1. INTRODUCTION
Rice is an essential crop globally, with an estimated 162 million hectares cultivated worldwide, producing approximately 738.1 million tons of rice annually, particularly in Asian countries, where it provides about 80% of the daily caloric intake [1]. Asia leads in rice cultivation, production, and consumption globally. Despite its significance, rice is highly susceptible to both biotic stresses (such as pests, insects, and diseases) and abiotic stresses (including extreme temperatures, drought, flooding, and salinity). Among these, drought stress is particularly destructive, severely impacting rice plants during their growth periods when there is insufficient water in the soil [2,3]. In India, rice is grown on about 43 million hectares, yielding around 118 million tons, making it the second-largest producer after China. The southern part of India, including states such as Tamil Nadu, Karnataka, Andhra Pradesh, and Telangana, are significant rice-producing states, contributing substantially to the country’s rice output. However, this region also faces substantial challenges due to its dependence on monsoon rains, with large areas of rice cultivation being rainfed and thus highly susceptible to drought stress.
Drought stress is a global issue, affecting rice-cultivating areas in developing countries (33%), developed countries (25%), and underdeveloped countries (42%) [4]. In Asia alone, about 34 million hectares of rainfed lowland and 8 million hectares of upland rice-cultivating areas experience drought stress [5]. In India, 68% of the total agricultural land is rainfed, with approximately 40 million hectares of rice-cultivating areas relying on rainfall and prone to drought stress [6]. In southern India, rainfed rice areas are particularly vulnerable to erratic rainfall patterns, leading to periods of drought that can severely affect crop yields. The Cauvery Delta Region, a critical rice-growing area, frequently experiences drought conditions due to unpredictable monsoon patterns and water scarcity. Drought-prone regions in southern India face significant yield losses, with the reproductive stage drought stress causing the most severe impacts. The degree and duration of drought stress during the reproductive stage can lead to considerable reductions in grain yield, often resulting in crop failure and substantial economic losses for farmers [7].
Grain yield loss due to drought stress at the reproductive stage is particularly severe, often resulting in male sterility and embryo abortion due to insufficient water [8,9]. In response, several quantitative trait loci (QTLs) associated with drought tolerance have been identified, including qDTY12.1 [10] and qDTY1.1 [11] for upland and lowland rice. Among these, the major effect locus qDTY1.1, located on Chromosome 1, has been shown to increase grain yield and is flanked by markers RM11943 and RM431 [11,12]. This locus, derived from the rice varieties Nagina 22 (N22) and Dhagaddeshi [13], contains several putative drought tolerance genes such as WRKY, serine/threonine protein kinase, and MYB [14]. The qDTY1.1 locus has demonstrated consistent performance across various genetic backgrounds of popular rice varieties like Swarna, IR64, and MTU1010 [11].
The economic impact of drought stress on rice cultivation is profound, leading to significant yield losses and threatening food security in regions heavily dependent on rice as a staple crop. The improvement of rice varieties for drought tolerance [15] not only ensures stable production but also contributes to the livelihoods of millions of smallholder farmers. The use of marker-assisted backcrossing (MABC) represents a significant advancement in breeding techniques, allowing for the precise introgression of desirable traits such as drought tolerance into elite rice varieties. This method accelerates the breeding process and enhances the efficiency of developing improved varieties. The development and deployment of drought-tolerant rice varieties like the improved ADT36 can lead to several benefits: Increased yield stability under water-limited conditions, reduced reliance on irrigation, conservation of water resources, enhanced resilience of rice farming systems to climate variability and change, and improved food security and economic stability for rice-growing communities.
Further research is needed to evaluate the performance of these improved rice lines under a wider range of environmental conditions and to understand the underlying genetic mechanisms of drought tolerance. In addition, integrating these improved varieties into sustainable agricultural practices and ensuring their adoption by farmers through effective extension services and policy support is crucial for maximizing their impact. By enhancing our understanding and improving the resilience of rice to drought stress, we can contribute to the global challenge of ensuring food security in the face of changing climatic conditions. This study addresses the significant yield loss in rice due to drought stress at the reproductive stage, which poses a severe threat to food security in drought-prone regions. Traditional breeding methods have been inadequate in developing rice varieties that can withstand the increasing frequency and intensity of droughts attributed to climate change. This research aims to enhance drought tolerance in the ADT36 rice variety, which is critical for sustaining rice production in the Cauvery Delta Region – a region highly vulnerable to erratic monsoon patterns.
The MABC method was employed to introduce the qDTY1.1 locus, known for its significant impact on drought tolerance, into the ADT36 variety. This precise and efficient breeding technique ensures the retention of the desirable agronomic traits of ADT36 while enhancing its drought tolerance. Furthermore, this study evaluates the field performance of the improved ADT36 lines under various environmental conditions, providing valuable insights into their stability and adaptability. By addressing the urgent need for drought-tolerant rice varieties, this research contributes to the broader goal of ensuring food security amidst changing climatic conditions. The primary focus of this study is to address the critical issue of reproductive stage drought stress in rice, which significantly affects grain yield and food security. This research aims to enhance the drought tolerance of the widely cultivated rice variety ADT36 by introgressing the qDTY1.1 locus through MABC. This variety is particularly important for the Cauvery Delta Region of southern India, an area highly susceptible to drought due to irregular monsoon rains. By improving ADT36 for drought tolerance, the study seeks to provide a viable solution to mitigate yield losses and enhance food security in drought-prone regions.
2. MATERIALS AND METHODS
2.1. Source of Rice Seeds and Development of Near Isogenic Lines
A small quantity of rice seeds of ADT36 from Tamil Nadu Rice Research Institute (TRRI), Aduthurai, Tamil Nadu, and CR Dhan 801 from National Rice Research Institute (NRRI), Cuttack, Odisha, were obtained. ADT36 was used as the female parent (Recipient), and CR Dhan 801 as the male parent (Donor) for improving drought tolerance at the reproductive stage. Hybridization took place between ADT36 and CR Dhan 801 in a Nethouse at Kandaswami Kandar’s College, Velur, Namakkal District, Tamil Nadu, during the Kharif season of 2019.
2.2. Polymerase Chain Reaction (PCR) amplification
2.2.1. Parental polymorphism and selection of positive F1 and BC3F1 progenies
Genomic DNA was isolated from the fresh leaf of ADT36, CR Dhan 801, F1, and BC3F1 progenies using CTAB method with minor modifications [16]. PCR was done with the volume of 10 μL containing 20–30 ng template DNA, 5 pmol of each primer (Forward - 5’-TCCTGCGAACTGAAGAGTTG-3’and Reverse - 5’-AGAGCAAAACCCTGGTTCAC-3’) of SSR marker, RM431 linked with qDTY1.1, 0.05 mM dNTPs, 10× PCR buffer (10 mM Tris, pH 8.4, 50 mM KCl and 1.8 mM MgCl2) and 0.5U of Taq DNA polymerase (Bangalore Genei Pvt. Ltd., Bengaluru, India).
2.3. Backcrossing and Selection
After confirmation of CR Dhan 801 as resistant and ADT36 as susceptible to drought stress at a phenotypic and genotypic level based on polymorphism, we went for cross-pollination process. For emasculation of anthers from the female parent (ADT36), we removed all six pre-matured anthers from each floret very carefully with the help of foreceps in the early morning (around 7 am). From each panicle, around 25 florets were emasculated and covered the panicle using a butter paper envelope after emasculation. Then, we collected matured anthers (light yellow in color) from the male plant (CR Dhan 801) at the time of opening of the palea and lemma part of the floret in the morning time between 9 am and 10.30 am carefully, and pollen grains were dusted on feathered stigma in the emasculated flowers. Thus, we gave due importance to the emasculation and pollination step to get true hybrid seeds.
F1 seeds derived from the cross were backcrossed with ADT36 as the recurrent parent (RP). Foreground selection using the SSR marker RM431 linked with qDTY1.1 was conducted to generate BC1F1 seeds during the Kharif season of 2020. Rice progenies of the BC1F1 generation were phenotyped in pots alongside both parental lines to select drought-tolerant progenies at the vegetative stage based on drought tolerance degree (DTD) [17] and International Rice Research Institute (IRRI’s) score (IRRI’s scale, 2002) for leaf drying.
2.4. Generation Advancement
Selected progenies were backcrossed with the RP to produce the BC2F1 generation during the Kharif season of 2021. During the Rabi season of 2021–2022, BC2F1 progenies exhibiting drought tolerance were further selected at the phenotypic level and backcrossed to produce the BC3F1 generation. From this population, four rice lines with heterozygous alleles for RM431 were selected and allowed to self-pollinate to produce BC3F2 seeds during the Kharif season of 2022.
2.5. Field Evaluation
Genotypically selected positive rice lines of the BC3F2 generation were evaluated at the field level under moisture conditions (MC) during the Rabi season of 2022–2023. Subsequently, the BC3F3 generation rice lines were evaluated in upland areas with red soil during the Rabi season of 2022–2023 and in clay soil during the Kharif season of 2023 under non-flooding conditions.
2.6. Evaluation of Improved Rice Lines at Field Level Under MC and Flood Condition (FC)
During the Rabi season of 2022–2023, seedlings of four improved rice lines (RL-1, RL-2, RL-3, and RL-4) along with the RP (ADT36) were raised in plastic pots for 21 days. They were then transplanted into an experimental field with 5 cm standing water enriched with cow dung and green manure. Ten seedlings from each rice line, including the RP, were randomly planted with 10 × 15 cm spacing. After allowing the seedlings to establish under flooding conditions for 10 days, irrigation was suspended until the soil moisture tension reached −70 kPa at a depth of 30 cm. Subsequent irrigation was initiated on observation of leaf rolling, with water being drained after 24 h. This wetting and drying cycle was repeated until harvest. During the Kharif season of 2023, seedlings of the improved rice lines and RP were transplanted as described above and maintained under continuous flooding until seed harvest.
2.7. Measurement of Growth Parameters and Yield
In assessing the growth parameters and yield components of rice plants, several critical measurements were taken to evaluate the plant’s performance under different environmental conditions.
Plant height (PH): PH was determined by measuring from the base of the plant to the top of the highest spikelet on the panicle, excluding the awn. This measurement is crucial as it reflects the overall growth and vigor of the plant. Taller plants often indicate better growth conditions and genetic potential, though excessively tall plants can be prone to lodging (falling over), which can affect yield [18].
Root length (RL): RL was assessed by measuring the entire length of the root system, spanning from the base of the plant to the tip of the longest root. This parameter is crucial for evaluating the plant’s capacity to uptake water and nutrients from the soil. Longer roots can indicate a more robust and efficient root system, which is especially important for drought tolerance as deeper roots can access water from deeper soil layers [19].
Number of tillers (NT): The NT per plant was counted, including both productive (those bearing panicles) and non-productive tillers. This measurement provides insights into the plant’s vigor and its ability to branch out. A higher number of productive tillers generally correlates with a higher potential grain yield, as each tiller can produce a panicle [20].
Number of spikelet (NS): The NS per panicle was counted in sampled panicles. This parameter assesses the potential grain yield of the plant. More spikelet per panicle usually translate to a higher yield potential, provided that a significant proportion of these spikelets develop into fertile seeds [21].
Number of fertile seeds (NFS): The NFS, indicating filled grains, was counted in sampled panicles. Fertile seeds are those that have successfully developed and filled with grain, as opposed to empty or partially filled spikelet. This measurement is critical for evaluating the actual yield, as it reflects the plant’s reproductive success [22].
Seed setting percentage (SS%): The SS% was determined by calculating the ratio of fertile seeds to the total NS, serving as an indicator of the plant’s reproductive efficiency. The SS% was categorized according to the IRRI scale [23]: Scale 1 (more than 80%), scale 3 (61–80%), scale 5 (41–60%), scale 7 (11–40%), and scale 9 (<11%) [24].
2.8. Statistical Analysis
Statistical analyses were carried out using analysis of variance techniques. Mean comparisons were performed using the least significant difference method at a 5% significance level. All statistical analyses were conducted using Statistix 8.0 software and Microsoft Excel (2010).
3. RESULTS
3.1. Parental Polymorphism at Genotypic Level
In the parental polymorphism study, we found a difference in banding pattern between ADT36 and CR Dhan801and PCR band size was 280bp in ADT36 and 250bp in CRDhan801 [Figure 1].
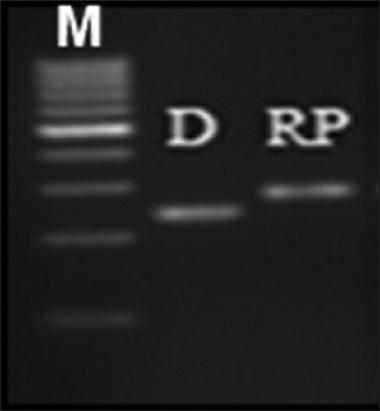 | Figure 1: Parental polymorphism at genotypic level using SSR marker RM431 linked with qDTY 1.1. M: Marker, D: Donor (CR Dhan 801), RP: Recurrent parent (ADT36). [Click here to view] |
Parental polymorphism study discloses that the genome of ADT36 rice variety differs from CRDhan801 for RM431 and is intolerant to drought stress at the reproductive stage. Likewise, in a previous study, RM431 has differentiated a drought-tolerant genotype, N22, which is harboring qDTY1.1 from intolerant rice cultivars, Swarna, IR64, and MTU1010 (Vikram et al., 2011 [11]). Using this marker, qDTY1.1 is incorporated into the genome of many mega rice varieties such as IR64, Swarna, MTU1010, etc. [11,25].
3.2. Selection of Drought-tolerant Progenies and Backcrossing with RP
In cross-pollination, we derived 43 F1 seeds from crosses between ADT36 and CRDhan801. From these, we identified 6 positive F1 plants (plant # 3, 4, 5, 6, 7, 10) having banding patterns in heterozygous conditions for RM431 in foreground selection [Figure 2].
 | Figure 2: Polymerase chain reaction amplification of F1 plants derived ADT36/CRDhan801 cross combination using SSR marker RM431 linked with DTY1.1 locus. M: 100 base pair DNA ladder, RP: ADT36, D: CR Dhan 801, Lane: 1–10 F1 plants from a cross between ADT36 and CR Dhan 801. [Click here to view] |
In four positive plants (# 3, 4, 5, 6) used for backcrossing with a RP, ADT36 (RP), 74 BC1F1 seeds were produced. In the phenotype selection under drought stress conditions, we observed the differential responses in three leaves for leaf drying, that is, highly resistant to highly susceptible reaction in 1st (from top) and 2nd leaf and susceptible to highly susceptible reaction in 3rd leaf. Progenies showing highly resistant or resistant reactions to stress accounted for a higher value for DTD and vice-versa. In phenotypic screening, lower and higher DTD value was noted in ADT36 (0.31) and CR Dhan801 (0.61), respectively. Among 52 progenies, the DTD value was noted in the range of 0.06–0.61, and from these, 7 progenies (plant # ADT36-2.5, 13; 4.5, 7, 16; 5.2; 8.2) were selected as drought tolerant based on higher DTD value than that of RP [Table 1].
Table 1: List of phenotypically selected plants from BC1F1 population under drought stress condition.
| BC1F1 population (ADT36/CR Dhan 801 combination) | ||||
|---|---|---|---|---|
| Rice progenies | Leaf position | DTD value | ||
| 1st Leaf score | 2nd Leaf score | 3rd Leaf score | ||
| CR Dhan 801(S) | 3 | 5 | 9 | 0.62 |
| ADT36 (S) | 7 | 5 | 9 | 0.31 |
| CR Dhan 801(C) | 0 | 0 | 0 | 1.00 |
| ADT36 (C) | 0 | 0 | 0 | 1.00 |
| ADT36-2.5 | 1 | 3 | 9 | 0.61 |
| ADT36-2.12 | 5 | 7 | 7 | 0.35 |
| ADT36-2.13 | 3 | 7 | 5 | 0.55 |
| ADT36-4.5 | 3 | 5 | 9 | 0.51 |
| ADT36-4.7 | 1 | 5 | 9 | 0.47 |
| ADT36-4.8 | 3 | 7 | 9 | 0.34 |
| ADT36-4.9 | 1 | 9 | 9 | 0.38 |
| ADT36-4.16 | 1 | 5 | 9 | 0.51 |
| ADT36-5.2 | 3 | 3 | 9 | 0.51 |
| ADT36-8.2 | 9 | 9 | 7 | 0.52 |
DTD: Drought tolerance degree
Similarly, in a previous study, Zu et al. [17] reported that 13 rice cultivars exhibiting the strongest drought tolerance in upland screening were identified based on higher DTD value. In this study, thus selected rice progenies with higher DTD value as drought tolerant reveal the effect of qDTY1.1 in the genetic background of ADT36 rice variety at the vegetative stage. Because some QTLs from a particular genetic background exhibit minor effects or remain silent in rice varieties with different genetic backgrounds [10,26,27]. Then, selected progenies of BC1F1 population were again backcrossed with RP and produced 92 BC2F1 seeds. In BC2F1 generation, 79 progenies were imposed drought stress at the vegetative stage along with both parents in pots. In this screening, the DTD values of ADT36 and CR Dhan 801 for drought tolerance were 0.43 and 0.76, respectively. In control, the DTD value of these parental lines was recorded as 1.0. In BC2F1 population, DTD value ranged from 0.33 to 0.66 and from these, 17 progenies (plant # ADT36-2-5.2, 3, 5, 7, 9, 14, 19; ADT36-2-13.7, 8, 11, 17; ADT36-2-4-5.3; ADT36-2-4-16.2, 6; ADT36-2-5-2.5; ADT36-2-8-2.4, 5) were selected based on higher DTD value than that of RP [Table 2].
Table 2: List of phenotypically selected rice plants from BC2F1 population under drought stress condition.
| BC2F1 population (ADT36/CR Dhan 801 combination) | ||||
|---|---|---|---|---|
| Rice progenies | Leaf position | DTD value | ||
| 1st Leaf score | 2nd Leaf score | 3rd Leaf score | ||
| CR Dhan 801 (S) | 0 | 0 | 5 | 0.76 |
| ADT36 (S) | 0 | 7 | 9 | 0.43 |
| CR Dhan 801 (C) | 0 | 0 | 0 | 1.00 |
| ADT36 (C) | 0 | 0 | 0 | 1.00 |
| ADT36-2-5.1 | 0 | 5 | 9 | 0.57 |
| ADT36-2-5.2 | 0 | 0 | 9 | 0.66 |
| ADT36-2-5.3 | 0 | 1 | 9 | 0.65 |
| ADT36-2-5.5 | 0 | 0 | 9 | 0.66 |
| ADT36-2-5.7 | 0 | 0 | 9 | 0.66 |
| ADT36-2-5.9 | 0 | 1 | 9 | 0.65 |
| ADT36-2-5.14 | 0 | 1 | 9 | 0.65 |
| ADT36-2-5.15 | 0 | 5 | 9 | 0.57 |
| ADT36-2-5-19 | 0 | 1 | 7 | 0.79 |
| ADT36-2-13.3 | 0 | 5 | 0 | 0.58 |
| ADT36-2-13.7 | 0 | 1 | 9 | 0.65 |
| ADT36-2-13-8 | 0 | 3 | 9 | 0.61 |
| ADT36-2-13-11 | 0 | 1 | 9 | 0.65 |
| ADT36-2-13-17 | 0 | 3 | 9 | 0.62 |
| ADT36-4-5.3 | 0 | 0 | 9 | 0.66 |
| ADT36-4-5.4 | 0 | 3 | 9 | 0.58 |
| ADT36-4-7.1 | 0 | 7 | 7 | 0.56 |
| ADT36-4-16.1 | 0 | 3 | 9 | 0.57 |
| ADT36-4-16.2 | 0 | 7 | 7 | 0.64 |
| ADT36-4-16.4 | 0 | 7 | 9 | 0.64 |
| ADT36-4-16.6 | 0 | 0 | 9 | 0.66 |
| ADT36-5-2.3 | 0 | 3 | 9 | 0.59 |
| ADT36-5-2.5 | 0 | 0 | 9 | 0.66 |
| ADT36-8-2.4 | 0 | 5 | 5 | 0.68 |
| ADT36-8-2.5 | 0 | 0 | 9 | 0.66 |
DTD: Drought tolerance degree
In this screening of BC2F1 population, the rate of DTD value was in the range of 0.33–0.79, respectively. From these, six plants (plant # ADT36-F12-5.5, ADT36-F12-5-19, ADT36-F14-5.3, ADT36-F15-2.5, ADT36-F18-2.4, ADT36-F18-2.5) having higher DTD value than that of RP (0.36) were selected. Following backcrossing of selected progenies, we produced 43 seeds for BC3F1 generation. Of these, 4 plants having heterozygous alleles were documented, and these plants were allowed for selfing to derive BC3F2 population [Figure 3].
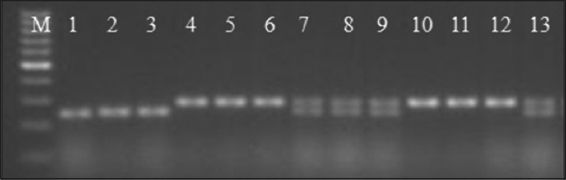 | Figure 3: Polymerase chain reaction amplification of BC3F1 progenies using RM431primer. M: 100 base pair DNA ladder; Lane 1–3: Donor line, Lane 4: ADT36 (RP); Lane 5–13: BC3F1 progenies derived from ADT36/CR dhan801 cross. [Click here to view] |
3.3. Growth and Yield Characteristics Under MC and FC
The growth and yield characteristics of four improved rice lines (RL-1, RL-2, RL-3, and RL-4) along with the RP (ADT36) were evaluated under both MC and FC during the Rabi season of 2022-23 and the Kharif season of 2023.
3.3.1. PH
In the field evaluation, significant differences were observed in plant growth parameters between MC and FC. In Table 3, the mean PH of parental and improved rice lines under MC and FC are compared. For the parental line (ADT36), the mean PH was 93.0 cm under FC and 71.0 cm under MC, reflecting a 23.6% reduction under MC. Improved lines under FC showed a mean PH range of 94.4–99.3 cm, with RL-4 being the tallest (99.3 cm) and RL-2 the shortest (94.4 cm). These lines exhibited up to 6.3% higher PH than the parental line under FC, with significant differences (P > 0.01 and P > 0.001), except RL-2, which showed no significant difference. Significant differences in PH were observed between RL-2 and RL-4 (P > 0.001) and between RL-3 and RL-4 (P > 0.01). Under MC, the mean PH of improved lines ranged from 67.2 to 73.0 cm, with RL-4 being the tallest (73.0 cm) and RL-1 the shortest (67.2 cm). These lines showed up to a 7.9% decrease in PH compared to the parental line, with RL-1 and RL-2 exhibiting a significant negative difference (P > 0.01). In addition, significant differences were found between RL-1 and RL-2 versus RL-3 and RL-4 (P > 0.01). The BC3F2 generation of improved lines exhibited a dwarf nature under MC compared to the RP. Overall, PH decreased by 32.3% under MC compared to FC, with a highly significant difference (P > 0.001).
Table 3: Mean value of plant height of parental and improved rice lines cultivated under moisture and flood condition.
| Rice lines | Flooding condition | Moisture condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RP (93.0) | RL1 (97.0) | RL2 (94.4) | RL3 (96.1) | RL4 (99.3) | RP (73.0) | RL1 (67.2) | RL2 (67.9) | RL3 (70.5) | RL4 (71.2) | |
| Flooding condition | ||||||||||
| RP (93.0) | 0 | 4.0** | 1.4ns | 3.1** | 6.3*** | |||||
| RL1 (97.0) | 0 | 3.4** | 0.9ns | 2.3* | 29.8*** | 29.1*** | 26.5*** | 25.8*** | ||
| RL2 (94.4) | 0 | 2.3* | 4.9*** | 27.2** | 26.5*** | 23.9*** | 23.2*** | |||
| RL3 (96.1) | 0 | 3.2** | 28.9*** | 28.2*** | 25.6*** | 24.9*** | ||||
| RL4 (99.3) | 0 | 32.1** | 31.4*** | 28.8*** | 28.1*** | |||||
| Moisture condition | ||||||||||
| RP (73.0) | 0 | 6.0*** | 4.9*** | 2.5* | 1.8ns | |||||
| RL1 (67.2) | 0 | 0.7ns | 3.3** | 4** | ||||||
| RL2 (67.9) | 0 | 2.6* | 3.3** | |||||||
| RL3 (70.5) | 0 | 0.7ns | ||||||||
| RL4 (71.2) | 0 | |||||||||
RP: Recurrent parent, RL: Rice line; (***) significantly different at P≤0.001, (**) significantly different at P≤0.01, (*) significantly different at P≤0.05, ns: Not significant
3.3.2. RL
Table 4 presents the mean RL of parental and improved rice lines under MC and FC, along with their significant differences. For the parental line under FC and MC, the mean RL was 19.2 cm and 11.1 cm, respectively, showing a 42.1% reduction under MC. In improved lines under FC, the mean RL ranged from 19.1 to 21.8 cm, with RL-3 having the longest (21.8 cm) and shortest (19.1 cm) RL. These lines showed up to an 11.9% increase in RL under FC compared to the parental line, with highly significant differences (P > 0.001), except for RL-3, which was non-significant. Under MC, the mean RL of improved lines ranged from 10.7 to 14.0 cm, with RL-1 having the longest (14.0 cm) and RL-4 the shortest (10.7 cm). These lines exhibited up to a 20.7% increase in RL compared to the parental line, with RL-1 showing a positive and highly significant difference (P > 0.001) compared to the parental line and other rice lines. Comparing RL in MC with FC, RL decreased by up to 43.7%, with all lines under FC showing positive and highly significant differences (P > 0.001) compared to MC. Root elongation was significant in many lines under FC but not under MC, except for RL-1.
Table 4: Mean value of root length of parental and improved rice lines cultivated under moisture and flood condition.
| Rice lines | Flooding condition | Moisture condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RP (19.2) | RL-1 (21.3) | RL-2 (21.8) | RL-3 (19.1) | RL-4 (20.6) | RP (11.1) | RL-1 (14.05) | RL-2 (10.85) | RL-3 (11.41) | RL-4 (10.75) | |
| Flooding condition | ||||||||||
| RP (19.2) | 0 | 2.1*** | 2.6*** | 0.1ns | 1.4*** | |||||
| RL-1 (21.3) | 0 | 0.5ns | 2.2*** | 1.3*** | 6.8*** | 10.45*** | 10.11*** | 11.45*** | ||
| RL-2 (21.8) | 0 | 2.7*** | 1.2*** | 7.75*** | 11.05*** | 10.41*** | 11.05*** | |||
| RL-3 (19.1) | 0 | 1.5*** | 5.05*** | 8.25*** | 7.69*** | 8.35*** | ||||
| RL-4 (20.6) | 0 | 6.55*** | 9.75*** | 9.19*** | 10.15*** | |||||
| Moisture condition | ||||||||||
| RP (11.1) | 0 | 2.95*** | 0.3ns | 0.31ns | 0.35ns | |||||
| RL-1 (14.05) | 0 | 3.2*** | 2.64*** | 3.3*** | ||||||
| RL-2 (10.85) | 0 | 0.56ns | 0.1ns | |||||||
| RL-3 (11.41) | 0 | 0.66* | ||||||||
| RL-4 (10.75) | 0 | |||||||||
RP: Recurrent parent, RL: Rice line, (***) Significantly different at P≤0.001, (**) Significantly different at P≤0.01, (*) Significantly different at P≤0.05, ns: Not significant
3.3.3. NTs
Table 5 presents the mean NTs for parental and improved rice lines under MC and FC, along with their significant differences. For the parental line, the mean NT under FC and MC was 23.5 and 2, respectively, showing a 91.5% reduction under MC. In improved lines under FC, the mean NT ranged from 23.9 to 27.6, with RL-3 having the highest (27.6) and RL-4 the lowest (23.9). These lines showed an increase in NT under FC by 1.67% to 14.8% compared to the parental line, with highly significant differences (P > 0.001), except for RL-4, which was non-significant. Under MC, the mean NT in improved lines ranged from 0.5 to 1.4, with RL-2 having the highest (1.4) and RL-4 the lowest (0.5). These lines exhibited up to a 75.0% decrease in NT compared to the parental line, with RL-1 and RL-4 showing negative and significant differences, while RL-2 and RL-3 were non-significant. Comparing NT in MC with FC, the reduction ranged from 94.9% to 97.9%, with differences being positive and highly significant (P > 0.001) between MC and FC.
Table 5: Mean value of number of tiller of parental and improved rice lines cultivated under moisture and flood condition.
| Rice lines | Flooding condition | Moisture condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RP (23.5) | RL-1 (24.3) | RL-2 (26.8) | RL-3 (27.6) | RL-4 (23.9) | RP (2.0) | RL-1 (1.2) | RL-2 (1.4) | RL-3 (0.8) | RL-4 (0.5) | |
| Flooding condition | ||||||||||
| RP (23.5) | 0 | 1.2** | 3.3*** | 4.1*** | 0.4ns | |||||
| RL-1 (24.3) | 0 | 2.5*** | 3.3*** | 0.4ns | 23.1*** | 22.9*** | 23.5*** | 3.8*** | ||
| RL-2 (26.8) | 0 | 0.8* | 22.9*** | 25.6*** | 25.4*** | 26.0*** | 26.3*** | |||
| RL-3 (27.6) | 0 | 3.7*** | 26.4*** | 26.2*** | 26.8*** | 27.1*** | ||||
| RL-4 (23.9) | 0 | 22.7*** | 22.5*** | 23.1*** | 23,4*** | |||||
| Moisture condition | ||||||||||
| RP (2.0) | 0 | 0.8* | 0.6ns | 0.2ns | 1.5** | |||||
| RL-1 (1.2) | 0 | 0.2ns | 0.4ns | 0.7ns | ||||||
| RL-2 (1.4) | 0 | 0.6ns | 0.9* | |||||||
| RL-3 (0.8) | 0 | 0.3ns | ||||||||
| RL-4 (0.5) | 0 | |||||||||
RP: Recurrent parent, RL: Rice line; (***) Significantly different at P≤0.001, (**) Significantly different at P≤0.01, (*) Significantly different at P≤0.05, ns: Not significant
3.3.4. NS
Table 6 presents the mean NS for parental and improved rice lines under MC and FC, along with their significant differences. In the parental line, the mean NS under FC and MC was 143.7 and 105.0, respectively, showing a 26.9% reduction under MC. In improved lines under FC, the mean NS ranged from 125.3 to 159.7, with RL-2 having the highest (159.7) and RL-4 the lowest (125.3). These lines showed an increase in NS under FC for RL-1 (4.4%), RL-2 (10.0%), and RL-3 (7.1%) compared to the parental line, with highly significant differences (P > 0.001), except for RL-4, which showed a negative and highly significant difference. Under MC, the mean NS in improved lines ranged from 79.1 to 83.5, with RL-4 having the highest (83.5) and RL-3 the lowest (79.1). In all lines, the NS rate decreased by 20.5% to 24.6% compared to the parental line, with negative and highly significant differences (P > 0.001). There were positive and highly significant differences between RL-1 and RL-3, as well as RL-3 and RL-4 (P > 0.001). Comparing NS in MC with FC, the reduction ranged from 41.9% to 44.9%, with negative and highly significant differences (P > 0.001).
Table 6: Mean value of number of spikelet of parental and improved rice linescultivated under moisture and flood condition.
| Rice lines | Flooding condition | Moisture condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RP (143.7) | RL-1 (150.4 | RL-2 (159.7) | RL-3 (154.7) | RL-4 (125.3) | RP (105) | RL-1 (82.5) | RL-2 (81.5) | RL-3 (79.1) | RL-4 (83.5) | |
| Flooding condition | ||||||||||
| RP (147.3) | 0 | 6.7*** | 12.4*** | 7.4*** | 22.0*** | |||||
| RL-1 (150.4) | 0 | 9.3*** | 4.7*** | 25.1*** | 67.9*** | 69.2*** | 71.3*** | 66.9*** | ||
| RL-2 (159.7) | 0 | 5.0*** | 34.4*** | 77.2*** | 78.5*** | 80.6*** | 76.2*** | |||
| RL-3 (154.7) | 0 | 29.4*** | 72.2*** | 73.2*** | 75.6*** | 71.2*** | ||||
| RL-4 (125.3) | 0 | 42.8*** | 43.8*** | 46.2*** | 41.8*** | |||||
| Moisture condition | ||||||||||
| RP (105.0) | 0 | 22.5*** | 23.5*** | 25.9*** | 21.5*** | |||||
| RL-1 (82.5) | 0 | 1.0ns | 3.4*** | 1.0ns | ||||||
| RL-2 (81.5) | 0 | 2.0* | 2.0* | |||||||
| RL-3 (79.1) | 0 | 4.4*** | ||||||||
| RL-4 (83.5) | 0 | |||||||||
RP: Recurrent parent, RL: Rice line, (***) Significantly different at P≤0.001, (**) Significantly different at P≤0.01, (*) Significantly different at P≤0.05, ns: Not significant
3.3.5. NFS
Table 7 presents the mean NFS for parental and improved rice lines under MC and FC, along with their significant differences. In the parental line, the mean NFS under FC and MC was 130.2 and 69.0, respectively, showing a 52.9% reduction under MC. In improved lines under FC, the mean NFS ranged from 105.5 to 143.4, with RL-3 having the highest (143.4) and RL-4 the lowest (105.5). These lines showed an increased NFS under FC for RL-1 (4.4%), RL-2 (10.0%), and RL-3 (7.1%) compared to the parental line, with positive and highly significant differences (P > 0.001), except for RL-4, which showed a negative and highly significant difference. Under MC, the mean NFS in improved lines ranged from 82.4 to 84.0, with RL-1 having the highest (84.0) and RL-2 the lowest (82.4). In all lines, the NFS rate increased by 16.3% to 17.8% compared to the parental line, with positive and highly significant differences (P > 0.001). Among improved lines, there was no significant difference under MC except between RL-1 and RL-3 (P > 0.05). Comparing NFS in improved lines under FC to those under MC, the NFS rate increased by 21.3% to 42.5%, with RL-1 (38.2%), RL-2 (39.6%), RL-3 (42.5%), and RL-4 (21.3%) showing positive and highly significant differences (P > 0.001).
Table 7: Mean value of number of fertile seed of parental and improved rice lines cultivated under moisture and flood condition.
| Rice lines | Flooding condition | Moisture condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RP (130.2) | RL-1 (135.9) | RL-2 (137.1) | RL-3 (143.3) | RL-4 (105.5) | RP (69.0) | RL-1 (84.0) | RL-2 (82.8) | RL-3 (82.4) | RL-4 (83.0) | |
| Flooding condition | ||||||||||
| RP (130.2) | 0 | 4.2*** | 6.9*** | 10.1*** | 24.7*** | |||||
| RL-1 (135.9) | 0 | 1.2ns | 7.5*** | 30.4*** | 51.9*** | 53.1*** | 53.5*** | 52.9*** | ||
| RL-2 (137.1) | 0 | 6.3*** | 31.6*** | 53.1*** | 54.8*** | 54.4*** | 54.1*** | |||
| RL-3 (143.4) | 0 | 37.9*** | 59.4*** | 60.6*** | 60.4*** | 60.4*** | ||||
| RL-4 (105.5) | 0 | 21.5*** | 22.7*** | 22.3*** | 22.5*** | |||||
| Moisture condition | ||||||||||
| RP (69.0) | 0 | 15.0*** | 13.8*** | 13.4*** | 14.0*** | |||||
| RL-1 (84.0) | 0 | 1.2ns | 1.6* | 1.0ns | ||||||
| RL-2 (82.8) | 0 | 0.4ns | 0.2ns | |||||||
| RL-3 (82.4) | 38.2 | 39.6 | 42.5 | 21.32 | 0 | 0.6ns | ||||
| RL-4 (83.0) | 0 | |||||||||
RP: Recurrent parent, RL: Rice line, (***) Significantly different at P≤0.001, (**) Significantly different at P≤0.01, (*) Significantly different at P≤0.05, ns: Not significant
4. DISCUSSION
The findings of this study on the field evaluation of improved rice lines with qDTY1.1 introgression into the ADT36 genetic background reveal significant advancements in enhancing rice productivity under varying environmental conditions, specifically moisture, and flood stress. These results align with previous studies that have investigated the role of qDTY1.1 in improving drought tolerance and grain yield in rice varieties. Earlier studies conducted by Vikram et al. [11] and Salomi et al. [28] have underscored the efficacy of qDTY1.1 in augmenting grain yield under drought stress, demonstrating its effectiveness across various genetic backgrounds. Our study corroborates these findings by demonstrating that the incorporation of qDTY1.1 into the ADT36 variety consistently improved traits such as PH, RL, NTs, spikelets, and fertile seeds under both MC and FC.
Drought stress triggers multiple physiological responses in rice, such as restricted leaf growth, decreased leaf area, leaf rolling, leaf desiccation, increased leaf thickness, premature senescence, stomatal closure, and the formation of a cutinized layer on the leaf surface [29-32]. These responses significantly impact rice growth and productivity, particularly affecting seed setting during the flowering stage due to spikelet sterility under severe water stress conditions [33]. The incorporation of qDTY1.1 into ADT36 effectively mitigated these adverse effects, resulting in improved yield and growth parameters.
Rice cultivation in the Cauvery Delta Zone (CDZ) of southern India is crucial, with farmers predominantly cultivating short and medium-duration varieties like those evaluated in our study. This preference stems from the need to mitigate water crises and capitalize on multiple harvests per year, aligning with findings by Arivelarasan et al. [34] and Arun et al. [35]. The reliance on monsoon rains for irrigation in this region makes it highly susceptible to cyclones and drought stress, underscoring the importance of drought-tolerant rice varieties like those developed with qDTY1.1.
Our study confirms the successful incorporation of qDTY1.1 along with sd-1, controlling PH in rice [11], into the genetic background of ADT36. Similar findings by Rai et al. [36] observed increased PH in improved lines under FC, suggesting a beneficial recombination effect. This aligns with studies by Rishav Kumar et al. [37], highlighting the potential of recombinant selection in retaining desirable traits across generations. The presence of sd-1 ensures shorter plant stature, which is advantageous for reducing lodging risks while maintaining robust growth and yield.
In addition, Ghimire et al. [13] reported consistent improvements in grain yield under drought conditions with qDTY1.1, indicating its heritability and significant differentiation from RPs [38]. Challenges in transferring QTLs across diverse genetic backgrounds [10,25,26] underscore the importance of selecting robust candidates like qDTY1.1 from N22, known for its major effect on grain yield enhancement under drought stress [11]. Recent advancements, such as the successful introgression of qDTY1.1 into modern rice varieties like ADT37 [28], further validate its utility and efficacy in improving grain yield under varying MC.
The observed improvements in PH, RL, NTs, spikelets, and fertile seeds indicate the successful incorporation of qDTY1.1 into ADT36, enhancing its overall performance under stress conditions. These enhancements are critical for sustaining rice production in the CDZ, a region highly vulnerable to erratic monsoon patterns. Future research should focus on further evaluating the field performance of these improved rice lines under a wider range of environmental conditions to confirm their stability and adaptability. Understanding the underlying genetic mechanisms of drought tolerance and integrating these improved varieties into sustainable agricultural practices through effective extension services and policy support is crucial for maximizing their impact. By enhancing our understanding and improving the resilience of rice to drought stress, we can contribute to the global challenge of ensuring food security in the face of changing climatic conditions.
5. CONCLUSION
In this study, the integration of qDTY1.1 into the genetic makeup of the ADT36 rice variety has demonstrated substantial enhancements in both grain yield and stress tolerance, particularly under conditions of moisture deficit and flooding. These results underscore the effectiveness of qDTY1.1 in bolstering rice productivity, especially in regions susceptible to drought stress, such as the CDZ in southern India. The consistent performance of these improved lines highlights the potential of marker-assisted breeding strategies for developing resilient rice varieties. Moving forward, further validation through extensive multi-location trials and wider adoption of these improved varieties through molecular techniques could significantly contribute to advancing sustainable rice farming and strengthening food security initiatives in drought-prone areas across the nation.