1. INTRODUCTION
Nanotechnology is the field that focuses on the design, development, and application of materials, structures, and devices by precisely controlling their size and shape at the 10-9 m scale. Richard Feynman, the 1965 recipient of the Nobel Prize in physics, is credited with creating modern nanotechnology [1]. Matter’s characteristics at the nanoscale differ from those at larger scales. Nanomaterials’ unique physical, chemical, biological, electrical, and optical properties can be used for new performance and commercial applications that benefit society [2]. The concept of “nanofood” encompasses food that is grown, produced, processed, or packaged using nanotechnology tools or techniques, or food enhanced with engineered nanomaterials [3]. Nanotechnology could enhance every aspect of the food chain, from storage and quality control to food processing and packaging [4]. As material science and technology advance, food packaging is evolving to address both environmental concerns and consumer expectations. In today’s competitive markets, consumers are drawn to foods that are naturally high-quality, safe, minimally refined, and convenient to consume, with the advantage of a longer shelf life [5].
In recent years, the incorporation of bio-polymers through nanotechnology in food packaging development has been rapidly advancing. Food packaging materials made from biomass (lipids, proteins, and polysaccharides) sourced from living organisms such as plants and animals are cost-effective and widely accessible. However, for the purpose of creating bio-nanocomposite materials for food packaging applications, polysaccharides and their derivatives (particularly starch) are the biopolymers that have been examined the most frequently, as apart from being edible, exhibiting good extensibility, and oxygen barrier properties, starch-based biofilms (SBBF) also share physical characteristics with traditional packaging plastics, such as transparency, odor, and taste. SBBF can also be used to create smart packaging materials by acting as a carrier for naturally occurring antioxidant and antibacterial agents. On the whole, SBBF has proven to improve the shelf-life, overall quality, and stability of food products, while also being less environmentally destructive [6,7].
However, as for any other existing materials, SBBF has several disadvantages as well. First, the hydroxyl groups make starch hydrophilic and brittle, which in turn renders any material developed out of it to have poor water barrier properties and makes the packing of dry and oxygen-sensitive food products by SBBF ineffective. Moreover, in a humid atmosphere, the physical integrity of packaging made of SBBF can also deteriorate. Second, SBBF has been shown to have poor mechanical properties, which might make them less suitable as packaging materials for the containment and protection of food from the external environment. Third, there is increasing concern among public and international agencies such as the Food and Agriculture Organization that food supply may decrease due to starch being used to produce bio-based products. Finally, although starch is generally inexpensive, the processing and modification required to enhance its qualities may result in higher end material costs [8].
The poor thermal and mechanical properties of SBBF can, however, be improved using different methods. One such method to improve the brittleness of SBBF is the addition of plasticizers such as fructose, maltitol, sorbitol, ethylene glycol, and glycerol, which make their way between the polymer chains and increase the amount of available free space, enabling them to move over each other more easily and thus making the films more flexible. Research shows that water and glycerol are the most effective plasticizers for starch, with glycerol usually used at 25–30% (w/w). Perry and Donald found that glycerol alone can gelatinize starch completely and raise the gelatinization temperature by about 60°C compared to a product plasticized with water [6,7].
Another method is to use nanotechnology, through which SBBF can be meshed or embedded with other organic and inorganic nanofillers (NF) to improve their characteristics and performance [5]. Organic NF includes nanofibers such as starch, cellulose, chitin, and chitosan. Inorganic NF includes nano-clays such as saponite, bentonite, hectorite, organically modified clay, and metallic nanoparticles (NPs) such as copper, platinum, titanium dioxide, and iron oxide [4].
Among the various NPs, ZnO NPs have drawn a lot of interest due to their distinctive morphology and structure, as well as their considerable and potent antibacterial and antifungal action against a variety of microorganisms. The white color, UV-blocking abilities, and greater versatility also add on to the interest. Zinc oxide (ZnO) is among five zinc compounds designated as generally recognized as safe by the U.S. Food and Drug Administration (2011). It is used in the food industry as a zinc supplement, an essential micronutrient vital for human and animal growth, development, and overall health.
It is, therefore, important to note that before completely integrating these materials into the food sector and consumer market, a deeper understanding of their health and environmental effects across the food chain is needed.
In this study, we aim to develop ZnO NPs embedded starch biofilms from potato starch and corn starch as alternatives to plastic packaging for food materials and evaluate their effectiveness and efficiency by testing their sensory characteristics and functional properties.
2. MATERIALS AND METHODS
This experimental study was designed to develop ZnO NPs embedded starch biofilms from potato starch and corn starch as alternatives to plastic packaging for food materials, followed by testing of their sensory characteristics and functional properties (moisture content, swelling index, solubility, and scanning electron microscopy [SEM], i.e., SEM Analysis). The research was conducted at the Sri Ramachandra Institute of Higher Education and Research in Porur, Chennai. The place of study and materials were chosen based on convenience for experimentation; materials which were accessible, affordable, and available were chosen for the study. Glycerol was used as the plasticizer, and 99.5% glacial acetic acid was used as the cross-linking agent. Potato starch and corn starch were purchased from the local market. The study was conducted during the period of January 2023–February 2023 and was approved by the Institutional Ethical Committee of Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai (Ref No: CSP/23/JAN/120/09).
2.1. Experimental Procedure
2.1.1. Phase I - standardization of the procedure
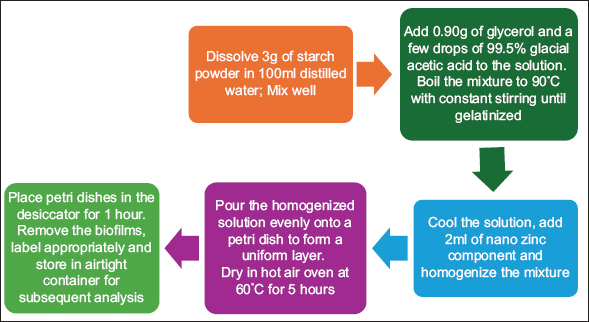
The initial protocol was performed as three trials for standardization of the procedure as shown in Figure 1. The first trial involved dissolving 10 g of the starch powder in 100 mL of distilled water, with addition of 0.90 g of glycerol and 2 mL of nano zinc component. The second trial involved dissolving 7 g of the starch powder in 100 mL of distilled water, with the addition of 1 g of glycerol and 1 mL of nano zinc component. The final trial involved dissolving 3 g of the starch powder in 100 mL of distilled water, with the addition of 0.90 g of glycerol, a few drops of 99.5% glacial acetic acid and 2 mL of nano zinc component.
 | Figure 1: Standardization of the procedure. [Click here to view] |
2.1.2. Phase II - development of starch biofilms
3 g of starch powder was added to 100 mL of distilled water and stirred until completely dissolved. To this solution, 0.90 g of glycerol and a few drops of 99.5% glacial acetic acid were added and the solution was boiled to 90°C with constant stirring until gelatinized. The solution was then cooled and 2 mL of nano zinc component was added to it and the mixture was homogenized. The homogenized solution was poured onto a petri dish to create a uniform layer, which was then dried in a hot air oven at 60°C for 5 h. After drying, the petri dishes were removed from the oven and positioned in a desiccator for an hour. The biofilms were then carefully removed from the petri dishes, labeled appropriately, and stored in an airtight container for subsequent analysis [Figures 2 and 3; Table 1].
 | Figure 2: Procedure for development of the starch biofilms. [Click here to view] |
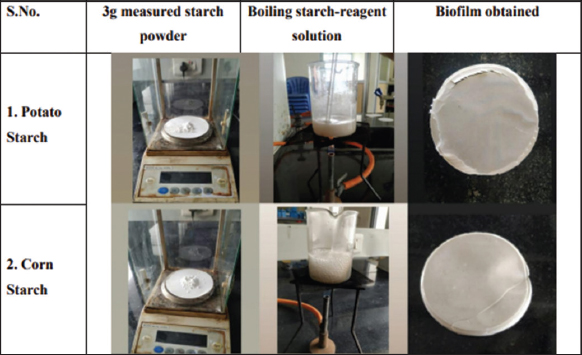 | Figure 3: Development of the starch biofilms. [Click here to view] |
Table 1: Sensory characteristics of the starch biofilms.
| Characteristic | Potato starch biofilm | Corn starch biofilm |
|---|---|---|
| Color | Off White | Cream |
| Odor | Slightly Unpleasant | No Characteristic Smell |
| Gloss and Transparency | Least Gloss, Fair Transparency | Good Gloss, Poor Transparency |
| Time to Gelatinize (minutes) | 35 | 31 |
| Texture and Flexibility | Rough, Brittle | Smooth, Inflexible |
| Image of a Grape covered by the Starch Biofilm |  |  |
2.1.3. Phase III - testing of the sensory characteristics and functional properties of the biofilms
2.1.3.1. Moisture content
Moisture Content = [(pre-dried weight/dried weight) – 1] × 100
The moisture content of the starch biofilms was assessed by calculating the weight loss. First, 2 g of each film sample were cut and weighed, following which the samples were placed in a hot air oven at 105°C for 1 day to remove moisture, and their weight was measured again after drying [6]. The moisture content was calculated using the formula [9]: Each measurement was performed in triplicate for consistency.
2.1.3.2. Swelling index
Swelling Index = [(w2 – w1)/w1] × 100, where w2 = weight of the swollen sample and w1 = initial weight of the dried sample
The swelling index evaluates how starch molecules absorb and interact with water. 2-g sample, dried in a hot air oven, was immersed in distilled water for 2 min, then removed. Any excess water on the sample was blotted away, and the swollen sample was weighed [6]. Following formula was used for calculation [10]: Each measurement was performed in triplicate for consistency.
2.1.3.3. Solubility
Solubility = [(s1 – s2)/s1] × 100, where s1 = initial weight of the dried sample and s2 = final dry weight after immersion and drying
2-g dried sample was placed in a beaker containing 15 mL of distilled water and left for 24 h at room temperature. After swelling, the sample was removed, dried again at 105°C for 24 h, and reweighed. The following formula was used for calculation [6]: Each measurement was performed in triplicate for consistency.
2.1.3.4. Scanning electron microscope analysis
SEM analysis helped analyze the starch biofilms’ surface structure by assessing their integrity and uniformity. The analysis was performed with an accelerating voltage of 30 kV, capturing images at magnifications ranging from ×200 to ×5,000, with spot sizes between 5 µm and 200 µm.
3. RESULTS AND DISCUSSION
3.1. Sensory Characteristics of the Starch Biofilms
The potato starch biofilm was off-white in color, had a slightly unpleasant smell, and exhibited the least gloss. It was also rough and brittle, and took the most time to gelatinize. However, it was fairly transparent. The cornstarch biofilm was cream-colored, had no characteristic smell, and possessed a good gloss. Although it had a smooth surface, it was inflexible and exhibited poor transparency; the grape, when covered was thus the least visible [Table 2]. Figure 4 refers to the images provided within
Table 2: Moisture content of the starch biofilms.
| Film | Moisture content % |
|---|---|
| F1: Potato starch | 11.03±0.78 |
| F2: Corn starch | 10.21±0.25 |
3.2. Functional Properties of the Starch Biofilms
3.2.1. Moisture content (MC)
Moisture content refers to the proportion of water mass in a material relative to its total mass, usually expressed as a percentage. The films’ moisture content was assessed by calculating weight loss. This property is crucial as it influences the packaging’s mechanical strength and can significantly impact microbial growth. According to McHugh and Krochta (1994), increasing the starch concentration enhances the moisture content [11]. Seligra et al. (2016) reported that citric acid boosts the moisture content of starch films [12], while Ma et al. found that higher glycerol or plasticizer content also raises moisture content [16]. Bergo et al. [15] similarly reported that cassava starch films exhibit moisture-dependent structural variations, underscoring the key role of water-polymer interactions in subsequent properties such as swelling behaviour.
In this study, the corn starch biofilm exhibited the least moisture content, whereas the potato starch biofilm had the highest [Table 3] [Figure 6].
Table 3: Swelling index of the starch biofilms.
| Film | Swelling index (%) |
|---|---|
| F1: Potato starch | 30.16±0.31 |
| F2: Corn starch | 28.37±0.14 |
 | Figure 4: Scanning electron microscopy analysis of zinc oxide nanoparticles embedded potato starch biofilm; ×5,000 (a), ×2,500 (b), ×1,000 (c) size of potato starch biofilm: 11.6 µm. [Click here to view] |
 | Figure 5: Scanning electron microscopy analysis of zinc oxide nanoparticles embedded cornstarch biofilm; ×5,000 (a), ×2,500 (b), ×1,000 (c) size of corn starch biofilm: 11.6 µm. [Click here to view] |
 | Figure 6: Moisture content of the Starch Biofilms. [Click here to view] |
3.2.2. Swelling index (SI)
The swelling index measures the volume (in milliliters) occupied by each gram of material under specific conditions. In this study, the swelling index of the films is quantified by the amount of excess water removed from the biofilms. This property is crucial as it influences the functional characteristics of packaging materials, such as their flexibility and brittleness. According to Bertuzzi et al. (2007), a rise in starch concentration leads to a higher swelling index [14,15].
In addition, Ghanbarzadeh et al. observed that increasing the concentration of acetic acid and plasticizers in starch films also raises the swelling index [17]. Furthermore, it has been noted that ZnO NPs reduce the swelling index of biofilms. In this study, the corn starch biofilm exhibited the least swelling index, while the potato starch biofilm had the highest [Table 4] [Figure 7].
 | Figure 7: Swelling Index of the Starch Biofilms. [Click here to view] |
3.2.3. Solubility (S)
Solubility refers to a material’s (solute) ability to dissolve in a solvent, such as water, to form a solution. The solubility of the films was measured by the change in the biofilms’ weight before and after immersion in water. This is a key property to assess, as it impacts the functional characteristics of the packaging material. Seligra et al. found that the solubility of starch films decreases in the presence of acetic acid and glycerol [12].
Similarly, the inclusion of ZnO NPs in the starch film also reduces its solubility. In this study, corn starch biofilm had the least solubility, whereas potato starch biofilm had the highest [Table 5].
Table 4: Solubility of the starch biofilms.
| Film | Solubility (%) |
|---|---|
| F1: Potato starch | 26.37±0.63 |
| F2: Corn starch | 21.63±0.42 |
3.3. Characterization of the ZnO NPs Embedded Starch Biofilms Using SEM Analysis
SEM analysis was performed to investigate the starch biofilms and evaluate their structural integrity and consistency, using an accelerating voltage of 30 kV. The images were captured at magnifications ranging from ×200 to ×5,000 with a spot size ranging from 5 µm to 200 µm. Potato and corn starch biofilms exhibited rough, porous textures with ZnO NPs clusters, enhancing durability and antimicrobial properties [Figure 8].
 | Figure 8: Schematic Illustration of Antimicrobial Effects of ZnO Nanoparticles on Biofilm Integrity. [Click here to view] |
However, the uneven distribution of ZnO NPs in both starch biofilms may create weak points, affecting mechanical strength and barrier effectiveness [Figures 4 and 5].
4. CONCLUSION
In this study, ZnO NPs embedded starch biofilms were developed from potato starch and corn starch to be tested as alternatives for plastic packaging of food products. The procedure for the development of biofilms was first standardized. The addition of the plasticizer glycerol provided physical and structural stability to the biofilm by entering between the polymer chains and increasing the amount of available free space. The addition of acetic acid helped in the stabilization of the pH to further enhance the biofilms’ performance. The films’ sensory characteristics and functional properties were tested. SEM analysis was conducted to characterize the ZnO NPs embedded in the SBBF, but no significant differences were observed. When comparing potato starch biofilm and corn starch biofilm, the former, characterized by its higher moisture content, swelling index, and solubility, may be more suitable for biodegradable packaging of fresh or moist foods. In contrast, the latter may be better suited for dry, shelf-stable foods, offering enhanced moisture resistance and structural integrity.
Moreover, the biofilm prepared from potato starch exhibited a slightly off-odor, was rough and brittle, but provided moderate transparency. On the other hand, the biofilm prepared from corn starch, while displaying limited flexibility and poor transparency, had no distinctive odor and offered a good glass along with a smooth texture. SEM Analysis of both potato and corn starch biofilms exhibited rough, porous textures with ZnO NPs clusters, which enhanced their durability and antimicrobial properties. However, the uneven distribution of ZnO NPs in both biofilms may create weak points, potentially compromising their mechanical strength and barrier effectiveness. Therefore, further development is needed to optimize both the physical properties and sensory characteristics of these biofilms, or an alternative material must be developed and tested for more effective food packaging applications.
5. ACKNOWLEDGMENTS
The authors are thankful to Sri Ramachandra Institute of Higher Education and Research (DU) and the Department of Biomedical Sciences, SRIHER, for their support.
6. AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
7. FUNDING
There is no funding to report.
8. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
9. ETHICAL APPROVALS
Ethical approval details are given in the ‘Materials and Methods’ section.
10. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
11. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
12. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Neethirajan S, Jayas DS. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol 2011;4:39-47. [CrossRef]
2. Bhushan B. Introduction to nanotechnology. In:Springer Handbook of Nanotechnology. Germany:Springer;2017. 1-9. [CrossRef]
3. Meghani N, Dave S, Kumar A. Introduction to nanofood. In:Nano-Food Engineering. Vol. 1. Germany:Springer;2020. 1-23. [CrossRef]
4. Shankar S, Rhim JW. Bionanocomposite films for Food Packaging Applications. Vol. 1. Reference Module Food Science. Netherlands:Elsevier;2018. 1-10. [CrossRef]
5. Tamimi N, Mohammadi Nafchi A, Hashemi-Moghaddam H, Baghaie H. The effects of nano-zinc oxide morphology on functional and antibacterial properties of tapioca starch bionanocomposite. Food Sci Nutr 2021;9:4497-508. [CrossRef]
6. Kumar P, Gautam S. Developing ZnO Nanoparticle Embedded Antimicrobial Starch Biofilm for Food Packaging [Preprint];2019.
7. Muller J, González-Martínez C, Chiralt A. Combination of poly(lactic) acid and starch for biodegradable food packaging. Materials (Basel) 2017;10:952. [CrossRef]
8. Samsudin H, Hani NM. Use of starch in food packaging. In:Starch-Based Materials in Food Packaging. United States:Academic Press;2017. 229-56. [CrossRef]
9. Chiumarelli M, Hubinger MD. Stability, solubility, mechanical, and barrier properties of cassava starch-carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll 2012;28:59-67. [CrossRef]
10. Cao N, Fu Y, He J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll 2007;21:575-84. [CrossRef]
11. McHugh TH, Krochta JM. Sorbitol-vs glycerol-plasticized whey protein edible films:Integrated oxygen permeability and tensile property evaluation. J Agric Food Chem 1994;42:841-5. [CrossRef]
12. Seligra PG, Jaramillo CM, FamáL, Goyanes S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr Polym 2016;138:66. [CrossRef]
13. Adjouman YD, Nindjin C, Tetchi FA, Dalcq AC, Amani NG, Sindic M. Water vapor permeability of edible films based on improved cassava (Manihot esculenta Crantz) native starches. J Food Process Technol 2017;8:1-6.
14. Bertuzzi MA, Armada M, Gottifredi JC. Physicochemical characterization of starch based films. J Food Eng 2007;82:17-25. [CrossRef]
15. Bergo PV, Sobral PJ, Prison JM. Physical Properties of Cassava Starch Films Containing Glycerol. Gene Conserve 2009;8(32).
16. Ma J, Zhu W, Tian Y, Wang Z. Preparation of zinc oxide-starch nanocomposite and its application on coating. Nanoscale Res Lett. 2016;11:200. https://doi.org/10.1186/s11671-016-1404-y
17. Ghanbarzadeh B, Almasi H, Entezami AA. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind Crops Prod. 2011;33:229-35. https://doi.org/10.1016/j.indcrop.2010.10.016