1. INTRODUCTION
Microbial biofilms are composed of up of closely-knit populations of bacteria that are affixed to surfaces and covered in the extracellular matrix in the environment. Amongst the greatest areas of interest are the ways in which microbes aggregate on a surface and how they can become resistant to pharmaceuticals. Microorganisms create a special structure called biofilm to live in severe environments, including those treated with existing antibiotics. Biofilms are any association of microorganisms where the cells adhere to one another on a surface and are often embedded in an extracellular polymeric substance (EPS) matrix that the organisms themselves produce. This substance, also known as slime, is primarily composed of exopolysaccharides and traces of other organic compounds such as proteins, DNA, and polysaccharides, and it provides a safe environment for the microorganisms to grow [1,2]. The concept of biofilm is originated in 1947 by Antonie van Leuwenhoek, using his primitive but effective microscope found aggregates of animalcule [3]. Nearly all surfaces, including those of medical equipment such as catheters, contact lenses, prosthetics, and surgical implants, frequently develop biofilm. These cells may colonize and spread from the contaminated devices, which could be harmful to human health and raise the possibility of microbial infection [2]. Certain traits of biofilm-forming bacteria include greater resistance to antibiotics [1,4]. The majority of the time, bacteria may coexist in mixed-species biofilms, which makes intra- and interspecies interactions more complex. The coexistence of different bacterial species affects the collective behavior in multispecies biofilms, where interspecies interactions are essential to the formation, morphology, and characteristics of the biofilms. Microbes immobilize themselves onto hard surfaces to produce biofilms, which are then utilized by a variety of surfaces [5–7]. In contrast to naturally existing bacteria, this technique of immobilization of microorganisms includes the adhesion of germs that are helpful for a variety of diverse functions [8,9]. The stability of biofilms, their toxicity tolerance against harmful chemicals, their efficiency in treating high volumetric loadings, and the simultaneous existence of anaerobic and anoxygenic metabolic processes within the same unit process are all important characteristics of biofilms that are possibly associated with wastewater treatment [10–12].
Any surface can produce biofilms in three primary stages. Cells attach to a surface in the first stage, assemble to create microcolonies, and then differentiate into a mature structure called a biofilm. After the complete development of biofilm, its disassembly or dispersion takes place through both mechanical and active processes [13–15]. External factors that affect the formation process include temperature, pH, gravitational and hydrodynamic pressures, Brownian motions, the type of surfaces that are inhabited, quorum sensing (QS), secondary messengers, and other signaling molecules. The microbes then proliferate and integrate into a network made of extracellular polymeric molecules that it has created by assembling its own parts. The formation of biofilms, especially during the early attachment stages, is facilitated by a number of surface-related proteins, including OmpA, fibronectin binding proteins, 31 protein A, 32 SasG, 33, 34, biofilm-associated protein (BAP), 35, 36, and numerous additional elements. Some species cannot attach to a surface but can anchor themselves to the matrix or directly to the earlier colonies. Small signaling molecules with the help of cell-cell communication systems mediate this colonization [16,17].
On the other hand, the presence of surface proteins has been observed during the first stages of bacterial adhesion to the biofilm matrix [18]. The location of the biofilm is the most important element influencing its growth. It is possible for biofilms to grow in almost any place where there is moisture and a surface that has at least a modest nutrition supply [4,2,19]. The options are unlimited, but they can be categorized into a number of groups that have been well-researched [20]. The selection of biofilms from them, some of which occur naturally, and others have been influenced by human intervention [21].
The uncontrollably persistent nature of microbial infections is caused by persistent cells and antibiotic resistance, both of which are facilitated by the development of biofilms [22]. Infections that are persistent and recurring are caused by biofilm, which confers increased resistance to antibiotics and becomes resistant to host immune responses. It greatly complicates the therapeutic management of biofilm infections. The most likely causes of antibiotic resistance are decreased antibiotic molecule penetration through EPS, target site mutation, buildup of enzymes that degrade antibiotics, and increased expression of efflux pump genes [23]. Biofilms can be found practically anywhere and are associated with a variety of clinical symptoms. They can be found in living tissues, water channels, pipes, hospital floors, food processing units, and other biotic and abiotic surfaces [24]. Biofilm-associated bacteria are characterized by changes in phenotypic and gene expressions together with resistance to recognized antibiotics, decreased metabolic activity and growth rate, and production of virulence-associated proteins [25].
The scientific community has given biofilm-coated electrodes for microbial fuel cells (MFC)-based bioelectricity production a great deal of attention. It is impossible to overlook the fact that MFC technology appears to be a partial answer to the current energy issue. MFCs are a sustainable energy source that can reliably power modern society while also treating wastewater. This technology can be utilized to provide solutions for powering household appliances, other electrical equipment, and recharging biomedical devices since it recognizes the potential for large-scale conversion of organic waste and biomass into bioenergy [26].
The United Nations established the 2030 sustainable development goals in January 2016 with the aim of attaining progress in the areas of the environment, society, and economy by utilizing cleaner and more environmentally friendly industrial techniques. Since the majority of people still lack access to basic essentials like food, clothing, shelter, and health care despite the rapid growth of the global economy, these aims' most important objectives are the fulfillment of basic human needs and desires. Furthermore, biofilm-producing microbes have a detrimental impact on a variety of food business sectors, including aquaculture, dairy, poultry, and ready-to-eat foods [27]. This can lead to food spoiling, disease outbreaks, and fatalities. Contaminants accumulate in milk processing equipment due to inadequate sanitization and cleaning, which leads to the formation of biofilm, which further becomes a major source of dairy product contamination. Due to the high frequency of biofilm-associated microorganisms and the ineffectiveness of the available antibiotics, it is necessary to develop non-toxic but highly effective antibiofilm agents that target signaling pathways that control a variety of biological processes, including QS, EPS synthesis, biofilm-related genes, microbial motility, adhesion, dispersion, and many others [24]. It will be helpful to examine all the traits connected to biofilm production in order to identify novel inhibitors for the treatment of biofilm and biofilm-forming illnesses. As a result, the microbial biofilm, its properties, and the range of surfaces on which it can grow are the main topics of this review article.
2. BIOFILMS ON SURFACES
It is possible for biofilms to grow in almost any place where there is moisture and a surface that has at least a modest nutrition supply. The options are unlimited, but they can be divided into a number of groups that have been well-researched. Biofilms have chemical and physical characteristics that can be studied. The biofilm matrix develops when the polymeric extracellular substances secreted by the organisms consist of proteins, polysaccharide macromolecules, lipids, nucleic acids, and other biopolymers [28]. They are highly hydrophilic molecules, as they form a three-dimensional (3D) [29]. It is possible for biodiversity to exist inside a biofilm because of the creation of the matrix along with homogeneous gradients, which provides a range of microhabitats. When microorganisms move from a free-living, i.e., nomadic stage to a multicellular sedentary state, continued development results in the establishment of organized communities characterized by cellular differentiation. Biofilm production happens as a result of extracellular environmental cues as well as signals produced by the organism itself [2,30–32]. Researchers who have looked into the production of biofilms have come to the conclusion that it is likely to create a global hypothetical model to describe how they arise.
There are five phases to this biofilm growth model: Individual plankton bacteria migrate and stick to the surface during the first phase [33]. The connected bacteria begin to build biofilms with a thin layer of exopolymeric material when the right conditions are met. A bacterial aggregation and matrix formation result from connected bacteria secreting extracellular matrix (EPS) and adhering to the surface during the second phase. Biofilms completely mature in the third phase, when they build water channel structures and microcolonies and becoming increasingly layered. Finalized biofilms attain the maximum density of cells and operate as three-dimensional communities during the fourth stage. Mature biofilms release bacterial microcolonies from the main population during the fifth phase, which allows the infection to spread to new locations. Antibiotics find it challenging to pierce the matrix and eradicate the buried bacteria because of these biofilms [4] (Fig. 1).
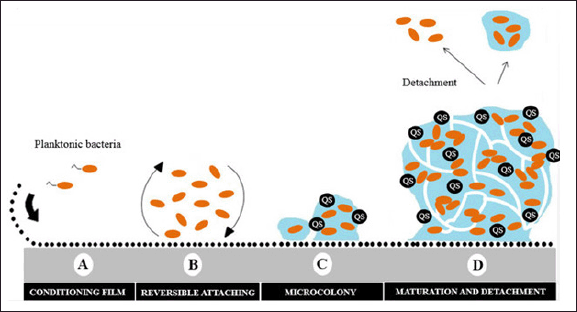 | Figure 1. Schematic representation of biofilm development on a solid surface. Adapted from Moura et al. [120]. [Click here to view] |
Individual microorganisms are placed on a surface in the case of mobile species, and this marks the beginning point for a major shift in their way of existence, from nomadic free to sedentary. Therefore, diverse constructions such as pilus, cilia, flagella, and fimbria, as well as sticky compounds, contribute to the development of the matrix, hence, the movement capability is reduced. In both scenarios (non-mobile and mobile microorganisms), tiny masses or microcolonies are created, resulting in increased cell-cell contact, cells clustered together are more likely to undergo adaptive phenotypic changes as a result of their increased cell-to-cell contact [34] (Table 1).
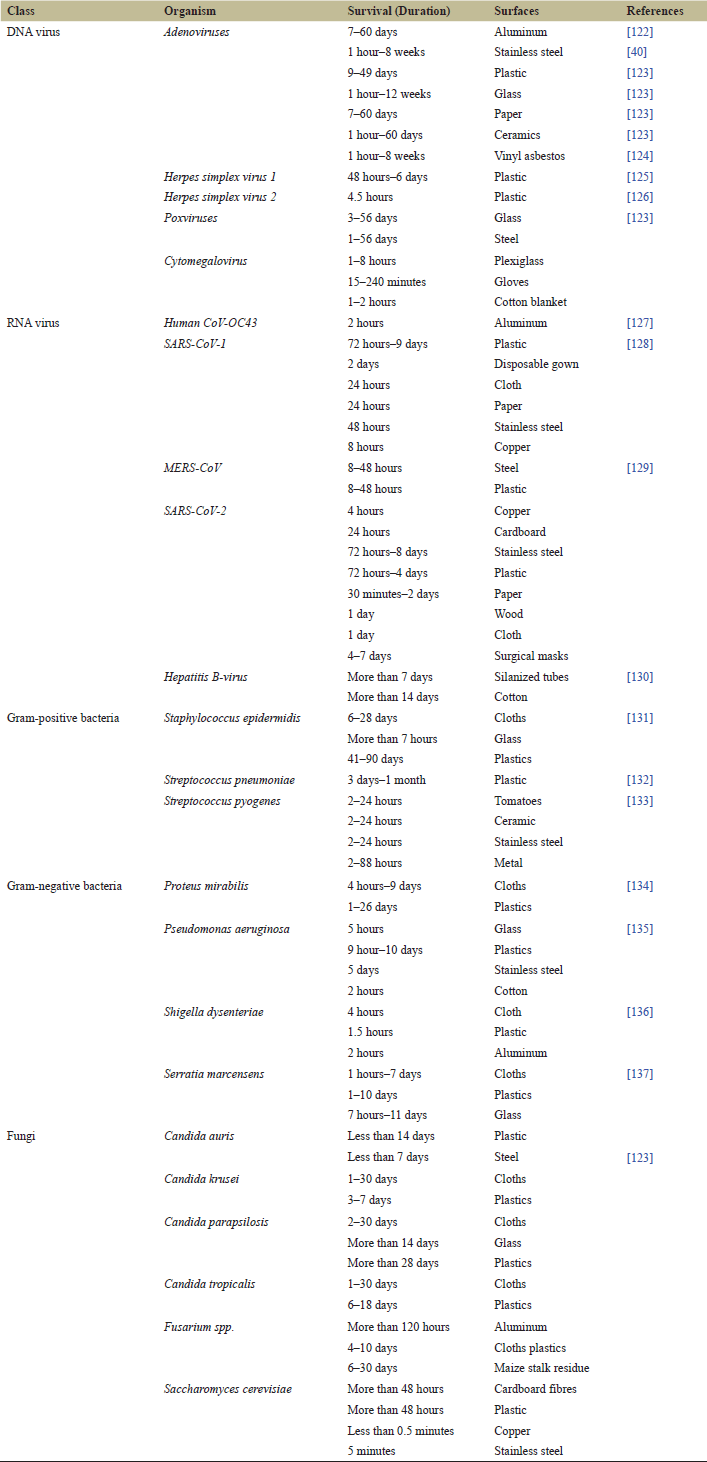 | Table 1. Survival of various microbes on different surfaces. [Click here to view] |
Hence, the formation of a monolayer resulted in the development of microcolonies in multi-layered systems. The creation of EPS starts, followed by the establishment of the first monolayer and the subsequent growth of the second and third layers. The formation of the extracellular matrix and the development of the 3D biofilm are two important steps [32]. Finally, the biofilm achieves its mature stage, exhibiting the existence of channels via which nutrients, water, communication chemicals, and nucleic acids may be transported [29]. The biofilm matrix keeps and holds the cells together, allowing for a greater degree of contact, intercellular communication, and the development of synergistic consortia to occur. Therefore, the cells of the biofilm cannot be totally immobilized as they have the ability to move inside it and to get disconnected from it.
Biofilms may also be classified as follows, depending upon the environment in which they are formed, such as natural, industrial, domestic, and hospitable [35]. It is also dependent on the kind of interface where they are created. They may be classified into the following categories, depending on the kind of contact at which they are created [36]. The genus Lactobacillus is composed of acidophilic bacteria that denature the proteins in dentin. Moreover, the genus Actinomyces contains bacteria that are aciduric and proteolytic in nature, such as Actinomyces viscosus, Actinomyces odontoliticus, and Actinomyces naeslundii, which are three of the species that have been identified. Biofilms are also present in a solution of black water such that treating home wastewater by nitrifying microorganisms can help in oxidizing nitrite, ammonium, and autotrophic nitrifying bacteria that dwell in biofilms adhering to tubes [37].
When it comes to the ammonium-oxidizing bacteria in these biofilms, the dominating species belongs to the genus Nitrosomonas, which can be found in abundance over the whole biofilm matrix [38]. The bulk of the components in this group of nitrite oxidants are members of the genus Nitrospira, which are found in the biofilm's interior. Unlike other types of biofilms, subaerial types of biofilms (SABs) are identified by patchy development on rock-solid material surfaces or urban structures. These biofilms include dominating families of algae, fungus, heterotrophic bacteria, protozoa, cyanobacteria, and tiny animals, among other microbes and fungi. SAB biofilms, are home to chemolithotrophic bacteria, which are capable of using inorganic mineral compounds as a source of food and energy [39,40].
The fact that the mineral–SAB interface affects ecosystem-scale processes like primary production, the stability and productivity of food webs, and biogeochemical cycling is also becoming more and more evident. These processes are governed by microscale interactions that take place within the mineralosphere. Thus, the ecological interactions between minerals and SABs within the framework of ecosystem function potentially reflect some of the most significant associations in dry terrestrial settings and land colonization, supporting a fundamental and pivotal shift in the development of microbes [41].
The Earth's crucial zone, a small layer where physical, chemical, and biological processes interact to support life on Earth, is home to the mineral SAB air interaction system [42]. The SAB's color, a ubiquitous phenotypic trait in the microbial communities near the mineral–air interface, is crucial for determining the nature and function of these survival strategies. SAB communities exhibit the functional ability to sustain a self-sustaining community at the community level, even in the face of the aforementioned circumstances and low biomass. The current suite of omics-based technologies can be fully utilized to fully understand the complicated complexity of interspecies interactions in SABs [41]. SAB contains a variety of microbial species, such as Blastococcus, Modestobacter, Apatococcus, Spirosoma, Rubellimicrobium, Thuepera, Deinococcus, Coccomyxa, Rubrobacter, Chroococcidiopsis, Halococcus, Kocuria, Salinimicrobium, Pontibacter, Halobacterium, Marinobacter, Halomarina, and Thuepera. Truepera, Chroococcidiopsis, Rubrobacter, Capnobotryella, Scytonema, Thiobacillus, Malikia, Ochrobactrum, Knufia, Leptolyngbya, Sarcinomyces, Nitrogenbacter, Thioclava, Thiobacillus, Rhodovulum, Desulfuromonas, Chroococcidiopsis, Leptolyngbya, Nostoc, Trebouxiophyceae, Nitrososphaera, Nitrospira, Novosphingobium, Nitrobacter, Stenotrophomonas, Pseudomonas, Crosiella, Rhodobacter, Aurantiamonas, Acidimicrobium, Ferrimicrobium Bacillus, Phormidium, Aurantiamonas, Thiobacillus, and Thioclava [43]. In a study, photocatalytically reactive subaerial surfaces revealed the presence of novel fungus strains recognized as Constantinomyces oldenburgensis [44].
Many of the bacteria that are known to be the causal agents of human illness may be found living in biofilms [45,46]. Vibrio parahaemolyticus, Vibrio cholerae, Vibrio fischeri, Streptococcus mutans, and Legionella pneumophyla are only a few of the bacteria that may cause disease [47]. Another class of microbes is present in venous catheters used in hospitals. Explanted central venous catheter biofilms constitute an incredible variety of gram-negative bacteria and gram-positive bacteria, as well as other microorganisms, which have been identified from the biofilm. Furthermore, biofilms formed by gram-positive bacteria have been found in venous catheters by several scientific studies, including Corynebacterium spp. Enterococcus faecium spp., Enterococcus spp., Staphylococcus spp. Staphylococcus aureus, and Streptococcus spp. [48].
In the world, we wonder how biofilms interfere with the functioning of industry, and we can say that they cause clogs in pipes, damage to equipment, interference with processes like heat transmission while covering exchanger surfaces, and corrosion of metal components. The formation of film in the food sector has the potential to cause serious public health and operational issues [49]. Pathogens associated with biofilms have the potential to infect food items with pathogenic microorganisms, resulting in major public health consequences for consumers. Flagella and membrane proteins are utilized by this pathogen in the early stages of biofilm development [50,51].
Food-borne diseases can result from infections or intoxications linked to bacterial biofilms on food matrixes or industrial equipment. Plants that digest food have biofilms that can produce toxins. As a result, plenty of food-borne bacteria might attach themselves to the contact surfaces found in these places, raising the possibility of bacterial food-borne disease [52]. From there, they have the potential to contaminate a food matrix, leading to one or more intoxications (in the event of an outbreak). Its potential as a foodborne pathogen in a number of food groups, including water, milk, meat, fruits, and vegetables, has been underappreciated. Food safety issues can arise from the use of chemical preservatives, which are frequently employed to inhibit the growth of microbes found in food sources [53].
Biofilm-associated diseases encompass both tissue- and device-related infections, such as endocarditis, meningitis, kidney infections, periodontitis, osteomyelitis, rhinosinusitis, and nonhealing chronic wounds [54]. The European Centre for Disease Prevention and Control reported that up to 37,000 persons die as a result of healthcare-associated infections, which affect approximately 4.1 million patients yearly on average in European hospitals [55]. The National Institutes of Health estimates that biofilm-forming microbes are responsible for around 65% and 80% of human acute and chronic infections, respectively [56–58]. As biofilms are formed on steel surfaces of slicing machines, preventing them from being cut. Biofilms formed by Listeria monocytogenes have been found in liquid milk and dairy products obtained from milk in the dairy sector. The presence of dairy wastes in containers, tanks, pipes, and other equipment encourages the formation of biofilms by this pathogen, which utilizes the residues as accessible nutrition [59,60]. It is possible to find bacterial biofilms in food industry facilities, like on floors and drains, as well as on food surfaces like vegetables, fruits, meats, and in low-acid dairy products such as yogurt [61,62].
When Pseudomonas aeruginosa produces extracellular chemicals, they are utilized in the production of the polymeric matrix that adheres to a significant quantity of inorganic materials, such as stainless steel, resulting in the formation of a biofilm. Pseudomonas may cohabit in a biofilm with other dangerous bacteria, such as Salmonella and Listeria, and this is known as coexistence [34]. They are the initial causative agents of bacterial etiology and outbreaks of foodborne illness because they are the most prevalent. Several scientific investigations have shown that Salmonella may attach itself to concrete, plastic surfaces, steel, and food processing plant facilities, forming biofilms on these surfaces [63].
One of the key elements in the development and maintenance of the structure and properties of the biofilm is the extracellular matrix. The extracellular matrix is made up of water and EPS, primarily polysaccharides, proteins, and DNA [64]. The rdar morphotype, so called because of the red, dry, and rough look of colonies formed on agar plates containing Congo red dye, has been identified as the most well-studied biofilm phenotype for Salmonella. Congo red concentrates within the rdar colony due to the presence of cellulose, the β1-4-linked glucose polymer, and proteinaceous curli fimbriae, which are functional amyloid structures resistant to pH, detergents, and proteases. Together, curli and cellulose serve as the extracellular matrix scaffold, facilitating short-range connections between cells and long-range interactions spanning the colony's whole length. It has been demonstrated that BapA, a large Salmonella protein with several repeating sequences, contributes to the pellicles' strength and integrity [65] (Fig. 2).
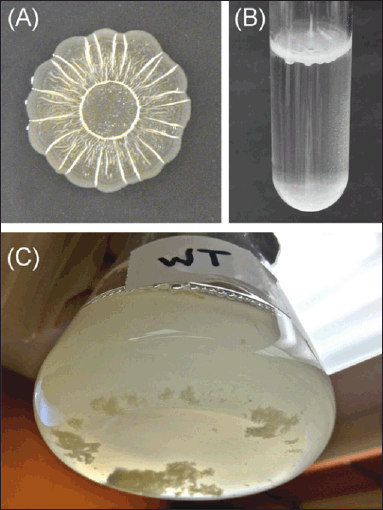 | Figure 2. Examples of Salmonella biofilm formation. (A) Colonies grown for 48 hours at 28°C on solid 1% tryptone media form the characteristic surface patterns of the red, dry, and rough (rdar) morphotype. The colony appears red when the media is supplemented with the dye Congo red. (B) Pellicle formation at the air–liquid interface of a 1% tryptone liquid culture. (C) Salmonella form multicellular aggregates and planktonic cells within the bulk liquid phase of a flask culture. Adapted from MacKenzie et al. [65]. [Click here to view] |
2.1. Surface-Associated Growth
The mechanism followed by microbes for adherence differs depending upon the method of attachment. There are three phases to the production of biofilms: early, medium, and late. Reversible and irreversible adhesion steps make up early stage biofilms. Planktonic bacteria use surface appendages like flagella and pili to approach and connect to the surface during the reversible adhesion stage. The bacteria-surface interaction can be readily overcome by the bacteria's motility, allowing them to revert to their planktonic condition. To covalently connect to the surface and gradually complete the firm attachment, initially attached bacteria release EPS during the irreversible adhesion stage [66]. EPSs are used by connected bacteria to attach to surface-associated cells, and they can also aggregate via type IV pili-mediated twitching motilities in the early stages. Bacteria grow in number and release more EPSs, which eventually coat the bacteria's surface in a thin layer of water and produce microcolonies [67]. Microcolonies develop into mature colonies. The final phase involves the biofilms reaching maturity and separating. When a biofilm reaches maturity, its compact structure and coordinated functions resemble a 3D network structure. Once fully developed, biofilms burst, allowing bacteria to spread out into planktonic forms and initiating a fresh cycle of biofilm formation. A similar report documented that Pseudomonas fluoresces has been observed on glass surfaces [68]. Further, in the case of continuous culture growth, kinetics on the surface differs from that of the bulk phase. In the case of high dilution rates, the productivity of microbes increases by itself [69,70]. It happens because of the fact that microbes still remain intact to the surface, i.e., beyond the maximum dilution rate. Bacteria in huge amounts can be utilized as a buffer to reimburse the biomass loss as it changes dilution rates.
2.2. Surface Properties and Mechanisms Used by Bacteria for Sensing Surfaces
The bacteria follow a chemotaxis system to measure the concentration of ions and small molecules and to study the mechanism affecting bacterial mobility. A study suggested that bacteria can sense variations in spatial changes in particular conditions [71]. The intriguing topic of "How does a microbe know it is on a surface?" is raised by the fact that the initial stage in the formation of a bacterial biofilm is contact with the surface on which the microbe would eventually build this community. For decades, researchers studying biofilms have been deeply intrigued by this query. According to Zobell and Allen [72] theory, bacteria may require a bacterial film or nutrients to convey the signal that allows them to choose an appropriate surface for attachment. Swarms and biofilms are examples of bacterial communities where cells interact with one another in a variety of ways.
The term "surface sensing" refers to a wide range of behaviors, such as the mechanisms underlying the device that permits the perception of a surface's close proximity, the device that selects various surfaces for attachment, the biochemical chain reaction, and the physical effects that ensue from surface recognition. Bacterial communities with tight cell packing allow for a concentration rise of tiny molecules that facilitate information flow between cells and cause physiological changes [73]. Certain bacteria changed how they produced polysaccharides and even how their cells looked. Numerous other physiological variations have been discovered thanks to developments in high-throughput screening techniques, global transcriptome and proteome analysis of bacteria, and identification of the genes necessary for biofilm formation. In addition, modifications in the extracellular polysaccharide and organelle formation that take place in bacteria in response to the biofilm community's presence and expansion linked with a surface [71].
The development of chemical gradients near surfaces facilitates the chemical information transmission between biofilms and surface-attached communities. Comparing biofilms to planktonic cells that are free to float in liquids, there is also an increase in lateral gene transfer. It should come as no surprise that surface sensing has historically been interpreted differently depending on the type of microorganism. Although the mechanics of surface sensing in microbes have not been thoroughly studied, this subject has been discussed in the literature using a range of model microorganisms [74]. Additionally, surface-associated growth induces phenotypes that promote “natural competence” in Vibrio cholerae. Myxobacteria cells that are associated with biofilms even exchange outer membrane proteins and lipids [75] (Figs. 3 and 4).
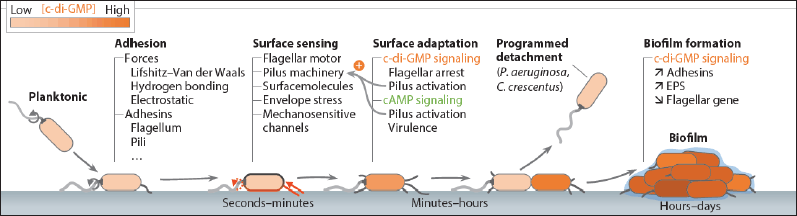 | Figure 3. Timeline of bacterial surface adaptation leading to biofilm formation. Adopted from Laventie and Jenal [121]. [Click here to view] |
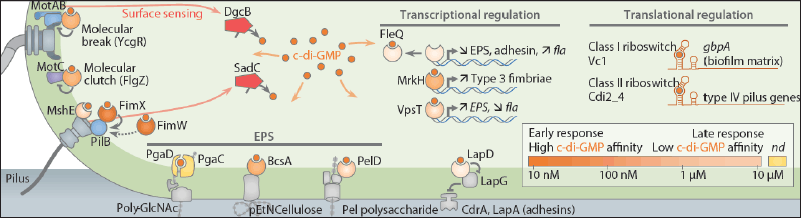 | Figure 4. Overview of surface-sensing and adaptation mechanisms. Adopted from Laventie and Jenal [121]. [Click here to view] |
McCarter et al. [76] concluded that Vibrio parahaemolyticus has a "flagellar dynamometer," or a mechanism by which, upon surface contact or in conditions of high viscosity, decreased rotation of the polar flagellum starts a signal transduction pathway that in turn causes swarming motility with lateral flagella. The concept behind this model is that when this appendage binds to the substratum, it restricts the rotation of the flagella, signaling that the microbe has made contact with a surface. This conclusion is corroborated by the finding that planktonic cell incubation in a highly viscous liquid also initiates the “surface” programme. Staphylococcus aureus makes strong binding with surface ligands to receptors on one side of the cell surface and further responds by localizing the receptors to the associated surface. It is also reported that cells have the ability to sense and allow spatial changes that modify the attachment of ligands to receptors by surrounding the receptors in nearby regions [77]. This indicates the ability of bacteria to recognize signals from different subsets where the receptor lies. This study truly explains the association of bacteria and chemical gradients during the formation of biofilm. As E. coli attaches to the surface, the pH shifts in decreasing order reaches below the bulk liquid phase, and stays for at least 72 hours. A Cpx two-component system plays a critical role in maintaining cell surfaces and pH-sensing responses [78].
Moreover, similar works have been concluded that E. coli controls the assembly of pili and regulates its expression [79]. Another study showed sensing of osmolality in gram-negative bacteria by using OmpA which brings variation in other genes that are involved in transcription. Furthermore, repression in cellulose production is regulated by the OmpA gene via the Cpx pathway and enhances the formation of E. coli [80–82]. The presence of extracellular fibrils is polymeric in nature that involved in attaching bacteria to different surfaces [83]. Water and EPS, mainly polysaccharides, proteins, and DNA, make up the extracellular matrix. Identification of the matrix's constituent parts is necessary for its characterization, as is the calculation of the relative concentration of each EPS component and an explanation of their physicochemical characteristics and interactions. Infrared spectroscopy examination of biofilm provides details on the chemical composition of the matrix and the relative amounts of various EPS. The biofilm's reactivity to several EPS-targeting hydrolytic enzymes provides information about the matrix's composition and the roles that matrix constituents play in maintaining the structure's integrity. Determining the matrix composition can also be accomplished through the extraction and purification of EPS from the biofilm using both chemical and physical methods [84].
Another study revealed that anionic material could be used for attaching freshwater bacteria and cations, which influences contractions in initial adhesives, thereby decreasing the distance between the cell and substratum [85]. Hence, cross-linking of cations with anionic polymer leads to contraction. However, a study suggests the role of lectins in inhibiting attachment, whereas glucosidases reduce attachment for Pseudomonas fluorescence. Also, lectins showed affinity to bind with polysaccharides on the cell walls and would decrease the attachment sites [86]. Another study revealed the effect of polysaccharides on studies and interaction with P. fragi [87]. Furthermore, a study on non-motile and motile strains of P. fluorescens depicted increased cell attachment and flow in motile strains as compared to non-motile. The study also showed vacant seed areas that no longer recognize substratum as mobile strains, which results in the formation of biofilm by non-motile organisms. This suggests a critical role of flagella attachment during the early stages, thereby turning off the force exerted by the substratum [88].
Further findings on different cell surfaces like EPS, LPS, proteins, and fimbriae display an essential role in the processes of attachment. There are different cell surfaces having nonpolar sites that are attached to hydrophobic substrata, whereas lipopolysaccharides play an essential role in attachment to materials that are hydrophilic in nature.
The hydrophobic–hydrophilic structure of interacting surfaces is a key factor in microbial adhesion, as demonstrated by a body of experimental evidence. Enhanced hydrophobicity of the cell surface may facilitate surface approaching and activate the specific forces responsible for the irreversible adhesion. There was an increase in cell-to-cell adhesion when bacteria became more hydrophobic; hence, cell surface hydrophobicity may have contributed to the cells' immobilizing power [89]. The role of hydrophobic interactions between microorganisms and supports in the microbial adhesion process has not received much attention in research to date, and little data is currently available to characterize quantitatively how much the hydrophobicities of bacterial and support surfaces contribute to microbial adhesion. A model that describes how microbial adherence depends on the system's relative hydrophobicity was created using the idea of the relative hydrophobicity of cell-to-support interaction. The suggested model has the ability to establish a clear link between microbial adherence and the surface thermodynamics related to hydrophobicity. It was found that increased cell surface hydrophobicity would favor cell adhesion on both hydrophilic and hydrophobic support surfaces [90].
However, the role of flagella is only highlighted to surpass the opposing forces rather than to perform as adhesives [91]. Therefore, the attachment will take place on surfaces that are more hydrophobic, rougher, and coated by surface “conditioning” films. An increament in water temperature, flow velocity, and nutrient concentration also adds to the increased attachment. Cell surface properties, mostly the presence of surface-associated polysaccharides, flagella, and fimbriae, are essential and can possibly give a competitive advantage for one organism where a mixed community is present [46,92].
Bacterial cell appendages adhere to surfaces when they get close to them. Flagella facilitate adhesion; they stick especially to hydrophobic surfaces because they are hydrophobic. Adhesion depends on both the rotational ability and the presence of flagella, since E. coli mutants without functioning flagella have trouble forming biofilms and separate more quickly than the wild-type. On the other hand, it was discovered that the presence of flagella decreased adherence in Caulobacter crescentus, demonstrating the intricacy of the adhesion mechanism. Once connected, flagella which result from impeded rotation can communicate with the cell to indicate surface contact [93].
3. PHYSICAL SURFACE PROPERTIES OF VIRAL PERSISTENCE
In 1892, the discovery of the first virus was accomplished [94]. Efforts have led to understand the viral survival in various environments and to evaluating the impact of surface properties on their viability. Various factors influencing surface properties are absorption, porosity, surface hydrophobicity, and so on. All viruses have their own way of interacting with the surface in a unique way. So, there is no prerequisite for designing a specific type of virus with an altered design having a specialized antiviral surface. The persistence of the virus is influenced by a number of factors that not only include environmental conditions but are also altered by relative humidity, temperature, and how they differ in absorbing onto different surfaces. These factors can be considered in designing an antiviral surface [95,96].
Biofilms have already been recognized as a common cause of bacterial infections from the perspective of public health [46]. Additionally, generated EPS has been proposed as a potential defense against viruses, particularly phage penetration, in biofilms [97]. Recent research has shown that viral particles can enter the EPS structure of mucoid biofilms even in the absence of particular enzyme processes. Once inside the polymeric matrix, the viruses may benefit from the unique "biofilm lifestyle" and defence against environmental stressors such desiccation or other antimicrobial agent effects [46]. Furthermore, protected immobilized viral particles may be released into the environment by biofilm erosion or sloughing. These particles will then come into touch with their intended host, starting the viral infectious cycle. The speeds at which viruses attach to biofilms can differ significantly and rely on a variety of parameters, including the properties of the biofilm or virus (size, shape, and isoelectric point), as well as the concentration of viral particles [98].
According to a variety of studies, biofilms have the ability to capture and hold onto virus-sized particles, creating a possible reservoir for bacterial or human infections. Biofilms are seen in natural settings where microbial cultures are typically composed primarily of prokaryotes with a little amount of eukaryotes. Though there has been experimental evidence of virus attachments to biofilms and very little pathogenic virus contamination of natural biofilms, biofilms should be viewed as a reservoir of protection from pathogenic viruses, which may be the cause of many chronic viral infections. Various studies have been reported the antiviral surface properties that show virus absorbance [95]. Also, absorbent surfaces like cardboard and cotton provide more protection against droplets containing the virus. A study reported the survival of SARS-CoV on two different personal protective equipment (PPE) gowns were in the hospital, gowns were in the hospital, which are cotton and fluid-repelling disposable gowns. The results confirmed the presence of virus droplets absorbed by cotton cloth, and there was no evidence of viable virus after 1 hour. However, the persistence of the virus was seen on disposable gowns after 24 hours. Further, an outer fluid layer in medical devices and PPE gowns can offer more advantages [99]. Another study on SARS-CoV-1 and SARS-CoV-2 showed virus persistence on cardboard at 21°C–23°C and 40% humidity as compared to stainless steel and plastic [100].
A significant role is played by porous inmate surfaces in the survival of the virus and studies have distinguished the time and persistence of viruses on different types of surfaces, such as porous and non-porous surfaces [101]. There are some reports which suggest longer persistence of the virus on non-porous materials than on porous surfaces, but few exceptions still exist. A study on the influenza A virus reported that in the case of humid conditions (35%–40%), the virus stayed longer than 24–48 hours on plastic and stainless-steel surfaces [102]. However, on porous surfaces, the number of particles was less after 8–12 hours, such as paper or cloth. A study concluded from the observations that because of complete drying on porous surfaces resulted in less virus persistence. The report further suggested the persistence of the SARS-CoV-2 virus on a surgical mask even after 7 days, while no virus was detected on surfaces like plastic or stainless steel after a week [31,103].
Surface hydrophobicity factor can also alter viral persistence on different surfaces [104]. It is also known that the outer layer hydrophobicity of proteins present in capsids can alter interactions with the environment and solid surfaces [105]. An understanding can be developed through these interactions, which is essential for regulating and designing antiviral strategies and environmental transmission. Different computational and environmental experiments have helped in determining the hydrophobicity of viruses [106]. Various studies have been reported the sorption of hydrophobic viruses on surfaces coated with hydrophobic sorbents preferred by viruses having hydrophobic protein outer layers. However, hydrophilic surfaces are favored by hydrophilic viruses for absorption [107].
Biofouling can be defined as the colonization of microorganisms such as bacteria in the aquatic environment [108]. An understanding of this process and how it can be prevented has been a keen interest in various biofilm studies, yet it still lacks more research so far [109]. Microorganisms like barnacles, mollusks, encrusting bryozoans, and tube worms are a few examples of calcareous fouling microbes, whereas non-calcareous fouling organisms include hydroids, seaweed, and slime [110]. It poses a serious threat to the maintenance of mariculture, cooling large industrial equipment by repeated water cycles. This phenomenon occurs in oil pipelines carrying oil, cutting oils, and hydraulic oils. The attachment of microorganisms can be prevented using nontoxic anti-sticking coatings, which are made of organic polymers [111].
4. MOLECULAR BIOLOGY PROCEDURES
Earlier, scanning confocal laser microscopy was used to scan the specimen in one plane using a laser beam. Also, the image is processed and analyzed by a computer. Nowadays, various reports display a variety of molecular methods to study the composition and diversity of biofilm communities [112]. Techniques such as hybridization with 16S/23S rRNA probes can be used to characterize bacteria forming biofilms in oil fields, trickling filters, and drinking water [113]. Furthermore, if biofilm structures can be preserved, then taxonomic types can be identified through their distribution, and the characterization of individuals will be possible within the community. Different approaches, diversity, and composition of a community are applied in a hydrothermal vent system, such as microbial mats made from sea sediments and wastewater treatment reactors. A study concluded by Muyzer and Ramsing [114] reported that, hydrothermal vent biofilms in an experiment where restriction fragment length polymorphism analysis was carried out using 16S rRNA genes.
Microelectrodes are very beneficial in providing information on biofilm activity and structure. A precision current is passed to provide a spatial resolution of concentration in the range of 25–100 [115]. Measurements from the microsensor include pH, sulfide, and oxygen. Moreover, physiological processes can be evaluated by measuring environmental and nutrient gradients from the sensor. These evaluations can help to link the chemical microenvironment in the presence of specific taxonomy of organisms. The levels of hydrogen sulfide, along with pH and oxygen gradients, are analyzed using microelectrodes followed by cold freezing the samples in liquid nitrogen. Later, the section probed with fluorescently labeled phylogenetic 16S rRNA probes [116].
5. NEW OPPORTUNITIES FOR MATERIAL SCIENTISTS, CHEMISTS, AND ENGINEERS
An understanding of microbes with different surfaces is yet not much studied. This topic deals with multidisciplinary approaches as it creates a platform for microbiologists, chemists, material scientists, and engineers to collaborate and study different areas such as classifying properties of surfaces sensed by microbes, exploring molecular mechanism and their biochemical responses to sense various surfaces, determining how to alter surface properties by changing morphology and varying energetics to get the desired response. The area that holds an advantage from the development of physical sciences is the conditioning layer of protein that promotes bacterial attachment to a surface. However, conditioning layers can lead to the rendering of surface chemistry, which results in the short lifespan of antimicrobial surfaces [117]. Studies require bacterial-surface interactions to prevent the formation of conditioning layers. So, engineers, along with material scientists, can solve this issue but might face difficulty in measuring cellular responses, at the time of microbe-surface interaction, i.e., changes in gene expression. However, with advancing technology, fluorescent reporters can be measure varying levels of gene expression. A study was conducted to measure the yellow fluorescent protein expression, which controls the changes in the gene coding for flagellin protein [118]. Nowadays, surface-enhanced Raman scattering helps in localizing peptide-guided nanoparticles to the bacterial membrane and exploring the chemistry of how bacterial communities and genetic profiling work. The role of microbes surface sending is not well understood at the cell biology, biochemistry, and physical chemistry levels. The use of physically and chemically defined substrates, along with the latest biochemical and analytical techniques, can help us guide applications in the fields of biomedicine, food safety, industrial processing, and agriculture [119].
6. CONCLUSION
The intricate process of bacterial adhesion and biofilm formation is governed by the interaction of topographical surface features, physicochemical, mechanical, and environmental factors. This review offers an in-depth overview and understanding of the characteristics that influence bacterial adhesion. The effects of various surface characteristics, bacterial motility, or the surrounding hydrodynamic conditions on the bacterial sensing and binding behavior on surfaces have not been taken into account in a large portion of the studies conducted to date. Crucially, before bacteria bind, bare surfaces are really covered in conditioning films of organic and inorganic materials. This has a substantial impact on the binding behaviors of bacteria. Thus, research projects that assess the effect of many surface parameters on bacterial adhesion are essential to improve understanding rather than focussing on a single surface characteristic and its effect on adhesion. Moreover, the identification of strategies and mechanisms that biofilms adapt to evade powerful antibiotics and the application of environmentally benign biological, physical, and chemical techniques to disrupt biofilm communities are equally noteworthy. Surface nanopatterning and its hybrid approach with bactericidal chemicals hold considerable potential to offer more sophisticated treatments for biofilm-related fouling in commercial or medical fields.
7. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
10. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
11. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
12. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
REFERENCES
1. Dohare S, Singh D, Sharma D, Agarwal V. Effect of Staphylococcus epidermidis on Pseudomonas aeruginosa biofilm in mixed-species culture. J Exp Biol Agric Sci 2021;9:325–34. CrossRef
2. Singh D, Pandey H, Singh V. Natural products that target cancer stem cells. In: Pandurangan AK, Anandasadagopan SK, Alhumaydhi FA (eds.). Handbook of research on natural products and their bioactive compounds as cancer therapeutics, IGI Global, Hershey, PA, pp 169–86, 2022; CrossRef
3. Girmaye D, Abdeta D, Tamiru Y. Review on bacterial biofilms and its impact. Int J Adv Microbiol Health Res 2018;2:22–30.
4. Kalia M, Singh D, Sharma D, Narvi SS, Agarwal V. Senna alexandriana mill as a potential inhibitor for quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Pharmacogn Mag 2020;16:797–802. CrossRef
5. Hadjiev D, Dimitrov D, Martinov M, Sire O. Enhancement of the biofilm formation on polymeric supports by surface conditioning. Enzyme Microb Technol 2007;40:840–8. CrossRef
6. Nicolas M, Beito B, Oliveira M, Tudela Martins M, Gallas B, Salmain M, et al. Strategies for antimicrobial peptides immobilization on surfaces to prevent biofilm growth on biomedical devices. Antibiotics 2021;11:13. CrossRef
7. Skerlavaj B, Boix-Lemonche G. The potential of surface-immobilized antimicrobial peptides for the enhancement of orthopaedic medical devices: a review. Antibiotics 2023;12:211. CrossRef
8. Alonso VPP, Lemos JG, Nascimento M. Yeast biofilms on abiotic surfaces: adhesion factors and control methods. Int J Food Microbiol 2023;400:110265. CrossRef
9. Alonso VPP, Gonçalves MPM, de Brito FAE, Barboza GR, Rocha LO, Silva NCC. Dry surface biofilms in the food processing industry: an overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods. Compr Rev Food Sci Food Saf 2023;22:688–713. CrossRef
10. Yang S, Ngwenya BT, Butler IB, Kurlanda H, Elphick SC. Coupled interactions between metals and bacterial biofilms in porous media: implications for biofilm stability, fluid flow and metal transport. Chem Geol 2013;337:20–9. CrossRef
11. Funari R, Shen AQ. Detection and characterization of bacterial biofilms and biofilm-based sensors. ACS Sens 2022;7:347–57. CrossRef
12. Saini S, Tewari S, Dwivedi J, Sharma V. Biofilm mediated wastewater treatment: a comprehensive review. Mater Adv 2023;4:1415–43. CrossRef
13. Paula AJ, Hwang G, Koo H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat Commun 2020;11:1354. CrossRef
14. Sharma S, Mohler J, Mahajan SD, Schwartz SA, Bruggemann L, Aalinkeel R. Microbial biofilm: a review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023;11:1614. CrossRef
15. Pal MK, Lavanya M. Microbial influenced corrosion: understanding bioadhesion and biofilm formation. J Bio Tribo-Corros 2022;8:76. CrossRef
16. Su Y, Yrastorza JT, Matis M, Cusick J, Zhao S, Wang G, et al. Biofilms: formation, research models, potential targets, and methods for prevention and treatment. Adv Sci 2022;9:2203291. CrossRef
17. Schulze A, Mitterer F, Pombo JP, Schild S. Biofilms by bacterial human pathogens: clinical relevance-development, composition and regulation-therapeutical strategies. Microb Cell 2021;8:28. CrossRef
18. Lasa I, Penadés JR. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol 2006;157:99–107. CrossRef
19. Jackson A, Murphy R, Underwood A. Biofilms on rocky shores: influences of rockpools, local moisture and temperature. J Exp Mar Bio Ecol 2013;443:46–55. CrossRef
20. Kushwaha A, Kumar V, Agarwal V. Pseudomonas quinolone signal induces organelle stress and dysregulates inflammation in human macrophages. Biochim Biophys Acta 2023;1867:130269. CrossRef
21. Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019;5:e02192. CrossRef
22. Michaelis C, Grohmann E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023;12:328. CrossRef
23. Singh A, Amod A, Pandey P, Bose P, Pingali MS, Shivalkar S, et al. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed Mater 2022;17:022003. CrossRef
24. Uruén C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020;10:3. CrossRef
25. Dutt Y, Dhiman R, Singh T, Vibhuti A, Gupta A, Pandey RP, et al. The association between biofilm formation and antimicrobial resistance with possible ingenious bio-remedial approaches. Antibiotics 2022;11:930. CrossRef
26. Naaz T, Kumar A, Vempaty A, Singhal N, Pandit S, Gautam P, et al. Recent advances in biological approaches towards anode biofilm engineering for improvement of extracellular electron transfer in microbial fuel cells. Environ Eng Res 2023;28:220666. CrossRef
27. Firoze S, Sami H, Azhar A, Asaad M, Khan PA, Khan HM. Microbial biofilms and the role of biotechnology as a solution. In: Ahmad F, Mohammad ZH, Ibrahim SA, Zaidi S (eds.). Microbial biotechnology in the food industry: advances, challenges, and potential solutions, Springer, Cham, Switzerland, pp 187–240, 2024; CrossRef
28. Hublikar LV, Ganachari SV, Raghavendra N, Banapurmath NR, Patil VB, Yunus Khan T, et al. Biogenesis of silver nanoparticles and its multifunctional anti-corrosion and anticancer studies. Coatings 2021;11:1215. CrossRef
29. Velichko N, Grinev V, Fedonenko Y. Characterization of biopolymers produced by planktonic and biofilm cells of Herbaspirillum lusitanum P6-12. J Appl Microbiol 2020;129:1349–63. CrossRef
30. López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol 2010;2:a000398; CrossRef
31. Chauhan V, Rakshit A, Dhiman VK, Mahajan G, Pnadey A, Kanwar SS, et al. Effectiveness of the vaccine (Covaxin®) on different age groups of people: a pilot study. Vacunas 2023;25:152–60. CrossRef
32. Singh D, Sharma D, Agarwal V. Screening of anti-microbial, anti-biofilm activity, and cytotoxicity analysis of a designed polyherbal formulation against shigellosis. J Ayurveda Integr Med 2021;12:601–6. CrossRef
33. Kragh KN, Tolker-Nielsen T, Lichtenberg M. The non-attached biofilm aggregate. Commun Biol 2023;6:898. CrossRef
34. Dohare S, Singh D, Sharma D, Agarwal V. The effect of Staphylococcus epidermidis cells on Pseudomonas aeruginosa-associated virulence factors. J Appl Biol Biotechnol 2021;9:122–7. CrossRef
35. Carrascosa C, Raheem D, Ramos F, Saraiva A, Raposo A. Microbial biofilms in the food industry—a comprehensive review. Int J Environ Res Public Health 2021;18:2014. CrossRef
36. Yang J, Monnot M, Ercolei L, Moulin P. Membrane-based processes used in municipal wastewater treatment for water reuse: state-of-the-art and performance analysis. Membranes 2020;10:131. CrossRef
37. Yu OY, Zhao IS, Mei ML, Lo ECM, Chu CH. Dental biofilm and laboratory microbial culture models for cariology research. Dent J 2017;5:21. CrossRef
38. Elumalai P, Parthipan P, AlSalhi MS, Huang M, Devanesan S, Karthikeyan OP, et al. Characterization of crude oil degrading bacterial communities and their impact on biofilm formation. Environ Pollut 2021;286:117556. CrossRef
39. Vázquez-Nion D, Fuentes E, Prieto B. Effect of inorganic carbon concentration on the development of subaerial phototrophic biofilms on granite. Coatings 2020;10:1049. CrossRef
40. Zhu HZ, Jiang CY, Liu SJ. Microbial roles in cave biogeochemical cycling. Front Microbiol 2022;13:950005. CrossRef
41. Villa F, Cappitelli F. The ecology of subaerial biofilms in dry and inhospitable terrestrial environments. Microorganisms 2019;7:380. CrossRef
42. Villa F, Wu YL, Zerboni A, Cappitelli F. In living color: pigment-based microbial ecology at the mineral–air interface. Bioscience 2022;72:1156–75. CrossRef
43. Vázquez-Nion D, Rodríguez-Castro J, López-Rodríguez M, Fernández-Silva I, Prieto B. Subaerial biofilms on granitic historic buildings: microbial diversity and development of phototrophic multi-species cultures. Biofouling 2016;32:657–69. CrossRef
44. Ruibal C, Selbmann L, Avci S, Martin-Sanchez PM, Gorbushina AA. Roof-inhabiting cousins of rock-inhabiting fungi: novel melanized microcolonial fungal species from photocatalytically reactive subaerial surfaces. Life 2018;8:30. CrossRef
45. Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020;9:59. CrossRef
46. Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, Jin X, et al. Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol 2020;11:928. CrossRef
47. Yildiz FH, Visick KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol 2009;17:109–18. CrossRef
48. Nair N, Biswas R, Götz F, Biswas L. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect Immun 2014;82:2162–9. CrossRef
49. Srey S, Jahid IK, Ha SD. Biofilm formation in food industries: a food safety concern. Food Control 2013;31:572–85. CrossRef
50. Giacomucci S, Cros CDN, Perron X, Mathieu-Denoncourt A, Duperthuy M. Flagella-dependent inhibition of biofilm formation by sub-inhibitory concentration of polymyxin B in Vibrio cholerae. PLoS One 2019;14:e0221431. CrossRef
51. Salazar-Sánchez A, Baztarrika I, Alonso R, Fernández-Astorga A, Martínez-Ballesteros I, Martinez-Malaxetxebarria I. Arcobacter butzleri biofilms: insights into the genes beneath their formation. Microorganisms 2022;10:1280. CrossRef
52. Liu X, Yao H, Zhao X, Ge C. Biofilm formation and control of foodborne pathogenic bacteria. Molecules 2023;28:2432. CrossRef
53. Galié S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F. Biofilms in the food industry: health aspects and control methods. Front Microbiol 2018;9:898. CrossRef
54. Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018;4:e01067. CrossRef
55. Latour K, Jans B, Cookson B, Moro M, Ricchizzi E, MacKenzie D, et al. Surveillance report: point prevalence survey of healthcare-associated infections and antimicrobial use in European long-term care facilities 2010. 2014.
56. Yaacob MF, Murata A, Nor NHM, Jesse FFA, Yahya MFZR. Biochemical composition, morphology and antimicrobial susceptibility pattern of Corynebacterium pseudotuberculosis biofilm. J King Saud Univ Sci 2021;33:101225. CrossRef
57. Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003;2:114–22. CrossRef
58. Barzegari A, Kheyrolahzadeh K, Hosseiniyan Khatibi SM, Sharifi S, Memar MY, Zununi Vahed S. The battle of probiotics and their derivatives against biofilms. Infect Drug Resist 2020;13:659–72. CrossRef
59. Byun KH, Kim HJ. Survival strategies of Listeria monocytogenes to environmental hostile stress: biofilm formation and stress responses. Food Sci Biotechnol 2023;32:1–21. CrossRef
60. Ribeiro AC, de Almeida FA, Medeiros MM, Miranda BR, Pinto UM, Alves VF. Listeria monocytogenes: an inconvenient hurdle for the dairy industry. Dairy 2023;4:316–44. CrossRef
61. Grigore-Gurgu L, Bucur FI, Borda D, Alexa EA, Neagu C, Nicolau AI. Biofilms formed by pathogens in food and food processing environments. In: Dincer S, Ozdenefe MS, Arkut A (eds.). Bacterial biofilms, IntechOpen, London, UK, pp 1–32, 2019; CrossRef
62. Olanbiwoninu A, Popoola B. Biofilms and their impact on the food industry. Saudi J Biol Sci 2022;30(2):103523. CrossRef
63. Thi MTT, Wibowo D, Rehm BH. Pseudomonas aeruginosa biofilms. Int J Mol Sci 2020;21:8671. CrossRef
64. Costa RC, Bertolini M, Costa Oliveira BE, Nagay BE, Dini C, Benso B, et al. Polymicrobial biofilms related to dental implant diseases: unravelling the critical role of extracellular biofilm matrix. Crit Rev Microbiol 2023;49:370–90. CrossRef
65. MacKenzie KD, Palmer MB, Köster WL, White AP. Examining the link between biofilm formation and the ability of pathogenic Salmonella strains to colonize multiple host species. Front Vet Sci 2017;4:138. CrossRef
66. Fu J, Zhang Y, Lin S, Zhang W, Shu G, Lin J, et al. Strategies for interfering with bacterial early stage biofilms. Front Microbiol 2021;12:675843. CrossRef
67. Wang W, Yan Y, Zhao Y, Shi Q, Wang Y. Characterization of stratified EPS and their role in the initial adhesion of anammox consortia. Water Res 2020;169:115223. CrossRef
68. Caldwell D, Korber D, Lawrence J. Analysis of biofilm formation using 2D vs 3D digital imaging. J Appl Bacteriol 1993;74:52S–66S. CrossRef
69. Legner M, McMillen DR, Cvitkovitch DG. Role of dilution rate and nutrient availability in the formation of microbial biofilms. Front Microbiol 2019;10:916. CrossRef
70. Brück HL, Coutte F, Dhulster P, Gofflot S, Jacques P, Delvigne F. Growth dynamics of bacterial populations in a two-compartment biofilm bioreactor designed for continuous surfactin biosynthesis. Microorganisms 2020;8:679. CrossRef
71. Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol 2008;6:199–210. CrossRef
72. Zobell CE, Allen EC. The significance of marine bacteria in the fouling of submerged surfaces. J Bacteriol 1935;29:239–51. CrossRef
73. O’Toole GA, Wong GC. Sensational biofilms: surface sensing in bacteria. Curr Opin Microbiol 2016;30:139–46. CrossRef
74. Tuson HH, Weibel DB. Bacteria–surface interactions. Soft Matter 2013;9:4368–80. CrossRef
75. Pathak DT, Wei X, Bucuvalas A, Haft DH, Gerloff DL, Wall D. Cell contact–dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genet 2012;8:e1002626. CrossRef
76. McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell 1988;54:345–51. CrossRef
77. Ekerdt BL, Segalman RA, Schaffer DV. Spatial organization of cell-adhesive ligands for advanced cell culture. Biotechnol J 2013;8:1411–23. CrossRef
78. Xu Y, Zhao Z, Tong W, Ding Y, Liu B, Shi Y, et al. An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat Commun 2020;11:1496. CrossRef
79. Claret L, Miquel S, Vieille N, Ryjenkov DA, Gomelsky M, Darfeuille-Michaud A. The flagellar sigma factor FliA regulates adhesion and invasion of Crohn disease-associated Escherichia coli via a cyclic dimeric GMP-dependent pathway. J Biol Chem 2007;282:33275–83. CrossRef
80. Ma Q, Wood TK. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol 2009;11:2735–46. CrossRef
81. Cho TH, Wang J, Raivio TL. NlpE is an OmpA-associated outer membrane sensor of the Cpx envelope stress response. J Bacteriol 2023;205:e00407–22. CrossRef
82. Yaeger LN, French S, Brown ED, Côté JP, Burrows LL. Central metabolism is a key player in E. coli biofilm stimulation by sub-MIC antibiotics. PLoS Genet 2023;19:e1011013. CrossRef
83. Chagnot C, Listrat A, Astruc T, Desvaux M. Bacterial adhesion to animal tissues: protein determinants for recognition of extracellular matrix components. Cell Microbiol 2012;14:1687–96. CrossRef
84. Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol 2018;4:274. CrossRef
85. Fletcher M, Lessmann JM, Loeb GI. Bacterial surface adhesives and biofilm matrix polymers of marine and freshwater bacteria. Biofouling 1991;4:129–40. CrossRef
86. Chemani C, Imberty A, de Bentzmann S, Pierre M, Wimmerová M, Guery BP, et al. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect Immun 2009;77:2065–75. CrossRef
87. Heredia-Ponce Z, de Vicente A, Cazorla FM, Gutiérrez-Barranquero JA. Beyond the wall: exopolysaccharides in the biofilm lifestyle of pathogenic and beneficial plant-associated Pseudomonas. Microorganisms 2021;9:445. CrossRef
88. Jain A, Gupta Y, Agrawal R, Jain SK, Khare P. Biofilms-A microbial life perspective: a critical review. Crit Rev Ther Drug Carrier Syst 2007;24:393–443. CrossRef
89. Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res 2015;94:1027–34. CrossRef
90. Liu Y, Yang SF, Li Y, Xu H, Qin L, Tay JH. The influence of cell and substratum surface hydrophobicities on microbial attachment. J Biotechnol 2004;110:251–6. CrossRef
91. BinAhmed S, Hasane A, Wang Z, Mansurov A, Romero-Vargas Castrillo?n S. Bacterial adhesion to ultrafiltration membranes: role of hydrophilicity, natural organic matter, and cell-surface macromolecules. Environ Sci Technol 2018;52:162–72. CrossRef
92. Elisabeth ZOM. The mechanisms of bacterial biofilm inhibition and eradication: the search for alternative antibiofilm agents. In: Das T (ed.). Focus on bacterial biofilms, IntechOpen, London, UK, 2022; CrossRef
93. Kimkes TE, Heinemann M. How bacteria recognise and respond to surface contact. FEMS Microbiol Rev 2020;44:106–22. CrossRef
94. Taubenberger JK, Hultin JV, Morens DM. Discovery and characterization of the 1918 pandemic influenza virus in historical context. Antivir Ther 2007;12:581–91. CrossRef
95. Rakowska PD, Tiddia M, Faruqui N, Bankier C, Pei Y, Pollard AJ, et al. Antiviral surfaces and coatings and their mechanisms of action. Commun Mater 2021;2:53. CrossRef
96. Bregnocchi A, Jafari R, Momen G. Design strategies for antiviral coatings and surfaces: a review. Appl Surf Sci Adv 2022;8:100224. CrossRef
97. Visnapuu A, Van der Gucht M, Wagemans J, Lavigne R. Deconstructing the phage–bacterial biofilm interaction as a basis to establish new antibiofilm strategies. Viruses 2022;14:1057. CrossRef
98. Vasickova P, Pavlik I, Verani M, Carducci A. Issues concerning survival of viruses on surfaces. Food Environ Virol 2010;2:24–34. CrossRef
99. Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open 2020;3:e203976. CrossRef
100. Bueckert M, Gupta R, Gupta A, Garg M, Mazumder A. Infectivity of SARS-CoV-2 and other coronaviruses on dry surfaces: potential for indirect transmission. Materials 2020;13:5211. CrossRef
101. Yu CC, Hlaing T, Tun KM. Influencing factors for the persistence of SARS-CoV-2 (COVID-19) exposed in environmental matrices and disinfection methods: systematic review. Int J Community Med Public Health 9(8) 3304–14. CrossRef
102. Weber TP, Stilianakis NI. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect 2008;57:361–73. CrossRef
103. Chauhan V, Pandey A, Rakshit A, Mahajan G, Kanwar SS, Dhiman VK, et al. Musculoskeletal and neuromuscular dysfunction due to covid-19 infection: a review. Vacunas 2023;25(3):355–66; CrossRef
104. Owen L, Shivkumar M, Cross RB, Laird K. Porous surfaces: stability and recovery of coronaviruses. Interface Focus 2021;12:20210039. CrossRef
105. Gerba CP. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol 1984;30:133–68. CrossRef
106. Xu F, Tanaka S, Watanabe H, Shimane Y, Iwasawa M, Ohishi K, et al. Computational analysis of the interaction energies between amino acid residues of the measles virus hemagglutinin and its receptors. Viruses 2018;10:236. CrossRef
107. Sellaoui L, Badawi M, Monari A, Tatarchuk T, Jemli S, Dotto GL, et al. Make it clean, make it safe: a review on virus elimination via adsorption. Chem Eng J 2021;412:128682. CrossRef
108. Varin T, Lovejoy C, Jungblut AD, Vincent WF, Corbeil J. Metagenomic analysis of stress genes in microbial mat communities from Antarctica and the high Arctic. Appl Environ Microbiol 2012;78:549–59. CrossRef
109. Karunakaran E, Mukherjee J, Ramalingam B, Biggs CA. “Biofilmology”: a multidisciplinary review of the study of microbial biofilms. Appl Microbiol Biotechnol 2011;90:1869–81. CrossRef
110. Reyes R, del Norte-Campos A, Añasco NC, Santander-de Leon SMS. Biofouling development in marine fish farm influenced by net colour, immersion period and environmental conditions. Aquac Res 2020;51:3129–38. CrossRef
111. Wol JO, Gorb SN. Attachment structures and adhesive secretions in arachnids. Springer, New York, NY, 2016.
112. Briones A, Raskin L. Diversity and dynamics of microbial communities in engineered environments and their implications for process stability. Curr Opin Biotechnol 2003;14:270–6. CrossRef
113. Poulsen LK, Ballard G, Stahl DA. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol 1993;59:1354–60. CrossRef
114. Muyzer G, Ramsing NB. Molecular methods to study the organization of microbial communities. Water Sci Technol 1995;32:1–9. CrossRef
115. Okabe S, Satoh H, Watanabe Y. Analysis of microbial structure and function of nitrifying biofilms. In: Doyle RJ (ed.). Methods in enzymology, Elsevier, pp 3–469, 2001. CrossRef
116. Lacroix EM, Aeppli M, Boye K, Brodie E, Fendorf S, Keiluweit M, et al. Consider the anoxic microsite: acknowledging and appreciating spatiotemporal redox heterogeneity in soils and sediments. ACS Earth Space Chem 2023;7:1592–609. CrossRef
117. Maan AM, Hofman AH, de Vos WM, Kamperman M. Recent developments and practical feasibility of polymer-based antifouling coatings. Adv Funct Mater 2020;30:2000936. CrossRef
118. Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, et al. A novel factor controlling bistability in Bacillus subtilis: the YmdB protein affects flagellin expression and biofilm formation. J Bacteriol 2011;193:5997–6007. CrossRef
119. McNay G, Eustace D, Smith WE, Faulds K, Graham D. Surface-enhanced Raman scattering (SERS) and surface-enhanced resonance Raman scattering (SERRS): a review of applications. Appl Spectrosc 2011;65:825–37. CrossRef
120. Moura MC, Napoleão TH, Paiva PM, Coelho L. Bacterial biofilms: the structure, development and potential of plant compounds for alternative control. Adv Med Biol 2017;122:1–34.
121. Laventie BJ, Jenal U. Surface sensing and adaptation in bacteria. Ann Rev Microbiol 2020;74:735–60. CrossRef
122. Zhuang Y, Zhu J, Shi L, Fu Q, Hu H, Huang Q. Influence mechanisms of iron, aluminum and manganese oxides on the mineralization of organic matter in paddy soil. J Environ Manag 2022;301:113916. CrossRef
123. Wißmann JE, Kirchhoff L, Brüggemann Y, Todt D, Steinmann J, Steinmann E. Persistence of pathogens on inanimate surfaces: a narrative review. Microorganisms 2021;9:343. CrossRef
124. Kasperson RE. Corporate management of health and safety hazards: a comparison of current practice. Routledge, Abingdon, UK, 2019. CrossRef
125. Castaño N, Cordts S, Jalil MK, Zhang K, Koppaka S, Bick A, et al. Fomite transmission and disinfection strategies for SARS-CoV-2 and related viruses. arXiv: 200511443. 2020.
126. Haftlang F, Zarei-Hanzaki A, Abedi HR. In-situ frictional grain refinement of Ti–29Nb–14Ta–4.5 Zr bio-alloy during high-speed sliding wear. Mater Lett 2020;261:127083. CrossRef
127. Upadhyay S, Siddiqui S, Ahmad R, Gupta A, Husain I. sewage water as indicator for transmission of SARS-COV-2. Era's J Med Res 2020;7:212–6. CrossRef
128. Corpet DE. Why does SARS-CoV-2 survive longer on plastic than on paper? Med Hypotheses 2021;146:110429. CrossRef
129. Abdelrahman Z, Li M, Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front Immunol 2020;11:552909. CrossRef
130. Wong GLH, Wong VWS, Yuen BWY, Tse YK, Yip TCF, Luk HWS, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol 2020;72:57–66. CrossRef
131. Regassa F, Noakes D. Acute phase protein response of ewes and the release of PGFM in relation to uterine involution and the presence of intrauterine bacteria. Vet Rec 1999;144:502–6. CrossRef
132. Gibson LE, Bittner EA, Chang MG. Handheld ultrasound devices: an emerging technology to reduce viral spread during the Covid-19 pandemic. Am J Infect Control 2020;48:968–9. CrossRef
133. Ingham AC, Kielsen K, Cilieborg MS, Lund O, Holmes S, Aarestrup FM, et al. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome 2019;7:1–22. CrossRef
134. Neely AN. A survey of gram-negative bacteria survival on hospital fabrics and plastics. J Burn Care Rehabil 2000;21:523–7. CrossRef
135. Hirai Y. Survival of bacteria under dry conditions; from a viewpoint of nosocomial infection. J Hosp Infect 1991;19:191–200. CrossRef
136. Islam MS, Hossain M, Khan S, Khan M, Sack R, Albert MJ, et al. Survival of Shigella dysenteriae type 1 on fomites. J Health Popul Nutr 2001;19(3):177–82.
137. Getchell-White SI, Donowitz LG, Groschel DH. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Cont Hosp Epidemiol 1989;10:402–7. CrossRef