1. INTRODUCTION
The world population is going to touch about 9.7 billion at the end of 2050 that imposes a great challenge to the sustainable development and availability of natural resources on Earth [1]. As a result, the food demand will increase drastically with the growing population [2]. In particular, the global food crisis is attracting unprecedented attention due to concerns regarding its safety and sustainability. Therefore, it is urgent to search for renewable and alternative sources for both traditional food and energy [3]. Microalgae are single celled prokaryotic (cyanobacteria) and eukaryotic (higher algal species) photoautotrophs that can produce up to 60% of their biomass in form of carbohydrate or oil [4]. These photoautotrophs grow in diverse habitats including lakes, pounds, rivers, oceans, and even wastewater. There are around 72,500 algal species, names for 44,000 of which have probably been published, and 33,248 names have been processed by AlgaeBase [5]. Beside the primary producer of aquatic ecosystem, the biomass of microalga is potent source of carbon compounds which can be utilized as biofuels, health supplements, and cosmetics [6]. Microalgae produce a wide range of bioactive products, including polysaccharides (PS), lipids, pigments, proteins, and vitamins [7]. On the other hand, microalgae also involve in waste water treatment by removing toxic substances by chelating/adsorbing with the help of exopolysaccharides [8,9]. A new emphasis in bio-refinery has been spurred by the interest in microalgae as a sustainable and renewable feedstock for the production of biofuels. They also help to preserve agricultural soil fertility and boost crop productivity and acts as bio-fertilizers [10]. Hence, to full-fill the demand of over-growing populations, there is a need of commercializing the algal bioproducts by mass culturing of microalgal species. While on the similar note, during stress condition microalgae have the capability to mitigate the stress condition and evolve itself in significant manner in such situations through their defensive mechanism [Figure 1]. Despite considerable variability in the literature and lack of long-term full-scale data, microalgae cultivation for food production generally appears to be more sustainable than terrestrial crop farming. Furthermore, compared to other crops, the cultivation of microalgae does not require fertile land, herbicides, or pesticides, so there will be no competition for these resources. Microalgae can be cultured and cultivated in ponds, shallow water, and artificial tanks with little pressure and their biomass is used in producing valuable bioproducts. Even wastewater from home sewage systems and palm oil milling effluents can be used to cultivate microalgae, which can help in wastewater treatment [11,12]. Beside many advantages in microalgae farming, there are still a number of challenges. For instance, the small size of the cells, negative charge on the cell surface, low cell density, and low biomass production of microalgae make the harvesting process extremely expensive [13]. Hence, to develop microalgae as a sustainable bio-resource, there is a need of cost-effective techniques for microalgae harvesting to achieve large-scale commercial production.
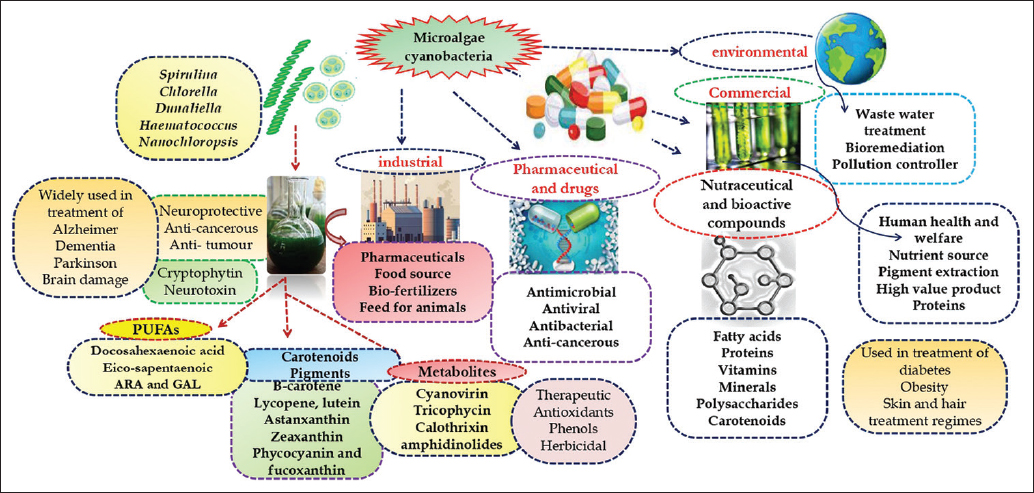 | Figure 1: Diagram showing potential applications of microalgae. [Click here to view] |
In addition, microalgae are also potent source of valuable non-enzymatic antioxidants such as cysteine, proline, non-protein thiols, and glutathione under normal and stress conditions [14]. Common microalgal species which are used in nutraceutical and biotechnological based applications are Spirulina platensis/maxima (Arthrospira), Chlorella vulgaris, Dunaliella salina, and Haematococcus pluvialis [15]. They grow in large scale with regard to high protein content molecule which is commonly known as “single cell protein (SCP).” On the other hand, there are some red algae such as Porphyridium, Rhodella, and Galdieria, which also contain the water soluble phycobiliproteins having anti-inflammatory, hepatoprotective, and neuroprotective activity [16]. The derivatives of polyunsaturated fatty acids (PUFAs) such as linoleic acid and arachidonic acid (AA) are extracted from the microalgae that show the cognitive property [17]. Further, in present condition, the extensive application of microalgae in pharmaceuticals and nutraceuticals industries is not economically feasible due to their high cost. In large-scale industrial applications of microalgae, the energy-intensive harvesting process occupies up to 30% of the total cost [18]. Microalgal bioactive metabolites are therefore of particular interest in the medical, pharmaceutical, cosmetic, and food industries [19]. Hence, further investigation regarding the potential benefits of microalgal-based metabolites to humans is necessary [20]. In light of this, this review investigates the bioactive metabolites and high-value compounds produced by microalgae and their possible applications in the life sciences, particularly in pharmaceutical, nutraceutical, and healthcare.
2. CULTIVATION AND HARVESTING OF MICROALGAE
Microalgae, being an aquatic photosynthetic organism, exhibit rapid growth rates compared to terrestrial plants and do not present a competitive threat to crops or lands [21]. Contrary to plants, they require less attention, low space, and minimum time for growth. They have inherent capability to accumulate valuable bioproducts within their cells that make them a suitable candidate for serving as raw material for industrial purpose [22]. In addition, the cultivation of microalgae does not require herbicides and pesticides, in contrast to other crops. As a result, microalgae cultivation does not engage in resource competition [23]. Despite the advantages of cultivating microalgae, the progress in its development is hindered by various challenges; one such issues are low biomass production and the small size of cells [23]. Addressing the limitations, over the years, various technologies have in consideration to increase the mass production of microalgae in highly controlled laboratory conditions [23]. For better microalgal growth, there is ample light, efficient gas exchange, easy operation, low contamination risk, and it is cost-effective [24]. Culturing and cultivation systems of microalgae can be categorized into two categories: (1) Open pond (further sub-categorized into deep ponds, shallow ponds, and artificial ponds) and (2) culturing in photobioreactor [25]. Open pond cultivation stands as one of the earliest and simplest methods for large-scale cultivation of microalgae and it requires lesser investment and man power. Cultivation typically occurs in open systems, including natural water bodies (ponds and lakes) and artificial setups such as raceway ponds, circular ponds, and tanks [26]. Due to its vast economic potential, abundance of bioactive compounds, low cost, and easy availability, it is well-suited for large-scale industrial use. For example, Spirulina and Dunaliella cultivated and harvested in open lakes in Texcoco and in Hutt Lagoon Australia, respectively, for production of the β-carotene [27,28]. While on the similar note, artificial open pond system mainly is recently implemented by some countries such as Japan and Taiwan to cultivate C. vulgaris as food supplement. The pond cultivation is the most frequent used approach for the cultivation of microalgae [29]. Beside this, various designs of closed photobioreactors have been developed to achieve better growth control and operating parameters. The three most common types for larger-scale cultivation systems are horizontal tubular reactors, bubble column vertical tubular reactors, and flat plate or panel (FP) type photobioreactors [30]. Improved growth rates and increased biomass yields are the outcome of photo-bioreactors, which reduce external contamination and enable precise control of light, temperature, pH, and nutrients [31]. Closed photo-bioreactor is a technological advancement for sustainable uses of microalgae in industries such as pharmaceuticals, nutraceuticals, bio-stimulants, biofuels, and aquaculture systems [25]. Flat plate photobioreactors are recommended for mass production of microalgae in both indoor and outdoor culture systems due to their high irradiation of plate surface. For example, closed photobioreactor is used for mass cultivation of H. pluvialis and C. vulgaris [32,33]. Harvesting of microalgae is considered as an important step of the processing of microalgae which makes it about 25–30% of the total cost production on the basis of high-energy demand and cost ratio. The grown biomass was harvested by gravitational settling and then centrifuged to obtain thick algal slurry. This algal slurry is further increased by cultivating the algal species beyond 12 days associated with increase in biomass production [34]. The supernatant was discarded, and the thickened microalgae were centrifuged and the thickened algal slurry was further used for metabolite extraction. There are major four types of the harvesting techniques by which we harvest the microalga (i) filtration, (ii) centrifugation, (iii) flocculation, and (iv) flotation; each step is discussed briefly.
2.1. Filtration
Harvesting of microalgae using a semi-permeable membrane, which permits the separation of culture medium while retaining algal biomass, is referred to as filtration. This method is generally employed to harvest such species of microalgae that are principally at risk to prone as well as delicate in nature and through adopting this method that the dense culture is obtained [35]. There are various other methods of filtration which is as follows, (i) cross flow filtration, (ii) tilted membrane panel filtration, (iii) axial vibration membrane filtration, (iv) ultra-filtration, and (v) polyacrylonitrile-based membrane filtration. The microalgal sp. such as C. vulgaris is harvested through the cross-flow filtration as well as axial vibration membrane filtration method for the large-scale production [36].
2.2. Centrifugation
Based on the difference in density between the particle and the surrounding medium, centrifugation is a physical dewatering technique that accelerates particle movement and separation by creating a centrifugal force. This technique can quickly lead to a high harvest efficiency and a higher biomass concentration [37]. The efficiency of the centrifugation depends on the settling characteristics of the cells and the retention time of the slurry in the centrifuge [38]. There are two main advantages of this process; firstly, centrifugation can be used effectively to recover microalgal biomass with 80%–90% of biomass recovery within 2–5 min, and secondly, there is no chemical additives are required. On comparing with sedimentation and magnetic separation, the centrifugation has the highest efficiency. The major disadvantage of this technique is that there is destruction of the cell structure of algae due to high gravitational force and shear stresses algae [39]. However, certain species (Tetraselmis suecica, Isochrysis galbana, and Skeletonema costatum) exhibited tolerance to shear stress as observed by Michiels et al. [40]. The main centrifugal devices that possess the potential to be utilized in biomass harvesting are (i) disk stack centrifuges, (ii) multi chamber centrifuges, (ii) tubular centrifuges, (iv) decanters centrifuge, and (v) hydro-cyclones [41]. Among these, the disc-stack centrifuge is the most common centrifuge used for separating algae biomass for various applications. Using extremely high centrifugal forces in a single continuous process, a disc-stack centrifuge has been successfully used to separate liquids from one another as well as solids from liquids [42].
2.3. Flocculation
The scattered free floating microalgal cells accumulate and form larger particle known as floc which on charge separation and with the help of flocculating agent in the presence of higher sedimentation rate facilitate the harvesting [43]. Based on the nature of flocculating agent, flocculation is mainly divided into two groups; one is chemical flocculants and second is bio-flocculants. Chemical flocculants are iron and aluminium salts which remove 90–95% of the biomass production of microalgae at normal situation [44]. In earlier study, it has been demonstrated that the cultures of Chlorella spp. are harvested by chemical flocculation method. However, this approach is not considered as eco-friendly regarding to the environment as well as for human welfare due to their toxic chemical effects [44]. Further, bio-flocculating method of harvesting is eco-friendly in nature due to their existence in nature. For example, chitosan biomolecule found in nature but it produces in artificial manner that helps to recover 90–95% of the cell in culture media. Some microalgae like Desmodesmus brasiliensis, Chlorella vulgaris and Scenedesmus are harvested by using the bio-flocculating method. [45].
2.4. Flotation
Flotation is the manifested technique of harvesting in which small particle as form of bubble can access the floating of microalgal cells that attach on the surface of the culture media [46]. This phenomenon shows the higher production efficiency of cells at low-cost ratio with easy availability. Scenedesmus spp., C. vulgaris, I. galbana, and T. suecica harvested through the flotation method [47,48].
The different farming and harvesting sequences are shown in Figure 2. Besides, reviewing the process of cultivation and harvesting the question ascends that what is the need for the production of the microalgae on such a large scale. The answer to this infers that the microalgae are the diverse group of microorganisms that are bestowed with the antioxidants, vitamins, minerals, and other potent bioactive molecules that are remarkably use for the human and animal welfare, as a food supplement, nutritional requirement, and also against various diseases. That is the reason the microalgae are exploited on large scale for the commercial purpose to meet the human need for food as well as for nutritional scarcity. The various nutraceutical and pharmaceutical product extracted from microalgae has been discussed briefly in below section.
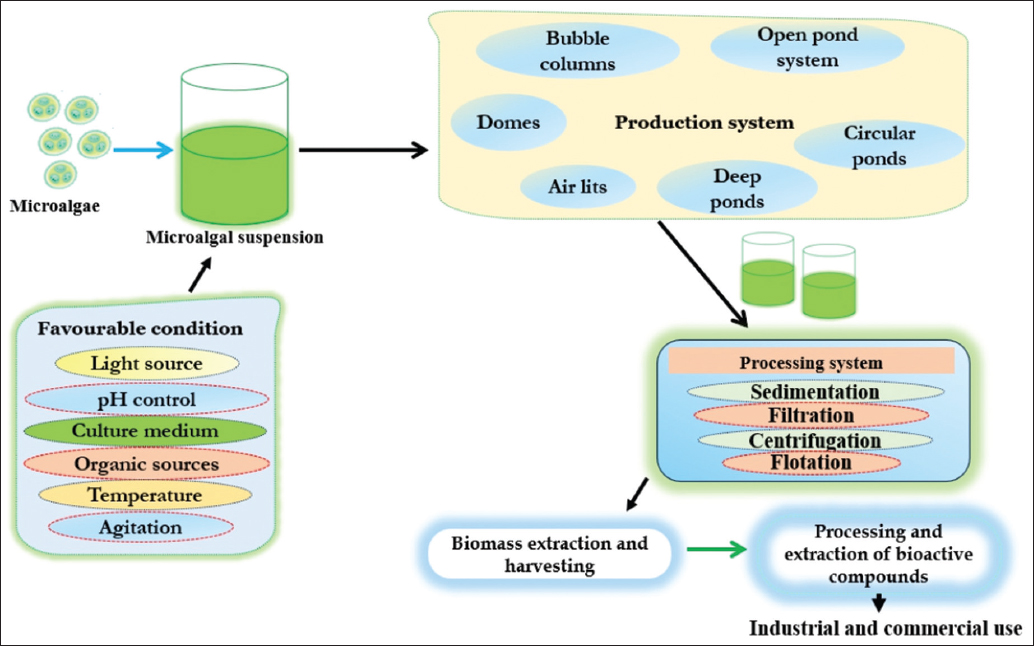 | Figure 2: Various steps involved in cultivation and culturing of microalgae. [Click here to view] |
3. ALGAL METABOLITES
Being the first photosynthetic organisms, the microalgal species play important role in aquatic ecosystems and covers up around 40% of global photosynthesis [49]. Due to their habitats, the microalgal species are in direct contact with the changing environmental conditions that positively or negatively alter the physiological and biochemical activities of cell. Several studies have been conducted and results are much more interesting that these microalgal species have immense potential to synthesize important metabolites in response to adverse environmental conditions with possible applications to humans in different fields of interest. Microalgae have been described as rich sources of various bio-compounds of commercial interest [50]. Microalgal origin bioactive compounds are categorized either from primary metabolites (proteins, lipids, carbohydrates, and nucleic acids) or from secondary metabolites. These bio-active compounds have antifungal, antiviral, anti-cancer, and antibiotic properties. Bioactive metabolites of microalgal origin are of special interest in the development of new products for medical, pharmaceutical, cosmetic, and food industries because from microalgae have been used as food by humans for thousands of years [51]. There are around 110 commercial producers of microalgae present in the Asia-Pacific region, with capacities ranging from 3 to 500 tons/year and around nine-tenths of algal cultivation is located in Asia. Major countries of commercial microalgae producers are located in China, Taiwan and India. Despite of high number of microalgal genus, only few have commercially importance such as Spirulina, Chlorella, Haematococcus, Dunaliella, Botryococcus, Phaeodactylum, Porphyridium, Crypthecodinium, Nannochloris, Schizochytrium, Tetraselmis, and Skeletonema [52]. Microalgae are a highly promising group of microorganisms for the development of new products and applications due to their vast biodiversity and the recent advancements in genetic engineering [53]. According to recent research, microalgae are capable of producing a wide range of chemical compounds having antimicrobial, antiviral, anticoagulant, antifungal, anti-inflammatory, and anticancer activity [54-56]. In this context, the further sections are firstly focused on role of micro-algae as bio-stimulants, bio-fertilizers and bio-pesticides and secondly use of bioactive compounds as pharmaceuticals and nutraceuticals.
4. MICROALGAE AS BIO-STIMULANTS, BIO-FERTILIZERS, AND BIO-PESTICIDES
Microalgae generally enhance plant growth through three main mechanisms: Functioning as bio-fertilizers, acting as bio-stimulants, and serving as bio-pesticides [25,57]. The most widespread use of microalgae biomass is as bio-fertilizers, making it the predominant method of application [57,58]. According to Swarnalakshmi et al. [59], applying a biofilm containing the cyanobacterium Anabaena torulosa to a wheat crop significantly increased the amount of available nitrogen in soil. Dineshkumar et al. [60] evaluated the impact of different concentrations of C. vulgaris and S. platensis on rice growth and yield to assess their potential as biofertilizers. Both microalgae species positively influenced key growth parameters, including plant height, leaf number, leaf area, and both fresh and dry biomass. Their application enabled a reduction of up to 50–75% in the recommended nitrogen fertilizer dosage without compromising plant performance. The use of S. platensis biomass as a biofertilizer for various leafy vegetables, including Brassica rapa, Eruca sativa, B. rapa, Amaranthus gangeticus, Brassica oleracea, and B. rapa. The results demonstrated that Spirulina-based biofertilizers significantly promoted plant growth by improving key biometric parameters such as leaf count, plant height, root length, and overall biomass, along with enhancing seed germination rates [61].
Microalgae, especially cyanobacteria, play a key role in managing soil-borne diseases and pathogenic fungi in plants through the production of biologically active compounds [62]. The green alga C. vulgaris produces chlorellin, a bioactive compound known to inhibit the growth of both Gram-negative and Gram-positive bacteria. This marked the first documented instance of an algal-derived substance exhibiting biopesticidal activity [63,64]. Methanolic extract of the brown seaweed Sargassum wightii was found to be effective against Xanthomonas oryzae, the bacterial pathogen responsible for rice blight [65]. A study by Kumar et al. [66] demonstrated that inoculating spice crop seeds with the cyanobacteria Anabaena laxa and Calothrix elenkinii enhanced the activity of the enzyme β-1,3-endoglucanase in both shoots and roots, which is known to degrade components of pathogen cell walls. In addition, the treatment led to increased fungicidal activity, along with improvements in plant dry weight, and shoots and root length. Microalgae consist of sesquiterpenes, alkaloids, and amines that have insecticidal properties [67]. Eremophilone, a sesquiterpene found in microalgae Calothrix spp., has insecticidal properties and can effectively control pests in Oryza sativa [68]. The cyanobacterium Nostoc spp. ATCC 53789 produced cryptophycin 1, an active depsipeptide. This chemical exhibits antiproliferative and antimitotic properties, primarily inhibiting the cell cycle during the metaphase of mitosis in yeasts of the Cryptococcus genus [69].
5. MICROALGAL NUTRACEUTICALS
Cyanobacteria and other microalgal species have inimitable property to perform photosynthesis and convert the solar energy into chemical energy in form of ATP which is further used in production of bioactive compounds [56]. The word “nutraceutical” in general is categorized as the combination of “nutrition” and “pharmaceutical,” that is, compounds that having properties of being used as food supplements and in prevention of chronic diseases. Nutraceutical compounds are mainly dietary derivatives extracted initially from plants but now shifted toward algae with easy cultivation and culturing and beneficial effects [70]. In China around 2000 years, the first use of algae (Nostoc) by humans is observed to survive during famine and after that a much research on algal derivatives nutraceutical compounds are still performed to feed over growing population [71]. Since the past 20 years, nutraceutical application of microalgae has focused specifically on four major microalgae: Spirulina (Arthrospira), Chlorella, Dunaliella, and Haematococcus. Beside this, Nostoc, Aulosira, Phormidium, and Anabaena are also potent source of bioactive compounds as well as secondary metabolites including fibers [9,14]. On the other hand, due to presence of minerals, vitamins (A, C, B1, B2, B6, and niacin) and micro/macro-nutrients make microalgae a valuable food supplements in Asian countries where population is not under control and also throughout the world [9,56].
Among different genera of blue-green algae, Spirulina is grown worldwide and used as a food supplement due to having highest protein content and thus also called as SCP [72]. Along with protein, Spirulina is also a rich source of PUFAs [73], pigments [74,75], and vitamins and phenolics [76]. There are two species of Spirulina having different geographical distributions, that is, S. platensis diversified in Africa, Asia, and South America while S. maxima is confined to Central America. Commercialization of Spirulina is common in India, Taiwan, China, Bangladesh, Pakistan, Burma (Myanmar), and USA. The species of Spirulina is commonly used as a protein supplement because having low purine concentration hence risk associated with high uric acid accumulation is very low in human beings [77] and it also lowers the cardiovascular diseases by reducing the cholesterol level [78]. Further, Spirulina also has good source of PUFA hence high oil content (7%) in form of α-linolenic acid, ?-linoleic acid (GLA), stearidonicacid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and AA. In study of [79], the nutraceutical property of Spirulina is further improved by fermenting it with the lactic acid bacterium Lactobacillus plantarum. The GLA, AA, EPA, and DHA are mainly extracted from Spirulina, Porphyridium, Nanochloropsis, Crypthecodinium, and Schizochytrium that extensively used as nutritional supplements for infants [80]. Besides, this major compound that is extracted from cyanobacteria is phycocyanin (a blue photosynthetic water-soluble pigment) and carotenoids (functions as photo-protection) that extensively used as food supplements and in cosmetic industries. Number of workers has reported high phycocyanin content in different species of cyanobacteria such as Nostoc, Anabaena, Spirulina, and Phormidium [81,82]. Cyanobacteria are also good source of PS having different glycosidic bonds that is used as an important food supplements. Cyanobacteria Aphanothece halophytica are good source of galactose and mannose [83] while immulina and immurella were isolated from S. platensis [84].
Another important microalga having nutraceutical property is the green algae Chlorella. The name Chlorella was derived from the Greek word “chloros” and the Latin “ella” that mean green and small, respectively. Chlorella is originated on earth during pre-Cambrian period about 2.5 billion years ago and in current situation Japan is the world leader in Chlorella microalgae consumption [85,86] and the dried biomass contains about 45–55% protein, 20% fat, 20% carbohydrate, 5% fiber, 9–18% dietary fiber, 10% minerals and vitamins, and other important bioactive compounds [87]. Being a green algae Chlorella contains both chlorophyll-a and chlorophyll-b as light harvesting pigment and considered as the richest source of chlorophyll available. Core of various species discovered–C. vulgaris is the most researched until now. Consumption of protein in human diet is very essential for building block of muscles because proteins are the ultimate source of protein and essential amino acids. As compared to protein present in beef meat, the proteins in Chlorella are 3 times more and easily digested due to low-molecular-weight and thus play an important preventive role on cellular damage [88]. Further, Chlorella is also a potential source of fatty acids in form of oleic, palmitic, and linolenic acid. Other species such as Chlorella emersonii, Dunaliella, Nannochloris, and Phaeodactylum tricornutum reported to contain more than 50% lipid content [89,90]. Ferreira et al. [91] compared to the fatty acid profile in form of production of long chain (LC) PUFAs in Chlorella homosphaera, Chlorella spp. and Chlorella minutissima grown in BG 11 or basal media led to the foundation that lipids in dry mass of these strains are 22.4% w/w, 21% w/w, and 21.5% w/w, respectively. This study mainly describes the role of Chlorella in production of PUFAs, particularly the essential fatty acids that can be applied in the food production. Chlorella also produces PS and major PS is the β-1,3-glucan that is used as an antioxidant, immune-stimulator, and effectively reduces the blood lipid levels [71]. Monosaccharide (glucose) along with galactose, mannose, rhamnose, N-acetylglucosamide, and N-acetylgalactosamine is some major PS excessively synthesized by Chlorella pyrenoidosa, and Chlorella ellipsoidea that is used as food supplement [92,93]. Chlorella is an important source of vitamins and minerals and it can be easily consumed as food supplement by both adults and children for the fulfillment of their daily dietary intake of vitamins. Vitamins that present in Chlorella spp. are Vitamin B1, B2, B3, B5, B6, E, K, α-carotene, and β-carotene. Beside this, trace amount of folic acid (Vitamin B9) is also present in Chlorella that plays an important role in regulation of basal metabolism of cells and also protects the skin and mucous membranes as well as strengths the bones and teeth [94].
Next to Chlorella, another important unicellular green alga is Dunaliella mainly D. salina that is a potent source of important nutraceutical compounds such as β-carotene, glycerol, and protein that can easily be dig out through its thin cell wall. Being a marine green algae culturing of Dunaliella is done in brackish water, marine water, and highly saline water and the annual production is around 1200 tons dry weight per year [95]. Dunaliella is mainly cultured for extraction of β-carotene and Israel, China, USA, and Australia are the leading contributors around the world. In recent time, more advance techniques are used for production of Betatene by AquaCarotene Ltd. Cyanotech Corp, Nature Beta Technologies, and Western Biotechnology [96]. Apart of β-carotene, Dunaliella also used in the production of trace amount of lutein and lycopene [97] and yields around 400 mg β-carotene/m2 of cultivation area under optimum environmental condition [98]. Beside consumptions as food supplements, carotenoids from Dunaliella are a good free radical scavenger which results in decrease membrane damage, enzyme inactivation, and thereby increased the production. Beta-carotene is used as an orange dye and as a Vitamin C supplement. Protein, fat, and carbohydrate content in the 100 mg dry mass of Dunaliella is around 7.4, 7.0, and 29.7 mg, respectively. Dunaliella also serve as potent source of nutrients such as magnesium (Mg) and some vitamins such as Vitamin B1 (thiamin), Vitamin B2 (riboflavin), Vitamin B3 (niacin), Vitamin B5 (pantothenic acid), Vitamin B6 (pyridoxine), Vitamin B9 (folic acid), and Vitamin B12 (cobalamine).
Another important unicellular biflagellate freshwater green microalga is H. pluvialis cultivated mainly for extraction of strong antioxidant astaxanthin (up to 2–3% on dry weight) under favorable or unfavorable conditions [99,100]. Consumption of astaxanthin is proved to be a good nutritional supplement and also act as an anti-inflammatory and anticancer agent for cardiovascular diseases. Due to this property, dry biomass of H. pluvialis has widely used in production of capsules, creams, granulated powders, oils, soft gels, syrups, and tablets [101]. Photoautotrophic culture of H. pluvialis is mainly carried out in open raceway ponds or closed photobioreactors. The cellular composition of H. pluvialis varies notably between its “green” and “red” stages of cultivation [102]. H. pluvialis can accumulate approximately 5% DW of astaxanthin which is considered as a natural source of this high-value carotenoid protein. Beside this, H. pluvialis is also potent source of proteins, lipids (phospholipids and glycolipids), carbohydrates, carotenoids, neoxanthin, violaxanthin, β-carotene, lutein, zeaxanthin, adonixanthin, adonirubin, canthaxanthin, echinenone, and chlorophylls [98]. The sources and impact of various bioactive compound of nutraceutical importance is listed in Table 1 [103-114] and their detail application is described in Figure 1.
Table 1: Bioactive compounds from microalgae and cyanobacteria and their potential role.
| Class of compounds | Bioactive from microalgae | Microalgae | Potential use | References |
|---|---|---|---|---|
| Polyunsaturated hydrocarbons | β-carotene Lutein | • Botryococcus braunii • Chlamydocapsa spp. • Chlorella sorokiniana • Chlorococcum spp. • Dunaliella | Used as cosmetics additives as natural food coloring agents and as health food, Possess properties like Anti-aging, help in curing coronary disease prevention, cancer, immune control, retinal and sensory disability enhancement and low-density lipoprotein | [103-105] |
| Lipids fatty acids | Arachidonic acid, Eicosapentaenoic acid, Docosahexaenoic acid | • Spirulina • Porphyridium spp. • Scenedesmus • Lobosphaera incisa • Crypthecodinium cohnii,Nannochloropsis spp. • Schizochytrium spp. • Ulkenia spp. • Phaeodactylum tricornutum | Enhancing cardiovascular health, fostering mental growth, and support, offering anti-inflammatory properties guarding against atherosclerosis, boosting nervous system and cognitive function, promoting infants’ growth, functional advancement, and vision improvement. | [106] |
| Phenolic | Chlorogenic acids and caffeic acids | • Isochrysis spp. • Chlorella vulgaris • Nannochloropsis spp. | Exhibiting anti-cancer effects, blocking HIV-1 integrase activity, reducing the mutagenicity of carcinogenic compounds, and containing antioxidant and antispasmodic properties. | [107] |
| Phenolic | Astaxanthin, Canthaxanthin, Violaxanthin | • Haematococcus spp. • Chlamydomonas nivalis • Chlorococcum braunii • Chlorella vulgaris • Chondria striolata • Monoraphidium spp. • Tetradesmus obliquus • Chlamydocapsa spp. • Chlorococcum spp. | Enhancing skin protection, promoting eye health, boosting muscle strength and endurance, shielding against oxidative damage, serving as additives in aquaculture feeds and nutraceuticals, defending against cancer, inflammation, metabolic syndrome, diabetes, neurodegenerative and ocular diseases, as well as lung injury, restraining alveolar wall swelling and myeloperoxidase activity. Additionally, it exhibits anti-proliferative activity, increases Vitamin E levels, and possesses antioxidative, anti-inflammatory, and neuroprotective properties. | [104,108] |
| Phycobili protein | Phycocyanin Phycoerythrin Porphyridium chlorophyll a Proteins | • Anabaena. flos-aquae • Caulerpa racemosa • Ulva lactuca • Caulerpa racemosa and Spirulina • Porphyridium • Scenedesmus • Chlorella spp. • Microcystis aeruginosa | Improves light utilization efficiency, food coloring agents, food antioxidants, humans and plants Functional foods, animal feed supplements, bioplastics production, antioxidant properties, immune activators, prevent atherosclerosis, cancer, and coronary diseases, and also used in photo-ageing protective formulations, cytotoxicity toward tumoral cells | [103,109] |
| Vitamins | Vitamins D and K B12, B9, B6 A, C, D, E | • Spirulina spp. • Chlamydomonas spp. • Chlorella spp. • Scenedesmus spp. • Dunaliella tertiolecta • Nannochloropsis oculata • Chaetoceros calcitrans | Antioxidants serve as food supplements and sources of essential vitamins, contributing to the reduction of breast cancer risks, promoting DNA repair and histone methylation, and exhibiting chemo-preventive activities. They are also utilized in nutraceuticals and cosmetics. | [110,111] |
| Polysaccharides | Starch, cellulose, hemicellulose, pectin Sulfated | • Chlorella vulgaris • Fucus vesiculosis • Margalefidinium polykrikoides • Porphyridium spp., • Turbinaria conoides • Sargassum wightii • Porphyra spp. | Nanocellulose finds applications in biofilters, biofuels, cosmeceuticals, bioplastics, and more. Extracts from Laminaria spp. offer preventive and therapeutic benefits across various stages of viral infections, including inhibiting reverse transcriptase in HIV infections and blocking cytopathic effects and cell adhesions during viral infections. Certain molecules derived from algae can be utilized in vaccines and antibody production for preventing and treating COVID-19. Additionally, these extracts demonstrate antioxidative, immunomodulatory, and anti-inflammatory properties. | [111,112] |
| Polysaccharides | Carrageenan naviculan, fucoidans, lectin, agar, laminaran, alginate, glycosaminoglycan | [113,114] |
6. MICROALGAL PHARMACEUTICAL
Microalgae are potent sources of various bioactive compounds (carbohydrates, lipids, PUFA, proteins, vitamins, and enzymes) having pharmaceutical properties and thus extensively used in pharmaceutical industry [115]. Further, fluctuating environmental condition such as deprivation of minerals and nutrient, light (high and low), temperature (heat and cold), and pH significantly enhance the synthesis of microalgae-based secondary metabolites [116]. Microalgae-based nutraceutical compound is β-carotene, omega-3 fatty acids, and algal oil, and due to this, microalgae are tremendous choice [56]. Thus, rising call for these compounds overlays the approach for sustainable microalgal technology [117]. The secondary metabolites were primarily extracted from plants but due to lesser production and seasonal dependency, the attention is engrossed toward the microalgae. The enormous kind of pharmaceutical products from microalgae is discussed below:
The extracts obtained from microalgal species possess defensive properties such as antimicrobial, antiviral, and antifungal properties, for example, certain species such as Chlorella and Spirulina are abundantly used in dermatology, as a constituent to the skin care product as well as also vastly used in sun protection regime and hair care treatments [118,119]. However, certain species along with pharmaceutical products also produce other toxic substances such as Ochromonas spp. The antibacterial activity to the microalgae is attributed due to presence of highly volatile compounds such as phenols and fatty acid [119]. Further, several current researches have geared toward the production of neuroprotective compounds to treat Alzheimers syndrome, memory loss, cognitive impairment, neural death, dementia, and brain damage [120]. The Neuroprotective products decelerate the progress of disease related to the nervous system by reducing the reactive oxygen species damage that causes to the brain in neurodegenerative diseases. The microalgal species are well-known to produce such compounds that are beneficial against the nerve persistence. For example, Spirulina spp. is seen to encompass 14.18% of phycocyanin (having neuroprotective properties) that providing protection and reducing cognitive impairment such as Alzheimer’s Disease or Parkinson’s Syndrome [121,122]. In recent years, microalgae have proven to be an ideal model for safer, efficient, and economic means of therapeutic production. Therapeutic proteins from microalgae are a cost-effective process which reduces the increasing burden on the treatment of cancer and other diseases that involve expensive treatments [123]. The green algae Chlamydomonas reinhardtii has been testified for large-scale production of certain protein such as vascular endothelial growth factor, high mobility group protein 1, Domain 14 human fibronectin, and Domain 10 of human fibronectin. Furthermore, certain studies specify that human proinsulin has also been produced by microalgae [124].
Cosmetological products such as eyeliners, lipsticks, eye shadows, moisturizers, facial cleansers, and beauty masks actively use compounds extracted from Chlorella and Spirulina [125]. Beside this, Chondrus crispus (Irish moss) is well known for the persuasive source of carrageenan (PS) which basically used in dermatological product primarily in skin and hair regime formulations. Similarly, non-toxic nature of cyano-phycocyanin is used as a potential substance for the topical treatment of various skin diseases in place of conventional medicine due to less side effects [126].
It has also been reported that algal extracts also used in manufacturing of drugs which are sustainable and cost-effective. For example, anti-cancer drugs such as cryptophycin 1, apratoxins, dolastatins, saxitoxin (local anesthetics), and brevetoxins (polyketide toxins) are typically isolated from blue-green algae (cyanobacteria) [127]. The various pharmaceutical compounds from microalgae are shown in
6.1. Emerging Biotechnology and Omics for Microalgal Metabolites
Microalgae have an incredible ability to synthesize a variety of metabolites during their growth, including pigments, LC-PUFAs, starch, proteins, bio-hydrogen, and other valuable compounds [140,141]. Various approaches such as omics, clustered regularly interspaced short palindromic repeats–CRISPR-associated protein (CSIPR/Cas), and genetic engineering (transcription activator-like effector nucleases [TALENs] and zinc-finger nucleases [ZFNs]) are commonly used to increase the production efficiency of these high-value metabolites [142-144]. Genome sequences are critical in reconstructing metabolic pathways. In contrast to genomics, metagenomics focuses on complete microbial populations, offering additional information from DNA data extracted directly from environmental samples [145]. An omics-based technology employs a systematic, stepwise strategy to engineer organisms for the efficient biosynthesis of targeted products [146]. A study was conducted to assess intracellular responses to the synthesis of 3-hydroxypropionate (3-HP), a valuable compound synthesized by Synechocystis spp. PCC 6803 using proteome and metabolomic methods. The study concluded that cellular processes were differentially controlled, and overexpressing particular transporter genes boosted 3-HP synthesis [147,148].
Recent progress in molecular technologies has provided the foundation for precise genome editing. The principal strategies developed for this purpose include ZFNs, TALENs, and the CRISPR-Cas [149,150]. Song et al. [151] employed RNP-mediated CRISPR/Cas9 technology to produce highly purified zeaxanthin by preventing lutein synthesis through knocking ZEP gene and α-branch of lycopene epsilon cyclase gene, resulting in 60% greater zeaxanthin yield than the parental strain in Chlamydomonas reinhardtii. Lin et al. [152] employed the CRISPRa/i system to regulate gene expression in Chlorella sorokiniana UTEX 1602. They found that gene regulation by dCas9-VP64 (CRISPRa) boosted protein content by about 60% (w/w), but gene regulation through dCas9-KRAB (CRISPRi) raised protein content to 65%, with lipid accumulation ranging from 150 to 250 mg/L (WT: 150 mg/L). Hao et al. [153] conducted a functional characterization of long-chain acyl-CoA synthetase (LACS) isozymes by generating CRISPR/Cas9-mediated knockouts of the ptACSL1–5 genes. Their study demonstrated the feasibility of producing gene knockout mutants through large-fragment deletions using multiplexed CRISPR/Cas9 and provided valuable insights into the functional roles of LACS isozymes in regulating lipid metabolism in oleaginous microalgae. A study by Daboussi et al. [154] reported that TALEN-mediated silencing of UDP-glucose pyrophosphorylase, a carbohydrate storage pathway gene, resulted in a 45-fold increase in triacylglycerol accumulation in P. tricornutum. Overexpression of FAX1, FAX2, and ABCA2 genes led to a 1.8-fold increase in starch accumulation under nitrogen-deprived conditions and a 53% increase under nutrient-replete conditions in Chlamydomonas reinhardtii [155]. Advances in genetic engineering technologies and metabolic engineering have enormous potential for the development of sustainable microalgal cell factories.
7. FUTURE PROSPECTS
Microalgae are a potent source of valuable bio-resource for pharmaceuticals and nutraceuticals due to their rich biochemical composition. However, several bottlenecks exist in the utilization of microalgae for these purposes, and future research directions aim to address these challenges and enhance their viability. Future research may involve genetic engineering, biotechnological approaches, and selective breeding to enhance traits such as growth, primary and secondary bio-active compounds. These modern technologies will optimize productivity, reduce costs, and minimize environmental risk. Moreover, recent cultivation systems such as photobioreactors, open ponds, and hybrid systems are under practice to enhance sustainability. Future research directions may include exploring novel extraction techniques such as supercritical fluid extraction, ultrasound-assisted extraction, and enzyme-assisted extraction to improve yields and purity. Research on the development of innovative microalgae-derived products and formulations tailored for pharmaceutical and nutraceutical applications is essential. This includes encapsulation techniques to improve stability and bioavailability, as well as formulation optimization to enhance sensory attributes and consumer acceptability. Addressing regulatory requirements and market demands is crucial for the commercialization of microalgae-based products. This will include conducting safety assessments, establish quality standards, and explore market trends to ensure compliance and competitiveness in the pharmaceutical and nutraceutical industries. Microalgae show promise as bio-resources for pharmaceuticals and nutraceuticals, but key research and bottleneck challenges must be addressed to unlock their full potential and enable mainstream adoption.
8. CONCLUSION
Microalgae are a diverse group of unicellular photosynthetic prokaryotic and eukaryotic organisms that are ubiquitous in nature ranging from usual to extreme habitats. Their economic importance worldwide is associated with a wide range of applications of microalgae, from the food industry to medicine, from immunostimulants to biofuels, from cosmetology to agriculture. In near future these microalgal species are uses as super food due to having valuable bioactive ingredients such as proteins, lipids, and carbohydrates as well as various phytochemicals such as carotenoids, PUFAs, sterols and phenolics. These bioactive compounds can exhibit considerable health promoting effects, such as antioxidant, anti-inflammatory, anti-cancer, and antimicrobial effects [Figure 1]. Microalgae biomass is a promising source of both nutritional and functional additives. The microalgal species behaves as commanding asset for renewable and highly quantitative production because they require minimum resources, land, and also easily cultivated under adverse environmental conditions. Furthermore, with the advancement in recombinant DNA technology, these microalgae can be easily manipulated genetically, thus enhancing their interest for production of valuable bioactive compounds. Thus, the large-scale production of microalgae may lead to fulfillment of human need for food and nutrition as well as various bioactive compounds.
9. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
10. FUNDING
There is no funding to report.
11. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
12. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
13. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
14. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
15. Use of artificial intelligence (AI)-assisted technology
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. United Nations Department of Economic and Social Affairs;2022. Available from: https://www.un.org/en/desa/highlights-report-2022-2023 [Last accessed on 2025 Sep 01].
2. Min M, Li H, Ma T, Miao C. Will agricultural land scale management aggravate non-point source pollution?-Chaohu Lake Basin, China as a case study. Appl Geogr. 2023;158:103056. [CrossRef]
3. Global Network Against Food Crises;2022. Available from: https://www.fightfoodcrises.net [Last accessed on 2025 Sep 01].
4. Okoro V, Azimov U, Munoz J, Hernandez HH, Phan AN. Microalgae cultivation and harvesting:Growth performance and use of flocculants-A review. Renew Sustain Energy Rev. 2019;115:109364. [CrossRef]
5. Guiry MD. How many species of algae are there?J Phycol. 2012; 48(5):1057-63. [CrossRef]
6. Das P, Aziz SS, Obbard JP. Two phase microalgae growth in the open system for enhanced lipid productivity. Renew Energy. 2011;36(9):2524-8. [CrossRef]
7. Ismail MM, Elkomy RG, El-Sheekh MM. Bioactive Compounds from Components of Marine Ecosystem. InMarine Ecosystems:A Unique Source of Valuable Bioactive Compounds. UAE:Bentham Science Publishers;2023. 206-56.[CrossRef]
8. Brennan L, Owende P. Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev. 2010;14(2):557-77. [CrossRef]
9. Tiwari S, Patel A, Prasad SM. Phytohormone up-regulates the biochemical constituent, exopolysaccharide and nitrogen metabolism in paddy-field cyanobacteria exposed to chromium stress. BMC Microbiol. 2020;20:1-5. [CrossRef]
10. Zehr JP. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 2011;19(4):162-73.[CrossRef]
11. Selmani N, Mirghani ME, Alam MZ. Study the growth of microalgae in palm oil mill effluent waste water. IOP Conf Ser Earth Environ Sci. 2013;16(1):012006.[CrossRef]
12. Posadas E, Alcántara C, García-Encina PA, Gouveia L, Guieysse B, Norvill Z, et al. Microalgae cultivation in wastewater. In:Microalgae-based Biofuels and Bioproducts. United Kingdom:Woodhead Publishing;2017. 67-91. [CrossRef]
13. Singh G, Patidar SK. Microalgae harvesting techniques:A review. J Environ Manage. 2018;217:499-508. [CrossRef]
14. Patel A, Tiwari S, Prasad SM. Modulation of salt stress in paddy field cyanobacteria with exogenous application of gibberellic acid:Growth behavior and antioxidative status. Physiol Mol Biol Plants. 2023;1:51-68. [CrossRef]
15. Mendes AR, Spínola MP, Lordelo M, Prates JA. Chemical compounds, bioactivities, and applications of Chlorella vulgaris in food, feed and medicine. Appl Sci. 2024;14(23):10810. [CrossRef]
16. Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K. Phycobiliprotein:Potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J. 2016;109:282-96. [CrossRef]
17. Abyor N, Dessy A, Hady H. Potential production of polyunsaturated fatty acids from microalgae. Int J Sci Eng. 2011;2(1):13-6.
18. Ogbonna CN, Nwoba EG. Bio-based flocculants for sustainable harvesting of microalgae for biofuel production. A review. Renew Sustain Energy Rev. 2021;139:110690. [CrossRef]
19. DupréC, Burrows HD, Campos MG, Delattre C, Encarnação T, Fauchon M, et al. Microalgal biomass of industrial interest:Methods of characterization. In:Handbook on Characterization of Biomass, Biowaste and Related by-products. Berlin:Springer;2020. 537-639. [CrossRef]
20. Olguín EJ, Sánchez-Galván G, Arias-Olguín II, Melo FJ, González-Portela RE, Cruz L, et al. Microalgae-based biorefineries:challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology (Basel). 2022;11(8):1146. [CrossRef]
21. Dickinson S, Mientus M, Frey D, Amini-Hajibashi A, Ozturk S, Shaikh F, et al. A review of biodiesel production from microalgae. Clean Technol Environ Policy. 2017;19:637-68. [CrossRef]
22. Randrianarison G, Ashraf MA. Microalgae:A potential plant for energy production. Geol Ecol Landscapes. 2017;1(2):104-20. [CrossRef]
23. Tan JS, Lee SY, Chew KW, Lam MK, Lim JW, Ho SH, et al. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered. 2020;11(1):116-29. [CrossRef]
24. Jegathese SJ, Farid M. [Retracted] microalgae as a renewable source of energy:A niche opportunity. J Renew Energy. 2014;2014(1):430203. [CrossRef]
25. Osorio-Reyes JG, Valenzuela-Amaro HM, Pizaña-Aranda JJ, Ramírez-Gamboa D, Meléndez-Sánchez ER, López-Arellanes ME, et al. Microalgae-based biotechnology as alternative biofertilizers for soil enhancement and carbon footprint reduction:Advantages and implications. Mar Drugs. 2023;21(2):93. [CrossRef]
26. Costa JA, de Morais MG. An open pond system for microalgal cultivation. In:Biofuels from Algae. Netherlands:Elsevier;2014. 1-22. [CrossRef]
27. Borowitzka LJ, Borowitzka MA. Commercial production of-carotene by Dunaliella salina in open ponds. Bull Mar Sci. 1990;47:244-52.
28. Hamed I. The evolution and versatility of microalgal biotechnology:A review. Compr Rev Food Sci Food Saf. 2016;15(6):1104-23. [CrossRef]
29. Show PL, Tang MS, Nagarajan D, Ling TC, Ooi CW, Chang JS. A holistic approach to managing microalgae for biofuel applications. Int J Mol Sci. 2017;18(1):215. [CrossRef]
30. Kunjapur AM, Eldridge RB. Photobioreactor design for commercial biofuel production from microalgae. Ind Eng Chem Res. 2010;49:3516-26.[CrossRef]
31. Wang SK, Stiles AR, Guo C, Liu CZ. Microalgae cultivation in photobioreactors:An overview of light characteristics. Eng Life Sci. 2014;14(6):550-9.[CrossRef]
32. Posten C. Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci. 2009;9(3):165-77. [CrossRef]
33. Torzillo G, Chini Zittelli G. Tubular photobioreactors. Algal Biorefineries:Products and Refinery Design. Vol. 2. Berlin:Springer International Publishing;2015. 187-212. [CrossRef]
34. Gani P, Sunar NM, Matias-Peralta HM. Cultivation system and harvesting techniques in Microalgae Biomass production. Quantum J Eng Sci Technol. 2020;1(1):33-44.
35. Al Hattab M, Ghaly A, Hammouda A. Microalgae harvesting methods for industrial production of biodiesel:Critical review and comparative analysis. J Fundam Renew Energy Appl. 2015;5(2):1000154.[CrossRef]
36. Zhao F, Chu H, Zhang Y, Jiang S, Yu Z, Zhou X, et al. Increasing the vibration frequency to mitigate reversible and irreversible membrane fouling using an axial vibration membrane in microalgae harvesting. J Membrane Sci. 2017;529:215-23.[CrossRef]
37. Najjar YS, Abu-Shamleh A. Harvesting of microalgae by centrifugation for biodiesel production:A review. Algal Res. 2020;51:102046. [CrossRef]
38. Fuad N, Omar R, Kamarudin S, Harun R, Idris A, Wan Azlina WA. Mass harvesting of marine microalgae using different techniques. Food Bioproducts Process. 2018;112:169-84. [CrossRef]
39. Junior WG, Gorgich M, Corrêa PS, Martins AA, Mata TM, Caetano NS. Microalgae for biotechnological applications:Cultivation, harvesting and biomass processing. Aquaculture. 2020;528:735562. [CrossRef]
40. Michels MH, Van der Goot AJ, VermuëMH, Wijffels RH. Cultivation of shear stress sensitive and tolerant microalgal species in a tubular photobioreactor equipped with a centrifugal pump. J Appl Phycol. 2016;28(1):53-62. [CrossRef]
41. Shelef G, Sukenik A, Green M. Microalgae Harvesting and Processing:A Literature Review. Technical Report, Solar Energy Research Institute;1984. [CrossRef]
42. Sharples AL, Doman Road C. Decanter and disc-stack centrifuge systems. Filtration+Separation. 1991;28(6):387-8.[CrossRef]
43. Muylaert K, Bastiaens L, Vandamme D, Gouveia L. Harvesting of Microalgae:Overview of Process Options and their Strengths and Drawbacks. Microalgae-based Biofuels and Bioproducts. Elsevier;2017. 113-32. [CrossRef]
44. Chatsungnoen T, Chisti Y. Harvesting microalgae by flocculation-sedimentation. Algal Res. 2016;13:271-83. [CrossRef]
45. Zhu L, Li Z, Hiltunen E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant:Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels. 2018;11:183. [CrossRef]
46. Laamanen CA, Ross GM, Scott JA. Flotation harvesting of microalgae. Renew Sustain Energy Rev. 2016;58:75-86. [CrossRef]
47. Oliveira GA, Carissimi E, Monje-Ramírez I, Velasquez-Orta SB, Rodrigues RT, Ledesma MT. Comparison between coagulation-flocculation and ozone-flotation for Scenedesmus microalgal biomolecule recovery and nutrient removal from wastewater in a high-rate algal pond. Bioresour Technol. 2018;259:334-42. [CrossRef]
48. Alkarawi MA, Caldwell GS, Lee JG. Continuous harvesting of microalgae biomass using foam flotation. Algal Res. 2018;36:125-38. [CrossRef]
49. Moreno-Garrido I. Microalgae immobilization:Current techniques and uses. Bioresour Technol. 2008;99(10):3949-64. [CrossRef]
50. Volk RB, Furkert FH. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res. 2006;161(2):180-6. [CrossRef]
51. Milledge JJ. Commercial application of microalgae other than as biofuels:A brief review. Rev Environ Sci Bio/Technol. 2011;10:31-41. [CrossRef]
52. Sathasivam R, Radhakrishnan R, Hashem A, Abd Allah EF. Microalgae metabolites:A rich source for food and medicine. Saudi J Biol Sci. 2019;26(4):709-22. [CrossRef]
53. Ibañez E, Cifuentes A. Benefits of using algae as natural sources of functional ingredients. J Sci Food Agric. 2013;93(4):703-9. [CrossRef]
54. Bhagavathy S, Sumathi P, Bell IJ. Green algae Chlorococcum humicola-a new source of bioactive compounds with antimicrobial activity. Asian Pac J Trop Biomed. 2011;1(1):S1-7. [CrossRef]
55. Markou G, Nerantzis E. Microalgae for high-value compounds and biofuels production:A review with focus on cultivation under stress conditions. Biotechnol Adv. 2013;31(8):1532-42. [CrossRef]
56. Priyadarshani I, Rath B. Bioactive compounds from microalgae and cyanobacteria:Utility and applications. Int J Pharm Sci Res. 2012;3(11):4123.
57. Parmar P, Kumar R, Neha Y, Srivatsan V. Microalgae as next generation plant growth additives:Functions, applications, challenges and circular bioeconomy based solutions. Front Plant Sci. 2023;14:1073546. [CrossRef]
58. Gonçalves J, Freitas J, Fernandes I, Silva P. Microalgae as biofertilizers:A sustainable way to improve soil fertility and plant growth. Sustainability. 2023;15(16):12413. [CrossRef]
59. Swarnalakshmi K, Prasanna R, Kumar A, Pattnaik S, Chakravarty K, Shivay YS, et al. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur J Soil Biol. 2013;55:107-16. [CrossRef]
60. Dineshkumar R, Kumaravel R, Gopalsamy J, Sikder MN, Sampathkumar P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valoriz. 2018;9(5):793-800. [CrossRef]
61. Wuang SC, Khin MC, Chua PQ, Luo YD. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016;15:59-64. [CrossRef]
62. El-Mougy NS, Abdel-Kader MM. Effect of commercial cyanobacteria products on the growth and antagonistic ability of some bioagents under laboratory conditions. J Pathog. 2013;2013(1):838329. [CrossRef]
63. Gupta V, Ratha SK, Sood A, Chaudhary V, Prasanna R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)-Prospects and challenges. Algal Res. 2013;2(2):79-97. [CrossRef]
64. Pratt R, Oneto JF, Pratt J. Studies on Chlorella vulgaris. X. Influence of the age of the culture on the accumulation of chlorellin. Am J Bot. 1945;32:405-8.[CrossRef]
65. Arunkumar K, Selvapalam N, Rengasamy R. The antibacterial compound sulphoglycerolipid 1-0 palmitoyl-3-0 (6′-sulpho-a-quinovopyranosyl)-glycerol from Sargassum wightii Greville (Phaeophyceae). Botanica Marina. 2005;48:441-5.[CrossRef]
66. Kumar M, Prasanna R, Bidyarani N, Babu S, Mishra BK, Kumar A, et al. Evaluating the plant growth promoting ability of thermotolerant bacteria and cyanobacteria and their interactions with seed spice crops. Sci Hortic. 2013;164:94-101. [CrossRef]
67. Costa JA, Freitas BC, Cruz CG, Silveira J, Morais MG. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J Environ Sci Health Part B. 2019;54(5):366-75. [CrossRef]
68. Hernández-Carlos B, Gamboa-Angulo MM. Metabolites from freshwater aquatic microalgae and fungi as potential natural pesticides. Phytochem Rev. 2011;10(2):261-86. [CrossRef]
69. Biondi N, Piccardi R, Margheri MC, Rodolfi L, Smith GD, Tredici MR. Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl Environ Microbiol. 2004;70(6):3313-20.[CrossRef]
70. Sivaramakrishnan R, Kanwal S, Incharoensakdi A, Nirmal N, Srimongkol P. Exploring the nutraceutical and functional food potential of microalgae:Implications for health and sustainability. J Agric Food Res. 2025;22:102148. [CrossRef]
71. Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J Biosci Bioeng. 2006;101(2):87-96. [CrossRef]
72. Colla LM, Reinehr CO, Reichert C, Costa JA. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol. 2007;98(7):1489-93. [CrossRef]
73. Sajilata MG, Singhal RS, Kamat MY. Fractionation of lipids and purification of g-linolenic acid (GLA) from Spirulina platensis. Food Chem. 2008;109(3):580-6. [CrossRef]
74. Oliveira Rangel-Yagui C, Danesi ED, De Carvalho JC, Sato S. Chlorophyll production from Spirulina platensis:Cultivation with urea addition by fed-batch process. Bioresour Technol. 2004;92(2):133-41. [CrossRef]
75. Madhyastha HK, Vatsala TM. Pigment production in Spirulina fussiformisin different photophysical conditions. Biomol Eng. 2007;24(3):301-5. [CrossRef]
76. Ogbonda KH, Aminigo RE, Abu GO. Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour Technol. 2007;98(11):2207-11. [CrossRef]
77. Pittman JK, Dean AP, Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol. 2011;102(1):17-25. [CrossRef]
78. Juárez-Oropeza MA, Mascher D, Torres-Duran PV, Farias JM, Paredes-Carbajal MC. Effects of dietary Spirulina on vascular reactivity. J Med Food. 2009;12(1):15-20. [CrossRef]
79. Marco Castro E, Shannon E, Abu-Ghannam N. Effect of fermentation on enhancing the nutraceutical properties of Arthrospira platensis (Spirulina). Fermentation. 2019;5(1):28. [CrossRef]
80. Wen ZY, Chen F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv. 2003;21(4):273-94. [CrossRef]
81. Kumar J, Parihar P, Singh R, Singh VP, Prasad SM. UV-B induces biomass production and nonenzymatic antioxidant compounds in three cyanobacteria. J Appl Phycol. 2016;28:131-40.[CrossRef]
82. Patel A, Tiwari S, Prasad SM. Toxicity assessment of arsenate and arsenite on growth, chlorophyll a fluorescence and antioxidant machinery in Nostoc muscorum. Ecotoxicol Environ Saf. 2018;157:369-79. [CrossRef]
83. Li P, Liu Z, Xu R. Chemical characterisation of the released polysaccharide from the cyanobacterium Aphanothece halophytica GR02. J Appl Phycol. 2001;13:71-7. [CrossRef]
84. Chaiklahan R, Chirasuwan N, Triratana P, Loha V, Tia S, Bunnag B. Polysaccharide extraction from Spirulina sp. its antioxidant capacity. Int J Biol Macromol. 2013;58:73-8. [CrossRef]
85. Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294-306. [CrossRef]
86. Wawrik B, Harriman BH. Rapid, colorimetric quantification of lipid from algal cultures. J Microbiol Methods. 2010;80(3):262-6. [CrossRef]
87. Shim JY, Shin HS, Han JG, Park HS, Lim BL, Chung KW, et al. Protective effects of Chlorella vulgaris on liver toxicity in cadmium-administered rats. J Med Food. 2008;11(3):479-85. [CrossRef]
88. Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients:Potential to reduce the incidence of chronic diseases. Mar Drugs. 2011;9(6):1056-100. [CrossRef]
89. Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications:A review. Renew Sustain Energy Rev. 2010;14(1):217-32. [CrossRef]
90. Upadhyay AK, Mandotra SK, Kumar N, Singh NK, Singh L, Rai UN. Augmentation of arsenic enhances lipid yield and defense responses in alga Nannochloropsis sp. Bioresour Technol. 2016;221:430-7. [CrossRef]
91. Ferreira SP, Holz JC, Lisboa CR, Costa JA. Fatty acid profile of Chlorellabiomass obtained by fed batch heterotrophic cultivation. Int Food Res J. 2017;24(1):284-91.
92. Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001;67(8):737-42.[CrossRef]
93. Shao B, Wang Z, Liu X, Yu J, Lan J, Wang J, et al. Breeding of a Chlorella strain with high yield of polysaccharide and its effect on growth and immunoregulation of Litopenaeus vannamei. J Nuclear Agric Sci. 2013;27(2):168-72.
94. Brown MR, Mular M, Miller I, Farmer C, Trenerry C. The vitamin content of microalgae used in aquaculture. J Appl Phycol. 1999;11:247-55.[CrossRef]
95. Bishop WM, Zubeck HM. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci. 2012;2(5):1-6.
96. Del Campo JA, García-González M, Guerrero MG. Outdoor cultivation of microalgae for carotenoid production:Current state and perspectives. Appl Microbiol Biotechnol. 2007;74:1163-74. [CrossRef]
97. Murthy KC, Vanitha A, Rajesha J, Swamy MM, Sowmya PR, Ravishankar GA. In vivo antioxidant activity of carotenoids from Dunaliella salina-a green microalga. Life Sci. 2005;76(12):1381-90. [CrossRef]
98. Finney KF, Pomeranz Y, Bruinsma BL. Use of algae Dunaliella as a protein supplement in bread. Cereal Chem. 1984;61(5):402-6.
99. Fritsch FE. The subaerial and freshwater algal flora of the tropics. A phytogeographical and ecological study. Ann Bot. 1907;21(82):235-75.[CrossRef]
100. Caire GD, Cano MD, Mule MD, Steyerthal N, Piantanida M. Effect of Spirulina platensis on glucose, uric acid and cholesterol levels in the blood of rodents. Int J Exp Bot. 1995;57(13):93-6.
101. Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin:Applications for human health and nutrition. Trends Biotechnol. 2003;21(5):210-6.[CrossRef]
102. Shah MM, Liang Y, Cheng JJ, Daroch M. Astaxanthin-producing green microalga Haematococcus pluvialis:From single cell to high value commercial products. Front Plant Sci. 2016;7:531. [CrossRef]
103. Eze CN, Onyejiaka CK, Ihim SA, Ayoka TO, Aduba CC, Ndukwe J, et al. Bioactive compounds by microalgae and potentials for the management of some human disease conditions. AIMS Microbiol. 2023;9(1):55.[CrossRef]
104. Galasso C, Gentile A, Orefice I, Ianora A, Bruno A, Noonan DM, et al. Microalgal derivatives as potential nutraceutical and food supplements for human health:A focus on cancer prevention and interception. Nutrients. 2019;11(6):1226. [CrossRef]
105. Neumann U, Derwenskus F, Gille A, Louis S, Schmid-Staiger U, Briviba K, et al. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients. 2018;10(8):965. [CrossRef]
106. Andrade LM, Andrade CJ, Dias M, Nascimento C, Mendes MA. Chlorella and Spirulina microalgae as sources of functional foods. Nutraceuticals Food Suppl. 2018;6(1):45-58.[CrossRef]
107. Barkia I, Saari N, Manning SR. Microalgae for high-value products towards human health and nutrition. Mar Drugs. 2019;17(5):304.[CrossRef]
108. Cai X, Chen Y, Xie X, Yao D, Ding C, Chen M. Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-kB. Am J Transl Res. 2019;11(3):1884.
109. Capelli B, Talbott S, Ding L. Astaxanthin sources:Suitability for human health and nutrition. Funct Foods Health Dis. 2019;9(6):430-45. [CrossRef]
110. Koller M, Muhr A, Braunegg G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014;6:52-63.[CrossRef]
111. Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A, et al. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health. 2018;15(11):2436. [CrossRef]
112. Raposo MF, Morais AM, Morais RM. Carotenoids from marine microalgae:A valuable natural source for the prevention of chronic diseases. Mar Drugs. 2015;13(8):5128-55.[CrossRef]
113. Singh R, Parihar P, Singh M, Bajguz A, Kumar J, Singh S, et al. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine:Current status and future prospects. Front Microbiol. 2017;8:515. [CrossRef]
114. Xia S, Gao B, Li A, Xiong J, Ao Z, Zhang C. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar Drugs. 2014;12(9):4883-97. [CrossRef]
115. Mimouni V, Ulmann L, Pasquet V, Mathieu M, Picot L, Bougaran G, et al. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr Pharm Biotechnol. 2012;13(15):2733-50. [CrossRef]
116. Paliwal C, Mitra M, Bhayani K, Bharadwaj SV, Ghosh T, Dubey S, et al. Abiotic stresses as tools for metabolites in microalgae. Bioresour Technol. 2017;244:1216-26. [CrossRef]
117. Jha D, Jain V, Sharma B, Kant A, Garlapati VK. Microalgae-based pharmaceuticals and nutraceuticals:An emerging field with immense market potential. ChemBioEng Rev. 2017;4(4):257-72. [CrossRef]
118. Katircioglu H, Beyatli Y, Aslim B, Yüksekdag Z, Atici T. Screening for antimicrobial agent production of some freshwater. Microbiology. 2006;2(2):1-9.[CrossRef]
119. Kamenarska Z, Serkedjieva J, Najdenski H, Stefanov K, Tsvetkova I, Dimitrova-Konaklieva S. Antibacterial, antiviral and cytotoxic activities of some red and brown seaweeds from black sea. Bot Mar. 2009;52:80-6.[CrossRef]
120. Olasehinde TA, Olaniran AO, Okoh AI. Therapeutic potentials of microalgae in the treatment of Alzheimer's disease. Molecules. 2017;22(3):480. [CrossRef]
121. Haoujar I, Haoujar M, Altemimi AB, Essafi A, Cacciola F. Nutritional, sustainable source of aqua feed and food from microalgae:A mini review. Int Aquat Res. 2022;14(3):157.
122. Sabat S, Bej S, Swain S, Bishoyi AK, Sahoo CR, Sabat G, et al. Phycochemistry and pharmacological significance of filamentous cyanobacterium Spirulina sp. Bioresour Bioproces. 2025;12:27. [CrossRef]
123. Carvajal F, Vargas-Torres V, Becerra D, González-Quezada N, Egaña JT. Effect of recombinant protein production and release on microalgal fitness and the impact of environmental conditions for localized therapeutic delivery. J Biol Eng. 2025;19(1):54. [CrossRef]
124. McDonald K. Study Shows Potential for Using Algae to Produce Human Therapeutic Proteins. University of California, Press Release, San Diego News;2010. Available from: https://biology.ucsd.edu/about/news/article_030910.html [Last accessed on 2025 Sep 01].
125. Abreu AP, Martins R, Nunes J. Emerging applications of Chlorella sp. Spirulina (Arthrospira) sp. Bioengineering. 2023;10(8):955. [CrossRef]
126. Dranseikien?D, Bal?i?nait?-Murzien?G, Karosien?J, Morudov D, Juodžiukynien?N, Hudz N, et al. Cyano-phycocyanin:Mechanisms of action on human skin and future perspectives in medicine. Plants. 2022;11(9):1249. [CrossRef]
127. Ricciardelli A, Pollio A, Costantini M, Zupo V. Harmful and beneficial properties of cyanotoxins:Two sides of the same coin. Biotechnol Adv. 2023;68:108235. [CrossRef]
128. Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2006;23(1):26-78. [CrossRef]
129. Mayer AM, Hamann MT. Marine pharmacology in 2001-2002:Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities;affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140(3-4):265-86. [CrossRef]
130. Rodríguez-Meizoso I, Jaime L, Santoyo S, Cifuentes A, Garcia-Blairsy Reina G, Senorans FJ, et al. Pressurized fluid extraction of bioactive compounds from Phormidium species. J Agric Food Chem. 2008;56(10):3517-23. [CrossRef]
131. Rastogi RP, Sinha RP. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv. 2009;27(4):521-39. [CrossRef]
132. El Semary NA. The characterisation of bioactive compounds from an Egyptian Leptolyngbya sp. . Ann Microbiol. 2012;62(1):55-9. [CrossRef]
133. Colla LM, Muccillo-Baisch AL, Costa JA. Spirulina platensis effects on the levels of total cholesterol, HDL and triacylglycerols in rabbits fed with a hypercholesterolemic diet. Braz Arch Biol Technol. 2008;51:405-11. [CrossRef]
134. Torres-Duran PV, Ferreira-Hermosillo A, Juarez-Oropeza MA. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population:A preliminary report. Lipids Health Dis. 2007;6:1-8. [CrossRef]
135. Singh U, Gandhi HA, Nikita, Bhattacharya J, Tandon R, Tiwari GL, et al. Cyanometabolites:Molecules with immense antiviral potential. Arch Microbiol. 2023;205(5):164. [CrossRef]
136. Deng Z, Hu Q, Lu F, Liu G, Hu Z. Colony development and physiological characterization of the edible blue-green alga, Nostoc sphaeroides (Nostocaceae, Cyanophyta). Prog Nat Sci. 2008;18(12):1475-83. [CrossRef]
137. Costa JA, de Morais MG. 16 Microalgae for Food Production. Fermentation Processes Engineering in the Food Industry. Boca Raton:CRC Press;2013. 405.
138. Medina-Jaritz NB, Carmona-Ugalde LF, Lopez-Cedillo JC, Leon FS. Antibacterial activity of methanolic extracts from Dunaliella salina and Chlorella vulgaris. The FASEB J. 2013;27:1167. [CrossRef]
139. Madkour FF, Abdel-Daim MM. Hepatoprotective and antioxidant activity of Dunaliella salina in paracetamol-induced acute toxicity in rats. Indian J Pharm Sci. 2013;75(6):642.
140. Hu J, Meng W, Su Y, Qian C, Fu W. Emerging technologies for advancing microalgal photosynthesis and metabolism toward sustainable production. Front Mar Sci. 2023;10:1260709. [CrossRef]
141. Prates JA. Applications of bioactive compounds from marine microalgae in health, cosmetics, and functional foods. Appl Sci. 2025;15(11):6144. [CrossRef]
142. Kang NK, Baek K, Koh HG, Atkinson CA, Ort DR, Jin YS. Microalgal metabolic engineering strategies for the production of fuels and chemicals. Bioresour Technol. 2022;345:126529. [CrossRef]
143. Dhokane D, Shaikh A, Yadav A, Giri N, Bandyopadhyay A, Dasgupta S, et al. CRISPR-based bioengineering in microalgae for production of industrially important biomolecules. Front Bioeng Biotechnol. 2023;11:1267826. [CrossRef]
144. Huang JJ, Xu W, Lin S, Cheung PC. The bioactivities and biotechnological production approaches of carotenoids derived from microalgae and cyanobacteria. Crit Rev Biotechnol. 2025;45(2):276-304. [CrossRef]
145. Maghembe R, Damian D, Makaranga A, Nyandoro SS, Lyantagaye SL, Kusari S, et al. Omics for bioprospecting and drug discovery from bacteria and microalgae. Antibiotics. 2020;9(5):229. [CrossRef]
146. Hassan S, Meenatchi R, Pachillu K, Bansal S, Brindangnanam P, Arockiaraj J, et al. Identification and characterization of the novel bioactive compounds from microalgae and cyanobacteria for pharmaceutical and nutraceutical applications. J Basic Microbiol. 2022;62(9):999-1029. [CrossRef]
147. Wang Y, Chen L, Zhang W. Proteomic and metabolomic analyses reveal metabolic responses to 3-hydroxypropionic acid synthesized internally in cyanobacterium Synechocystis sp. PCC 6803. Biotechnol Biofuels. 2016;9(1):209. [CrossRef]
148. Pathania R, Srivastava A, Srivastava S, Shukla P. Metabolic systems biology and multi-omics of cyanobacteria:Perspect future directions. Bioresour Technol. 2022;343:126007. [CrossRef]
149. Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397-405.[CrossRef]
150. Aljabali AA, El-Tanani M, Tambuwala MM. Principles of CRISPR-Cas9 technology:Advancements in genome editing and emerging trends in drug delivery. J Drug Deliv Sci Technol. 2024;92:105338. [CrossRef]
151. Song I, Kim J, Baek K, Choi Y, Shin B, Jin E. The generation of metabolic changes for the production of high-purity zeaxanthin mediated by CRISPR-Cas9 in Chlamydomonas reinhardtii. Microbial Cell Fact. 2020;19(1):220. [CrossRef]
152. Lin JY, Lin WR, Ng IS. CRISPRa/i with Adaptive Single Guide Assisted Regulation D NA (ASGARD) mediated control of Chlorella sorokiniana to enhance lipid and protein production. Biotechnol J. 2022;17(10):2100514. [CrossRef]
153. Hao X, Chen W, Amato A, Jouhet J, Maréchal E, Moog D, et al. Multiplexed CRISPR/Cas9 editing of the long-chain acyl-CoA synthetase family in the diatom Phaeodactylum tricornutum reveals that mitochondrial ptACSL3 is involved in the synthesis of storage lipids. New Phytol. 2022;233(4):1797-812. [CrossRef]
154. Daboussi F, Leduc S, Maréchal A, Dubois G, Guyot V, Perez-Michaut C, et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat Commun. 2014;5(1):3831. [CrossRef]
155. Chen R, Yamaoka Y, Feng Y, Chi Z, Xue S, Kong F. Co-expression of lipid transporters simultaneously enhances oil and starch accumulation in the green microalga Chlamydomonas reinhardtii under nitrogen starvation. Metabolites. 2023;13(1):115. [CrossRef]