1. INTRODUCTION
The rapid expansion of society and technological advancement have significantly impacted our planet, bringing both positive developments and serious challenges. The invention of plastic, initially perceived as a wonderful development, has instead resulted in a significant global negative impact. Particularly, the wrongful disposal of plastics, which persist in the environment for extended periods, poses a threat to the well-being of people, animals, aquatic life, and other living organisms [1]. Plastic microparticles or microplastics (MPs), measuring <5 mm, formed because of their continuous exposure to degradation by certain factors such as ultraviolet (UV) light and thermal and microbial agents, have recently emerged as a pressing environmental concern. These MPs comprise smaller particles stemming from degraded plastic sources, especially from wrongful handling and disposal of daily plastic waste and industrial discharge. The persistence of MPs in the environment extends for decades, during which time they inflict damage on the ecosystem. They have the potential to exert adverse effects on various environments, including soil, freshwater bodies, lakes, rivers, reservoirs, oceans, and seashores. These plastics accumulate in the human body with potential DNA damage, inflammation risk, oxidative stress, and endocrine disruption, causing metabolic disorders, neurotoxicity, and a heightened risk of cancer [1].
Therefore, there is an extreme need to remove MPs from the environment. Effective ways to mitigate the presence of MPs involve minimizing the consumption of traditional plastic products such as bags, covers, and bottles and encouraging the use of biodegradable bags, clothes, and refillable bottles. The remediation methods for MPs include physical methods in the form of adsorption, filtration, heat treatment, and microbiological decomposition. Some of the chemical removal methods of MPs include oxidative ditch, marine microbial interactions, and biological methods, including reverse osmosis (RO), membrane pore size, size distribution, and particle charge method. In addition, the discovery of nanomaterials has paved the way for a method called bio-nano-remediation, which could potentially be utilized in dealing with environmental contaminants such as MPs. The unusual properties of nanomaterials, especially in terms of structure, chemical stability, availability, and precision production, have become a research area of much interest to researchers in environmental science, especially for those interested in bioremediation. Some benefits of nanomaterials are size, shape, surface plasmon resonance phenomena, and quantum effects, which lower the stimulation energies [2]. Such characteristics make nanomaterials excellent tools for curing, eliminating, and remediating MPs [3]. Carbon nano springs, for example, have been used to successfully breakdown microplastics from cosmetics due to their outstanding stability, high graphitic content, and spiral shape [4]. The growth of nanotechnology, combined with the incorporation of nanomaterial units with 100 nm diameter, has opened doors to new strategies that transcend the restrictions of traditional bioremediation technologies. The integrated approach promises great hope in the treatment of MPs in groundwater, wastewater, sediments, and soil with reduced costs and adverse environmental impacts [5]. This study aims to fill a gap in the research by looking at MP pollution from all angles, focusing on its sources, how it ends up in the environment, health concerns, and remediation approaches. This review adopts a multidisciplinary approach by merging environmental sciences, biotechnology, material sciences, and policy research to address the issue comprehensively while building upon the limitations of prior studies that focused solely on isolated areas of MP pollution. This study primarily emphasizes the significant facets of MP pollution but also offers a structured comparison of physical, chemical, biological, and nanotechnological methods. Each method is evaluated based on its efficacy, cost, scalability, and environmental impact, providing readers with a comprehensive understanding of MP remediation techniques. This review not only emphasizes the comparison of various remediation strategies but also enlightens the audience on innovative biotechnological interventions that merge bio-nanotechnology with environmentally sustainable degradation mechanisms, including adsorption, photodegradation, and catalytic transformations, all of which prioritize human safety and yield enduring advantages for the environment. To investigate bio-nanotechnology as a sustainable, scalable, and environmentally friendly method for tackling MP pollution, it is essential to connect scientific innovation with practical applications.
2. SOURCES OF MPs
MPs are minute plastic elements measuring <5 mm (0.2 inch) in length [6]. They are introduced through various consumer products, including cosmetics, synthetic clothing, plastic bags, and bottles [7]. MPs are categorized as primary or secondary. Primary MPs consist of microbeads (commonly found in personal care products), plastic pellets (used in the manufacturing industry), and plastic strings (commonly present in synthetic textiles such as nylon) [8]. On the other hand, secondary MPs are formed when larger plastic items naturally degrade through exposure to environmental factors such as wave action, air abrasion, and UV radiation, leading to their fragmentation [9]. It is important to note that plastics come in two primary forms worldwide: synthetic and bio-based plastics. Goods made from bioplastics have a significantly lower environmental impact. Therefore, both the plastics industry and consumers should aim to adopt bio-based plastic goods to safeguard the ecosystem [Figure 1].
 | Figure 1: Representation of microplastics reaching the environment. [Click here to view] |
MPs have been examined in various environments, including beach sediments, seabed residues, shorelines, wastewater effluents, ice, and surface water [10]. Wind and ocean currents transport some MPs to remote regions such as the Arctic and Antarctic, a phenomenon highlighted in [11] study. The presence of MPs in marine ecosystems poses a significant threat to the diverse range of aquatic life, including bivalves, zooplankton, mussels, fish, shrimp, oysters, copepods, lugworms, and whales, all of which ingest these minute particles [12]. In fact, it was discovered in 2018 that more than 114 aquatic species contained MPs [13]. Various detrimental effects on marine creatures due to the consumption of MPs include oxidative stress, reduced growth rates, false satiation, and reproductive issues [14]. In addition, high levels of MPs have been found in rivers and lakes worldwide, and they have even been identified in consumables such as beer, water, marine food, and table salt. A pilot study revealed the intriguing discovery of MPs in human feces [15]. Recently, scientists have found MPs in human tissues and organs, indicating how detrimental MPs are to human fitness and marine life. This has seen MPs represent the critical class of materials posing a major threat to both humans and marine ecosystems on a global scale. Over the period from 1950 to 2015, the production of MPs managed to surpass the benchmark of 6 billion metric tons. Now, around 4.9 billion metric tons are already present in the environment and landfills. Despite these concerns, the global production of MPs continues to increase, and it is projected to reach 12 billion metric tons in landfills and the natural environment by 2050 [16]. MP pollution comes from various sources, and they can be broadly categorized into primary and secondary sources.
2.1. Land-based Sources
The textile industry produces and trades a significant number of MPs, with three main types of textile fibers: natural, regenerated, and synthetic. The MPs found in textile waste are primarily composed of synthetic fibers, accounting for >170% of the total. The textile industry produces an estimated 42 million tons of synthetic fibers annually, of which 80% are part of PES [17]. Because of the significant harm that synthetic textile fibers have caused to the marine environment, awareness of them has increased [18]. The fundamental source of microfibers is the industrial and household washing of synthetic fabrics. Studies demonstrated that microfibers originating from textile waste were responsible for 35% of the MPs found in the aquatic environment. Under home washing conditions, a 6 kg wash load of acrylic fabric could discharge more than 7,00,000 microfibers. Every garment may shed up to 1900 fibers in a single wash, and every pre-proof liter of effluent discharges more than 100 fibers from every clothing. During the initial machine washing, up to 13 million microfibers were released from PES and cotton textiles. Investigated the number of microfibers that are shed from PES, nylon, and acrylic textiles [19]. Averaging 7360 fibers/m2/L, PES fleece fabrics shed the highest quantity of microfibers. When it came to woven PES, as opposed to knitted PES and woven PP, the wash of woven PES produced the greatest amount of microfibers, more than 6 million per average 5-kg wash load of PES fabrics [20]. Wastewater treatment plants (WWPTs) are unable to prevent the release of MPs due to mechanical and chemical stressors. First estimates from the same research group showed that each washing released 2.98 × 108 PES microfibers annually into the water, whereas wearing PES garments released 1.03 × 109 microfibers into the air. The sea receives an order of magnitude more microfibers than those released directly into the air. Microfiber release during washing appears to be influenced by consumer cycles, detergent, temperature, water hardness, and textile structures. For example, compared to the initial wash, the abundance of microfibers was generally lower after multiple wash cycles. 0.8 million more microfibers were liberated by higher water-volume-to-fabric ratios than by standard wash conditions. Hernandez also showed that washing with liquid or powder detergent produced a noticeably greater number of microfibers than using deionized water. Furthermore, longer washing times, greater temperatures, and mechanical action improved the release of microfibers. Hard water increases the rates of abrasive damage. However, using softeners can reduce microfiber production by 35%. Furthermore, worn textiles and other loose textile structures release more microfibers upon washing, and high-twist yarns are chosen for minimizing shed [17].
2.2. Industrial Sources
This review section looks at several industrial sources of MPs, with a particular emphasis on industries including rubber manufacture, textiles, and cosmetics. MPs are frequently used as exfoliants in cosmetic products such as toothpaste, body washes, and face scrubs, which release plastic particles into water systems. The main source of MPs from the textile sector is synthetic fibers that are shed during washing and can end up in wastewater and natural water bodies. MPs are produced by the rubber industry, which includes the production of tyres. These sectors constitute substantial MP sources, adding to the particles found in marine and terrestrial environments, as briefed in the below section:
2.2.1. Cosmetic industry
A wide range of personal care products, such as scrubs and face exfoliating soaps, shower gel and shampoos, skin creams, and liquid makeup, have plastic microbeads added to them as an abrasive agent [21]. Because of their inadequate clearance, these microbeads can escape from WWPTs and be released directly into household sewage. Zitko and Hanlon first recognized that the microbeads connected to MPs in PPCPs are hazardous to the environment [22]. Due to their appropriately small sizes, aquatic organisms can easily consume them and amass them along the food chain. According to the European Cosmetic Industry Association, 4130 tons of microbeads are used annually in soap in the countries of the European Union, Norway, and Switzerland [23]. A study conducted in 2019 examined 37 popular face and body washes sold in the United Arab Emirates. Of the 37, 11 products had MPs, ranging in size from 12.3 to 273.4 μm, and they also underwent physical changes, such as fragmentation, when heated [24].
2.2.2. Rubber Industry
Rubber is regarded as a type of plastic in addition to thermoplastics such as PE, PP, PVC, and polyethylene terephthalate. There were 12.6 million tons of natural rubber and 14.6 tons of synthetic rubber in the 26.9 million tons of rubber market in 2016 [25]. One of the most significant sources of rubber emissions is automobile tyres. Tyres generate a significant number of particles and trash ranging in size from nano to micrometers. The fact that tyres are a covert source of MPs has gone unnoticed; estimates suggest that 26–74% of MPs originate from tire rubber [26]. Tire-derived MP emissions per capita per year ranged from 0.23 kg in India to 4.7 kg in the United States, with a global average of 0.81 kg, based on data currently available from 13 countries worldwide. The emissions from artificial turf, car tyres, brake wear, and road pre-proof markings were all substantially greater than those from airplane tyres. They account for 5–10% of all plastics that wind up in the water worldwide [27]. MPs were found in water and sediment from the stormwater floating treatment wetland on the Gold Coast of Queensland [28]. It was discovered that synthetic rubber carbon-filled particles, which primarily originate from automobile tyres and road runoff, made up 15–38% of the MPs in the sediment. The mass of tire and road wear particles emitted in the Seine watershed was 1.8 kg/person/year (−1) based on a baseline watershed-scale MPs mass balance model. Out of all the tire-derived MPs that were found, 2% were discharged into the estuary, and 18% were transferred to freshwater [25]. Rubber was used in tyres, but it was also recycled through the shredding of rubber granules (sized between 0.7 and 3 mm) for artificial turf infill and refill material. These recycling operations were significant sources of rubber in the environment. In Switzerland, 357 and 10600 ± 3800 t of rubber was freed from road tire wear. Furthermore, dyes, mostly red and yellow pigments that are frequently applied to thermoplastic road-surface marking paints, were found in these MPs, according to Raman analysis [24].
3. ENVIRONMENTAL TRANSPORT OF MPs
MPs can come from direct emissions that are not treated, although most sources from residential and urban areas send large amounts of their municipal or industrial effluents to WWTPs. Wastewater is often treated in three stages, and the amount of metal pollutants that were removed from different WWTPs ranged from 64% to 99.56% [29]. For partial elimination, minor and light MPs may dodge from WWTPs into surface runoff [21]. PPCPs release an average of 209.7 trillion microbeads into mainland China’s aquatic ecosystem. Merely 50% of them originate from direct emissions, with the remainder coming from the insufficient elimination of microbeads in WWTPs through their wastewater. Sewage sludge is another source of MPs entering the environment, in addition to WWTPs. The application of sewage sludge in landfills as fertilizer and soil amendment signifies an obvious addition of MPs to agricultural soils [25]. According to Fytili and Zabaniotou, fertilizer and landfills made up 70% of the reclaimed sewage sludge in Europe [30]. As of 2019, Hudcová et al. reported that up to 80% of the sewage sludge was used in Irish agriculture [31]. Journal pre-proof estimated that from 125 to 850 tons of MPs were added per million people directly applied annually to European agricultural soils through processed biosolids and sewage sludge. However, since deposited sewage sludge can be washed away and dispersed by wind and rain, further comprehensive research is needed to establish whether sewage sludge is a source or a sink [32].
4. EFFECTS OF MPs ON HUMAN HEALTH
In fact, MPs have been detected in the environment and can easily penetrate the human body through ingestion, inhalation, and contact with the skin. Health risks associated with MPs include carrying toxic chemicals such as bisphenol A and phthalates that disrupt endocrine functions. Once inside the body, MPs can trigger inflammation, oxidative stress, and immune responses, which may have adverse effects on the respiratory, digestive, and circulatory systems and their effects on human health are described below:
4.1. Respiratory Effects
Atmospheric MPs constitute a relatively new source of pollution, which in recent years has attracted broad attention. MPs were found in the atmospheric environment of megacities characterized by high population density, such as Beijing, Shanghai, and London. Species composition and concentration of airborne MPs are related to lifestyles in these communities, anthropological activities, and meteorological conditions [33]. There are MPs in the indoor and outdoor atmosphere, but the concentration of MPs in the indoor atmosphere is much higher than the concentration of MPs in the outdoor atmosphere. Plastic debris is also formed through abrasion by indoor objects, materials, and furnishings, and MPs from these sources have much greater impacts on humans than polymers within food and beverages [34]. Inhaled plastic fibers and particles cause mostly respiratory discomfort in exposed workers because of inflammation in the airways and interstitials. At ambient concentrations very near background levels, this could expose such individuals to the risk of lesion development [35].
Recently, it was reported that MPs were found in the lungs of humans and the sputum of people with lung diseases [36,37]. A maximum number of 565 particles/10 mL with 21 types of MPs (polyurethane, polyester, chlorinated polyethylene, and alkyd varnish with 78.36% of the total found in sputum) was identified. Some MPs are transported through the respiratory tract, and some come out through sputum, but others have been associated with smoking behaviors and advanced tracheal intubation [38]. MP exposures affect the biophysical properties of pulmonary surfactants with its structural changes and barrier function in airways at alveoli. Lung epithelial cells with MPs inhibit the proliferation of epithelial cells and induce apoptosis of epithelial cells. MPs stimulate the production of pro-inflammatory cytokines, which involve tumor necrosis factor-alpha, interleukin (IL)-6, IL-1β, and transforming growth factor-β produced by lung epithelial cells. Glutathione peroxidase, catalase, and superoxide dismutase activities relative to oxidative stress were highly suppressed in MP-exposed samples, accompanied by increasing reactive oxygen species (ROS) production and redox imbalance, as shown in the figure 2.
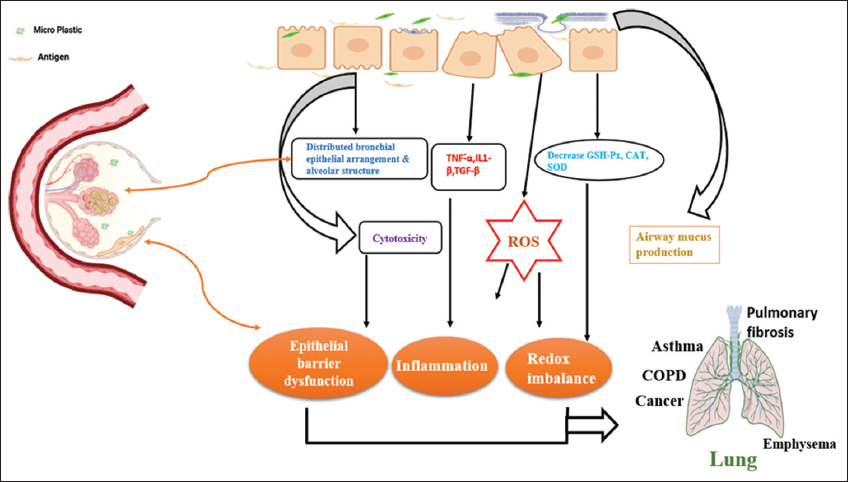 | Figure 2: Mechanisms of microplastics on the respiratory system. [Click here to view] |
4.2. Dermal Effects
MPs pose potential risks to human health, including dangers associated with human skin health. Ingestion, inhalation, and dermal exposure can enter the human body, causing significant damage to the system [39]. Figure 3 shows the entry of MPs through the dermal pathway, inducing inflammation and fostering chronic skin diseases. Furthermore, their ability to absorb carcinogens may lead to increased risks of cancer and even DNA damage, and cosmetics used by humans contain MPs, potentially leading to the development of skin cancer. Furthermore, specific studies focusing on MP exposure on the skin and how it can affect overall health in humans are to be conducted for further understanding of the possible mechanisms of action.
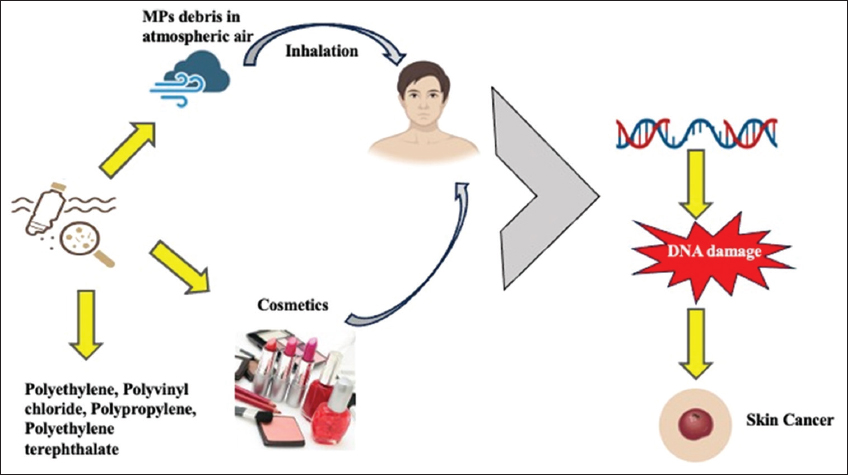 | Figure 3: Schematic representation depicts the pathways of microplastics in the human skin. [Click here to view] |
4.3. Digestive Effects
The cytotoxic effects of polystyrene-nano plastic (PS-NPs) on human gastric fibroblasts were tested to find out the relationship between size and concentration dependence [40]. Size-dependent increased apoptosis and necrosis were observed due to cell death by larger PS particles. This study reveals serious cellular membrane potential alterations as well as a marked increase in the level of ROS after exposure to PS particles, meaning that PS-NPs severely compromise cellular health and function, leading to oxidative stress and mitochondrial dysfunction. The involvement of large PS particles, to a significant extent, was observed in mitochondrial dysfunction, which is vital for energy production and general cell survival [41]. A simultaneous intracellular calcium burst further deranges cellular structure. Furthermore, the interaction with PS particles has been correlated with the activation of pathways of DNA damage and cell death; thus, it may serve as a basis for the long-term consequences of cellular integrity and health in the context of the gastrointestinal tract.
5. REMOVAL OF MP
There are two primary methods of removing technologies for MPs, which include separation and degradation. These three types are based on physical, chemical, or biological processes [42].
5.1. Physical Remediation Techniques
Separation of MPs from environmental samples generally follows from physical properties that differentiate MPs from other matter, with the most common currently applied techniques based on:
5.1.1. Screens
The purposeful elimination of MPs often involves screens and grids as effective devices in the cleaning process. Screens, a material usually composed of mesh, are used primarily to manually filter larger debris and particles from water or soil. Screens are constructed with pore sizes that do not allow water or other fluids to penetrate but capture MPs [43]. In constructing physical barriers that can direct the flow, focus it, and collect MPs at certain points, grid systems can also be applied. To remediate such water bodies or treatment systems while effectively capturing and isolating MPs from their environment, practitioners strategically locate screens and grids to minimize the effects of pervasive pollutants. It is an applied solution that marries the use of screens and grids for MP remediation, whereby physical filtration mechanisms can be used to address the difficult issue of getting rid of such minute plastic particles from ecosystems [43].
5.1.2. Grids
In MP removal, grids play an important role as effective filtration systems. These grids usually consist of fine meshes or nets strategically deployed in water bodies where MP pollution is common. As the water flows through these grids, the mesh acts as a physical barrier, capturing and retaining the MP particles present in the water. This method allows MPs to be extracted efficiently from many aquatic environments, such as rivers and oceans. The grids effectively catch and collect these very small pieces of plastic. This stops them from spreading further and, by extension, any possible environmental or ecological effects that might come with MP pollution. Although grids are only one portion of a larger cleanup effort of MPs from the environment, they comprise an in-situ cleaning agent for removal and are a process in environmental conservation and pollution management [44]. The two most common methods used for the removal of MPs from wastewater are primary sedimentation and floatation. In primary sedimentation, wastewater is allowed to settle inside a tank, allowing larger and denser particles, which include MPs, to settle at the bottom. This process works on the principle of gravity to separate solid particles from the water. Floatation allows small air bubbles to be released into the wastewater; it causes MPs to bind with air bubbles, become buoyant, float on the surface, and form a froth layer that can be skimmed off. Both of them use the physical treatment processes for the differentiation of MPs from water, which is the first remediation step aimed at reducing the harmful effects of MP pollution in freshwater and marine bodies [29].
5.1.3. Disk filters
Due to its efficiency and practicality, a disk filter represents one of the prime solutions from the vast amounts of research performed on efficient removal techniques caused by the intensifying concern surrounding MPs. Disk filters often consist of several stacked membranes with different pore sizes to catch such a range of MP particles – from more significant fragments toward finer fibers – and are notoriously difficult to filter out through traditional methods. Researchers have found that disk filters can greatly reduce the amount of MPs in treated wastewater. Some types of MPs can be removed from treated wastewater with a removal efficiency of more than 99% [14]. Because disk filters are flexible in their operation, this technology can, therefore, easily be integrated into existing WWPTs without requiring significant decampments of the filter. The disk filters can be made of various materials, including ceramics and polymers, which can be modified to enhance their filtration properties and optimize their hydrodynamic qualities. Besides, studies have also shown that surface coatings or electrostatic treatments may enhance the attraction of the filter material to MPs, thus increasing capture rates. Another type is the disk filter, which applies filtration technology in the process of removing MPs from water systems. The filters involve a set of disks with either fine mesh or pores that assist in capturing and holding MP particles as they filter through the passing water. Thus, the filter design allows suspended solids, mainly tiny plastic pieces, to get separated from water. MPs become retained on the surface or in the filter media as water flows through the filter and, therefore, cannot enter the treated water stream. Disk filters are valued for their ability to provide efficient and reliable MP removal and thus contribute toward efforts to mitigate the environmental effects of these persistent pollutants in water sources [45].
5.1.4. Rapid gravity sand filtration
The ease with which the traditional filtration methods could be used, along with their effectiveness, enabled rapid gravity sand filtration to take over as a method of MPs removal from water resources. The various techniques applied range from straining sedimentation and adsorption by letting water pass through layers of sand and gravel. Many factors are perceived to affect the efficiency of MP removal by Rapid Gravity Sedimentation and Flocculation (RGSF). These key factors are sand grain size, filtration rate, and biofilms on the sand grains that enhance particle catch. The type of MP that could be removed may vary with the filter design and operating conditions, and the range of its removal efficiency is reported to lie within 80% to over 95% [46]. Compared with other filtration techniques, RGSF can continuously filter with the primary advantage of having high flow rates and minimizing idle time. However, there are disadvantages, such as the possibility of clogging and the necessity of frequent maintenance in order to ensure optimal operation. Clogging caused by collected MPs and other particles requires constant backwashing or replacement of filter media. Furthermore, while RGSF drastically lowers the concentration of MPs, there is also an interest in the fate of those particles post-filtration since some research has raised concern over the release of hazardous additives or even the possibility of MPs resuspending when backwashing processes are carried out [47].
5.1.5. Dynamic membranes (DMs)
DMs have recently received much attention as a flexible and effective means to remove MPs from water sources, especially in wastewater treatment. DMs are formed in situ by a layer of particles that accumulate onto a porous support layer, an active filtering layer that removes pollutants, including MPs. This special configuration makes it possible to have lower operating costs because the self-forming layer is continuously renewed, hence eliminating the expensive membrane replacement cost. According to studies, DMs are very good at getting rid of MPs of all shapes and sizes because the dynamic layer can catch particles through physical straining and adsorption. In addition, DMs can operate at pressures lower than the traditional membranes, which reduces energy requirements and makes them suitable for application on a large scale [48]. A positive aspect of DMs is that they might self-heal; backwashing or changes in operation can rejuvenate the filtration layer after fouling or clogging, thereby prolonging the useful life of the system. This would imply that high organic matter concentration or other suspended materials compromise the formation and stability of the dynamic layer; hence, the process becomes susceptible to feed water properties. Optimizing DM systems through alterations in support material, operating conditions, or incorporation of coagulants or adsorbents is under investigation to improve MP capture with consistent filtration performance. DMs present a flexible and promising solution to remove MPs in areas where expensive filtration techniques are inaccessible [49]. They usually refer to a filtration system where the membrane undergoes constant changes or adaptations during the filtration process. In the case of MP removal, DMs could be innovative concepts to improve the efficiency of the capture of MPs from water sources. This can include the application of membranes with dynamic properties or configurations that could optimize filtration in accordance with specific characteristics of MPs. The dynamic nature of these membranes could improve the overall performance and sustainability of MP removal processes in water treatment applications [47].
5.1.6. Dissolved air floatation (DAF)
Dissolved air flotation can eliminate MPs from drinking water sources and wastewater. This highly established water treatment method dissolves air under pressure into water, produces microbubbles once the pressure is released, allows suspended particles, including MPs, to stick to bubbles and then rise to the surface, and can be removed as a sludge layer. This process highly captures low-density MPs due to their floatation tendencies and because, generally, such a method will not be of use for conventional separation through methods such as sedimentation or filtration. It has been reported in different literature reports that DAF has the capability of removing around 85% and above 95% depending upon factors, such as bubble size and time retention, as well as those of water, for instance [50]. It has many advantages, including the capacity to process high flow rates, making it appropriate for large-scale treatment plants. It is often used in combination with coagulants or flocculants to improve the removal of MPs as a way of agglomerating particles into bigger flocs, which are easier to separate and float. The operating flexibility of DAF also allows it to be used as a standalone treatment for the removal of MPs from water or as a pre-treatment before filtration. The sustainability of the process may be affected by issues that still need to be addressed, such as the proper recycling or disposal of sludge containing MPs and the energy requirements of the air saturation system. By improving air bubble formation techniques, adjusting chemical dosages, and creating hybrid processes that combine DAF with other treatments to improve overall MP capture, research is being done to increase the efficiency of DAF. DAF has great potential as a dependable and flexible way to lower MP concentrations in various water sources, whereas MP pollution keeps increasing [51].
5.2. Chemical Remediation Method
Chemical separation methods for MPs often aim to dissolve or break down other non-plastic materials, such as organic substances, to enrich and isolate the MPs. Some common procedures include:
5.2.1. Ligand-exchange-initiated uptake-complexation mechanisms
Ligand-exchange-initiated uptake-complexation mechanisms are a novel and extremely focused approach to combating MP pollution, which is becoming a more significant and worldwide environmental concern. Because they are generally <5 mL in size, MPs are widespread pollutants that exist in both aquatic and drinking water environments. They are, therefore, difficult to remove through traditional filtration. This method offers the possibility of molecularly capturing MP particles through the special capacity of ligand-exchange processes to chemically bind and immobilize them [52]. A ligand-metal complex is formed at the center of this mechanism, which is particularly made to recognize and bond with the hydrophobic surfaces of MPs, often with the help of transition metals such as copper, iron, or zinc. The metal ion and the ligand form a strong link that integrates the MP into the complex’s larger structure through an exchange with functional groups on the MP’s surface. The step of complexation can even be tailored depending on the type of plastic because, depending upon polymers, each has different types of functional groups, such as hydroxyl, carboxyl, amine, etc. As an example, depending upon surface properties, there are some specific ligands bearing carboxylate groups with which oxidized polyethylene MPs will interact to support selective binding. Isolation of MPs will be effective only when they bond.
The size of the complexes has now become bigger, allowing for filtration. The elimination method also involves precipitation, a condition under which the complex separates from the solution. However, magnetic separation can be effective if the metal complex possesses any magnetic properties. By changing the pH or adding certain salts, these complexes can also help MPs stick together and be cleaned up in wastewater or industrial settings. In view of the potential for such applications in rivers, lakes, and even ocean habitats where MPs are very common, the technique has immense potential for large-scale environmental applications. There are still issues today despite this commitment. It is as hard to ensure that the technique is economical and environment-friendly on a large scale as it is to design highly selective ligand-metal complexes that are capable of binding exclusively to MPs without damaging other organic particles. Selection and testing must be done carefully so that there would not be a potential toxicity in aquatic life or ecosystems where such complexes may be used because they depend on metals. Future research in the field of ligand design and selection of metal can lead to much more efficient and long-lasting methods of removing MPs, and improvements to this technology applied to a range of water systems may prove very useful. Scientists are also considering means of making these complexes biodegradable or reusable, thus further reducing their impact on the environment. Consequently, ligand-exchange-initiated uptake-complexation represents a technological advancement in environmental cleaning that can catch and remove MPs in the water that otherwise appeared to be practically impossible for their removal [53].
5.2.2. Activated sludge and oxidative ditch
Researchers have examined the effectiveness of wastewater treatment methods, such as activated sludge and oxidative ditch systems, in removing MPs, given the rising concern over MP pollution. MPs are the accumulation of plastics in freshwater and marine habitats and pose serious ecological and human health hazards. MPs are defined as plastic particles smaller than 5 mm in diameter. MPs can be efficiently removed in activated sludge systems, which are widely used in wastewater treatment facilities, through processes such as adsorption onto sludge flocs, aggregation, and subsequent sedimentation in clarifiers. The removal efficiency in these systems is typically between 50% and 90%, depending on the sludge retention time, hydraulic loading, and particle size distribution [54,55]. Since larger rates of adsorption and settling are allowed by the extended biological treatment, longer sludge retention periods appear to increase the collection of MPs [42]. Similarly, continuous circular flow, which promotes higher contact between MPs, sludge, and microbial biofilms, enhances the removal of MPs in the oxidative ditch method, which is characterized by its unique ring-shaped, extended aeration design. By enhancing microbial activity, this extended aeration condition provides MPs with increased opportunities to adhere to sludge and eventually settle out of the water column. Studies revealed that when parameters are well managed to extend the contact period of sludge and increase the intensity of mixing, it will be possible to ensure the removal efficiencies in the oxidative ditch that is at par with or even higher than that which is observed in conventional activated sludge systems [56]. This closed-loop design also decreases the possibility of re-release of MPs, which is a problem in other intermittent flow systems. These procedures combined provide useful information on how to improve the removal of MPs from treatment plants, which may reduce the emission of these long-lasting pollutants into the environment. Treatment plants are becoming increasingly crucial in the global fight against MP pollution, so it is essential that these techniques are better understood and optimized [28].
5.2.3. Marine microbial interactions
Marine microbial interactions are very important in excluding MPs from the oceanic environment. MPs are small plastic particles <5 mL in size, and their existence is becoming a concern because they are widespread in marine ecosystems. Microbes, including bacteria and fungi, interact with MPs in different ways. Some microorganisms can bind to the surface of MPs and secrete enzymes that degrade the plastic polymers, which is called biodegradation. Besides, microbial biofilms may be formed on MP surfaces, promoting the aggregation and sinking of these particles. Moreover, some microorganisms are also reported to utilize MPs as a carbon source for their degradation. Understanding and utilizing these microbial interactions offers promise in the development of environmentally friendly approaches to reduce the effects of MPs on marine ecosystems and improve their removal from the oceans [57].
6. BIOLOGICAL REMEDIATION METHODS
The methods of the biological separation of MPs from environmental samples mainly rely on breaking down the organic matter present, leaving the tougher plastic particles through enzymatic or microbiological means. So far, several biological approaches that have been developed include:
6.1. RO
RO is the process of water purification that applies a semi-permeable membrane to remove ions, molecules, and larger particles from water. It is a common method applied for the desalination of seawater and the purification of drinking water. Even though RO removes a wide variety of contaminants including some MPs, its efficiency for removing MPs will depend on the size of the particles and characteristics of the membrane. MPs are small pieces of plastic <5 mm in diameter and can be present in water sources due to the fragmentation of larger plastic items or as microbeads in some personal care products. RO membranes are usually around 0.1–0.0001 μm in pore size. Although this is small enough to capture many MPs, it may not be effective for removing the smallest particles [28]. Some considerations for the use of RO in MP removal include: The membrane’s pore size determines the effectiveness of RO in removing MPs. The smaller the pore size, the higher the chances of trapping smaller particles, including some MPs. Pre-treatment steps are necessary depending on the source of water to prevent fouling of the RO membrane. Suspended solids and other contaminants may accumulate on the surface of the membrane, thus reducing their effectiveness. Pre-filtration or other pre-treatment methods can help solve this problem. The charge of the MP particles may influence their interaction with the RO membrane. Some membranes are negatively charged, which repels negatively charged MPs. Adjustment of the pH of the water or using different membrane materials may enhance removal. RO may be more effective in removing larger MPs than smaller ones. Evaluating the efficiency of RO also includes considering the distribution of sizes of MPs in the water source.
6.2. Membrane Bioreactors (MBRs)
MBRs are high-efficiency wastewater treatment systems combining biological treatment processes with membrane filtration technology. The microorganisms present in the MBR break down organic pollutants found in the wastewater into biomass and gases. Ultrafiltration or microfiltration membranes are used in MBRs to separate the treated water from the activated sludge. This creates a barrier against microorganisms and suspended solids. This membrane-based separation improves the quality of the treated water while yielding effluent with low turbidity and a high degree of clarity. MBRs include many advantages, such as a small footprint, good effluent quality, and possible reuse of produced water, making them suitable for numerous applications in industrial and municipal wastewater treatment [47]. These remediation technologies collectively address the challenge of MP pollution in wastewater, each with its benefits and constraints given in Table 1.
Table 1: Microplastics remediation techniques.
| Remediation Techniques | Illustration | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Physical remediation techniques | ||||
| Screens | Acts as effective physical barriers, efficiently capturing plastic particles | Simplicity and low maintenance | Potential clogging and limited effectiveness for smaller particles | [43] |
| Grids | These are structures that facilitate size-based separation | Scalability and minimal energy requirements | Clogging and reduced efficiency for smaller particles | [58] |
| Primary sedimentation | Involves allowing wastewater to settle, facilitating the settling of larger particles, including microplastics | Simplicity and cost-effective | Limited effectiveness for smaller particles and potential resuspension | [59] |
| Rapid gravity sand filtration | Removes microplastics from water through sedimentation | Cost-effectiveness and simplicity | Potential clogging and limited effectiveness for smaller particles | [60] |
| Dynamic membranes | Involve the use of adjustable filtration systems for enhanced efficiency | Adaptability to varying conditions | Potential complexity and maintenance challenges | [11] |
| Dissolved air floatation | Utilizes tiny air bubbles to float particles for easier separation | High removal efficiency | Operational complexity and the need for chemical additives | [61] |
| Chemical remediation techniques | ||||
| Activated sludge | This process in microplastic removal uses biological treatment | Organic matter degradation | High energy consumption and potential for incomplete microplastic removal | [62] |
| Marine microbial interactions | Leverage microbial activity to break down and degrade microplastics | Ecological compatibility | Varying microbial effectiveness and potential ecosystem impacts | [63] |
| Oxidative ditch | It is an anaerobic treatment system with rotating ditches | Efficient organic matter degradation | High energy consumption and limited effectiveness for certain microplastics | [64] |
| Biological remediation techniques | ||||
| Reverse Osmosis | Eliminates MPs from water by applying a semi-permeable membrane | Provides efficient purification | High energy utilization | [65] |
| Membrane bioreactors | Combine biological treatment and membrane filtration to effectively remove microplastics from water | Offering high removal efficiency | Membrane fouling and high operational costs | [66] |
7. NANO-BIOREMEDIATION
Plastics are subjected to all forms of degradation under natural weather conditions, primarily including photodegradation, followed by thermo-oxidative, hydrolytic, and especially microbial biodegradation. Thus, the exposure to UV energy from the sunshine acts as a starting energy event, allowing photodegradation processes that can, in turn, lead the plastics to an easier microbial deterioration into smaller, lower molecular weight components of the structure. However, this natural degradation process is slow and takes more than 50 years to complete [67]. Nano-bioremediation technology offers a way to significantly expedite this process. While conventional bioremediation is environmentally friendly, it lacks mechanical and chemical steadiness, making it slow and less efficient [11]. The integration of nanotechnology with bioremediation opens new opportunities. Nanoparticles are highly reactive and can provide in situ treatment, making remediation with tailored nanomaterials more efficient and economical than current processes [68].
8. MECHANISM OF BIOREMEDIATION OF MPs USING NANOMATERIALS
Nanomaterials serve as membrane filters, flocculants, catalysts, and adsorbents [Table 2]. Adsorption is a mass transfer process that involves molecules from a liquid or gas interface physically or chemically bonding to a solid surface. Membrane filters can strip off MPs from water, while catalysts aid in their breakdown. Flocculants accelerate the aggregation of colloidal particles, allowing for easier filter separation [69]. Adsorption is an exothermic surface process where an adsorbate stage is transferred on a solid surface, forming a monomolecular adsorbent film under specific conditions [70]. Activated carbon is commonly used as a solid adsorbent because of its high porosity and substantial surface area, but its cost restricts widespread use. Consequently, various carbon allotropes and functionalized carbon nanomaterials have become preferred alternatives for their affordability and effectiveness [71]. The removal of MPs from wastewater is highly effective when using green nanoscale semiconductors as photocatalytic drivers. Nanomaterials have superior catalytic activity and a higher surface-to-volume ratio, thus excelling in photocatalysis by oxidizing contaminants more efficiently than traditional bulk materials [72]. Photocatalysis involves two reactions: photo-oxidation and photoreduction. Semiconductor nanoparticles having empty conduction bands and full valence bands tend to stimulate electron transfer when light energy matches or exceeds the band gap of the semiconductor. This leads to a series of photo-oxidation and photoreduction reactions that ultimately degrade MPs [73]. Hexagonal nanorods, known for their larger surface area and crystalline strength, have proven effective in this context [74]. Conventionally, inorganic substances such as AL2(SO4)3 and FeCl have served as flocculants, but they produce sludge and are sensitive to pH fluctuations, potentially contaminating groundwater. Polymer flocculants, on the other hand, have gained attention as they create more coherent aggregates, particularly in low-temperature, slow-settling conditions due to their large surface area. However, synthetic polymers, although effective, are not biodegradable, poorly soluble, and challenging to recycle. They may also pose ecological concerns. In comparison, biopolymers, or natural polymers, flocculate based on the dimension of their random coils in solution. These biopolymers can be simply improved to increase their performance but are sheerly stable and less effective at low doses [75]. Therefore, incorporating nanoscale components into biopolymers can significantly enhance their efficiency, as shown in (Figure 4 and Table 2).
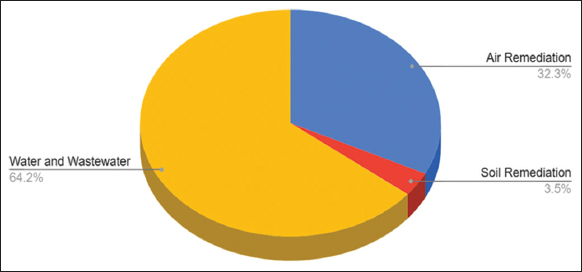 | Figure 4: Pie chart shows the distribution of nanomaterials production for environmental remediation of various categories, which include products, companies, and countries. [Click here to view] |
Table 2: Process of nanoparticle bioremediation of MPs.
| Nanomaterials | Mechanism |
|---|---|
| Adsorption | MPs and nano-adsorbent interactions may result in adsorption. Hydrophobic, hydrogen-bond, electrostatic, electron, electron-electron conjugation, and complexation are some examples of this interaction [76] |
| Photo catalysis | When exposed to UV rays, they produce reactive species, which lead to the MPs’ damage [87] |
| Flocculation | Charge neutralization is the foundation of this procedure. The production of flocs results from the positively charged flocculant neutralizing the negative charge of the MPs. These flocs can be separated using filtration or sedimentation [77] |
| Filtration | MPs and wastewater can be effectively split by means of nano filters utilizing nanoscale sieves [77] |
9. BIONANOMATERIALS IN MP REMEDIATION
Bionanomaterials encompass materials of biological origin combined with organic pieces. The field of utilizing bionanomaterials for MP bioremediation is in its early stages, but researchers worldwide are striving to advance it to an industrial scale, showing promise for the future. Bio-nano remediation, owing to its simplicity, enhanced performance, reduced energy consumption, and prolonged service life, has demonstrated significant effectiveness at the laboratory level [78].
Researchers introduced a membrane for water purification built on cellulose non-woven fabric. They employed a cast coating process to integrate several polysaccharide nanocrystals, including chitin nanocrystals (ChNCs), 2,2,6,6-tetramethylpiperidine-1-oxyl radical-oxidized cellulose nanofibers (T-CNF), and cellulose nanocrystals (CNCs). This coating substantially improved the fabric’s hydrophilicity, tensile strength, and elastic modulus, altering the surface charge to be more negative or positive, depending on the cellulose-based (chitin-based) modifications [81]. CNC and ChNC modifications enhanced membrane permeance, whereas T-CNF impregnation increased stiffness under dry conditions [78]. These biomaterials exhibited the ability to cutoff MPs as small as 500 nm, with T-CNF capable of separating particles as minor as 2 μm. Moreover, electro-spun lignin-zeolite composite nanofiber membranes outperformed other commercial options in terms of flux and penetration rates. The uniform dispersion of zeolite nanoparticles and heating post-treatment significantly improved the nanocomposite’s mechanical properties. This bionanomaterial successfully pre-filtered various MPs [79]. Researchers found a new method for MP removal using magnetic extraction. They designed hydrophobic iron nanoparticles (Fe) to adhere to plastic for magnetic recovery. This approach achieved impressive results, with 92–93% of MPs in ocean water, 78% in residues, and 84% in freshwater recoverable [43]. Recently, hydrophobic magnetite nanoparticles, created using an aerial component of the Anthemis pseudocotula plant extract, showed comparable benefits with reduced environmental impact [80]. Further research involved the one-pot microwave synthesis of highly porous carbon Fe3O4 composites from glucose and Fe3O4 solution, which served as an absorbent. An external magnet effectively removed MPs attached to the composite’s surface [81]. In addition, researchers developed a unique photocatalytic Au-Ni-Ti micromotor-based passive particle removal system. The micromotor, made of TiO2 particles coated with nickel and gold, offered two methods for MP removal: Photoreceptive interactions for individual micromotors and pushing/shoveling interactions for chained assemblies, making it suitable for real-world applications [82,83]. However, the nanomaterial in this case is not only based on bionanomaterials; there are other researches on environmentally friendly synthesis of Au, Ni, and TiO2 particles [84]. An important challenge in green synthesis is limiting nanomaterial mass, especially for photocatalysis. Some methods of remediation for heavy metals have been explored.
10. NANOSCALE ZERO-VALENT IRON (nZVI) FOR HEAVY METAL REMEDIATION
Heavy metals, due to their non-biodegradability and the ability for bioaccumulation, still exist in the environment and threaten to cause major health hazards; these include arsenic, chromium, lead, and cadmium. ZVI particles, usually smaller than 100 nm, have unique features that make them outstandingly good at adsorbing and reducing different heavy metal ions, with very high surface area-to-volume ratios and increased surface reactivity. For instance, nZVI assists in reducing toxic As(V) into less harmful forms of As(III), which can be precipitated and removed during the process of cleaning water contaminated with arsenic. As observed in some research, nZVI assists in immobilizing chromium in subsurface environments due to its transformation from Cr(VI) into Cr(III), which is much less dangerous and less soluble [85]. Similarly, the transformation of lead and cadmium to a less soluble form or allowing adsorption onto a particle surface has well proved the merit of ZVI in removing both of these elements from aqueous solutions [86]. The reasons for the effectiveness of nZVI in heavy metal cleanup are credited to both its distinct structure and electrochemical reactivity. The iron core can accelerate reduction reactions by donating electrons to heavy metal ions. Heavy metals are also recycled from contaminated surroundings via the oxide layer that gathers on the surface, which offers sorption sites. Although nZVI has great potential in lab-scale research, there are a few limitations to its further usage. Since nZVI particles might be poisonous to some aquatic and terrestrial animals, they incorporate particle agglomeration, reduced mobility in porous media, and possible ecotoxicological effects. Researchers have studied several surface modifications and stabilizing techniques to enhance the reactivity and dispersion of nZVI while minimizing its detrimental environmental impacts to address these issues. There is potential for enhancing nZVI stability and mobility by modifying its surface with compounds such as polymers and carbon nanotubes [87]. Considering these challenges, the continuous development of nZVI is promising for successful heavy metal remediation, especially when used in combination with other treatment technologies. The implementation of nZVI-based systems may be crucial in dealing with soil and groundwater contamination, offering a practical and long-term solution to a critical environmental problem. Scaling nZVI technology for real-world uses will require more investigation and pilot projects, guaranteeing its effective and safe use in a variety of environmental contexts [86].
11. BIODEGRADABLE POLYMERS FOR ORGANIC POLLUTANT ENCAPSULATION
Biodegradable polymers, having sustainability, flexibility, and a low ecological mark, are highly attractive for the encapsulation and removal of organic contaminants from different situations. These polymers can be synthesized to degrade into non-toxic byproducts, or they can be of biological origin, such as proteins, polysaccharides, and other bio-based materials, which may trap the organic pollutants within their matrix and permit containment and slow degradation in the presence of environmental conditions. Some common biodegradable polymers include alginates, polylactic acid, polyhydroxyalkanoates, and polycaprolactone. These polymers can be formulated into a wide variety of structures, including hydrogels, nanofibers, and microcapsules, to enhance their interaction with organic pollutants [88]. Particularly these polymeric systems have proven to be invaluable, especially in their formation of stable complexes with difficult-to-remove hydrophobic organic molecules, such as pesticides, dyes, pharmaceuticals, and polycyclic aromatic hydrocarbons, through conventional remediation methods. These pollutants can be effectively immobilized by biodegradable polymer matrices, which will restrict their diffusion and lessen their toxicity in both aquatic and terrestrial ecosystems [89]. These polymers could also be functionalized with selected chemical groups so that they provide better selectivity and affinity to specific organic pollutants in controlled degradation applications. This generally results in longer release and more efficient uptake of pollutants. These secondary pollution issues, usually associated with synthetic polymers, may be significantly diminished because these materials can decompose naturally after finishing their intended roles, leaving hardly any residue at all. Still, research on these materials is ongoing for practical applications such as soil remediation and wastewater treatment, especially when the combination of biodegradability and the effectiveness of pollutant encapsulation is crucial. Biodegradable polymers are, hence, crucial elements in this toolbox of green chemistry, which manages the environment through sustainable means and reduces pollution [90].
12. NANOCELLULOSE-BASED BIONANOMATERIALS FOR POLLUTANT ADSORPTION
The nanocellulose-based bionanomaterials received recent interest as a feasible and effective method for the adsorption of environmental pollutants. The bionanomaterials were found to have unique properties that made them suitable for the adsorption process. These bionanomaterials come from natural cellulose sources, including wood, algae, and bacterial fermentation. Due to its gigantic surface area, high tensile strength, and biodegradability, nanocellulose can provide an ideal scaffold for the adsorption of pollutants. CNC and CNF are the two primary forms of nanocellulose; each has specific morphologies and uses as follows. Surface modification is rather easy, thus making it easily alterable to enhance their attraction toward various contaminants such as organic chemicals, dyes, and heavy metals [91]. Bionanomaterials based on nanocellulose have also shown potential in the adsorption of organic contaminants, especially those with complex chemical structures that cannot be treated by conventional methods. Scientists have been able to develop hybrid bionanomaterials with complementary adsorption capabilities by effectively combining nanocellulose with other substances such as chitosan, activated carbon, and magnetic nanoparticles. For example, chitosan-nanocellulose composites enhance their versatility using the hydroxyl groups in nanocellulose and the amino groups in chitosan to gather both cationic and anionic pollutants. In addition, magnetic nanocellulose composites enable the easy use of magnetic fields to separate adsorbents from treated water, enhancing reusability and making treatment processes easier [92]. Compared to synthetic adsorbents that can, with time, introduce secondary contaminants, nanocellulose is biodegradable, thus ideal for sustainable environmental cleanup. Hence, bionanomaterials based on nanocellulose represent a flexible and sustainable alternative to pollutant adsorption, with an opportunity toward cleaner water systems and reduced environmental impact [93].
13. PLANT-ASSISTED PHOTO REMEDIATION USING BIONANOMATERIALS
Plant-assisted photo remediation is a new method of cleaning up the environment using bionanomaterials and phytoremediation, treating contaminated air, water, and soil. The process of phytoremediation utilizes the inherent ability of plants to absorb, collect, or alter contaminants. Bionanomaterials, which are biodegradable nanoparticles derived from natural materials, enhance the remediation capabilities of plants by many folds. Bionanomaterials, including polymers, carbon-based nanomaterials, and nanoscale metals, when exposed to sunlight, analyze chemical reactions and increase light absorption, which can improve the breakdown of contaminants. Nanoparticles can break down organic pollutants such as pesticides or hydrocarbons by producing ROS upon exposure to sunlight. ROS quickly breaks down complicated pollutants into non-toxic byproducts. Furthermore, some bionanomaterials can enhance the growth of plants by improving nutrient uptake, root development, and durability to environmental stress, thereby making it possible for plants to more efficiently absorb and decompose toxins in contaminated environments [94]. Moreover, since bionanomaterials are biocompatible and have a lower ecological impact than synthetic chemical treatments, their use is considered environmentally friendly. Recent studies have shown that customized bio-nanocomposites can serve as both photocatalysts, which degrade pollutants upon exposure to light, and adsorbents, which trap pollutants [95]. For example, because of their photocatalytic properties, which help in the degradation of various pollutants, ZnO and TiO2 nanoparticles are often used in conjunction with plants. In addition to this, silver nanoparticles (AgNPs) have also shown promising results in enhancing microbial interactions in the rhizosphere by further assisting in the degradation of pollutants. Although promising, further research is necessary to ascertain the long-term impact on the ecosystem, the dosage of nanoparticles to be optimized, and any potential hazardous effects in order to avoid unforeseen repercussions. Thus, bionanomaterials-based plant-assisted photo remediation is a flexible and sustainable approach to dealing with difficult pollution problems in several settings [96]. Plant-assisted phytoremediation with bionanomaterials integrates the natural capability of plants to remove contaminants from soil or water with engineered bionanomaterials, providing a promising, sustainable means of cleaning up polluted areas while minimizing ecological impact. Ongoing research and risk assessment are essential to ensure safe and effective implementation [97].
14. NANOMATERIAL ENVIRONMENTAL REMEDIATION PRODUCTS
The nanotechnology products database, available at statnano.com, contains information on 637 bioremediation products based on nanotechnology, which fall into 50 different categories. These innovative products have been launched worldwide by 316 companies whose headquarters are in 36 different countries. Many companies are engaged in the manufacture of nano-based products for the environmental industrial sector worldwide. Among the prominent contributors are Dow Chemical Company, Samsung Electronics Co., Pentair, Lenntech, Nanoxi Technologies, Shin-Ah Electronics Co., Great Lakes Filters LLC, Chungpung Co., SGX Sensortech, AAF Suzhou Co., Craft Brew Water, Nanostone Water, Pro-reach biotech (Suzhou), Ausclimate Ply, Bionomic Technologies, Caware Int’l Co., APRIA System, Yosemite Technologies Co., Applied Membranes, Chanson Water Ionizers USA, TriSep Corporation, Suzhou Leanstar Electronic, and Hunan Keensen Technology. Upon analyzing the locations of these companies, it is evident that the USA, China, S. Korea, Sweden, Germany, Netherlands, Canada, and Taiwan emerge as the most active countries in championing nanotechnology within the environmental industrial sector. The products are categorized into three sub-industrial sectors: air remediation, soil remediation, and water and wastewater. In the air remediation sub-industrial sector, a range of products is available, including air filters and filtration media, dust collector bags and cartridges, cleaners, sensors, air conditioners, catalysts, scavengers, humidifiers, and Dura socks. These products are designed to enhance air quality and efficiently eliminate airborne contaminants. Liquid nano-clay and soil stabilizers are some products in the remediation sector for soils. It is used to reduce contaminant concentrations in soil, which fosters the growth of vegetation and promotes the improvement of air and groundwater quality. Similarly, a variety of products are produced and marketed in the global market to make clean water accessible. These products aid in the cleaning and purification of urban and industrial wastewater. Membranes, RO systems, nanofiltration plants, water treatment systems and electrodes, water coolers, ionizers, water dispensers, flocculants, additives, surfactants, and limestone removers are examples. The objective is healthy water for the people.
15. TOXICITY ASSESSMENT AND REGULATIONS ON BIONANOMATERIALS
Long-term environmental fate and potential toxicity of bionanomaterials have been researched. These interactions with organisms and ecosystems have been studied to evaluate the safety of such materials [98]. The regulatory landscape for the usage of nanomaterials in ecological applications is changing as researchers and policymakers work together to set guidelines and standards for safe and responsible use. Due to vast research on green synthesis techniques, exciting developments in green technology are expected [99]. There is a need for scientists to identify new approaches of controlling MP pollution on the planet. It will be extremely hard to remove all MPs from water bodies since MPs are very small. However, it is possible to reduce the devastating effects of MP contamination on the environment by decreasing the use of plastic, recycling plastics, enforcing prohibitions on using microbeads in cosmetics and improving the plants purifying water from sewers [35]. Identifying the primary sources of MPs and raising public awareness about their detrimental effects is also an important step in curbing their proliferation. Notably, the UN panel of experts for the UNEP has played a vital role in raising awareness of MPs’ impact on marine organisms, reaching more than 40 million people and promoting the reduction of plastic use and increased recycling. In addition, the Baltic Marine Environment Protection Commission-Helsinki Commission, the convention for the protection of the marine environment of the North-East Atlantic (OSPAR), and the UNEP/Mediterranean Action Plan have set procedures for measuring marine waste, including MPs. In 2011, the plastics industry initiated a joint declaration of marine litter through global associations of the plastics industry, promising support for numerous reviews [100]. Besides, many NGOs play an essential role in determining the global number of MPs produced and their implications on a different scale ranging from the local to global scale while creating public awareness as well. All these efforts work together toward the establishment of a safer environment for both humans and marine life.
16. CONCLUSION
MP environmental pollution is an issue that needs proactive approaches. Scientists have been finding novel ways to combat this problem, and one such promising approach is bioremediation with a nanotechnology perspective. The union of bionanomaterials and nanotechnology has presented sophisticated tools for combating the challenge of MP pollution. Nanotechnology enhances the efficiency of bioremediation, a process that uses biological agents to remove pollutants. Bio-nanomaterials are materials at the nanoscale that are derived from biological sources, and they have been found to be safe for the environment since no negative impacts have been reported. These materials possess characteristics such as adsorption and photodegradation, which can be used to efficiently remove MPs from nature. Large-scale efforts toward the intelligent degradation of MPs through advancements in nanotechnology are now becoming feasible. These technologies could be integrated into remediation strategies that can alleviate the impact of MPs on the environment. On the other hand, though, no adverse effects have been reported thus far; ongoing monitoring and evaluation of the environmental safety of these technologies are warranted. Despite the lack of complete scientific understanding, addressing the issue of MPs requires a multifaceted approach. Political interventions, technological advancements, cultural activities, and an understanding of the environmental implications of MPs should all be considered. This holistic approach is crucial for reducing plastic contamination comprehensively. Failure to do so may result in the persistence of this ecological issue for decades. With these advancements in nanotechnology, large-scale attempts for the intelligent degradation of MPs can now be possible.
17. ACKNOWLEDGMENT
The authors are thankful to Shoolini University for providing the appropriate facilities and data to complete this review.
18. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
19. FUNDING
There is no funding to report.
20. CONFLICTS OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
21. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
22. DATA AVAILABILITY
The data to support the findings for this study are available with the corresponding author upon request.
23. PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
24. USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
25. INFORMED CONSENT
All the authors have consented to participate in the study.
REFERENCES
1. Rahman A, Sarkar A, Yadav OP, Achari G, Slobodnik J. Potential human health risks due to environmental exposure to nano- and MPs and knowledge gaps:A scoping review. Sci Total Environ. 2021;757:143872. [CrossRef]
2. Rizwan M, Singh M, Mitra CK, Morve RK. Ecofriendly application of nanomaterials:Nanobioremediation. J Nanopart. 2014;2014:1-7. [CrossRef]
3. Gong X, Huang D, Liu Y, Peng Z, Zeng G, Xu P, et al. Remediation of contaminated soils by biotechnology with nanomaterials:Bio-behaviour, applications, and perspectives. Crit Rev Biotechnol. 2017;38(3):455468. [CrossRef]
4. Kang J, Zhou L, Duan X, Sun H, Ao Z, Wang S. Degradation of cosmetic MPs via functionalized carbon nanosprings. Matter. 2019;1(3):745-58. [CrossRef]
5. Vázquez-Núñez E, Molina-Guerrero CE, Peña-Castro JM, Fernández-Luqueño F, de la Rosa-Álvarez Ma G. Use of nanotechnology for the bioremediation of contaminants: A review. Processes. 2020;8(7):826. [CrossRef]
6. Reimonn G, Lu T, Gandhi N, Chen WT. Review of microplastic pollution in the environment and emerging recycling solutions. J Renew Mater. 2019;7(12):1251-68. [CrossRef]
7. Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health:current consensus and future trends. Philos Trans R Soc B Biol Sci. 2009;364(1526):2153-66. [CrossRef]
8. Coyle R, Hardiman G, Driscoll KO. MPs in the marine environment:A review of their sources, distribution processes, uptake and exchange in ecosystems. Case Stud Chem Environ Eng. 2020;2:100010. [CrossRef]
9. Cole M, Lindeque P, Halsband C, Galloway TS. MPs as contaminants in the marine environment:A review. Mar Pollut Bull. 2011;62(12):2588-97. [CrossRef]
10. Waller CL, Griffiths HJ, Waluda CM, Thorpe SE, Loaiza I, Moreno B, et al. MPs in the Antarctic marine system:An emerging area of research. Sci Total Environ. 2017;598:220-7. [CrossRef]
11. Mishra A, Kumar J, Melo JS. Silica based bio-hybrid materials and their relevance to bionanotechnology. Austin J Plant Biol. 2020;6(1):1024.
12. Madhav NV, Gopinath KP, Krishnan A, Rajendran N, Krishnan A. A critical review on various trophic transfer routes of MPs in the context of the Indian coastal ecosystem. Watershed Ecol Environ. 2020;2:25-41. [CrossRef]
13. Szymanska M, Obolewski K. MPs as contaminants in freshwater environments:A multidisciplinary review. Ecohydrol Hydrobiol. 2020;20(3):333-45. [CrossRef]
14. Auta HS, Emenike CU, Fauziah SH. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut. 2017;231:1552-9. [CrossRef]
15. Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, et al. Detection of various MPs in human stool. Ann Intern Med. 2019;171(7):453-7. [CrossRef]
16. Borrelle SB, Rochman CM, Liboiron M, Bond AL, Lusher A, Bradshaw H, et al. Why we need an international agreement on marine plastic pollution. Proc Natl Acad Sci. 2017;114(38):9994-7. [CrossRef]
17. Kelly MR, Lant NJ, Kurr M, Burgess JG. Importance of water-volume on the release of microplastic fibers from laundry. Environ Sci Technol. 2019;53(20):11735-44. [CrossRef]
18. Stanton T, Johnson M, Nathanail P, MacNaughtan W, Gomes RL. Freshwater and airborne textile fibre populations are dominated by “natural”, not microplastic, fibres. Sci Total Environ. 2019;666:377-89. [CrossRef]
19. Napper IE, Thompson RC. Release of synthetic microplastic plastic fibres from domestic washing machines:Effects of fabric type and washing conditions. Mar Pollut Bull. 2016;112(1-2):39-45. [CrossRef]
20. Carney Almroth BM, Åström L, Roslund S, Petersson H, Johansson M, Persson NK. Quantifying shedding of synthetic fibers from textiles;a source of MPs released into the environment. Environ Sci Pollut Res. 2017;25(2):1191-9. [CrossRef]
21. Cheung PK, Fok L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017;122:53-61. [CrossRef]
22. Zitko V, Hanlon M. Another source of pollution by plastics:Skin cleaners with plastic scrubbers. Mar Pollut Bull. 1991;22(1):41-2. [CrossRef]
23. Gouin T, Avalos J, Brunning I, Brzuska K, De Graaf J, Kaumanns J, et al. Use of micro-plastic beads in cosmetic products in Europe and their estimated emissions to the North Sea environment. SOFW J. 2015;141(4):40-6.
24. Habib RZ, Salim Abdoon MM, Al Meqbaali RM, Ghebremedhin F, Elkashlan M, Kittaneh WF, et al. Analysis of microbeads in cosmetic products in the United Arab Emirates. Environ Pollut. 2020;258:113831. [CrossRef]
25. Galafassi S, Nizzetto L, Volta P. Plastic sources:A survey across scientific and grey literature for their inventory and relative contribution to MPs pollution in natural environments, with an emphasis on surface water. Sci Total Environ. 2019;693:133499. [CrossRef]
26. Kole PJ, Löhr AJ, Van Belleghem F, Ragas A. Wear and tear of tyres:A stealthy source of MPs in the environment. Int J Environ Res Public Health. 2017;14(10):1265. [CrossRef]
27. Unice KM, Weeber MP, Abramson MM, Reid RC, van Gils JA, Markus AA, et al. Characterizing export of land-based MPs to the estuary - Part I:Application of integrated geospatial microplastic transport models to assess tire and road wear particles in the Seine watershed. Sci Total Environ. 2019;646:1639-49. [CrossRef]
28. Ziajahromi S, Neale PA, Rintoul L, Leusch FD. Wastewater treatment plants as a pathway for MPs:Development of a new approach to sample wastewater-based MPs. Water Res. 2017;112:93-9. [CrossRef]
29. Zhang X, Chen J, Li J. The removal of MPs in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere. 2020b;251:126360. [CrossRef]
30. Fytili D, Zabaniotou A. Utilization of sewage sludge in EU application of old and new methods-A review. Renew Sustain Energy Rev. 2008;12(1):116-40. [CrossRef]
31. HudcováH, Vymazal J, RozkošnýM. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019;14(2):104-20. [CrossRef]
32. Nizzetto L, Futter M, Langaas S. Are agricultural soils dumps for MPs of urban origin? Environ Sci Technol. 2016;50(20):10777-9. [CrossRef]
33. Wright SL, Ulke J, Font A, Chan KL, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2020;136:105411. [CrossRef]
34. Rist S, Almroth BC, Hartmann NB, Karlsson TM. A critical perspective on early communications concerning human health aspects of MPs. Sci Total Environ. 2018;626:720-6. [CrossRef]
35. Prata JC. MPs in wastewater:State of the knowledge on sources, fate and solutions. Mar Pollut Bull. 2018;129(1):262-5. [CrossRef]
36. Amato-Lourenco LF, Carvalho-Oliveira R, Junior GR, Dos Santos Galvao L, Ando RA, Mauad T. Presence of airborne MPs in human lung tissue. J Hazard Mat. 2021;416:126124. [CrossRef]
37. Jenner LC, Rotchell JM, Bennett RT, Cowen M, Tentzeris V, Sadofsky LR. Detection of MPs in human lung tissue using mFTIR spectroscopy. Sci Total Environ. 2022;831:154907. [CrossRef]
38. Huang S, Huang X, Bi R, Guo Q, Yu X, Zeng Q, et al. Detection and analysis of MPs in human sputum. Environ Sci Technol. 2022;56:2476-86. [CrossRef]
39. Parthasarathy S, Arumugam S, Ajith N. Comment on “cancer may be induced by MPs-sorbed polycyclic aromatic hydrocarbons? Oral Oncol Rep. 2024;11:100555. [CrossRef]
40. Yee MS, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY, et al. Impact of MPs and nanoplastics on human health. Nanomaterials. 2021;11(2):496. [CrossRef]
41. Kadac-Czapska K, Osko J, Knez E, Grembecka M. MPs and oxidative stress-current problems and prospects. Antioxidants (Basel). 2024;13(5):579. [CrossRef]
42. Pico Y, Alfarhan A, Barcelo D. Nano- and microplastic analysis:Focus on their occurrence in freshwater ecosystems and remediation technologies. TrAC Trends Anal Chem. 2019;113:409-25. [CrossRef]
43. Shen M, Song B, Zhu Y, Zeng G, Zhang Y, Yang Y, et al. Removal of MPs via drinking water treatment:Current knowledge and future directions. Chemosphere. 2020;251:126612. [CrossRef]
44. Blair RM, Waldron S, Gauchotte-Lindsay C. Average daily flow of MPs through a tertiary wastewater treatment plant over a ten-month period. Water Res. 2019;163:114909. [CrossRef]
45. Hidayaturrahman H, Lee TG. A study on characteristics of microplastic in wastewater of South Korea:Identification, quantification, and fate of MPs during treatment process. Mar Pollut Bull. 2019;146:696-702. [CrossRef]
46. Zhang Y, Jiang H, Bian K, Wang H, Wang C. A critical review of control and removal strategies for microplastics from aquatic environments. J Environ Chem Eng. 20212;9(4):105463. [CrossRef]
47. Sol D, Laca A, Laca A, Díaz M. Approaching the environmental problem of MPs:Importance of WWTP treatments. Sci Total Environ. 2020;740:140016. [CrossRef]
48. Shen M, Zhao Y, Liu S, Hu T, Zheng K, Wang Y, et al. Recent advances on micro/nanoplastic pollution and membrane fouling during water treatment:A review. Sci Total Environ. 2023;881:163467. [CrossRef]
49. Poerio T, Piacentini E, Mazzei R. Membrane processes for microplastic removal. Molecules. 2019;24(22):4148. [CrossRef]
50. Henderson RK, Parsons SA, Jefferson B. Polymers as bubble surface modifiers in the flotation of algae. Environ Technol. 2010;31(7):781-90. [CrossRef]
51. Feilin H, Mingwei S. Ecofriendly removing microplastics from rivers:A novel air flotation approach crafted with positively charged carrier. Process Saf Environ Protect. 2022;168:613-23. [CrossRef]
52. Goveas LC, Nayak S, Kumar PS, Rangasamy G, Vidya SM, Vinayagam R, et al. Microplastics occurrence, detection and removal with emphasis on insect larvae gut microbiota. Mar Pollut Bull. 2023;188:114580. [CrossRef]
53. Padervand M, Lichtfouse E, Robert D, Wang C. Removal of MPs from the environment. A review. Environ Chem Lett. 2020;18(3):807-28. [CrossRef]
54. Lv X, Dong Q, Zuo Z, Liu Y, Huang X, Wu WM. Microplastics in a municipal wastewater treatment plant:Fate, dynamic distribution, removal efficiencies, and control strategies. J Clean Prod. 2019;225:579-86. [CrossRef]
55. Murphy F, Ewins C, Carbonnier F, Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol. 2016;50(11):5800-8. [CrossRef]
56. Iyare PU, Ouki SK, Bond T. Microplastics removal in wastewater treatment plants:A critical review. Environ Sci Water Res Technol. 2020;6(10):2664-75. [CrossRef]
57. Harrison JP, Sapp M, Schratzberger M, Osborn AM. Interactions between microorganisms and marine MPs:A call for research. Mar Technol Soc J. 2011;45(2):12-20. [CrossRef]
58. Coppock RL, Cole M, Lindeque PK, Queirós AM, Galloway TS. A small-scale, portable method for extracting MPs from marine sediments. Environ Pollut. 2017;230:829-37. [CrossRef]
59. Magni S, Binelli A, Pittura L, Avio CG, Della Torre C, Parenti CC, et al. The fate of MPs in an Italian wastewater treatment plant. Sci Total Environ. 2019;652:602-10. [CrossRef]
60. Nuelle MT, Dekiff JH, Remy D, Fries E. A new analytical approach for monitoring MPs in marine sediments. Environ Pollut. 2014;184:161-9. [CrossRef]
61. Wang Y, Li Y, Tian L, Ju L, Liu Y. The removal efficiency and mechanism of microplastic enhancement by positive modification dissolved air flotation. Water Environ Res. 2020;93(5):693-702. [CrossRef]
62. Lares M, Ncibi MC, SillanpääM, SillanpääM. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236-46. [CrossRef]
63. Xiang JK, Bairoliya S, Cho ZT, Cao B. Plastic-microbe interaction in the marine environment:Research methods and opportunities. Environ Int. 2023;171:107716. [CrossRef]
64. Li X, Chen L, Mei Q, Dong B, Dai X, Ding G, et al. MPs in sewage sludge from the wastewater treatment plants in China. Water Res. 2018;142:75-85. [CrossRef]
65. Cai Y, Wu J, Lu J, Wang J, Zhang C. Fate of MPs in a coastal wastewater treatment plant:Microfibers could partially break through the integrated membrane system. Front Environ Sci Eng. 2021;16(7):96. [CrossRef]
66. Bayo J, López-Castellanos J, Olmos S. Membrane bioreactor and rapid sand filtration for the removal of MPs in an urban wastewater treatment plant. Mar Pollut Bull. 2020;156:111211. [CrossRef]
67. Sethi B. Recycling of polymers in the presence of nanocatalysts:A green approach towards sustainable environment. Int J Chem Mol Eng. 2016;10(5):525-31.
68. Mueller NC, Nowack B. Nanoparticles for remediation:Solving big problems with little particles. Elements. 2010;6(6):395-400. [CrossRef]
69. Macczak P, Kaczmarek H, Ziegler-Borowska M. Recent achievements in polymer bio-based flocculants for water treatment. Materials. 2020;13(18):3951. [CrossRef]
70. Jain K, Patel AS, Pardhi VP, Flora SJ. Nanotechnology in wastewater management:A new paradigm towards wastewater treatment. Molecules. 2021;26(6):1797. [CrossRef]
71. Awasthi A, Jadhao P, Kumari K. Clay nano-adsorbent:Structures, applications and mechanism for water treatment. SN Appl Sci. 2019;1(9):1076. [CrossRef]
72. Tofa TS, Kunjali KL, Paul S, Dutta J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ Chem Lett. 2019;17(3):1341-6. [CrossRef]
73. Kiriyanthan RM, Maharajan T, Radha A, Pandikumar P. A review on the role of nanotechnology in enhancing environmental sustainability. Chem Biol Interface. 2021;11(1):13.
74. Baruah S, Mahmood MA, Myint MT, Bora T, Dutta J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J Nanotechnol. 2010;1:14-20. [CrossRef]
75. Shaikh SM, Nasser MS, Magzoub M, Benamor A, Hussein IA, El-Naas MH, et al. Effect of electrolytes on electrokinetics and flocculation behavior of bentonite-polyacrylamide dispersions. Appl Clay Sci. 2018;158:46-54. [CrossRef]
76. Tang Y, Zhang S, Su Y, Wu D, Zhao Y, Xie B. Removal of MPs from aqueous solutions by magnetic carbon nanotubes. Chem Eng J. 2021;406:126804. [CrossRef]
77. Krystynik P, Strunakova K, Syc M, Kluson P. Notes on common misconceptions in MPs removal from water. Appl Sci. 2021;11(13):5833. [CrossRef]
78. Jalvo B, Aguilar-Sanchez A, Ruiz-Caldas MX, Mathew AP. Water filtration membranes based on non-woven cellulose fabrics:Effect of nanopolysaccharide coatings on selective particle rejection, antifouling, and antibacterial properties. Nanomaterials. 2021;11(7):1752. [CrossRef]
79. Bahi A, Shao J, Mohseni M, Ko FK. Membranes based on electrospun lignin-zeolite composite nanofibers. Sep Purif Technol. 2017;187:207-13. [CrossRef]
80. Abdullah M, Atta A, Allohedan H, Alkhathlan H, Khan M, Ezzat A. Green synthesis of hydrophobic magnetite nanoparticles coated with plant extract and their application as petroleum oil spill collectors. Nanomaterials. 2018;8(10):855. [CrossRef]
81. Elmaci G. Microwave-assisted rapid synthesis of C@Fe3O4 composite for removal of MPs from drinking water. Adiyaman Univ J Sci. 2020;10:207-17. [CrossRef]
82. Wang L, Popescu MN, Stavale F, Ali A, Gemming T, Simmchen J. Cu@TiO2 Janus micro swimmers with a versatile motion mechanism. Soft Matter. 2018;14(34):6969-73. [CrossRef]
83. Wang L, Kaeppler A, Fischer D, Simmchen J. Photocatalytic TiO2 micromotors for removal of MPs and suspended matter. ACS Appl Mater Interfaces. 2019;11(36):32937-44. [CrossRef]
84. Sharma M, Behl K, Nigam S, Joshi M. TiO2-GO nanocomposite for photocatalysis and environmental applications:A green synthesis approach. Vacuum. 2018;156:434-9. [CrossRef]
85. Li XQ, Zhang WX. Iron nanoparticles:The core-shell structure and unique properties for Ni (II) sequestration. Langmuir. 2006;22(10):4638-42. [CrossRef]
86. Zhang WX. Nanoscale iron particles for environmental remediation:An overview. J Nanopart Res. 2003;5:323-32. [CrossRef]
87. Samir A, Ashour FH, Hakim AA, Bassyouni M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater Degrad. 2022;6(1):68. [CrossRef]
88. Xu X, Yang Y, Liu T, Chu B. Cost-effective polymer-based membranes for drinking water purification. Giant. 2022;10:100099. [CrossRef]
89. Mistretta MC, La Mantia FP, Titone V, Megna B, Botta L, MorrealeM. Durability of biodegradable polymers for the conservation of cultural heritage. Front Mater. 2019;6:151. [CrossRef]
90. Tortorella S, Vetri Buratti V, Maturi M, Sambri L, Comes Franchini M, Locatelli E. Surface-modified nanocellulose for application in biomedical engineering and nanomedicine:A review. Int J Nanomed. 2020;15:9909-37. [CrossRef]
91. Abdelhamid HN. Nanocellulose-based materials for water pollutant removal:A review. Int J Mol Sci. 2024;25(15):8529. [CrossRef]
92. Mahfoudhi N, Boufi S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation:A review. Cellulose. 2017;24:1171-97. [CrossRef]
93. Sharma N, Singh G, Sharma M, Mandzhieva S, Minkina T, Rajput VD. Sustainable use of nano-assisted remediation for mitigation of heavy metals and mine spills. Water. 2022;14(23):3972. [CrossRef]
94. Chellasamy G, Kiriyanthan RM, Maharajan T, Radha A, Yun K. Remediation of microplastics using bionanomaterials:A review. Environ Res. 2022;208:112724. [CrossRef]
95. Gangwar J, Sebastian JK, Chandran P. Advanced bionanomaterials for environmental remediation. In:Sustainable Nanomaterials for Biosystems Engineering. Florida:Apple Academic Press;2023. p193-211. [CrossRef]
96. Mani D, Kumar C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems:An overview with special reference to phytoremediation. Int J Environ Sci Technol. 2013;11(3):843-72. [CrossRef]
97. Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4(10):634-41. [CrossRef]
98. Handy RD, van den Brink N, Chappell M, Mühling M, Behra R, DušinskáM, et al. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials:What have we learnt so far? Ecotoxicology. 2012;21(4):933-72. [CrossRef]
99. Vince J, Hardesty BD. Governance solutions to the tragedy of the commons that marine plastics have become. Front Mar Sci. 2018;5:214. [CrossRef]
100. Gold M, Mika K, Horowitz C, Herzog M. Stemming the tide of plastic litter:a global action agenda. Tulane Environ Law J. 2013;27:165.