1. INTRODUCTION
Cancer constitutes a growth and spread of abnormal cells which enable to invade most parts of organs. It is caused by accumulated abnormalities in cells due to deoxyribonucleic acid (DNA) mutation. Its presence can be recognized by changes in properties of normal cells such as more aggressive, growing and dividing unregularly since these cells are able to respond to their own growth signals. Cancer cells could penetrate and invade their surrounding normal tissues, then eventually spreading throughout the body through vascular and lymphatic system called as metastasis [1].
Cancer treatments have gained importance in response to the increasing prevalence over the world. The most common cancer treatments included surgery, radiation, and chemotherapy. The surgery is ineffective for removing cancer cells that are at metastatic stage, while radiation is often ineffective and unsafe to normal cells. In addition, chemotherapy was reported to have less optimum effects as the drugs used did not destroy specific targets [2]. Till date, the cancer drug that is completely effective is not found, which may be linked with a low selectivity of cancer drugs and unclear mechanism of carcinogenesis. The carcinogenesis consists of the following stages, that is, initiation, promotion, progression, and metastasis. Initiation stage begins with the existence of initiator compounds which induce destruction and mutation of DNA. At this stage, histological changes do not occur, but the cell necrosis increases with elevating cell proliferation. At promotion stage, these initiated cells become premalignant stimulated by other promoters, thereby increasing gene expression, cell proliferation, and generating abnormal histology at the end of phase. After this stage, the cells progressively become malignant that invades the surrounding tissues, leading to angiogenesis and metastasis [1].
Unfortunately, cancer disease is commonly recognizable after progression stage, which makes it difficult to remove. Cancer treatments must be carried out at all stages, enabling to reduce the growth of tumor cells and progressive cancer cells. Therefore, the development of cancer therapy should consider the preventive action using plant source which allows regulating cancer cells selectively and effectively without generating detrimental effects on normal cells. Exploration and use of anticarcinogen-promoting herbal materials are necessary to prevent and inhibit the growth of cancer cells. Herbal-based treatment was reported to have advantageous properties such as safe, less harmful, and non-addictive [3]. The herbal material was not only beneficial for anticancer treatment but also related to exploration of bioactive compounds for developing new drugs [4].
As widely known, marine resources including its biota are rich in potential compounds being investigated by research studies for the treatment of various diseases. Marine sponge Neopetrosia chaliniformis, an endemic sponge found along the Indonesian coastline, was reported to show cytotoxicity activity against WiDr cell line [5], while bacteria Lactobacilli was also found capable of lowering the induction of gut cancer [6]. In addition, microalgae Scytonemin demonstrated inhibitory activity of protein kinase against antiproliferative and anti-inflammatory activity [7] and also showed antioxidant, antitumor, and immunomodulatory properties [8].
Furthermore, brown algae Padina australis were also reported to have anticancer-promoting activity [9]. The algae belong to class Heterokontophyta and a member of Phaeophyceae. Brown algae P. australis contained alginate and iodine useful for industries such as food, pharmacy, cosmetics, and textile [10]. In general, the brown algae contained polyphenol, carotene, polysaccharide sulfate, omega-3, fatty acid, and anthocyanin. Carotenoid compounds could induce apoptosis on the living cells, and the presence of steroid compounds was also responsible for cytotoxic activity [9]. However, studies pertaining to cytotoxicity and antiproliferative activity of P. australis are rather scarce.
Therefore, this present study aimed to investigate the cytotoxicity of P. australis water and ethanol 96% extract, as well as its n-hexane, ethyl acetate, and ethanol fraction against Artemia salina and evaluate its antiproliferative activity against MCM-B2 and K562 cells. The water and ethanol extract of P. australis were expected to have cytotoxicity against A. salina and show antiproliferative effect.
2. MATERIALS AND METHODS
2.1. Plants Materials
The fresh brown algae P. australis were collected from Pari Island, Kepulauan Seribu, Jakarta, Indonesia, on September 2017.
2.2. Extraction
Brown algae P. australis were sundried for 3 days and further oven dried at 50°C for 2 days. The dried algae were powdered using electrical blender and extracted using water (using reflux method) and ethanol 96% (using maceration). The water and ethanol extracts were then fractioned using liquid-liquid fractionation with various solvents, that is, n-hexane, ethyl acetate, and ethanol. The resulting extracts and fractions were evaporated using rotary evaporator.
2.3. Brine Shrimp Lethality Test (BSLT)
BSLT was carried out to investigate cytotoxic activity of the extracts and fractions. Brine shrimp (A. salina) larvae were placed and hatched for 48 h with constant oxygen supply and under the light at room temperature (25°C). The extract (10 mg) was transferred into a test tube containing 50 μl Tween 80 in 10 ml of seawater to produce stock solution (1000 ppm). The concentration of extracts was 10 ppm, 100 ppm, 500 ppm, and 1000 ppm, and experiment for each extract was carried out at triplicates. The surviving larvae were counted after incubation under TL light 14 Watt for 24 h. The similar procedure (without adding extracts) was applied as the negative control. The larvae with no movements during 10 seconds of observation were considered dead, while toxicity against larvae was set at lethal concentration 50 (LC50) <1000 ppm [11]. LC50 was determined using SPSS 24.0 for probit analysis at confidence interval of 95%.
2.4. Antioxidant Activity Assay
Antioxidant activity was tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. This method considers the capability of sample to reduce stable free radical DPPH. The extract (1 mg) and butylated hydroxyl toluene as the positive control were weighed and added with ethanol at 1:1000. DPPH (1.3 g) was dissolved using 25 ml ethanol. Ethanol (1 μl) was pipetted into microwell plate, followed by the extracts and DPPH. The mixture was homogenized and incubated at 37°C for 30 min, then analyzed for absorbance using spectrophotometer at wavelength of 517 nm. Percentage of free radical activity inhibition was calculated from the absorbance values. Regression equation was established from the correlation of sample concentration and inhibitory activity. The concentration for inhibitory activity of 50% (IC50) was determined using regression. The IC50 value was obtained at y = 50 with known value of A and B. The axis (x) as IC50 was determined using following equation: y = A + B Ln (x) [12].
2.5. Antiproliferative Activity Assay
The antiproliferative activity was determined using an in vitro protocol [13]. In vitro experiment was arranged using MCM-B2 and K562 cancer cell lines obtained from laboratory of tissue culture, Department of Veterinary Clinic, Reproduction, and Pathology, Faculty of Veterinary Medicine, Bogor Agricultural University, Indonesia. The cell lines were cultured in the 24-well microplates at density of 7 × 10
2.6. Statistical Analysis
Differences were determined using ANOVA one-way with the Tukey test. P = 0.05 or less was reported as statistically significant.
3. RESULTS AND DISCUSSION
BSLT was commonly used to assess bioactivity of an extract. In this current work, BSLT was applied to evaluate toxicity of P. australis extracts that potentially showed antiproliferative activity against cancer cells, allowing it as a promising candidate for ingredient of anticancer drugs [14]. Our experiment compared the mortality of A. salina larvae and further investigated the inhibitory activity against nasofaring carcinoma, and we found a positive correlation of the extract toxicity to the both studied tests.
BSLT experiment observed the mortality of A. salina larvae after exposed to the extract during incubation for 24 h. The result was expressed as LC50 referring to the required concentration of the extract to reduce 50% population of A. salina larvae. An extract is regarded as “active” and “toxic” when having LC50 of <1000 ppm and vice versa [11].
As presented in Table 1, water and ethanol extracts of P. australis were found to be non-toxic over A. salina larvae, as indicated by LC50 >1000 ppm. Both extracts were considered not having anticancer properties and were not used in the next stage. This is in accordance with the result reported by Francisco and Uy [15] finding that crude ethanol and water (polar solvents) extracts had LC50 >1000 ppm over A. salina larvae, which means that they are not toxic.
The results exhibited that ethyl acetate fraction of water extract showed the lowest LC50, that is, 200.53 ppm [Table 1]. This suggested that the ethyl acetate fraction of water extract possessed toxicity against A. salina larvae, indicating that the fraction showed anticancer properties. Furthermore, this fraction presumably contains the best secondary metabolite. Nursid et al. [16] also studied cytotoxicity of brown algae (obtained from Binuangeun Beach, Banten) extracts using n-hexane, ethyl acetate, and ethanol and found that ethyl acetate extract demonstrated the best cytotoxicity due to the presence of fucoxanthin as an anticancer compound.
The toxicity of ethyl acetate fraction of water extract of P. australis related to the presence of secondary metabolites, at a particular level resulting in acute toxicity and mortality over A. salina larvae. The mortality of A. salina larvae was associated with the role of bioactive compounds through decreasing the feeding activity (antifeedant) of the larvae. Maharany et al. [17] reported that phytochemicals in P. australis consisted of flavonoid, phenol, hydroquinone, triterpenoid, tannin, and saponin, in which these compounds induced mortality on A. salina larvae through stomach poisoning.
The result of antioxidant activity test showed that ethyl acetate P. australis fraction had IC50 of 113.37 ppm. This value was much lower than that reported by Setha et al. [18] finding that IC50 of ethyl acetate Padina sp. extract was 483.09 ppm. Murugan et al. [19] reported that screening of 10 green algae species (Chlorophyta) and 25 brown algae species (Phaeophyta) yielded IC50 of 1.63–530.14 ppm. Nagarajan and Mathaiyan [20] also found that, in general, cytotoxicity and antioxidant activity (IC50) of brown algae were relatively low. Antioxidant activity of ethyl acetate P. australis fraction was associated with the presence of phlorotannin, a member of phenolic compounds [21]. The antioxidant properties of polyphenolic compound were attributed to the reducing reaction by hydrogen donors, while phlorotannin was capable of scavenging free radicals stronger than other phenolic compounds.
P. australis constitutes shallow water algae found in Pari Island of Kepulauan Seribu, enabling it to be exposed to ultraviolet (UV) light from the sun. Sunlight exposure leads to the formation of free radicals or reactive oxygen species (ROS) which cause cellular biomolecule oxidation, contributing to cell death. Even though exposed to harmful ROS, the structural components (such as fatty acid) of the algae were unaltered due to oxidative reaction. This displays protective properties in cellular system of the algae against oxidative stress. The cells donated an electron and antioxidant compounds enabled to neutralize free radicals and ROS [22]. The seaweed can synthesize bioactive compounds that may serve as antioxidant agent for its defensive system against free radicals. As described by Budhiyanti et al. [23], seaweed is capable of adapting to UV light from the sun.
Antiproliferative activity of ethyl acetate P. australis fraction was tested against MCM-B2 (canine benign mammary gland mixed tumor) and K562 (chronic myelogenous leukemia) cell line in vitro, as depicted in Figures 1 and 2. The growth of MCM-B2 and K562 cell line decreased as the higher concentration of the studied fraction was added. Nursid et al. [16] reported that higher mortality of HeLa and T47D cells was found with increasing dose of acetone Sargassum sp. extract. In our experiment, even though at the lowest concentration (50 ppm), ethyl acetate fraction could demonstrate the inhibition of both cancer cells.
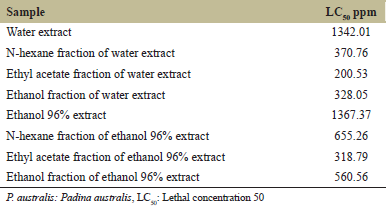 | Table 1: Cytotoxic activity of P. australis extracts and fractions against brine shrimps [Click here to view] |
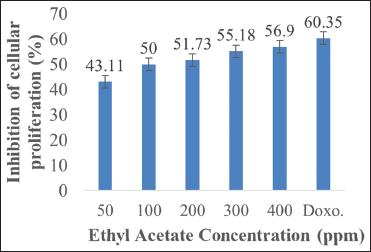 | Figure 1: Inhibitory activity of MCM-B2 cell proliferation by ethyl acetate Padina australis fraction. The data are expressed as percentage of proliferation inhibition, as compared to the negative control (100%). Level of significance is denoted as follows: P < 0.05. [Click here to view] |
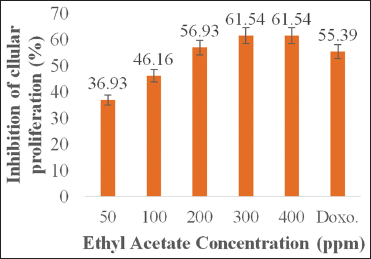 | Figure 2: Inhibitory activity of K562 cell proliferation by ethyl acetate Padina australis fraction. The data are expressed as percentage of proliferation inhibition, as compared to the negative control (100 %). Level of significance is denoted as follows: P < 0.05. [Click here to view] |
Ethyl acetate P. australis fraction at a concentration of 400 ppm showed optimum inhibition of MCM-B2 and K562 cell line. Interestingly, the fraction demonstrated dissimilar effects on both cancer cells. Antiproliferative effect of the fraction on K562 cell (61.54%) was higher than that of the positive control doxorubicin (55.39%) while its effect on MCM-B2 (55.18%) was lower than that of the positive control doxorubicin (60.35%). The previous study revealed that ethanol P. australis extract at concentration of 2.84, 5.69, 11.37, and 22.75 mM inhibited growth of H1299 cells after 48 h of incubation, resulting in IC50 of 2.45 mM [24]. Furthermore, methanol P. australis extract exhibited cytotoxic activity at IC50 of 86.45 μg/ml and 74.59 μg/ml against HeLa (cervical cancer cell line) and MDA-MB-453 (breast cancer cell line), respectively [25]. Dichloromethane P. australis extract at concentration of 8.2 ± 0.4 μg/ml also showed effective anticancer activity against HEp-2 (laryngeal epidermal carcinoma) cells [26], while ethanol Padina gymnospora extract at dose of 15.0 ± 2.8 μg/ml could show effective cytotoxicity against NCI-H292 (human lung mucoepidermal carcinoma) cells [26]. As previously stated by Priosoeryanto et al. [27], anticancer activity of plant extracts could be different, depending on types of cancer cells used.
Cancer cell K562 is a blast cell that has very low cell differentiation characteristics (highly undifferentiated) in granulocytic cells and is difficult to mature into functional cells [28]. [29] reported that MCM-B2 cancer-sustaining cells may originate from stem cells or atypical cells so that the cells have not been differentiated. The results of in vitro observations show that MCM-B2 cells have homogeneous morphology and immunohistochemistry. MCM-B2 cell differentiation in vivo can occur in several ways, but its differentiation is low in cell culture in vitro [30]. The characteristics of cancer cells MCM-B2 and K562 are suspected to be the cause of inhibition of cell growth, so the number of cells each decreased after inhibited cell proliferation in vitro using ethyl acetate fraction of water extract P. australis.
Percentage inhibition data of cancer cell proliferation of MCM-B2 and K562 were further analyzed to know which treatment concentration was significantly different statistically using Tukey’s advanced test. The result of post hoc statistical analysis test of Tukey at 95% confidence level in Table 2 shows that inhibition activity of cancer cells MCM-B2 and K562 by ethyl acetate fraction at concentration of 300 ppm and 400 ppm was not significantly different (statistically) where P > 0.05. The results of this study indicate that there are differences in antiproliferation activity of extracts on both types of sustainable cells. This is accordingly reported by Priosoeryanto et al. [13] that the secondary metabolite compounds contained in seaweed have different anticancer potentials depending on the type of cancer cell. This is presumably because each sustainable cell has a different response to the extract of a plant associated with cellular structures and components as well as the mechanisms of cell metabolism [31]. Other assumptions that result in the existence of differences in antiproliferation activity are the difference in the number of receptors P. australis compounds in cancer cells. It is suspected that the K562 cancer cell has more number of receptors than MCM-B2, so the ethyl acetate fraction is more tied to K562 receptor. This causes increased antiproliferative activity in cancer cells K562, so the number of cancer cells K562 more decreased.
Budhiyanti et al. [23] reported that bioactive compounds in P. australis responsible for anticancer activity were fucoxanthin. This compound shows a strong antioxidant activity, which makes it possible as anticancer agent. However, fucoxanthin may target the specific cells and inhibit cancer cells through several mechanisms. It plays a role in cancer proliferation pathway, retards cellular cycles, induces apoptosis, and inhibits angiogenesis [32]. Gunasekaran et al. [33] also found that bioactive compounds in brown macroalgae extract were able to induce cell shrinkage, plasma membrane deterioration, and cellular apoptosis. The results of this study successfully revealed that P. australis contained anticancer compounds, and further study was needed to develop it as anticancer drug.
 | Table 2: Inhibitory activity of cancer cells MCM‑B2 and K562 effects of the ethyl acetate fraction of P. australis [Click here to view] |
4. CONCLUSION
The ethyl acetate fraction of water P. australis extract has cytotoxic activity against shrimp larvae of A. salina at LC50 of 200.53 ppm and antioxidant activity against DPPH at IC50 of 113.370 ppm. The ethyl acetate fraction of the water extract P. australis also has antiproliferation activity against the growth of cell MCM-B2 and cell K562 in vitro. The highest inhibitory activity was achieved in the treatment of ethyl acetate fraction of water P. australis extract at a concentration of 300 ppm, that is, 61.54% on cell K562 and at a concentration of 400 ppm, that is, 56.90% on cell MCM-B2. Thus, it was suggested that brown algae P. australis had a potent natural anticancer activity.
5. ACKNOWLEDGMENTS
The authors are thankful to the Indonesia Endowment Fund for Education (LPDP) Scholarship from Ministry of Finance of the Republic of Indonesia for financial support and Faculty of Veterinary Medicine, Bogor Agricultural University, Indonesia, for providing cancer cell lines and all facilities to conduct this research.
6. REFERENCES
1. Arimbi AA, Darsono R, Widiyatno TV, Legowo D. Buku Ajar Patologi Umum Veteriner. Surabaya: Airlangga University Press; 2013.
2. Fadhli H, Teruna HY, Jose C. Uji toksisitas ekstrak kulit batang pulai basung (Alstonia spatulata BL) dengan metode brine shrimp lethality test. J Indones Chem Acta 2012;3:10-5.
3. Olaku O, White JD. Herbal therapy use by cancer patient: A literature review on case report. Eur J Cancer 2011;47:508-14. CrossRef
4. Wahyuni FS, Lusianti M, Dachriyanus A. Isolasi senyawa sitotoksik terhadap sel kanker payudara dari kulit batang Garcinia griffithii T. Daners. J Farm Indonesia 2009;4:177-87.
5. Artasasta MA, Yanwirasti AD, Handayani S. Cytotoxic activity screening of ethyl acetate fungal extracts derived from the marine sponge Neopetrosia chaliniformis AR-01. J App Pharm Sci 2017;7:174-8.
6. Devine DA, Marsh P. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol 2012;1:1-11.
7. Stevenson CS, Capper EA, Roshak AK. The identification and characterization of the marine natural product Scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Ther 2011;303:858-66. CrossRef
8. Furusawa E, Furusawa S. Anticancer activity of a natural product, viva-natural, extracted from Undaria pinnantifida on intraperitoneally implanted lewis lung carcinoma. Oncology 2009;42:364-9.
9. Barrung E. Isolation and identification of proteins from brown macroalgae (Padina asutralis) and their potential as anticancer. JIMKI 2015;1:11-5.
10. Kadi A. Some records of the presence of the Sargassum clan in Indonesian waters, the field of marine resources. LIPI Jakarta 2008;30:19-29.
11. Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 1982;45:31-4. CrossRef
12. Salazar AR, Perez-Lopez LA, Lopez-Arroyo J, Alanis-Garza BA, De Torres JL. Antimicrobial and antioxidant activities of plants from northeast of Mexico. J Alternat Med 2009;2011:1-6.
13. Priosoeryanto BP, Tateyama S, Yamaguchi R, Uchida K. Transplantation of a cell line derived from a canine benign mixed mammary tumour into nude mice. J Comp Pathol 1995b;113:383-8. CrossRef
14. Kumar PS, Febriyanti RM, Sofyan FF, Luftimas DE, Abdulah R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacogn Res 2014;6:350-4. CrossRef
15. Francisco JT, Uy MM. Toxicity and antioxidant potential screening of extracts from five marine sponges collected off Zamboanga Peninsula, Philippines. Asian J Biol Life Sci 2016;5:233-6.
16. Nursid M, Wikanta T, Susilowati R. Aktivitas Antioksidan, Sitotoksisitas, dan Kandungan Fukosantin Ekstrak Rumput Laut Coklat Dari Pantai Binuangeun, Banten. Jakarta: Balai Besar Penelitian dan Pengembangan Pengolahan Produk dan Bioteknologi Kelautan dan Perikanan, KKP; 2015.
17. Maharany F, Nurjanah N, Suwandi R, Anwar E, Hidayat T. Bioactive compounds of seaweed Padina australis and Eucheuma cottonii as sunscreen raw materials. JPHP Indonesia 2017;20:10-7. CrossRef
18. Setha B, Gaspersz FF, Idris AP, Rahman S, Mailoa MN. Potential of seaweed Padina sp. as a source of antioxidant. Int J Sci Technol Res 2013;2:221-4.
19. Murugan AC, Vallal D, Karim MR, Govindan N, Yusoff MB, Rahman MM. In vitro antiradical and neuroprotective activity of polyphenolic extract from marine algae Padina australis H. J Chem Pharm Res 2015;7:355-62.
20. Nagarajan S, Mathaiyan M. Emerging novel anti HIV biomolecules from marine algae: An overview. J App Pharm Sci 2015;5:153-8. CrossRef
21. Shakambari G, Ashokkumar B, Varalakshmi P. Phlorotannins from brown algae: Inhibition of advanced glycation end products formation in high glucose induced Caenorhabditis elegans. Indian J Exp Biol 2015;53:371-9.
22. Kelman D, Posner EK, McDermid KJ, Tabandera NK, Wright PR, Wright AD. Antioxidant activity of Hawaiian marine algae. Mar Drugs 2012;10:403-16. CrossRef
23. Budhiyanti SA, Raharjo S, Marseno DW, Lelana YB. Antioxidant activity of brown algae Sargassum species extract from the coastline of Java Island. Am J Agric Biol Sci 2012;7:337-46.
24. Jaswir I, Dedi N, Reno FH, Fitri O. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. A review. J Med Plant Res 2011;5:7119-31.
25. Stanojković TP, Šavikin K, Zdunić G, Kljajić Z, Grozdanić N, Antić J. In vitro antitumoral activities of Padina australis on human cervix and breast cancer cell lines. J Med Plants Res 2013;7:419-24.
26. Guedes EA, da Silva GT, Aguiar JS, Barros LD, Pinotti LM, Sant’Ana AS. Cytotoxic activity of marine algae against cancerous cells. Rev Bras Farmacogn 2013;23:668-73.
27. Priosoeryanto BP, Huminto H, Sumarny R. In vitro anti-proliferation and anti-invasion activities of chloroform and methanol extract Mussaenda philippica on derived tumor cell lines. National Seminar on Animal Technology and Veterinary 2004;9:782-788.
28. Koeffler HP, Golde DW. Human myeloid leukemia cell linea: A review. Blood 1980;56:344-50.
29. Priosoeryanto BP, Tateyama S, Yamaguchi R, Uchida K. Establishment of a cell line derived from a canine benign mixed mammary tumour into nude mice. J Comp Path 1995a;113:383-8. CrossRef
30. Priosoeryanto BP, Tateyama S, Yamaguchi R, Uchida K. Transplantation of a cell line derived from a canine benign mixed mammary tumour into nude mice. J Comp Pathol 1995c;113:383-8.
31. Saputra V. Dasar-dasar stem cell dan potensi aplikasinya dalam ilmu kedokteran. Cermin Dunia Kedokt 2006;153:21-5.
32. Wang J, Chen S, Xu S, Xu X, Ma D, Hu X, et al. In vivo induction of apoptosis by fucoxanthin a marine carotenoid, associated with down regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs 2012;10:2055-68. CrossRef
33. Gunasekaran S, Thirumalairaj VK, Shanmugasokan LS, Pitchai S, Yesudas R, Chacko A. Screening of in vitro cytotoxic activity of brown seaweeds against hepatocellular carcinoma. J App Pharm Sci 2017;7:51-5.