1. INTRODUCTION
Plastics are man-made, non-biodegradable composites which are of substantially petrochemical origin [1]. They come from plants like corn and sugarcane, as well as from natural gas and oil. About 4% of the petroleum produced worldwide is utilized to generate plastic, and an additional 4% is required to fuel the processes used to make plastic [2]. They are simply made up of hydrogen and carbon with some other organic and inorganic materials [3]. Plastics have become universal due to its dual nature as it is a widely used material as well as considered as an environmental contaminant [4]. In the past few decades plastic materials have covered each and every sector of human need. It has replaced the other material such as glass, wood, and metal that were used in varied applications, due to its distinct properties that have created the way for its use in enormous sectors [5]. Low-density polyethylene (LDPE), high-density polyethylene (HDPE), polyethylene terephthalate (PET), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polycarbonate (PC), and polyurethane (PU) are the most widely used polymers The commonly used plastics are LDPE, HDPE, PET, PP, PS, PVC, PC, PU, etc. [6,7] [Figure 1]. Widespread applications of plastic leads to large scale production which is creating an issue of their disposal and management [4]. Over the course of at least 500 years, about 90% of all plastic created worldwide persists in the environment [8]. The health of the biotic community in both terrestrial and aquatic habitats could be severely threatened by plastic [9-11]. Both the incineration of plastic waste and the dumping of it in landfills produce significant amounts of CO2 and contribute to global warming [12]. Air, water, and soil pollution result from the environmental implications of dumping so much plastic into the environment [8]. In attempts to develop novel strategies for managing plastic waste, significant research expenditure has explored whether bacteria might utilize the commonly contaminated polymers. By doing so, they could provide a sustainable and eco-friendly alternative to the current extreme usage of plastic [4]. Biodegradation is the most beneficial approach for plastic degradation compared to other methods since it is non-polluting in nature. Multiple environmental parameters and numerous microbial strains are involved in biodegradation [13]. According to reports, the most effective technique to minimize plastic trash in an environmentally acceptable manner is through bioremediation, which uses biological agents such as bacteria, fungi, and algae [14]. Fungi are a varied group of eukaryotic organisms that can act as saprobes, symbionts, and parasites in nearly all aerobic and some anaerobic conditions [15].
 | Figure 1: Types of plastic. [Click here to view] |
The significance of fungus in the biodegradation of plastic is considerable. The presence of pro-oxidant ions and the secretion of enzymes that aid in degradation, such as proteases, cutinases, and lipases, has been reported to cause effective degradation by fungi. By generating functional groups through oxidation or hydrolysis by the action of enzymes, higher molecular weight compounds can be converted into lower one by making polymers more hydrophilic [13]. Several fungal strains including Aspergillus clavatus, Trichoderma viride, Aspergillus nomius, Penicillium sp. have been known to potentially degrading this alarming plastic waste and providing a way to get rid of this plastic waste management issue in an eco-friendly way [16-18]. There is a need to create innovative solutions to both reduce and degrade this waste using a green approach, as the generation of plastic garbage is a problem that affects the entire globe. This review article gives a systematic view at the various fungal strains involved in plastic biodegradation, process of biodegradation, and various assessment techniques involved.
2. BIODEGRADATION
Plastic polymers are a big threat to the entire world and are not biodegradable. It would take decades for these plastics to break down. Biodegradation is the most efficient and ideal method for plastic breakdown due to its non-polluting mechanism, environmental friendliness, and economic viability [13]. Microbes are the intermediaries in a challenging physicochemical process that breaks down polymers into smaller components [19,20]. Complex organic molecules can be biochemically broken down, assimilated, and metabolized by microorganisms, specifically fungi [21,22].
Biodeterioration, biofragmentation, assimilation, and mineralization are a few of the biochemical degradative routes for plastic biodegradation. These procedures all rely on different enzyme functions and bond cleavage [14,23]. Biodeterioration is the chemical and physical activity of microbes that results in a plastic polymer’s surface degradation and alteration of its mechanical, physical, and chemical properties [24]. Adhesion and colonization of microbes on the surface of the polymer initiates this first step, the only goal of which is to reduce the resistance and durability of plastic materials. It is frequently required to add hydrophilic functional groups to plastic surfaces to promote microbe adherence because plastics are naturally hydrophobic in nature [25]. The polymers are used by microbes as their main source of carbon as they attach to the polymer’s surface and keep multiplying. Next is the depolymerization process known as “biofragmentation,” extracellular enzymes and bacterially generated free radicals catalyze the breakdown of biodegraded polymers into smaller pieces [26]. Next step is assimilation in which the biofragmented smaller molecular weight compounds are then transported into the microbial cytoplasm [27,28]. The last step is mineralization, which involves the successful delivery of these plastic derivatives into cells and a sequence of enzymatic reactions that cause them to completely decompose into oxidized metabolites including CO2, N2, CH4, and H2O [29]. Numerous enzyme activities, including peroxidases, lipases, esterase, cutinase, and laccase, are necessary for the complete mineralization process [30].
3. FUNGAL STRAINS INVOLVED IN BIODEGRADATION
The world’s fungus species range from 2.2 to 3.8 million from harmless free-living bacteria to dangerous diseases that may survive in a variety of host and environmental niches such soil, water, plants, and animals [31]. Fungi vary in their morphology and can be unicellular, filamentous or dimorphic [32]. They can exist independently or in mutualistic symbiotic relationships or as parasitic pathogens of diverse plants and animals, including humans [33,34]. Numerous fungi inhabit terrestrial, freshwater, and aquatic habitats [35-37].
Numerous fungi species have been found to be able to degrade a wide range of plastic polymers due to their potential to use these synthetic polymers as their primary or only source of energy. In this context, it has been demonstrated that a vast variety of fungi, representing different classes, ecologies, and morphologies, are capable of degrading plastics. Due to its enormous advantage over chemical and physical degradation approaches, the biodegradation of these man-made compounds by microorganisms seems to be one of the important techniques to control the problem of plastic waste [38]. Biological degradation is thought to be a more effective and strong solution to this global issue. Biodegradation involves many kinds of plastic degrading microorganisms [39,40]. Many chemicals are transformed into simpler compounds by microorganisms through biochemical processes. An indicator of the biodegradation of plastic polymers is a change in the physical properties of the polymers, such as a reduction in molecular weight, a loss of mechanical strength, or a change in the surface properties of the plastic [29] [Figure 2].
 | Figure 2: System overview of biodegradation of plastic by fungal community. [Click here to view] |
A. clavatus isolated from landfill soil has been found to degrade LDPE [16]. There are numerous Aspergillus species, including Aspergillus terreus, Aspergillus sydowii, Aspergillus tubingensis, Aspergillus fumigates isolated from Mangrove dumpsite. The coastal environment of the Gulf of Mannar and seawater is known to degrade polyethylene effectively [18,41,42]. Various fungal strains have been discovered so far and showing degradation of different types of plastic in an accomplished way. Various fungal strains isolated from different habitats and degrading different types of plastics have been reported worldwide [Table 1].
Table 1: Various fungal strains showing plastic biodegradation.
| Substrate | Sample location | Fungal strains | Weight loss (%) | Assessment techniques | References | |
|---|---|---|---|---|---|---|
| PSc | 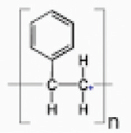 | NCIM | Mucor sp. | 1.81±0.13 | Weight reduction measurement, FTIR, SEM, TGA–DTG, GC-MS and GPC | [43] |
| PS | 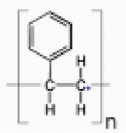 | NCIM | Cephalosporium sp. | 2.17±0.16 | Weight reduction measurement, FTIR, SEM, TGA–DTG, GC-MS and GPC | [43] |
| LDPE | 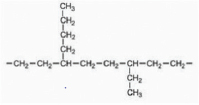 | Sewage disposal ground | A. nomius | 4.9 | Determination of Dry Weight of Residual LDPE, AFM, GC-MS and FTIR | [44] |
| LDPE |  | Landfill soil | T. viride | 5.13 | Weight loss and tensile strength analyses | [17] |
| LDPE |  | Sewage disposal ground | Streptomyces sp., | 5.2 | Determination of dry weight of residual LDPE, AFM, GC-MS and FTIR | [44] |
| HDPE |  | Marine environmental site dumped with plastic waste | A. tubingensis | 6.02±0.2 | dry weight of the residual HDPE, FTIR analysis, fungal cell surface hydrophobicity and SEM analysis | [42] |
| LDPE |  | Landfill soil | A. nomius | 6.63 | Weight loss and tensile strength analyses | [17] |
| HDPE |  | Marine environmental site dumped with plastic waste | A. flavus | 8.51±0.1 | Dry weight of the residual HDPE, FTIR analysis, fungal cell surface hydrophobicity and SEM analysis | [42] |
| PU |  | Solid waste-dumping site | Aspergillus sp. | 15–20 | SEM, FTIR and DSC measurement | [45] |
| PE |  | Seawater | A. niger | 19.5 | Weight loss, tensile Strength, SEM and FTIR | [18] |
| PE |  | Seawater | A. fumigatus | 20.5 | Weight loss, tensile Strength, SEM and FTIR | [18] |
| PE |  | Seawater | A. terreus | 21.8 | Weight loss, tensile Strength, SEM and FTIR | [18] |
| PET | 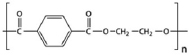 | Solid waste litter site | Aspergillus sp. | 22 | Weight loss, FTIR, SEM and XRD | [46] |
| LDPE |  | Landfill soil | A. clavatus | 35 | Weight loss, CO2 evolution measured by Strum test, infrared spectra and morphological changes measured by SEM and AFM analysis | [16] |
| PE |  | Mangrove Dumpsite | A. sydowii | 37.94 | Weight loss, tensile strength, SEM and FTIR | [41] |
| PE | 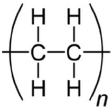 | Dumpsite soil | T. harzianum | 40. | SEM, FTIR, NMR analyses and enzymatic assay | [47] |
| PE | 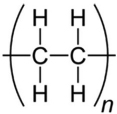 | Mangrove Dumpsite | A. terreus | 41.82 | Weight loss, tensile Strength, SEM and FTIR | [41] |
| LDPE |  | Sea water samples | Penicillium sp. | 43.4 | Weight reduction and SEM | [18] |
| LDPE |  | Culture Collection of the Institute of Excellence in Fungal Research | D. italiana | 43.90 | Weight loss, tensile strength, FTIR, SEM and GC-MS | [48] |
| LDPE |  | Culture Collection of the Institute of Excellence in Fungal Research | S. citrulli | 45.12 | Weight loss, tensile strength, FTIR, SEM and GC-MS | [48] |
| LDPE |  | Culture Collection of the Institute of Excellence in Fungal Research | T. jaczewskii | 46.34 | Weight loss, tensile strength, FTIR, SEM and GC-MS | [48] |
| LDPE | 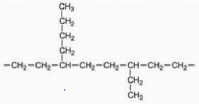 | Culture Collection of the Institute of Excellence in Fungal Research | C. fructicola | 48.78 | Weight loss, tensile strength, FTIR, SEM and GC-MS | [48] |
PS: Polystyrene, PE: Polyethylene, LDPE: Low-density PE, PET: PE terephthalate, HDPE: High-density PE, PU: Polyurethane, NCIM: National Collection of Industrial Microorganism, FTIR: Fourier-transform infrared spectroscopy, SEM: Scanning electron microscopy, GC-MS: Gas chromatography mass spectrometry, AFM: Atomic force microscopy, DSC: Differential scanning calorimetry, GPC: Gel-permeation chromatography, A. nomius: Aspergillus nomius, T. viride: Trichoderma viride, A. tubingensis: Aspergillus tubingensis, A. flavus: Aspergillus flavus, A. niger: Aspergillus niger, A. fumigatus: Aspergillus fumigatus, A. terreus: Aspergillus terreus, A. clavatus: Aspergillus clavatus, A. sydowii: Aspergillus sydowii, T. harzianum: Trichoderma harzianum, D. italiana: Diaporthe italiana, S. citrulli: Stagonosporopsis citrulli, T. jaczewskii: Thyrostroma jaczewskii, C. fructicola: Colletotrichum fructicola, TGA–DTG: Thermogravimetric analysis-derivative thermogravimetry, NRM: Nuclear magnetic resonance, XRD: X-ray diffraction.
4. ASSESSMENT TECHNIQUES
To measure the degree of plastic biodegradation, a variety of assessment techniques have been used. Previously, the gravimetric assessment of polymer weight/mass loss over time when exposed to cultured microbes was the most widely used method for assessing plastic biodegradation [49,50]. Although nowadays, various new assessment methodologies are currently being employed to assess the level of plastic biodegradation. Scanning electron microscopy (SEM), which creates a surface image by illuminating a surface with a high-intensity electron beam and scanning across it. High magnification and hence good resolution are provided by SEM at the nanoscale range. SEM observations are utilized to study and evaluate the colonization of plastic films or particles by microorganisms and at the same time visualizing fractures, trenches, and deformations on the plastic surface [51,52], Which can indicate if the polymer is damaged. SEM has been used in a number of studies to examine fungi on polymers. SEM is a fast technique for observing surface attachment and morphological microstructures [38]. Atomic force microscopy (AFM) is another technique that can be used to identify surface alteration of polymers during degradation [53]. Using this method, topographical changes at the polymer surface, such as the emergence of pits and crevices, the adhesion of microbes to the polymer surface, and an increase in surface roughness, can be directly observed [54].
Fourier-transform infrared spectroscopy (FTIR) is used to identify functional groups contained in polymer films. FTIR spectrum detects and semi-quantifies changes in initial polymer arrangement, such as the addition of carbonyl groups during oxidation [55,56]. The crystallinity of polymer films was measured using X-ray diffraction examination. It was carried out using an X-ray diffractometer. By examining the diffraction patterns produced by polymer films, the structure of such films was discovered [57]. Due to their high water content, hydrophilic surfaces have higher surface energies and yield smaller contact angles with water. As a result, polar functional groups that develop in polymers as a result of environmental degradation cause the contact angle to decrease. The rate of disintegration is further accelerated by increased hydrophilicity because it encourages microbe adhesion to the polymer surface [58]. Differential scanning calorimetry is a method for carrying out thermal analysis (DSC). Its analysis provides capacity to track phase transitions, solid state transformations, and thermodynamic parameters during controlled sample heating and cooling. DSC analysis can be used to measure a variety of characteristics, including the glass transition temperature, crystallization temperature, melting temperature, polymer crystallinity percentage, specific heat capacity, transformation enthalpy, and many others [59]. Evolution of CO2 is typically taken into account as a sign of biological decay [14,56]. By monitoring the CO2 released during biotic or abiotic mineralization in a controlled setting, the rate of polymer degradation can be estimated [61-63].
The oligomeric fraction produced during polymer breakdown or the detection of low-molecular-weight metabolites can also be studied using gas chromatography (GC) with flame ionization detection (GC-FID) or mass spectrometry (GC-MS) [64-66]. Another chromatographic method utilized for the examination of complex oligomeric mixtures created during biodegradation is LC-MS [66]. Gel-permeation chromatography (GPC) is used to quantify molar mass and molecular weight shifts [67,68]. High-performance liquid chromatography (HPLC) is also used to identify certain homologues of low-molecular-weight polymers [69].
5. APPLICATIONS
In general, fungi are more effective in degrading polymers than bacteria because they can stick to the hydrophobic surfaces of polymers, produce extracellular enzymes that target insoluble fibers, and endure challenging growth environments [24,70,71]. Fungi produce a wide range of enzymes that have the potential to break down the chemical bonds of the plastic polymers [72]. According to a study, enzymes called laccases from fungi like Aspergillus flavus and Pleurotus ostreatus significantly degraded polyethylene [73,74]. According to another study, PS can be broken down by an extracellular esterase from Lentinus tigrinus [75]. Serine and cysteine hydrolase from Pestalotiopsis microspore was shown in another investigation to be active in degrading PU [76]. The breakdown of lignin is frequently correlated with the enzymes manganese peroxidase (MnP) and lignin peroxidase (LiP) [77]. Several compounds converted into oxidized or polymerized products by these enzymes, which catalyze oxidation-reduction reactions [78]. Numerous fungi species, including Humicola insolens, Penicillium citrinum, and Fusarium oxysporum, produce enzymes such as polyesterase, cutinase, and hydrolase that are effective PET degraders [79-81]. Another study showed that F. solani was converted to terephthalic acid (TPA) from a hydrolyzed PET collection through a cutinase [82].
6. CONCLUSION
Several fungal microbes have the ability to breakdown the plastic polymer under the right conditions. The capacity of various fungal species can cause chemical and physical changes in various plastic polymers such as LDPE, HDPE, PET, PS, and others. Biodeterioration (Adhesion and colonization of microbes on the surface), biofragmentation (fragmentation of plastic polymer into monomer), assimilation (transport into microbial cytoplasm), and mineralization (enzymatic reactions that result in the breakdown into various oxidized metabolites such as CO2, N2, CH4, and H2O) are the four stages of biodegradation of plastic by fungal strains. Microbiological activities that use enzymes including peroxidases, lipases, esterase, cutinase, and laccase to catalyze plastic breakdown are growing as an environmental sustainable alternative to physicochemical depolymerization. Biodegradation, on the other hand, has a significant downside in terms of rate of degradation. Future research will put a lot of emphasis on the biological systems or biodegradation mechanism that are accountable for the detected chemical and physical change. With more efficient degradation on plastic polymer, it is important to identify novel fungal strains. The primary goal of upcoming study will be to understand and identify the biological systems that appear to be causing the apparent chemical and physical damage. Only few enzymes of a fungal origin have been associated to polymer breakdown thus far. However, these enzymes’ biochemical and structural features have received little attention. These details are necessary to comprehend the mechanisms underlying the biodegradation of resistant polymers. This knowledge will be useful for creating novel biodegradable plastic polymers, designing microbial cell factories with improved breakdown efficiency, and genetically altering enzymes through protein engineering. The goal is to isolate and discover potent microbial consortia directly from plastic contamination locations, as well as purify potent microbial enzymes for commercial usage and increase catalytic activity through genetic engineering.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
All authors declare that they have no conflict of interest.
10. ETHICAL APPROVALS
This article does not contain any studies with human participants or animals performed by any of the author.
11. DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wayman C, Niemann H. The fate of plastic in the ocean environment-a minireview. Environ Sci Processes Impacts 2021;23:198-212. [CrossRef]
2. Gourmelon G. Global plastic production rises, recycling lags. Vital Signs 2015;22:91-5.
3. Kumari NA, Kumari P, Murthy NS. A novel mathematical approach for optimization of plastic degradation. Int J Eng Trends Technol 2013;4:3539-42.
4. Cowan AR, Costanzo CM, Benham R, Loveridge EJ, Moody SC. Fungal bioremediation of polyethylene:Challenges and perspectives. J Appl Microbiol 2022;132:78-89. [CrossRef]
5. Feil A, Pretz T. Mechanical recycling of packaging waste. In:Letcher T, editor. Plastic Waste and Recycling. Amsterdam:Elsevier;2020. 283-319. [CrossRef]
6. Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv 2017;3:e1700782. [CrossRef]
7. Proshad R, Kormoker T, Islam MS, Haque MA, Rahman MM, Mithu MM. Toxic effects of plastic on human health and environment:A consequences of health risk assessment in Bangladesh. Int J Health 2018;6:1-5. [CrossRef]
8. Galloway T, Haward M, Mason SA, Babayemi JO, Hardesty BD, Krause S,
9. Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut 2019;254:112983. [CrossRef]
10. Stapleton PA. Toxicological considerations of nano-sized plastics. AIMS Environ Sci 2019;6:367-78. [CrossRef]
11. Mercogliano R, Avio CG, Regoli F, Anastasio A, Colavita G, Santonicola S. Occurrence of microplastics in commercial seafood under the perspective of the human food chain. A review. J Agric Food Chem 2020;68:5296-301. [CrossRef]
12. Eriksson O, Finnveden G. Plastic waste as a fuel-CO 2-neutral or not?Energy Environ Sci 2009;2:907-14. [CrossRef]
13. Srikanth M, Sandeep TS, Sucharitha K, Godi S. Biodegradation of plastic polymers by fungi:A brief review. Bioresour Bioprocess 2022;9:42. [CrossRef]
14. Pathak VM, Navneet. Review on the current status of polymer degradation:A microbial approach. Bioresour Bioprocess 2017;4:15. [CrossRef]
15. Peay KG, Kennedy PG, Talbot JM. Dimensions of biodiversity in the Earth mycobiome. Nat Rev Microbiol 2016;14:434-47. [CrossRef]
16. Gajendiran A, Krishnamoorthy S, Abraham J. Microbial degradation of low-density polyethylene (LDPE) by
17. Munir E, Harefa RS, Priyani N, Suryanto D. Plastic degrading fungi
18. Alshehrei F. Biodegradation of low density polyethylene by fungi isolated from Red Sea water. Int J Curr Microbiol Appl Sci 2017;6:1703-9. [CrossRef]
19. Chiellini E, Solaro R. Biodegradable polymeric materials. Adv Mater 1996;8:305-13. [CrossRef]
20. Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics:A comprehensive review. Biotechnol Adv 2008;26:246-65. [CrossRef]
21. Amobonye A, Bhagwat P, Singh S, Pillai S. Plastic biodegradation:Frontline microbes and their enzymes. Sci Total Environ 2021;759:143536. [CrossRef]
22. Harms H, Schlosser D, Wick LY. Untapped potential:Exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol 2011;9:177-92. [CrossRef]
23. Gu JD. Microbiological deterioration and degradation of synthetic polymeric materials:Recent research advances. Int Biodeterioration Biodegradation 2003;52:69-91. [CrossRef]
24. Anjana K, Hinduja M, Sujitha K, Dharani G. Review on plastic wastes in marine environment-biodegradation and biotechnological solutions. Mar Pollut Bull 2020;150:110733. [CrossRef]
25. Nauendorf A, Krause S, Bigalke NK, Gorb EV, Gorb SN, Haeckel M,
26. Jenkins S, Quer AM, Fonseca C, Varrone C. Microbial degradation of plastics:New plastic degraders, mixed cultures and engineering strategies. In:Soil Microenvironment for Bioremediation and Polymer Production. New Delhi:Whiley;2019. 213-38. [CrossRef]
27. Marjayandari L, Shovitri M. Potential bacteria of
28. Shahnawaz M, Sangale MK, Ade AB. Analysis of the plastic degradation products. In:Bioremediation Technology for Plastic Waste. Singapore:Springer;2019. 93-101. [CrossRef]
29. Ho BT, Roberts TK, Lucas S. An overview on biodegradation of polystyrene and modified polystyrene:The microbial approach. Crit Rev Biotechnol 2018;38:308-20. [CrossRef]
30. Alshehrei F. Biodegradation of synthetic and natural plastic by microorganisms. J Appl Environ Microbiol 2017;5:8-19.
31. Heitman J, Howlett BJ, Crous PW, Stukenbrock EH, James TY, Gow NA. The Fungal Kingdom. United States:John Wiley and Sons;2020.
32. Boyce KJ, Andrianopoulos A. Fungal dimorphism:The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev 2015;39:797-811. [CrossRef]
33. Watkinson SC. Mutualistic symbiosis between fungi and autotrophs. In:The Fungi. Cambridge:Academic Press;2016. 205-43. [CrossRef]
34. Szabo LJ, Bushnell WR. Hidden robbers:The role of fungal haustoria in parasitism of plants. Proc Natl Acad Sci 2001;98:7654-5. [CrossRef]
35. Raghukumar S. The marine environment and the role of fungi. In:Fungi in Coastal and Oceanic Marine Ecosystems. Champaign:Springer;2017. 17-38. [CrossRef]
36. Walker AK, Vélez P, González MC. Marine fungi. eLS. 2017:1-6.
37. Gladfelter AS, James TY, Amend AS. Marine fungi. Curr Biol 2019;29:R191-5. [CrossRef]
38. Ojha N, Pradhan N, Singh S, Barla A, Shrivastava A, Khatua P,
39. Okamoto K, Izawa M, Yanase H. Isolation and application of a styrene-degrading strain of
40. Agrawal P, Singh RK. Breaking down of polyethylene by
41. Sangale MK, Shahnawaz M, Ade AB. Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci Rep 2019;9:5390. [CrossRef]
42. Devi RS, Kannan VR, Nivas D, Kannan K, Chandru S, Antony AR. Biodegradation of HDPE by
43. Chaudhary AK, Vijayakumar RP. Studies on biological degradation of polystyrene by pure fungal cultures. Environ Dev Sustainability 2020;22:4495-508. [CrossRef]
44. Abraham J, Ghosh E, Mukherjee P, Gajendiran A. Microbial degradation of low density polyethylene. Environ Progress Sustainable Energy 2017;36:147-54. [CrossRef]
45. Osman M, Satti SM, Luqman A, Hasan F, Shah Z, Shah AA. Degradation of polyester polyurethane by
46. Sarkhel R, Sengupta S, Das P, Bhowal A. Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J Polym Res 2020;27:1-8. [CrossRef]
47. Sowmya HV, Krishnappa M, Thippeswamy B. Degradation of polyethylene by
48. Khruengsai S, Sripahco T, Pripdeevech P. Low-density polyethylene film biodegradation potential by fungal species from Thailand. J Fungi (Basel) 2021;7:594. [CrossRef]
49. Syranidou E, Karkanorachaki K, Amorotti F, Franchini M, Repouskou E, Kaliva M,
50. Welden NA, Cowie PR. Degradation of common polymer ropes in a sublittoral marine environment. Mar Pollut Bull 2017;118:248-53. [CrossRef]
51. Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the “plastisphere“:Microbial communities on plastic marine debris. Environ Sci Technol 2013;47:7137-46. [CrossRef]
52. Vaksmaa A, Hernando-Morales V, Zeghal E, Niemann H. Microbial degradation of marine plastics:Current state and future prospects. Biotechnol Sustainable Environ 2021;111-54. [CrossRef]
53. Araújo MA, Cunha AM, Mota M. Changes on surface morphology of corn starch blend films. J Biomed Mater Res A 2010;94:720-9.
54. Müller RJ. Biodegradability of polymers:Regulations and methods for testing. In:Steinbüchel A, editor. Biopolymers Online. Weinheim, Germany:Wiley-VCH;2005.
55. Xu JL, Thomas KV, Luo Z, Gowen AA. FTIR and Raman imaging for microplastics analysis:State of the art, challenges and prospects. TrAC Trends Anal Chem 2019;119:115629. [CrossRef]
56. Almond J, Sugumaar P, Wenzel MN, Hill G, Wallis C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e Polymers 2020;20:369-81. [CrossRef]
57. Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium
58. Suresh B, Maruthamuthu S, Kannan M, Chandramohan A. Mechanical and surface properties of low-density polyethylene film modified by photo-oxidation. Polym J 2011;43:398-406. [CrossRef]
59. Kodre KV, Attarde SR, Yendhe PR, Patil RY, Barge VU. Differential scanning calorimetry:A review. J Pharm Anal 2014;3:11-22.
60. Harshvardhan K, Jha B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar Pollut Bull 2013;77:100-6. [CrossRef]
61. De Wilde B. Biodegradation testing protocols. In:Khemani K, Scholz C, editors. Degradable Polymers and Materials:Principles and Practice. ACS Symposium Series 1114. 2nd ed. Washington, DC:American Chemical Society;2012. 33-43. [CrossRef]
62. Strotmann U, Reuschenbach P, Schwarz H, Pagga U. Development and evaluation of an online CO2 evolution test and a multicomponent biodegradation test system. Appl Environ Microbiol 2004;70:4621-8. [CrossRef]
63. Yabannavar AV, Bartha R. Methods for assessment of biodegradability of plastic films in soil. Appl Environ Microbiol 1994;60:3608-14. [CrossRef]
64. D?ímal P, Hrn?i?ík J, Hoffmann J. Assessing aerobic biodegradability of plastics in aqueous environment by GC-analyzing composition of equilibrium gaseous phase. J Polym Environ 2006;14:309-16. [CrossRef]
65. D?ímal P, Hoffmann J, Družbík M. Evaluating the aerobic biodegradability of plastics in soil environments through GC and IR analysis of gaseous phase. Polym Test 2007;26:729-41. [CrossRef]
66. Eyheraguibel B, Traikia M, Fontanella S, Sancelme M, Bonhomme S, Fromageot D,
67. Eubeler JP, Zok S, Bernhard M, Knepper TP. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. TrAC Trends Anal Chem 2009;28:1057-72. [CrossRef]
68. Trimpin S, Eichhorn P, Räder HJ, Müllen K, Knepper TP. Recalcitrance of poly (vinylpyrrolidone):Evidence through matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. J Chromatogr A 2001;938:67-77. [CrossRef]
69. Zgo?a-Grze?kowiak A, Grze?kowiak T, Zembrzuska J, ?ukaszewski Z. Comparison of biodegradation of poly (ethylene glycol) s and poly (propylene glycol) s. Chemosphere 2006;64:803-9. [CrossRef]
70. Seneviratne G, Tennakoon NS, Weerasekara ML, Nandasena KA. Polyethylene biodegradation by a developed
71. Sen SK, Raut S. Microbial degradation of low density polyethylene (LDPE):A review. J Environ Chem Eng 2015;3:462-73. [CrossRef]
72. Zeghal E, Vaksmaa A, Vielfaure H, Boekhout T, Niemann H. The potential role of marine fungi in plastic degradation-a review. Front Mar Sci 2021;8:1-17. [CrossRef]
73. Zhang J, Gao D, Li Q, Zhao Y, Li L, Lin H,
74. Gómez-Méndez LD, Moreno-Bayona DA, Poutou-Pinales RA, Salcedo-Reyes JC, Pedroza-Rodríguez AM, Vargas A,
75. Tahir L, Ali MI, Zia M, Atiq N, Hasan F, Ahmed S. Production and characterization of Esterase in
76. Russell JR, Huang J, Anand P, Kucera K, Sandoval AG, Dantzler KW,
77. Xu JZ, Zhang JL, Hu KH, Zhang WG. The relationship between lignin peroxidase and manganese peroxidase production capacities and cultivation periods of mushrooms. Microb Biotechnol 2013;6:241-7. [CrossRef]
78. Wei R, Zimmermann W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics:How far are we?Microb Biotechnol 2017;10:1308-22. [CrossRef]
79. Da Costa AM, Lopes VR, Vidal L, Nicaud JM, De Castro AM, Coelho MA. Poly (ethylene terephthalate) (PET) degradation by
80. Kawai F, Kawabata T, Oda M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl Microbiol Biotechnol 2019;103:4253-68. [CrossRef]
81. Palm GJ, Reisky L, Böttcher D, Müller H, Michels EA, Walczak MC,
82. Ronkvist ÅM, Xie W, Lu W, Gross RA. Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules 2009;42:5128-38. https://doi.org/10.1021/ma9005318