6. Statistical Analysis
A statistical analysis of variance (ANOVA) was performed to see whether the process parameters for color removal and T-N reduction were statistically significant or not.
3. RESULTS AND DISCUSSIONS
3.1. Characteristic of Untreated Batik Effluent
The batik effluent generated from batik processing was characterized before testing with microalgae. For this characterization, the pH value, COD, BOD, and TN were characterized. The COD and BOD are among the important information that represent the presence of chemicals such as organic and inorganic carbons in the effluent that could pollute the ecosystem. Table 1 show that the COD and BOD for the batik effluent were 661.5 mg/L and 439.5 mg/L, respectively. The pH value and TN for the sample were 11.6 and 63 mg/L, respectively. Based on this analysis, it was found that all the parameters tested were higher compared to the value permitted by the Standard Limit Environment Quality Act. The high COD value and nitrogen content in the effluent indicated this sample could lead to environmental issues such as algae bloom when the water is discharged into a river.
 | Table 1: Characteristic of the batik effluent used in the study [Click here to view] |
3.2. Effect of Immobilized Carrier
To determine the potential of immobilized Chlorella sp. to be used for batik effluent, three microalgal immobilized matrix carriers, namely alginate, CMC, and starch have been evaluated. These are common carriers that are used to immobilize microorganisms for various applications [12]. Figure 1 shows the color removal profile by various types of immobilized matrixes. The present study finds that the presence of immobilized-Chlorella sp. could increase the color removal in the batik effluent compared to that of free cells. The highest color removal was observed from the experiment using microalgae immobilized with alginate. The color removal in the batik effluent starts to increase after 6 hours of cultivation and excellerate to the maximum reduction of 77.9% after 3 days of cultivation. On the other hand, the present study indicates that there is no significant difference on the color removal of batik effluent when the treatment was carried out using CMC and starch as an immobilizing carrier. The lowest color removal is shown in the experiment that was conducted using free cells. Overall, it can be observed that the presence of immobilized cells could help remove color in batik effluent.
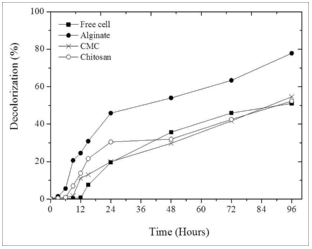 | Figure 1: Decolorization profile of batik effluent using different immobilized matrix carrier. [Click here to view] |
The immobilization technique provides better options to maintain high cell concentration and this method of wastewater treatment have gained great attention in the past few decades. Selecting the most suitable immobilized carrier matrix is important to ensure the success of the process. There are many types of immobilized carrier matrix that have been used for wastewater treatment such as porous polymeric materials such as alginate, agar, polyacrylamide, carrageenan, cellulose acetate, and polyvinyl alcohol to confine the migration of microorganisms [16]. The selection criteria for the most suitable immobilized carrier are based on their characteristics, in which the support materials need to meet the following criteria: Insoluble, not biodegradable, high mechanical stability, high diffusivity, simple immobilization procedure, high biomass retention, minimal attachment of other organisms, and preferably low cost [17]. The utilization of various immobilized microorganisms such as fungi Ganoderma lucidum, Clostridium sp., microalgae Chlorella sp., Desmodesmus sp., Spirogyra sp., Chlamydomonas sp., Haematococcus sp., and Scenedesmus obliquus Chlorella sp., Spirulina sp., and Desmodesmus sp has been tested for decolorization and nutrient removal [1,9,18,19]. In comparison with free cells, it is reported that the immobilized microorganisms exhibit higher decolorization rate [20]. Previous studies demonstrate that alginate immobilized Spirulina platensis, Chlamydomonas reinhardtii, and Chlorella sp. exhibit a promising capability to remove dye color from textile wastewater [10,11].
On the other hand, this present study found that alginate- immobilized Chlorella sp. exhibited the most excellent decolorization compared to the CMC and starch immobilized Chlorella sp. The high efficiency of decolorization by alginate immobilized Chlorella sp. can be attributed to few factors such as the functional group of the chemical used for the immobilization process [21]. The different natures of anionic groups associated with the carrier such as the sulfonate group are found in carrageenan, while the carboxyl group in alginate is important for absorption efficiency during the process [21,22]. Interaction of polysaccharide matrix and nutrient in the effluent could enhance nutrient removal efficiency during the treatment process. Apart from that, the present study also indicates that alginate- immobilized Chlorella sp. is more stable compared to CMC and starch as support materials. It is found that the microalgae cells are released from CMC and starch materials after 3 days of incubation. This serious leakage is observed from cultivation of microalgae using CMC and starch as support materials. Thus, the alginate is selected as an immobilized carrier for immobilization of Chlorella sp for batik effluent treatment.
3.3. Effect of Cultivation Condition on Decolorization and TN Removal
Decolorization and TN removal in wastewater by microalgae can be influenced by various factors such as pH, temperature, light intensity, and cell concentration. Hence, the effect of initial pH, light intensity, and microalgal bead concentration on the decolorization and TN removal from batik effluent was evaluated in this study. Figure 2 shows the decolorization of batik effluent profile cultivated in different cultivation conditions.
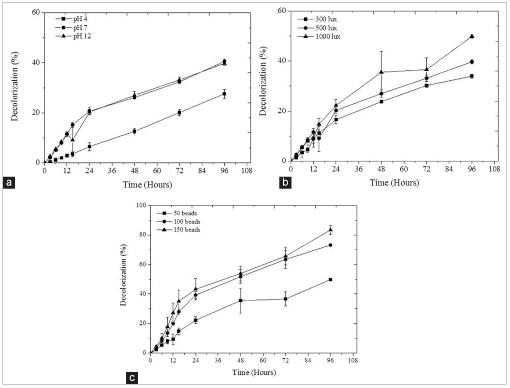 | Figure 2: Decolorization profile of batik effluent at different treatment condition. (a) Different pH (b), different light intensity, and (c) different microalgal bead concentration. [Click here to view] |
Changes of pH value have indirectly influenced the nutrient removal and decolorization in the effluent [9,23]. Figure 2a indicates that a significant decolorization increased when the treatment was increased from pH 4 to 12. The maximum decolorization is obtained from treatment at neutral to alkaline condition. It is observed that the decolorization of batik effluent by immobilizing Chlorella sp. at pH 7 and 12 increased rapidly in the first 12 h and starts to decrease after that period. The maximum decolorization reached 40.59% and 39.72% for the treatment using pH 7 and 12, respectively. Similar observation has been reported on the removal of cationic dye from aqueous sample by Chlorella sp. and Chlamydomonas sp., which the maximum decolorization was achieved under alkaline condition pH range 9–11 [9,24]. High decolonization obtained at this condition is contributed by zero point of both immobilized carrier and microalgae cell surface. In general, for the zero point of charge for algae species and alginate, their surfaces are presumably positively charged in acidic solution and negatively charged in alkaline solution. Thus, the surface of alginate and microalgae need more negatively charged ions that lead to adsorption of dye on to the alginate and microalgae cell surface [24].
Light intensity plays a major role on microalgae photosynthesis. Over exposure of microalgae to light intensity could inhibit microalgal growth and decolorization activity. Figure 2b shows the decolorization of batik effluent from immobilized Chlorella sp. It was found that the increase of light intensity could increase decolorization and TN removal from batik effluent. The maximum decolorization of 49.87% was attained when the treatment was carried out at 1000 lux. The treatment of batik effluent using immobilized Chlorella sp. at 300 lux showed the least decolorization and nitrogen removal at only 33%. These results were similar to previous studies, that report that C. sorokiniana XJK show the least decolorization at low light intensity [23].
High decolorization at high light intensity could be due to the fact that this condition provides a good growth for Chlorella sp. generally; suitable light intensity provides better growth condition that leads to high microalgae cell growth, resulting in an increase in metabolic activity and indirectly improves color removal from the medium [23]. An appropriately increased light intensity can boost the metabolism of Chlorella sp. to enhance the color removal efficiency. Moreover, a light–dark regime for efficient photosynthesis can encourage good color removal efficiency [25].
Figure 2c shows the effects of microalgal beads concentration on decolorization profile percentage from batik effluent. The results show that the decolorization increases with the increase of microalgal bead concentration. In this study, the maximum decolorization of 83.34% is obtained from the treatment using 150 bead concentration, followed by 100 bead concentration with 73% removal. Batik effluent treatment at low microalgal bead concentration is found to have least decolorization activity with a decolorization percentage of 19.88%.
High decolorization of batik effluent by immobilized microalgae could be attributed to the introduction of high cell concentration during treatment, which may accelerate metabolic activity and lead to an increment of color removal in the medium [26]. Similar observations have been reported by other studies that indicate that high color removal by C. sokokiniana XJK is obtained when the treatment is performed using high microalgae bead concentration [23].
Table 2 shows the percentage of TN removal in batik effluent at different cultivation conditions, i.e., initial pH value, light intensity, and microalgal bead concentration. Based on the present study, a significant increase of nitrogen removal is achieved by increasing of pH value from 4 to 7 with TN removal percentage from 28.1% to 57.1%, respectively. Further increase of pH value to pH 12 is found to reduce the percentage of TN removal with only a 42.9% reduction. This suggests that the most effective nitrogen removal happens under neutral and alkaline conditions. This result is in agreement with other studies that report that the suitable pH for nitrogen removal is between 6 and 10 [27,28]. It is suggested that high nitrogen removal by microalgae is due to the assimilation and biological nitrification in the medium [29]. Significant nitrogen removal at different pH values happens because the equilibrium between ammonium ions and free ammonia is controlled by the pH level [30]. An increase of pH value will result in the conversion of ammonium ion to free ammonia, which will be utilize by microalgae for its growth.
 | Table 2: Total nitrogen concentration before and after treatment with immobilized Chlorella sp. [Click here to view] |
Apart from pH value, tests showed that light intensity can also affect TN removal in effluent. It was found that there is no significant difference between the percentage TN removal under all tested light intensities. Approximately 57.1% of TN available in the batik effluent was removed when the cultivation was performed under the light intensity of 300, 500, and 1000 lux. This indicates that the light intensity supplied for Chlorella sp. is sufficient enough to support its growth in the medium. These results contradict the results reported by other researchers. According to Zhang et al. [31], nitrogen removal rate by microalgae Scenedesmus dimorphus were significantly enhanced when the light intensity was raised from 50 to 400/umol/s. Similar observation has been made for the nitrogen removal by Chlorella kesseleri and Chlorella protothecoides, which indicates that the best nitrogen consumption by microalgae is obtained at the light intensity of 200/umol/s [32]. A study by da Fontoura [33] reported that in nutrient removal in tannery wastewater, the maximum amount of nitrogen removed using Scendesmus sp. was obtained when the treatment was performed under a high light intensity of 200/umol/s.
Bead concentration is also found to be a profound factor of nitrogen removal in batik effluent. This study found that an increase of bead concentration could increase nitrogen removal in batik effluent [Table 2]. The maximum TN removal is obtained when the cultivation is carried out using 150 microalgal bead. Cultivation of microalgae using less bead concentration such as 50 and 100 beads only removed 42.9% and 57.1% of TN from the effluent, respectively. High TN removal is observed from cultivation using a high number of microalgal bead concentration because high cell concentration can accelerate the microalgae growth and nitrogen removal in the effluent. In general, nitrogen in the effluent is be utilized by microalgae for their growth through uptake and assimilation/nitrification process [34]. The nutrient uptake by immobilized microorganisms involve two steps. First, nutrients are absorbed onto the surface of the beads over a short period of time, then followed by a slow penetration of the nutrients into alginate and continually absorb into the cells [35]. Studies on the effects of bead concentration on nitrogen removal in various wastewater have been reported elsewhere [13,36]. Introducing high concentration of Chlorella sp. is found to increase the removal of BOD, COD, and TN up to 84.81% in wastewater samples [37]. The suitable cell and bead concentration is also important for the treatment feasibility. Treatment using cell and bead concentration that is too high could lead to serious leakage problems [13,36]. Furthermore, dense beads would reduce the amount of light penetrating through the bioreactor and enhance self-shading effects, limiting growth, and metabolic activity [35]. Based on this study, the most suitable bead concentration to obtain maximum nitrogen removal is 150 beads at pH value 12.
Further optimization study on the effect of treatment conditions such as pH, light intensity, and microalgal bead concentration on decolorization and nitrogen removal was carried out using Box-Behnken response surface design (BBD). The design matrix of the variable in the uncoded units by the BBD is presented in Table 3.
 | Table 3: Experimental design matrix on decolorization and TN removal of batik effluent (%) proposed by BBD [Click here to view] |
Multiple regression analysis for determining the relationship between parameters on the decolorization and TN removal was carried out and the quadratic model equations for both responses are generated as shown in following Eq:
Where X1, X2, and X3 are coded value for pH, light intensity, and microalgal bead concentration, respectively. The coefficient of determination (R2) of the regression equation for both decolorization and TN removal was 0.99 (Adj = 0.98 and 0.97). The R2 value is close to 1, indicating that the predictability of the value is at 95% confidence level and the response function for predicted value agreed well with the experimental data.
The present study indicates that the quadratic model was the most suitable fit for decolorization and TN removal from batik effluent with P > F value of <0.005 [Table 4]. As shown in Table 4, P value for each parameter is P < 0.05, implying that these parameters have a significant effect on the decolorization and TN removal. Parameters such as pH (X1), light intensity (X2), and microalgal bead concentration (X3) were the significant model terms that affect decolorization of batik effluent, whereas pH (X1) and microalgal bead concentration (X2) had significant effect on TN removal.
 | Table 4: ANOVA for the decolorization and TN removal of batik effluent [Click here to view] |
3.4. Effect of Variables on Decolorization and TN Removal
The 3D response surface plot simulated from the Equations 4 and 5 and Figure 3 describes the effects of pH, light intensity, and microalgal bead concentration on decolorization and TN removal. As shown in Figure 3a and b, decolorization and TN removal are significantly influenced by initial pH and light intensity provided during the treatment (P < 0.05). High color removal is obtained when the treatment was performed in alkaline medium range 8–10 using high light intensity ranges of 500–1000 lux. Furthermore, the maximum TN removal is obtained when the treatment is performed at pH range 8–10 at light intensity range from 300 to 1000 lux. Further treatment under acidic and beyond pH 10 condition are shown to reduce TN removal.
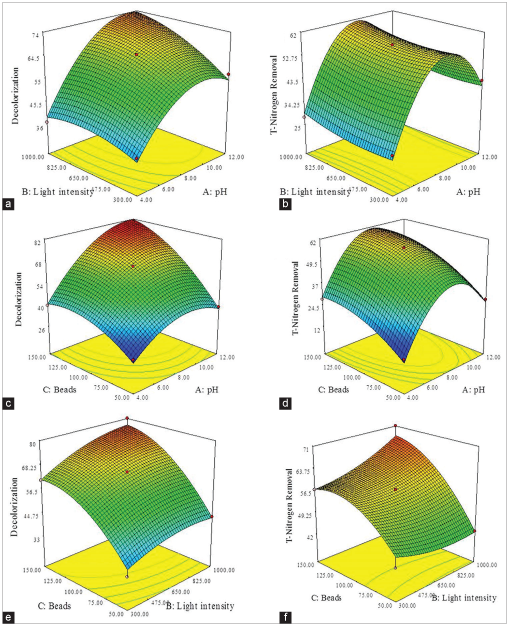 | Figure 3: 3D contour plot showing the interaction between variables on decolorization and total nitrogen (TN) removal of batik effluent (a) Effect of light intensity and pH on decolorization, (b) effect of light intensity and pH on TN removal, (c) effect of microalgal bead concentration and pH on decolorization, (d) effect of microalgal bead concentration and pH on TN removal, (e) effect of microalgal bead concentration and light intensity on decolorization and (f) effect of microalgal bead concentration and light intensity on TN removal. [Click here to view] |
Interactions with pH level and light intensity are found to show a significant effect on decolorization of batik effluent. Providing high light intensity will reduce the pH value of the medium that resulting to high biosorption during the treatment, causing decolorization. As the pH of the system decreases, the anionic polymer will protonate and provide high electrostatic attraction of the negatively charged alginate surface and positively charged cationic dye. Likewise, low pH levels will cause low decolorization as the alginate matrix will shrink, resulting in the reduction of pore size of the matrix. As a result, low diffusion and decolorization is obtained from treatment at this condition.
The effects of microalgal bead concentration and pH interaction on decolorization and TN removal are shown in Figure 3c and d, respectively. The results indicate that the decolorization and TN removal increases with an increase of pH and microalgal bead concentration. A significant decolorization of batik effluent was achieved when the cultivation was performed using high pH value with high microalgal bead. The maximum decolorization of ~82% is obtained when the cultivation was performed between pH 6 and 12 using 100–150 microalgal beads [Figure 3c]. Likewise, the maximum TN removal of ~62% could be achieved at initial pH between 6 and 10 using 75–150 microalgal beads [Figure 3d]. The percentage of TN removal decreases with further increase of pH value beyond results show that a combination of high microalgal bead concentration at alkaline conditions gives maximum decolorization and TN removal. This is due to the fact that this condition can increase microalgal metabolic activity that leads to the increase of nutrient uptake during the treatment process.
Figure 3e and f shows the effects of microalgal bead concentration and light intensity on the decolorization and TN removal of batik effluent. As shown in Figure 3e, it is found that the maximum decolorization was obtained when the treatment was carried out using a high microalgal bead concentration of 150 and a light intensity of 1000 lux. The results clearly indicate that changes in light intensity and bead concentration can significantly affect the rate color removal. High decolorization observed for cultivation at high light intensity and beads concentration could be due to the fact that high light intensity will increase the temperature in the cultivation medium, which can decrease the color viscosity in the samples. As a result, more color will diffuse into the bead active site and led to higher decolorization. Cultivation at low light intensity using less microalgal beads exhibits low decolorization. This could be due to the fact that the condition is unsufficient to increase the microalgal metabolic activity. Similarly, for TN removal, it was found that cultivation of immobilized Chlorella sp. requires high number of microalgal beads to obtain maximum TN removal efficiency. A significant TN removal of ~71% was achieved when the cultivation was performed with 150 microalgal bead concentration and 1000 lux [Figure 3f].
Overall, this study has found that the cultivation parameters such as initial pH, light intensity, and microalgal bead concentration have a significant effect on the decolorization and TN removal of batik effluent. Cultivation below and beyond optimum conditions can exhibit low decolorization and TN removal efficiency.
3.5. Feasibility of Decolorization and TN Removal of Real Batik Effluent using Alginate-immobilized Chlorella sp.
Further batik effluent treatment evaluation was carried out to determine the potential of immobilized Chlorella sp. to remediate real batik effluent at optimum conditions. For this study, the treatment was performed using 150 microalgal bead concentration at pH 12 and 1000 lux. Initially, the chemical characteristics of batik effluent were determined and are shown in Table 5. A significant color and nitrogen removal were observed for both samples. It is found that most of the BOD, COD, and TN of real batik effluent are at 49%, 49.72%, and 43.75% removal. The color removal for real batik effluent and synthetic batik effluent is also compared and the removal profile is shown in Figure 4. It is found that the color and nitrogen removal percentage in real batik effluent sample is slightly lower compared than that from synthetic batik effluent. The color removal for real and synthetic batik effluent are at 78% and 85.2%, respectively. Meanwhile, approximately 71% and 43.7% of nitrogen were removed from the simulated and real batik effluent. It is clear that decolorization and TN removal from real batik effluent are lower than that from the simulated sample. The lower decolorization and nitrogen removal in the real batik effluent can be attributed to the fact that real batik effluent is strong in color, and contains several undesirable organic and inorganic constituents that may affect the microalgae growth and color removal activity [38].
 | Table 5: Characteristic of the real batik effluent before and after treatment at optimum condition (pH=8, beads: 150 and light intensity: 1000 lux) [Click here to view] |
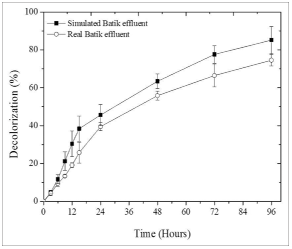 | Figure 4: Decolorization profile in simulated and real batik effluent performed using 150 microalgal bead concentration at pH 12 and light intensity of 1000 lux. [Click here to view] |
4. CONCLUSION
The capability of immobilized Chlorella sp. for decolorization and TN removal of batik effluent is investigated. The present study demonstrates that alginate-immobilized Chlorella sp. exhibits a great potential to be applied for batik effluent treatment. It can be said that decolorization and TN removal by immobilized Chlorella sp. can be influenced by the initial pH, light intensity, and number of beads used during the treatment process. The immobilized Chlorella sp. was found to be able to remove ~ 80% of color in the batik effluent when the treatment was performed using 150 microalgal bead concentration, at pH 12, and 1000 lux. Whereas, the maximum TN removal of ~ 71% with nitrogen uptake rates of 4.9 mgL-1d-1 was observed for the cultivation at pH 7 with 150 microalgae bead concentration, and 1000 lux. On the other hand, the findings from this study also indicate that immobilized microalgae can be an alternative treatment option for salt- alkaline wastewater such as batik effluent and has potential to be a low cost method to remove the pollutants from the effluent. Even though this study shows promising results, further investigation at a bioreactor scale is required to ensure the feasibility of this approach in actual industrial application.
5. ACKNOWLEDGMENT
Author wants to acknowledge the manager of Ruzz Gahaza factory for their help in collecting the samples from the discharge and School of Industrial Technology, Universiti Sains Malaysia for the facilities to carry out this project.
REFERENCES
1. Lim SL, Chu WL, Phang SM. Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour Technol 2010;101:7314-22. Crossref
2. Subki N, Rohasliney H. A Preliminary Study on Batik Effluent in Kelantan State: A Water Quality Perspective. International Conference on Management, Social Science, Biology and Pharmaceutical Science; 2011. p. 274-6.
3. Yaacob MR, Ismail M, Zakaria MN, Zainol FA, Zain NF. Environmental awareness of batik entrepreneurs in Kelantan, Malaysia: An early insight. Int J Acad Res Bus Soc Sci 2015;5:273-86. Crossref
4. Khalik W, Li-Ngee, H, Soon-An O, Yee-Shian W, Yusoff N, Ridwan F. Decolorization and mineralization of batik wastewater through solar photocatalytic process. Sains Malay 2015;44:607-12. Crossref
5. Siti-Zuraida M, Nurhaslina C, Halim KK. Influence of agitation, pH and temperature on growth and decolorization of batik wastewater by bacteria Lactobacillus delbruckii. Int J Rec Res Appl Stud 2013;14.
6. Ahmad A, Harris W, Ooi-Boon S. Removal of dye from wastewater of textile industry using membrane technology. J Teknol 2002;36:31-44. Crossref
7. Sarayu K, Sandhya S. Current technologies for biological treatment of textile wastewater-A Review. Appl Biochem Biotechnol 2012;167:645-661. Crossref
8. Wang Y, Ho SH, Cheng CL, Guo WQ, Nagarajan D, Ren NQ, et al. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour Technol 2016;222:485-97. Crossref
9. Khataee AR, Zarei M, Pourhassan M. Application of microalga Chlamydomonas sp. For biosorptive removal of a textile dye from contaminated water: Modelling by a neural network. Environ Technol 2009;30:1615-23. Crossref
10. Chu WL, See YC, Phang SM. Use of immobilised Chlorella vulgaris for the removal of colour from textile dyes. J Appl Phycol 2008;21:641. Crossref
11. Patnaik S, Sarkar R, Mitra A. Alginate immobilization of Spirulina platensis for wastewater treatment. Indian J Exp Biol 2001;39:824-6.
12. Steffan S, Bardi L, Marzona M. Azo dye biodegradation by microbial cultures immobilized in alginate beads. Environ Int 2005;31:201-5. Crossref
13. Lau PS, Tam NF, Wong YS. Effect of algal density on nutrient removal from primary settled wastewater. Environ Pollut 1995;89:59-66. Crossref
14. Al-Fawwaz AT, Abdullah M. Decolorization of methylene blue and malachite green by immobilized Desmodesmus sp. isolated from North Jordan. Int J Environ Sci Dev 2016;7:95-9. Crossref
15. APHA. Standard Methods for the Examination of Water and Wastewater. Baltimore: American Public Health Association. American Water Works Association. Water Pollution Control Federation, United Book; 1998.
16. Chang IS, Kim CI, Nam BU. The influence of poly-vinyl-alcohol (PVA) characteristics on the physical stability of encapsulated immobilization media for advanced wastewater treatment. Process Biochem 2005;40:3050-4. Crossref
17. Leenen EJ, Dos Santos VA, Grolle KC, Tramper J, Wijffels R. Characteristics of and selection criteria for support materials for cell immobilization in wastewater treatment. Water Res 1996;30:2985-96. Crossref
18. Ekambaram SP, Perumal SS, Annamalai U. Decolorization and biodegradation of remazol reactive dyes by Clostridium species 3 Biotech 2016;6:20.
19. Selvakumar S, Manivasagan R, Chinnappan K. Biodegradation and decolourization of textile dye wastewater using Ganoderma lucidum. 3 Biotech 2013;3:71-9. Crossref
20. Vijayakumar S. Treatment of dye industry effluent using free and immobilized Cyanobacteria. J Bioremediat Biodegrad 2012;3:1-6. Crossref
21. Lau PS, Tam NF, Wong YS. Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris. Environ Technol 1997;18:945-51. Crossref
22. Li M, Elder T, Buschle-Diller G. Alginate-based polysaccharide beads for cationic contaminant sorption from water. Polym Bull 2016;74:1267-81. Crossref
23. Xie L, Zhou L, Liu T, Xu X. Degradation of disperse blue 2BLN by oleaginous C. Sorokiniana XJK. RSC Adv 2016;6:106935-44. Crossref
24. Tsai WT, Chen HR. Removal of malachite green from aqueous solution using low-cost Chlorella-based biomass. J Hazard Mater 2010;175:844-9. Crossref
25. Waqas R, Arshad M, Asghar HN, Asghar M. Optimization of factors for enhanced phycoremediation of reactive blue azo dye. Int J Agric Biol 2015;17:803-8. Crossref
26. Patel Ka MP. Microbial decolorization and degradation of orange 16 dye by a newly isolated Aeromonas spp. Etl-1949. J Bioremediat Biodegrad 2013;4:194. Crossref
27. Kim J, Lingaraju BP, Rheaume R, Lee JY, Siddiqui KF. Removal of ammonia from wastewater effluent by Chlorella vulgaris. Tsinghua Sci Technol 2010;15:391-6. Crossref
28. Raheek II, Wong ZH, Mohammad AW. Optimization and performance evaluation for nutrient removal from palm oil mill effluent wastewater using microalgae. IOP Conf Ser Mater Sci Eng 2015;78:12006.
29. Perez-Garcia O, Escalante FM, de-Bashan LE, Bashan Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res 2011;45:11-36. Crossref
30. Sayadi M, Ahmadpour N, Fallahi-Capoorchali M, Rezaei M. Removal of nitrate and phosphate from aqueous solution by microalgae: An experimental study. Glob J Environ Sci Manage 2016;2:357-64.
31. Zhang S, Kim TH, Han TH, Hwang SJ. Influence of light conditions of a mixture of red and blue light sources on nitrogen and phosphorus removal in advanced wastewater treatment using Scenedesmus dimorphus. Biotechnol Bioprocess Eng 2015;20:760-5. Crossref
32. Li Y, Zhou W, Hu B, Min M, Chen P, Ruan RR, et al. Effect of light intensity on algal biomass accumulation and biodiesel production for mixotrophic strains Chlorella kessleri and Chlorella protothecoide cultivated in highly concentrated municipal wastewater. Biotechnol Bioeng 2012;109:2222-9. Crossref
33. da Fontoura JT, Rolim GS, Farenzena M, Gutterres M. Influence of light intensity and tannery wastewater concentration on biomass production and nutrient removal by microalgae Scenedesmus sp. Process Saf Environ Protect 2017;111:355-62. Crossref
34. Whitton R, Ometto F, Pidou M, Jarvis P, Villa R, Jefferson B. Microalgae for municipal wastewater nutrient remediation: Mechanisms, reactors and outlook for tertiary treatment. Environ Technol Rev 2015;4:133-48. Crossref
35. Tam NF, Wong YS. Effect of immobilized microalgal bead concentrations on wastewater nutrient removal. Environ Pollut 2000;107:145-51. Crossref
36. Abdel-Hameed M. Effect of algal density in bead, bead size and bead concentrations on wastewater nutrient removal. Afri J Biotechnol 2007;6:1185-91.
37. Choi HJ, Lee SM. Effects of microalgae on the removal of nutrients from wastewater: Various concentrations of Chlorella vulgaris. Environ Eng Res 2012;17:3-8. Crossref
38. Firmino PI, da Silva ME, Cervantes FJ, dos Santos AB. Colour removal of dyes from synthetic and real textile wastewaters in one- and two-stage anaerobic systems. Bioresour Technol 2010;101:7773-9. Crossref