1. INTRODUCTION
Numerous microorganisms present in soil play essential roles, such as aiding nutrient absorption, cycling organic matter, enhancing plant and soil health, restoring soil quality, and contributing to primary ecosystem productivity. Hence, they are considered beneficial organisms [17]. These microorganisms provide several benefits to farming systems, including improving crop yield and quality and protecting plants from various biotic and abiotic stresses [21]. Moreover, the rising demand for high-quality food has driven the development of environmentally friendly and sustainable agricultural methods. These approaches focus on reducing chemical usage and promoting the use of beneficial microbes [27,10]. Endophytic fungi are particularly important due to their ability to provide significant benefits to plants, such as enhancing growth, improving quality, and increasing resistance to biotic and abiotic stresses. This highlights their importance in agriculture [9,38].
Serendipita indica, previously known as Piriformospora indica, is a beneficial endophytic fungus belonging to the order Sebacinales (Basidiomycota) and has been widely studied for many years. Research has shown that S. indica significantly improves the growth and development of various plant species under both normal and stressful conditions. This fungus has been found to benefit plants such as rice, tobacco, chilli, cabbage, cucumber, aubergine, maize, okra, and spinach, enhancing their germination rates. In addition, it has been observed that seeds of beetroot, carrot, cauliflower, onion, and radish germinate more effectively in extremely cold conditions when this fungus is introduced into the soil [6,36]. Studies have demonstrated that the application of S. indica enhances biomass, root and shoot growth, photosynthetic pigment production, and seedling vigor, all of which contribute significantly to the growth and development of seedlings [18,23]. This fungus supports vegetative development in plants by promoting height, increasing the number of leaves, enhancing shoot and root growth, and boosting fresh and dry weight, as well as the production of phytohormones and photosynthetic pigments [28]. S. indica colonization has also been shown to improve nutrient uptake, leading to enhanced plant growth, quality, and yield [24,25]. Furthermore, the use of S. indica positively impacts plant physiological traits, including inflorescence growth, blooming duration, flower size, and flower quantity, and facilitates earlier reproduction in host plants. The fungus has been found to significantly increase agricultural yields in crops such as rice, sweet potatoes, sunflower, tomatoes, black pepper, fennel, and rapeseed by improving chlorophyll content, flowering duration, grain yield, and the size and number of pods [4]. Azotobacter sp. is a heterotrophic, non-symbiotic part of the plant growth-promoting rhizobacteria that mostly lives in neutral or alkaline soils and fixes nitrogen and phosphate [20]. The rhizosphere of agricultural plants typically contains low population of Azotobacter in wheat, maize, and rice. It is a proven fact that using Azotobacter as a biofertilizer is essential for improving soil and plant nutrient levels, increasing agricultural yields, and preserving the environment [16,42]. In some agricultural crops, S. indica and A. chroococcum have been shown to improve mineral nutrition and promote growth and production under pot experiments [8,31].
The production of phytohormones and the modulation of physiological features by S. indica are known to improve stress tolerance and increase root and shoot development with enhanced nutrient absorption [30]. Similarly, A. chroococcum also enhances the soil nutrient profile and promotes plant development by fixing atmospheric nitrogen and solubilizing phosphate. On combination, these microbes may provide a variety of advantages, including better nutrient uptake, higher photosynthetic efficiency, and increased resistance to biotic and abiotic stressors. The combination of S. indica and A. chroococcum has been found to show complementing advantages of both microbes in our studies.
In the present study, the combined effects of S. indica and A. chroococcum under field conditions have revealed that crop productivity, water retention, and chlorophyll content are enhanced under field conditions while the previous studies have mainly been focused on the effects of microbial treatments under controlled environments such as pot experiments or greenhouses. Controlled studies, while useful, fail to reflect real-world scenarios where factors such as soil heterogeneity, weather fluctuations, and interactions with native soil microbiota that play a significant role. Differential effect of microbial treatment on two rice varieties (PB1718 and PB1121) in the present study suggests varietal-specific responses to microbial inoculation. It also explains the reasons for the differential response of biofertilizers on different crops. Hence, the aim of this study was to assess the impact of A. chroococcum, S. indica, and their combined treatments on the photosynthetic pigment content, relative water content (RWC), morpho-agronomic traits, and yield in two rice varieties i.e. PB1718 and PB1121 under field conditions.
2. MATERIALS AND METHODS
2.1 Mass Preparation of Microbial Inoculum
4% (w/v) Jaggery solution was prepared, and the pH was adjusted to 6.5 and then autoclaved at 15 p.s.i for 15 min in a liter Erlenmeyer flask containing 100 mL medium and A. chroococcum and S. indica were cultured aseptically in shake flasks at 120 rpm for bulk multiplication of both species separately at 37 and 28°C, respectively, for 4 days and 10–14 days, respectively [3].
2.2. Soil Treatment with Microbial Biomass
Twenty-four fields (each measuring 2 × 2 m) with at least 60 plants each field were prepared using standard agronomical practices for the cultivation of two varieties of rice in a research farm at Shobhit Institute of Engineering and Technology, Meerut. Sowing was done in May 2023 and transplantation in July 2023 when the seedlings were 40-day old and 8–10 cm in size. The rice varieties PB 1121 and PB 1718 were obtained from Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut, and both are long-duration varieties. All the recommended standard cultivation practices for PB 1121 and PB 1718 were used. The recommended intercultural operations (weeding, water management, and plant protection measures) were also followed.
In this study, a Completely Randomized Design (CRD) was used, with each treatment repeated 3 times. To ensure randomization, treatments were assigned to plots individually. Fourteen-day-old microbial biomass of S. indica to achieve a concentration of 105 spores/mL and 4-day-old biomass of A. chroococcum to reach a concentration of 107 CFU/mL cultured in 100 mL medium were added to 500 g of sterile saw dust as carrier and spread in 2 × 2 m field where transplantation was done. The control (C) field was treated similarly but without microbial inoculum. Treatment 1 (T1) was treated with A. chroococcum alone, Treatment 2 (T2) with S. indica alone while Treatment (T3) included combination of both A. chroococcum and S. indica.
2.3. Determination of Photosynthetic Pigments and RWC
The total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid contents in rice leaves were measured using the method outlined by Hiscox and Israelstam [12]. Fresh rice leaves (50 mg) were cut into small pieces and placed in 5 mL of dimethyl sulfoxide (DMSO), then incubated at 37°C for 4 h or until the tissue turned translucent. The absorbance of the extract was measured at 470, 645, and 663 nm using a double-beam spectrophotometer (model SP-UV1100), with DMSO as the blank. Chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll, and carotenoid levels were calculated using the following equations:
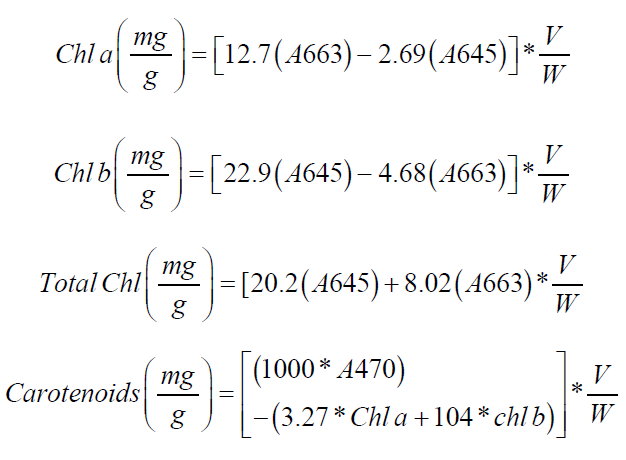
where, A=Optical density; V=Volume of DMSO (in mL); W=Sample weight.
The RWC of the leaves was measured using the method described by Barrs and Weatherley [7]. A 100 mg sample of fully expanded fresh leaves (FW) was placed in a Petri dish with double-distilled water and allowed to sit at room temperature for 4 h. The turgid weight (TW) was then recorded, and the samples were removed and dried. After placing the samples in an oven at 70°C overnight, the dry weight (DW) was noted. The RWC was then calculated as follows:
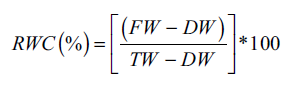
2.4. Morphological Studies
Morpho-agronomic data for both qualitative and quantitative traits were collected at various growth stages of the rice plants. Leaf length was measured from the base to the tip of the flag leaf at maturity, and plant height was measured from the soil surface to the panicle tip during the grain filling stage, both using a meter scale. Averages were calculated from 10 randomly selected plants per field. The number of panicles per plant was counted at the grain-filling stage by counting the panicles per hill, with the average data taken from ten hills per field. Similarly, the number of grains per panicle was counted at the same stage, with averages calculated from 10 randomly selected panicles per field. Root length was measured at maturity, and the number of leaves per plant was determined by counting the leaves on 10 randomly selected plants in each field.
2.5. Microbial Biomass Yield
The microbial biomass yield was evaluated for S. indica, A. chroococcum, and their combination in the Jaggery culture medium. 25 mL of broth was used in 100 mL Erlenmeyer flasks for each inoculum in 3 replicates and were incubated as described in para 2.1 above. After incubation, the cultures were centrifuged at 5000 rpm for 5 min, and the liquid (supernatant) was removed. The biomass yield was then measured by determining the volume of the pellet.
2.6. Heat Map Analysis
Pearson’s heat map analysis was used to find the strength of relationship between different parameters. The data were fed on Pearson’s website and followed the instructions that finally showed the relationship in the form of heat map.
2.7. Statistical Analysis
Analysis of variance (ANOVA) was used to analyze the experimental data with IBM Statistical Package for the Social Sciences Statistics 20. The ANOVA was performed to determine if there were significant differences in the effects of treatments on the photosynthetic pigments, RWC, and morpho-agronomic traits of rice. Duncan’s Multiple Range test (DMRT) was used to identify these differences, with a 5% level of significance (α = 0.05). The DMRT was selected because it can identify minute variations in treatment means, which makes it ideal for biological research where precise comparisons are essential. Since the main goal of this investigation was to find statistically significant differences between treatments rather than to quantify the magnitude of these effects, effect sizes such as η2 or Cohen’s d were not employed. The study focused on thorough pairwise comparisons using DMRT to identify minute treatment differences, even though effect sizes provide useful context for P-values.
3. RESULTS
3.1. Photosynthetic Pigments
The present study showed that all treatments in the rice variety PB1718 significantly improved the leaf photosynthetic pigment content compared to the control [Figure 1]. The treatment with A. chroococcum increased the total chlorophyll, chlorophyll a and b, and carotenoid content by 33, 29, 43, and 58%, respectively, compared to the control. When used alone, S. indica also significantly increased the total chlorophyll, chlorophyll a and b, and carotenoid content by 15, 21, 14, and 5%, respectively. The combination of S. indica and A. chroococcum had a synergistic effect, increasing total chlorophyll, chlorophyll a and b, and carotenoids by 63, 43, 150, and 67%, respectively, over the control [Figure 1].
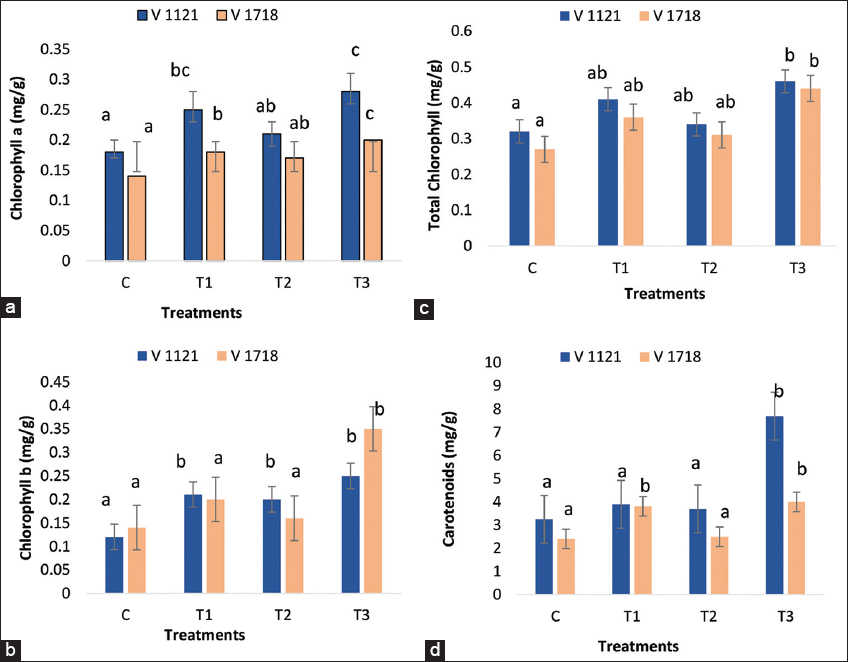 | Figure 1: S i.ndica and A. chroococcum for the improvement of the photosynthetic pigment content of leaf (A) chlorophyll a (Chl a) content, (B) chlorophyll b (Chl b) content, (C) total chlorophyll content, and (D) carotenoid content. Data are the means of three replicates (n = 3). (C=Control; T1=Treatment with Azotobacter chroococcum alone; T2= Treatment with Serendipita indica alone and T3 = Treatment with combination of Azotobacter chroococcum and Serendipita indica). [Click here to view] |
In the rice variety PB1121, all treatments also improved the leaf photosynthetic pigment content compared to the control. The A. chroococcum treatment significantly increased the total chlorophyll, chlorophyll a and b, and carotenoid content by 28, 39, 75, and 20%, respectively, compared to the control [Figure 1]. Similarly, S. indica alone increased the total chlorophyll, chlorophyll a and b, and carotenoid content by 6, 17, 67, and 14%, respectively. The combined treatment of S. indica and A. chroococcum also had a synergistic effect, increasing the total chlorophyll, chlorophyll a and b, and carotenoids by 44, 56, 18, and 137%, respectively, over the control. Overall treatments, whether with A. chroococcum, S. indica alone, or in combination, significantly boosted the photosynthetic pigment content of the leaves compared to the control.
3.2. RWC
In the PB1718 variety, all treatments – A. chroococcum, S. indica, and the combination of S. indica and A. chroococcum – significantly increased the RWC of the leaves compared to the control. The combined treatment with S. indica and A. chroococcum resulted in a 100% increase in RWC compared to the control. The A. chroococcum treatment increased the RWC by 75%, while S. indica increased it by 67% compared to the control [Figure 2]. In the PB1121 variety, the combined treatment with S. indica and A. chroococcum also significantly boosted the RWC by 84% compared to the control. The treatment with A. chroococcum increased the RWC by 71%, and S. indica increased it by 53% compared to the control [Figure 2].
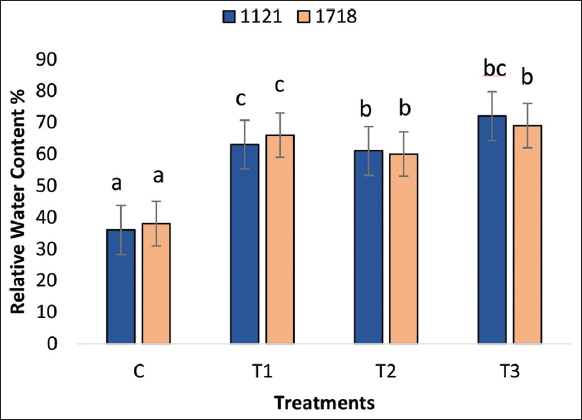 | Figure 2: Relative water content of the leaf. Data are the means of three replicates (n = 3). (C=Control; T1=Treatment with A. chroococcum alone; T2=Treatment with S. indica alone and T3=Treatment with combination of A. chroococcum and S. indica). S. indica: Serendipita indica, A. chroococcum: Azotobacter chroococcum. [Click here to view] |
3.3. Morpho-Agronomic Traits
The present study showed that in rice variety PB1121, all treatments improved the morpho-agronomic traits compared to the control [Figure 3]. A. chroococcum significantly increased plant height, number of leaves, leaf length, and root length by 3.25, 12.22, 5.04, and 9.5%, respectively, compared to the control. S. indica also significantly increased plant height, number of leaves, leaf length, and root length by 2.3, 9.44, 2.5, and 2.5%, respectively. The combined treatment with S. indica and A. chroococcum had a synergistic effect, increasing plant height, number of leaves, leaf length, and root length by 4.8, 22.22, 8.4, and 20%, respectively, compared to the control [Figure 3].
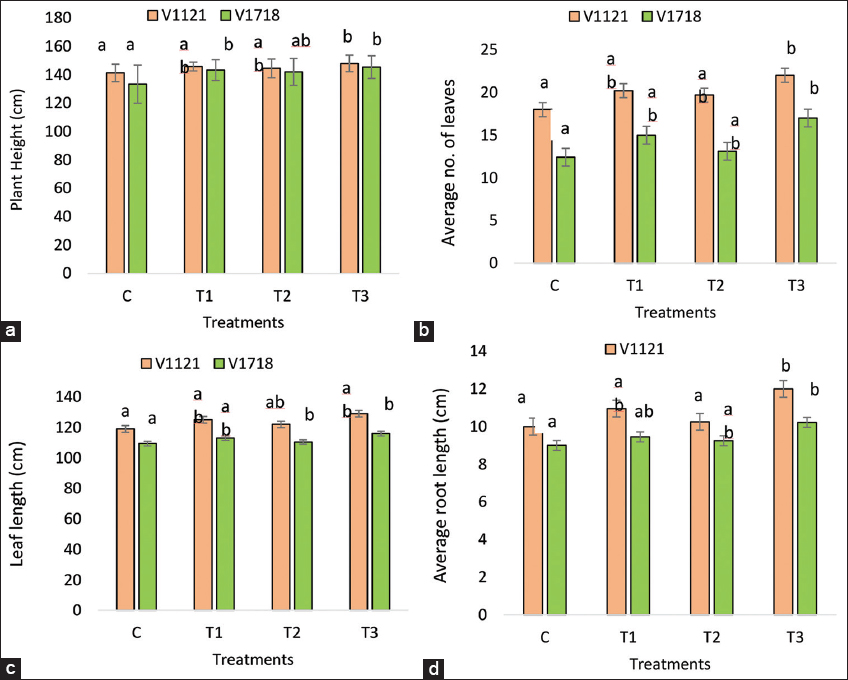 | Figure 3: Morphological traits (a) PH (b) LN (c) LL (d) RL. Data are the means of ten replicates (n = 10). (C=Control; T1=Treatment with A. chroococcum alone; T2=Treatment with S. indica alone and T3=Treatment with combination of A. chroococcum and S. indica). S. indica: Serendipita indica, A. chroococcum: Azotobacter chroococcum, PH: Plant height, LN: Leaf number, LL: Leaf length, RL: Root length. [Click here to view] |
In rice variety PB1718, all treatments significantly improved the morpho-agronomic traits compared to the control. A. chroococcum increased plant height, number of leaves, leaf length, and root length by 7.34, 21, 2.4, and 5%, respectively, compared to the control [Figure 3]. S. indica increased plant height, number of leaves, leaf length, and root length by 6.4, 5.64, 0.91, and 2.77%, respectively, compared to the control. The combined treatment with S. indica and A. chroococcum had a synergistic effect, increasing plant height, number of leaves, leaf length, and root length by 9, 37.1, 6.03, and 13.55%, respectively, compared to the control. All treatments, whether with A. chroococcum, S. indica alone, or in combination, significantly improved the morpho-agronomic traits compared to the control.
In rice variety PB1718, all treatments significantly increased the number of panicles and grains per panicle compared to the control. The combined treatment with S. indica and A. chroococcum increased the number of panicles and grains per panicle by 25 and 9.8%, respectively, compared to the control. The treatments with A. chroococcum and S. indica alone also increased the number of panicles and grains per panicle by 13.5, 5.6%, and 8.7%, 1.5%, respectively, compared to the control [Figure 4]. In rice variety PB1121, the combined treatment with S. indica and A. chroococcum increased the number of panicles and grains per panicle by 8 and 11.9%, respectively, compared to the control. The treatments with A. chroococcum and S. indica alone also significantly increased the number of panicles and grains per panicle by 4%, 8.9%, and 1%, 5%, respectively, compared to the control [Figure 4].
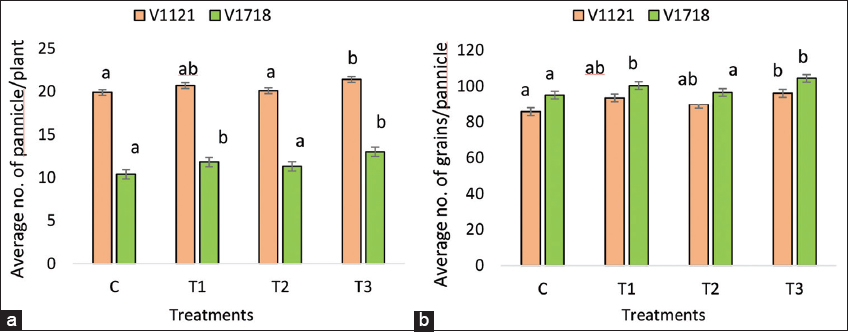 | Figure 4: Morphological traits (a) PPP (b) GPP. Data are the means of ten replicates (n = 10). (C=Control; T1=Treatment with A. chroococcum alone; T2=Treatment with S. indica alone; and T3=Treatment with combination of A. chroococcum and S. indica). S. indica: Serendipita indica, A. chroococcum: Azotobacter chroococcum, PPP: Panicle per plant, GPP: Grain per panicle. [Click here to view] |
3.4. Yield Analysis
Analysis of microbial biomass revealed that A. chroococcum produced a higher yield of 8 mL compared to S. indica (4.5 mL), suggesting that A. chroococcum has a more robust growth and higher biomass-producing capacity under the given conditions. The combination of S. indica and A. chroococcum produced 10.5 mL biomass suggesting that both microbes are growing well in co-culture [Table 1] and logically will exhibit synergistic growth under field conditions [Figure 5].
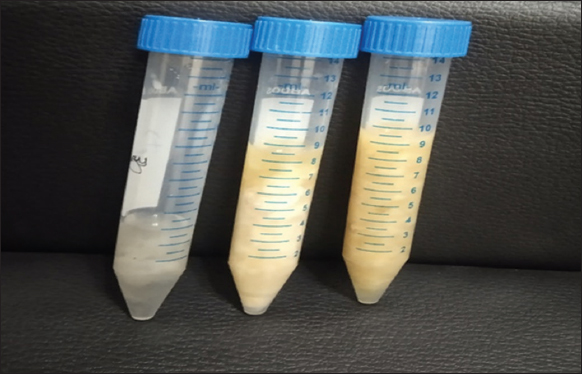 | Figure 5: Biomass of microbial cultures after centrifugation from 100 mL culture medium. [Click here to view] |
Table 1: Microbial biomass yield in liquid culture on Jaggery (4% w/v) medium
| S. n | Microbe | Biomass volume (mL/25 mL of medium) |
|---|---|---|
| 1 | S. indica (14-day-old) | 4 mL |
| 2 | A. chroococcum (4-day-old) | 8 mL |
| 3 | S. indica + A. chroococcum (14-day-old) | 10.5 mL |
S. indica: Serendipita indica, A. chroococcum: Azotobacter chroococcum
3.5. Heat Map Analysis of Various Treatments
The effect of microbial inoculation on photosynthetic traits, RWC, and morphological traits in rice varieties was also analyzed using a heat map hierarchal clustering. Hierarchical clustering was indeed employed in the heat map analysis, utilizing Ward’s method to determine distances. The heat map, based on Pearson and Ward for determining distance and clustering, shows how microbial inoculation affects these traits in rice varieties. The interrelationship between the study variables was plotted using Pearson’s correlation test as a heat map [Figure 6]. Red boxes indicate a strong positive correlation between variables, salmon boxes show a medium positive correlation, and blue boxes represent a low positive correlation. For the PB1718 variety, chlorophyll value had a strong positive correlation with RWC, root length, plant height, and panicles per plant. Total chlorophyll showed a medium positive correlation with chlorophyll b and panicles per plant. Chlorophyll b had a medium positive correlation with carotenoid content [Figure 7]. Total chlorophyll values were positively correlated with chlorophyll a, while chlorophyll b values showed a strong positive correlation with RWC. RWC had a medium positive correlation with panicles per plant, and grains per plant had a strong positive correlation with panicles per plant. Leaf length showed a medium correlation with panicles per plant [Figure 6].
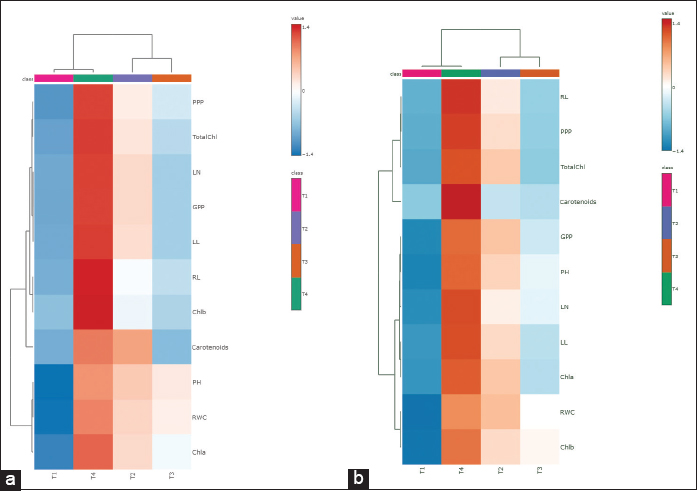 | Figure 6: The heat map based on Pearson and Ward for determining distance and clustering shows the effect of microbial treatments on physiological properties and morpho-agronomic traits of Rice Note: Chla- Chlorophyll a, Chlb- Chlorophyll b, TotalChl- Total Chlorophyll, Carotenoid Content (mg/g), RWC: Relative water content (%), PPP: Panicle per plant, GPP: Grain per panicle, PH: Plant height, LN: Leave number, LL: Leaf length, RL: Root length (a) Variety PB1718 and (b) Variety PB1121. [Click here to view] |
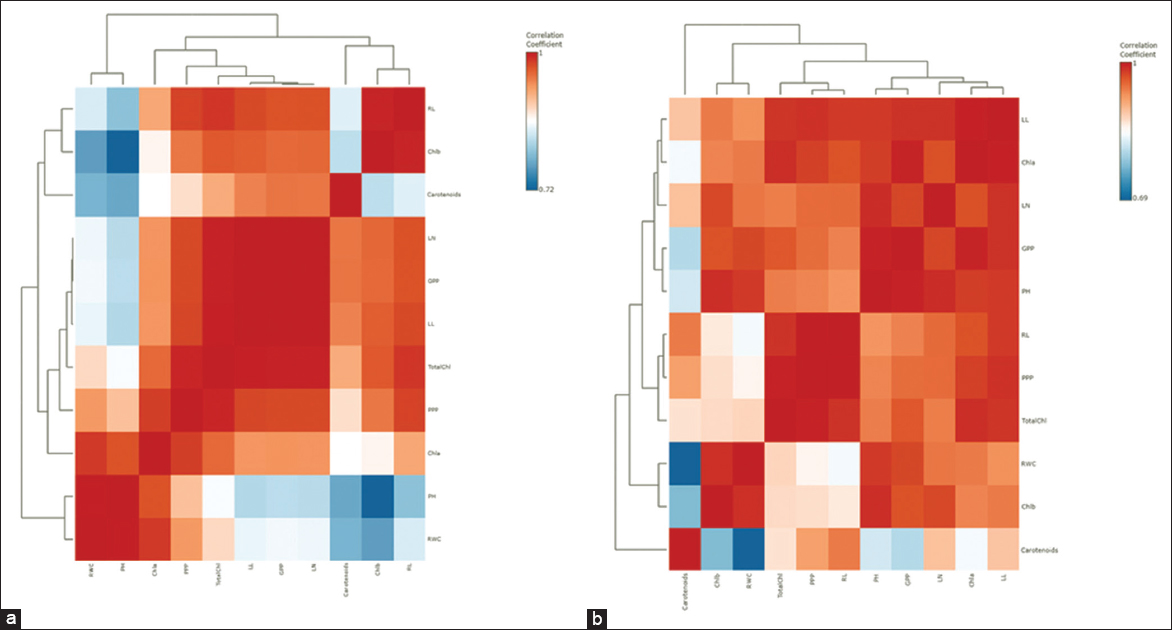 | Figure 7: Correlation Heatmaps (Pearson r) of variety (a) PB1718 and (b) PB1121. [Click here to view] |
For the PB1121 variety, chlorophyll value showed a medium positive correlation with carotenoid content. Total chlorophyll had a strong positive correlation with grains per panicle. Chlorophyll a was strongly positively correlated with RWC and chlorophyll b. RWC had a strong positive correlation with total chlorophyll, chlorophyll b, and leaf number [Figure 7]. Panicles per plant were strongly positively correlated with plant height. Carotenoid content had a medium positive correlation with leaf length [Figure 6].
4. DISCUSSION
The aim of this study was to examine how treating soils with A. chroococcum, S. indica, and a combination of both affects photosynthetic pigments, RWC, morphological traits, and yield in two rice varieties, PB1718 and PB1121. Both rice varieties showed a significant increase in photosynthetic pigments and RWC compared to the control. It has been reported that S. indica improves rice ability to withstand drought by postponing leaf curl and raising leaf temperature along with colonization [33,37]. Variety PB1718 responded more robustly to the treatments than variety PB1121 in terms of photosynthetic pigment content. According to Chaudhary et al. [49], the influence of soil conditions on RWC and photosynthetic components was clearly seen in our investigation. Water retention capacity and photosynthetic efficiency in plants are directly impacted by soil properties such as microbial population density, organic matter concentration, and nutrient availability. Higher RWC and increased levels of chlorophyll and carotenoid in rice leaves were accompanied by better soil structure and enhanced nutrient cycling after microbial inoculation with Azotobacter chroococcum and S. indica. These findings, which have been supported by previous research, show how enhanced soil characteristics enhance the physiological advantages of microbial treatments, especially in field settings where microbial interactions and soil variability are important factors.
Rice variety PB1718 exhibited a greater response to microbial treatments compared to PB1121 that may be attributed to different genetic makeup and physiological traits of both varieties. The bacterial blight-resistant cultivar PB1718 was created using marker-assisted selection, which could have added genes that improve its abilities for greater response to microbial inoculants such as S. indica and A. chroococcum. These genetic characteristics include increased expression of genes related to photosynthetic efficiency, stress tolerance, and nutrient absorption. In terms of physiology, PB1718 has a more effective root system, which also enables greater microbial colonization and enhanced absorption of nutrients and water. Different bio-fertilizers are known to produce different effects on different varieties of crops under different soil and environmental conditions that have been correlated by various scientists with the genetic composition of the crops. The microbial interaction with the host plant depends upon the genetic composition of the plants [43].
S. indica significantly enhances the growth of cabbage by promoting plant development and disease resistance. The application of S. indica, either through carrier or seed inoculation, resulted in increased plant height, leaf number, chlorophyll content, carotenoids, and antioxidant capacity, contributing to better photosynthetic efficiency and resilience under biotic stress conditions [29,34]. Under water stress conditions, S. indica has been shown to influence protein expression in the photosynthetic pathways of barley [1,2,11]. The most significant improvements were observed with the combined treatment of A. chroococcum and S. indica in both rice varieties, PB1718 and PB1121. This treatment greatly increased total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content compared to the control and also improved the RWC of the leaves in all treatments, with variety PB1718 showing a stronger response. Microbial inoculations are reported to enhance water retention, a beneficial trait in water-limited environments [19]. It has been reported in several plants that S. indica colonization increases the RWC and improves their resistance to drought [4,15,26,35] enabling them to withstand environmental stress [13,14,39,40,41]. Furthermore, PB1718’s increased chlorophyll content with microbial treatments suggests more resilient photosynthetic apparatus, which may be a result of improved control over the pathways involved in chlorophyll production and the activity of antioxidant enzymes [43,44]. PB1718 treated with S. indica and A. chroococcum showed increased chlorophyll content and water retention, which may be attributed to a number of processes. Phytohormones including auxins and cytokinins, which increase chlorophyll biosynthesis and postpone leaf senescence, are produced in greater quantities by S. indica. In addition, under stressful conditions, this fungus protects the photosynthetic machinery from oxidative damage by increasing the activity of antioxidant enzymes. In contrast, A. chroococcum fixes nitrogen from the atmosphere and solubilizes phosphate, increasing nutrient availability and promoting the formation of chlorophyll. These effects are enhanced by the combination treatment, which raises both photosynthetic and water-use efficiency. The genetic susceptibility of PB1718 to stress also amplifies these advantages, therefore leading to increased yield potential [45,46].
The synergistic increase in the yield in the combined treatment of S. indica and A. chroococcum may be attributed to mutual benefits provided by the microbes, such as enhanced nutrient availability, improved growth conditions, or more efficient utilization of resources. This suggests that co-inoculation with S. indica and A. chroococcum could be a promising strategy for improving microbial biomass production in biotechnological applications.
The heat map analysis provided better understanding of the links between the different relationships of treatments with other characteristics of the two types of rice. The variation in response between the two varieties could be attributed to their genetic makeup and physiological adaptations. Rice variety PB1718 appears to be more responsive to microbial inoculation, especially in terms of water retention and total chlorophyll content, which could offer it an advantage under stress conditions. Liu et al. [22] and Singh et al. [32] have also correlated the effects of microbial inoculations with drought resistance. Microbial inoculations protect the photosynthetic machinery from oxidative damage, especially under stress [5]. These findings suggest that different microbial treatments can be tailored to specific rice varieties to optimize growth, photosynthesis, and water-use efficiency. The combined treatment of A. chroococcum and S. indica appears to be the most effective for both varieties, though the specific benefits varied, demonstrating the importance of considering varietal responses when designing bio-inoculant strategies for crop improvement.
The results of this study can be used to create bio-inoculant techniques that are specifically targeted to particular types of rice. These methods can improve soil health and reduce dependency on synthetic fertilizers when included in sustainable farming methods. However, there are obstacles to scaling up such uses, including making sure microbial inoculants are consistently viable and of high quality, dealing with field circumstances that might change, and getting past logistical and regulatory obstacles. The acceptance and understanding of bio-inoculants by farmers continue to be significant obstacles. Field tests in a variety of settings, strong quality control procedures, and efficient extension services to inform farmers are all necessary to meet these difficulties [47,48].
Our results emphasize the potential benefits of co-inoculation strategies in sustainable agriculture and biotechnological applications.
5. CONCLUSION
The study shows that the combined treatment with S. indica and A. chroococcum increased chlorophyll content, relative water, and all plant growth parameters including the yield of Oryza sativa. However, the variety 1718 responded better overall, while the variety 1121 showed specific improvements in chlorophyll a, carotenoid content, and root length. The study suggests that the co-inoculation with A. chroococcum and S. indica is better for rice cultivation and exhibits a high potential for drought resistance. It is also suggested that the combined use of the fungus S. indica and the bacterium A. chroococcum could help reduce the negative effects of climate change. The synergistic effects of these two organisms provide a promising approach for sustainable agriculture and increased crop productivity.
Our studies reveal that co-inoculation with S. indica and A. chroococcum is a promising approach for growing rice sustainably, providing a viable way to boost crop resilience under stressful situations. However, further studies are required to address these issues including farmer acceptance, environmental variability, and varietal uniqueness. Through the optimization of microbial formulations and their integration with precision agriculture techniques, this strategy may be successfully applied to improve sustainability and food security in a variety of agricultural systems.
6. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Aletaha R, Sinegani AA. Water availability in soil affect performance of different root fungal colonizers on metabolism of wheat. Iran J Sci Technol Trans. 2020;4:919-31. [CrossRef]
2. Arora M, Saxena P, Abdin MZ, Varma A. Interaction between Piriformospora indica and Azotobacter chroococcum governs better plant physiological and biochemical parameters in Artemisia annua L. Plants grown under in vitro conditions. Symbiosis. 2018;75:103-12. [CrossRef]
3. Attri MK, Varma A. Comparative study of growth of Piriformospora indica by using different sources of jaggery. J Pure Appl Microbiol. 2018;12:933-42. [CrossRef]
4. Azizi M, Fard EM, Ghabooli M. Piriformospora indica affect drought tolerance by regulation of genes expression and some morphophysiological parameters in tomato (Solanum lycopersicum L). Sci Hortic. 2021;287:110260. [CrossRef]
5. Bandyopadhyay P, Yadav BG, Kumar SG, Kumar R, Kogel KH, Kumar S. Piriformospora indica and Azotobacter chroococcum consortium facilitates higher acquisition of N, P with improved carbon allocation and enhanced plant growth in Oryza sativa. J Fungi (Basel). 2022;8:453. [CrossRef]
6. Barazani O, Benderoth M, Groten K, Kuhlemeier C, Baldwin IT. Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia. 2005;146:234-43. [CrossRef]
7. Barrs HD, Weatherley PE. A Re-examination of the relative turgidity techniques for estimating water deficits in leaves Australian. J Biolo Sci. 1962;15:413-28. [CrossRef]
8. Beltayef H, Melki M, Saidi W, Hajri R, Cruz C, Muscolo A, et al. Potential Piriformospora indica effect on growth and mineral nutrition of Phaseolus vulgaris crop under low phosphorus intake. J Plant Nutr. 2021;44:498-507. [CrossRef]
9. Ye B, Wu Y, Zhai X, Zhang R, Wu J, Zhang C, et al. Beneficial effects of endophytic fungi from the Anoectochilus and Ludisia species on the growth and secondary metabolism of Anoectochilus Roxburghii. ACS Omega. 2020;5:3487-97. [CrossRef]
10. Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V, et al. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front Nutr. 2018;5:12. [CrossRef]
11. Gayatri GP, Ajith Kumar KG, Parvathy S, Pillai Somasekharan MS. GA/ABA antagonism in the physiology of seed germination in the recalcitrant and vulnerable tree- Vateria indica L. Indian J Agric Res. 2022;56:12-7. [CrossRef]
12. Hiscox JD, Israelstam GF. Method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332-4. [CrossRef]
13. Hosseini F, Mosaddeghi MR, Dexter AR. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Biochem. 2017;118:107-20. [CrossRef]
14. Hosseini F, Mosaddeghi MR, Dexter AR, Sepehri M. Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta Physiol Biochem. 2018;247:1229-45. [CrossRef]
15. Hui F, Liu J, Gao Q, Lou B. Effects of Piriformospora indica on drought resistance of Nicotiana tabacum. Tob Sci Technol. 2017;50:1-7.
16. Ibrahim HM, El-Sawah AM. The mode of integration between Azotobacter and Rhizobium affect plant growth, yield, and physiological responses of pea (Pisum sativum L). J Soil Sci Plant Nutr. 2022;22:1238-51. [CrossRef]
17. Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci. 2017;8:1617. [CrossRef]
18. Jisha S, Sabu KK, Manjula S. Multifunctional aspects of Piriformospora indica in plant endosymbiosis. Mycology. 2019;10:182. [CrossRef]
19. Kantwa CR, Saras PK, Vyas KG, Chaudhari HL, Choudhary RR, Patel SA, et al. Effect of wheat varieties and integrated nutrient management practices on nutrient content, Uptake and soil nutrient status. Indian J Agric Res. 2023;59:239-431. [CrossRef]
20. Khan M, Garg PA. Phenotypic trait analysis of tomato (Solanum lysopersicum) genotypes in Qatar and Indian agro-climatic conditions Indian. J Agric Res. 2024;58:969-78. [CrossRef]
21. Koskey G, Mburu SW, Awino R, Njeru EM, Maingi JM. Potential use of beneficial microorganisms for soil amelioration, phytopathogen biocontrol, and sustainable crop production in smallholder agroecosystems. Front Sustain Food Syst. 2021;5:606308. [CrossRef]
22. Liu B, Jing D, Liu F, Ma H, Xinghong L, Lin P. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) seedlings by stimulating osmotic adjustment and antioxidant defense system. Appl Microbiol Biotechnol. 2021;105:8951-68. [CrossRef]
23. Liu B, Liu X, Yu L, Dun X, Ma H, Liu F, et al. Serendipita indica improve seed germination and seedling growth of Lolium multiflorum Lam. Through amelioration of osmotic adjustment, nutrient accumulation and Na+/K+homoeostasis under salinity conditions. Plant Soil Environ. 2024;70:644-55. [CrossRef]
24. Liu B, Liu X, Liu F, Ma H, Ma B, Zhang W, et al. Growth improvement of Lolium multiflorum Lam. Induced by seed inoculation with fungus suspension of Xerocomus badius and Serendipita indica. AMB Express. 2019;9:145. [CrossRef]
25. Qu P, Zhang Z, Li R, Liu R, Zhang Y, Cheng C. Insights into the rooting and growth-promoting effects of endophytic fungus Serendipita indica in Blueberry (Vaccinium corymbosum). J Plant Growth Regulation. 2024;43:1-12. [CrossRef]
26. Saddique MA, Ali Z, Khan AS, Rana IA, Shamsi IH. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice (N Y). 2018;11:34. [CrossRef]
27. Saleem S, BytešníkováZ, Richtera L, Pokluda R. The effects of Serendipita indica and guanidine-modified nanomaterial on growth and development of cabbage seedlings and black spot infestation. Agriculture.2021;11:1295. [CrossRef]
28. Saleem S, Sekara A, Pokluda R. Serendipita indica-a review from agricultural point of view. Plants. 2022;11:3417. [CrossRef]
29. Saleem S, BytešníkováZ, Richtera L, Pokluda R. The effects of Serendipita indica and guanidine-modified nanomaterial on growth and development of cabbage seedlings and black spot infestation. Agriculture. 2021;11:1295. [CrossRef]
30. Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmuller R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem. 2005;280:26241-7. [CrossRef]
31. Silletti S, Di Stasio E, Van Oosten MJ, Ventorino V, Pepe O, Napolitano M, et al. Biostimulant activity of Azotobacter Chroococcum and Trichoderma Harzianum in durum wheat under water and nitrogen deficiency. Agronomy. 2021;11:380. [CrossRef]
32. Singh M, Jaswal A, Sarkar S, Singh A. Influence of integrated use of organic manures and inorganic fertilizers on physio-chemical properties of soil and yield of kharif maize in coarse loamy typic haplustept soil. Indian J Agric Res. 2024;58:616-21. [CrossRef]
33. Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol. 2010;167:1009-17. [CrossRef]
34. Tahiri AI, Raklami A, Bechtaoui N, Anli M, Boutasknit A, Oufdou K, et al. Beneficial effects of plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and compost on lettuce (Lactuca Sativa) growth under field conditions. Gesunde Pflanz. 2022;74:219-35. [CrossRef]
35. Tariq A, Pan K, Olatunji OA, Graciano C, Li Z, Sun F, et al. Phosphorous application improves drought tolerance of phoebe zhennan. Front Plant Sci. 2017;8:1561. [CrossRef]
36. Varma A, Bakshi M, Lou B, Hartmann A, Oelmüller R. Piriformospora indica:A novel plant growth-promoting mycorrhizal fungus. Agric Res. 2012;1:117-31. [CrossRef]
37. Wei Q, Wu M, Zhang W, Xu L, Chen J, Pan R, et al. Effect of the endophytic fungus Piriformospora indica on the growth and drought tolerance of rice seedling under drought stress. Chin J Ecol. 2018;37:2642-8.
38. Yang L, Zou YN, Tian ZH, Wu QS, Kuca K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci Hortic. 2021;277:109815. [CrossRef]
39. Yang R. In Response to Drought and Low Temperature stress Induced by Piriformospora indica Master's Thesis Fujian. Fuzhou, China:Agriculture and Forestry University;2019.
40. Yang Y, Dong S, Wang Y, Luo Z, Zhu J. Effects of adding Pirformaspora Indica to soil at different growth stages of oilseed on the prevention of waterlogged disaster Hubei. Agric Sci. 2015;54:790-4.
41. Yang Y, Luo Z, Dong S, Zhang J, Zhu J. Effects of Pirformaspora indica on yield and resistance to waterlogging of cotton. J Henan Agric Sci. 2015;44:46-9.
42. Zahedi H, Abbasi S. Effect of plant growth promoting rhizobacteria (PGPR) and water stress on phytohormones and polyamines of soybean. Indian J Agric Res. 2024;49:427-31. [CrossRef]
43. Chetry P, Sharma SS. Differential impact of aluminium stress on photosynthetic pigment contents of certain rice (Oryza sativa L.) landraces from Sikkim Himalaya. J Indian Bot Soc. 2024;31.
44. Srikanth B, Subrahmanyam D, Sanjeeva Rao D, Narender Reddy S, Supriya K, Raghuveer Rao P, et al. Promising physiological traits associated with nitrogen use efficiency in rice under reduced N application. Front Plant Sci. 2023;14:1268739. [CrossRef]
45. Tsai YC, Chen KC, Cheng TS, Lee C, Lin SH, Tung CW. Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol. 2019;19:403. [CrossRef]
46. Vanisri S, Sreedhar M, Jeevan L, Pavani A, Chaturvedi A, Aparna M, et al. Evaluation of rice genotypes for chlorophyll content and scavenging enzyme activity under the influence of mannitol stress towards drought tolerance. Int J Curr Microbiol Appl Sci. 2017;6:2907-17. [CrossRef]
47. Kahle P. Challenges and solutions in scaling up biofertilizer production. J Biodivers Biopros Dev. 2023;9:59.
48. Díaz-Rodríguez AM, Parra Cota FI, Cira Chávez LA, García Ortega LF, Estrada Alvarado MI, Santoyo G, et al. Microbial inoculants in sustainable agriculture:Advancements, challenges, and future directions. Plants. 2025;14:191. [CrossRef]
49. Chaudhary M, Garg AP, Jabborova D. Combinatorial Effect of Soil Treatment with Serendipita Indica and Azotobacter Chrococcum on Physiological Parameters and Yield of Orzya Sativa l. BBRA;2025. [CrossRef]