1. INTRODUCTION
Probiotic strains with longer shelf lives, which are also more acid and bile-tolerant, are constantly sought by the food industry [1]. They are essentially live microbes that, when given in sufficient quantities, provide positive impacts on their host’s health [2]. Probiotic bacteria produce beneficial effects on the host’s gastrointestinal tract (GIT) by producing nutritive essential substances and exhibit antimicrobial activity against bacterial pathogens. Further, probiotic strains such as lactic acid bacteria improve the colonization propensity of other beneficial probiotic strains by producing polymeric substances [3]. In most cases, probiotics are regarded as “Generally Recognized As Safe” [2], which means they could be applied in place of antibiotics and possibly hazardous chemicals.
The supply of these probiotics is through dairy products, fermented dairy products, or pharmaceuticals. However, the higher incidence of gluten sensitivity and evolution has rationalized keen popularity in dairy-free foods of probiotics such as dried fruits, jams, granolas, meats, juices, and some foods from plant sources as probiotic carriers (vegetarian and vegetarian diets) [4,5]. Also with the increased utilization of non-dairy probiotics, it has now become more and more essential to find beneficial bacteria from low-cost, alternative, or unique sources without any health complications; thus, leaves of edible crop plants were explored as sources of helpful bacteria.
Several authors have currently introduced fruit as vehicles for probiotic bacteria and probiotic delivery, with an emphasis on probiotic culture viability and sensory properties [6,7]. Hence, fruits, in this regard, are healthier options of foods that are welcomed by consumers, have an acceptable sensory quality, and can be applied in unique non-dairy probiotic food formulations [6,7]. Because of the difficulty in extracting edible arils, eating fresh fruit is somewhat inconvenient. Fruits that have been processed include grenadine syrup, jellies, and especially pomegranate juices. Many bioactive compounds are found in pomegranate (Punica granatum) fruit, including antioxidants, phytochemicals, anthocyanins, gallic acid, caffeic acid, organic acids, glucose, quercetin, vitamins, catechin, minerals, and rutin. Pomegranate’s well-being characteristics have made it a preferred fruit and it has been mentioned as a “superfood.” A study from the market survey (2021) reveals that the international demand for products from pomegranates is predicted to grow at a compound annual growth rate of 5.4%. Thus, the purpose of our research is to screen and investigate the beneficial bacterial strains from the leaf surface of lemon and orange plants. Moreover, we aim to examine the viability of isolated bacterial strains in fortified pomegranate juices and assess its quality.
2. MATERIALS AND METHODS
2.1. Sampling Site and Procedure
Fresh orange (Citrus sinensis) and lemon (Citrus limon) leaves were collected from our campus, School of Pharmaceutical Sciences, Bhubaneswar, India. The leaves were washed twice with tap water and then with distilled water. Then the leaves were surface sterilized using 70% ethanol followed by soaking in 0.85% NaCl solution for 5 min to clean contaminant microbes from the surface. Then 10 g of surface sterilized leaves were squashed using a clean, sterilized mortar and pestle followed by serial dilutions using distilled water. Then, sub-culturing was done in a nutrient agar medium and incubated at 35°C for 24 h to obtain pure cultures of the bacterial isolates. Earlier defined methods (biochemical, morphological, physiological, and molecular) were followed for the primary characterization of the isolates [8].
2.2. Hemolytic Activity Determination
The bacterial isolates cultured on blood agar 5% (v/v) plates were incubated at 35°C for 48–72 h to determine their hemolytic activity. The zones of inhibition formed around the bacterial colonies as a result of surfactant synthesis clearly showed that the pathogen has positive hemolytic activity [9]. Additional probiotic screening was performed in this study on isolates with negative hemolytic activity. Escherichia coli and Lactobacillus casei were used as positive and negative controls respectively.
2.3. Tolerance to Acids and Bile Salts
Based on the method described previously, individual isolates were tested for their ability to tolerate low pH and bile salts under simulated stomach secretions and GI travel (3 h) [10]. The pH tolerance test of bacterial isolate (SOR9) has been performed by inoculating in nutrient broth at different pH levels ranging from 2 to 9 (pH: 2, 3, 4, 5, 6, 8, and 9) and at 37°C for 24 h. An ultraviolet (UV)-visible spectrophotometer was used to measure the absorbance of bacterial isolate growth at 600 nm. The tolerance of bacterial isolate SOR9 to bile salts was investigated by inoculating the isolate in nutritional broth supplemented with bile salts of various concentrations (0.1%, 0.2%, 0.3%, 0.5%, and 1.0%) and incubating it at 37°C for 24 h. Later on, the isolate from the nutrient broth supplemented with bile salts was inoculated on a nutrient agar plate and incubated at 37°C for 24 h.
2.4. Lysozyme Resistance Test
The procedure suggested by Zago et al. has been adopted with minimal changes to evaluate the tolerance ability of the isolate to lysozyme [11]. The overnight grown bacterial suspension (10 mL) was harvested by centrifugation; the cells were then rinsed twice in PBS (phosphate-buffer saline) solution before being resuspended in Ringer’s solution (8.5 g/L NaCl, 0.4 g/L KCl, and 0.34 g/L hydrated CaCl2) of 0.1 M concentration at pH 7.0. The bacterial culture was injected in a sterile electrolyte solution (SES) (0.22 g/L CaCl2, 6.2 g/L NaCl, 2.2 g/L KCl, and 1.2 g/L NaHCO3) comprising lysozyme (100 g/L) for in vitro saliva dissemination. As a control, SES without lysozyme was used. After incubation in SES for 30 min and 180 min, the survival of the isolate was determined by inoculation on nutrient agar plates for 24 h at 37°C. The experiment was carried out in triplicate.
2.5. Pepsin and Pancreatin Tolerance
After completion of 24 h of incubation, the isolate SOR9 was centrifuged at 10,000 × g for 10 min at 5°C to separate the supernatant and pellets. The pellet was washed two times with PBS buffer (pH = 7.3–7.5) and added to 1 mL pepsin solution with different pH (3 mg/mL pepsin at pH2 and pH3 in PBS buffer) and the solution without pepsin was taken as control. The solution was kept at room temperature (RT) and inoculated on nutrient agar plates at 0 h, 1 h, and 3 h intervals for its viability check by incubating at 37°C for 24 h. To check the tolerance for pancreatin, the pellet was added to 1 mL pancreatin solution (1 mg/mL pancreatin in PBS buffer with pH = 8) and the solution without pancreatin was taken as control. The solution was kept at RT and inoculated at 0 h and 4 h intervals for its viability check and then incubated the nutrient agar plates at 37°C for 24 h.
2.6. Biofilm Analysis
Test tube experiments were performed on isolate SOR9 to evaluate its capability to form biofilms. The bacterial inoculants (2%) were injected into a sterilized liquid nutrient medium and cultured for 48 h at 35°C and 120 rpm. After that, a PBS solution (0.1 M) was used to wash the test tubes twice, followed by 0.1% crystal violet staining for 30 min and then distilled water was used to remove excess stain. Biofilm accumulation was detected after drying the test tubes. There was an observable biofilm along the bottom and wall of the test tube, indicating positive biofilm production. The walls of the test tubes were examined for violet coloring; the strength of the coloration was used to classify the film development as strong, moderate, or absent [12]. The test was carried out in triplicate.
2.7. Cell Surface Hydrophobicity
Microbial adhesion to solvents was evaluated by assessing hydrophobicity employing Rosenberg’s procedure with slight changes [13]. As part of the stationary phase, the bacterial liquid culture was centrifuged at 5000 × g for 15 min to separate the supernatant and pellets followed by rinsing of the pellets and resuspending in 0.1 mol/L KNO3 solution at pH = 6.2. The absorbance of bacterial suspension was read at OD600 nm (A0). 1 mL of suspension has been mixed with 3 mL of cell suspension. An incubation of 10 min at RT was followed by a 2 min vortexing of the two-phase system. The solution was incubated at RT for another 20 min. After removing the precipitate, the absorbance at 600 nm was evaluated (A1).
Calculation of bacterial adherence (%) to solvents was performed using eqn. 1:
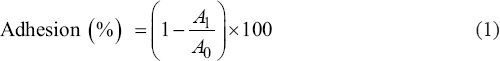 |
Where A0 = Initial absorbance.
A1 = Final absorbance (post-incubation).
This study was conducted with two distinct solvents: methanol (a polar solvent) and n-hexane (a non-polar solvent).
2.8. Assay for Auto-Aggregation
The auto-aggregation assay was carried out using the process approached by Del Re et al., with slight alternations [14]. The nutrient broth inoculated with isolate Y4 was incubated for 24 h at 35°C and 120 rpm. Then, the bacterial inoculum was centrifuged for 15 min at 5000 × g, the pellets were collected, rinsed and re-suspended in supernatant. To measure auto-aggregation (%), 4 mL of bacterial cell suspension was mixed thoroughly for 10 s and incubated at RT for varying periods (1 h, 2 h, 3 h, 24 h, and 28 h). After each time interval, the top layer of the solution (0.1 mL) was collected in a collection tube with 3.9 mL PBS, and the absorbance was measured (600 nm).
Eqn. 2 has been used to calculate the auto-aggregation percentage.
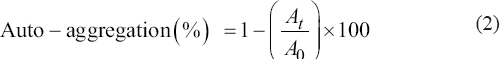 |
Where, At indicates absorbance at time t = 1, 2, 3, 4, 5, 24, or 28 h.
A0 denotes absorbance at t = 0.
2.9. Identification of Phenotypes and Molecular Characterization
Gram staining was performed to characterize the isolates phenotypically, while catalase and oxidase assays were used to characterize the isolates biochemically [15]. After overnight growth in nutrient broth, isolate SOR9 culture was centrifuged for 10 min at 5000 × g. The pellet was thoroughly rinsed with TE buffer before lysing in enzymatic lysis buffer [25 mM Tris-HCl (pH = 8), 2 mg/mL lysozyme, 25% sucrose, 10 mM EDTA] and incubated for 30 min at 37°C. The pellet was vortexed and incubated at 56°C for 30 min after being treated with extraction buffer and Proteinase K. To precipitate DNA, 96–100% (v/v) ethanol was taken, which has been then decanted to the DNeasy Mini spin column, rinsed with rinsing buffers and treated with a 200 μL elution buffer. The storage of the isolated DNA was done at 20°C. PCR amplification was performed on the supernatant using universal primers 27F (5′-AGA GTTTGATCCTGGCTCAG-3’) and 1492R (5′GGTTAC CTTGTTACGACTT-3′) [16]. Heredity Life Sciences Pvt. Ltd. performed the sequencing. BLAST and CLUSTAL W were used to generate the phylogenetic distance matrix and sequence homologies. MEGA4 and bootstrap trials have been applied for multiple alignments. Mostly parsimony procedure was used to make the phylogenetic tree [14].
2.10. Fortification of Juice
High-quality ripe pomegranate fruits were purchased from the Unit 1 market of Bhubaneswar, Odisha, India. The fruits were rinsed, pulped, and squashed for the extraction of juice. The resulting juice was filtered to extract pure juice. Out of that, 100 mL of the pure juice was pasteurized for 15 min in a Schott Duran bottle with the probe sonicator adjusted to an amplitude of 80% (on/off-3 s). Initial experimental results were used to select the parameters. The microbial load in pasteurized pomegranate juice was acceptable upto 50 CFU/mL [17]. Microbial cell loads were 8 logs CFU/mL for each inoculum with a volume of 2 mL and centrifuged at 4000 × g for 10 min. Before being inoculated into pomegranate juice (100 mL), the obtained biomasses were rinsed with PBS buffer (100 mL). The sample with the primary bacterial cell loads was fortified in an incubator at 37°C for 72 h. The viable cells were calculated following customary procedure [18].
2.11. Determination of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) Radical Scavenging Activity
By slightly altering the method described by Mousavi et al., the pomegranate juice was evaluated for its DPPH radical scavenging ability [18]. In 2.9 mL of DPPH solution, 0.1 mL of sample was added. After vigorously mixing the solution, it was kept for 30 min in dark. An UV-Vis spectrophotometer was used to measure the absorbance of the samples at 517 nm against a blank to evaluate the depletion of DPPH in the solution.
As a control, the DPPH solution was used without the sample.
 |
2.12. Total Phenolic Content (TPC)
TPC assessment was performed by Cassani et al., (2018) with slight modifications [19]. In brief, 15 μL of the harvest was introduced to a well containing 75 μL of diluted Folin-Ciocalteu reagent (1:10). After incubating for 3 min at RT, 60 μL of 7.5% Na2CO3 suspension was mixed and the solution was kept for 120 min. Followed by incubation, the absorbance of the mixture was monitored in triplicate by a spectrophotometric microplate reader at 765 nm. To compare the absorbance measurements, a gallic acid calibration graph was employed. The outcomes are given in milligrams of gallic acid equivalents (GAE) per 100 g of fresh product.
2.13. Impact of Storage Conditions on the Viability of Probiotic Cells in Juice
The probiotic-fortified juice has been preserved at 4 ± 1 °C. Probiotic survival (log CFU/mL) in the beverage has been investigated at 7-day intervals till the cell load reached 6 log CFU/mL [20].
2.14. Statistical Analysis
The investigations have been performed in triplicate. To represent the statistical variance among the samples and fortification period, a one-way analysis of variance (ANOVA) was done by applying the SPSS statistical program (Version 22.0). The data were analyzed using the Duncan multiple comparison test with a 95% level of confidence.
3. RESULTS
3.1. Isolation and Screening of Beneficial Bacteria
A total of 73strains were isolated from the leaves of two edible plants (lemon and orange). One isolate (SOR9) was found to have negative hemolytic activity. The isolate was then examined for its capability to coat the epithelial surface and to survive in the GIT.
3.2. Viability in a human GIT Simulation
Instead of analyzing the impact of each component separately, it is vital to assess all circumstances (low pH, enzymes, and bile salts) in a single system. In vitro survival tests showed that isolate SOR9 was resistant to low pH (2–4), with 4 being the optimum [Figure 1a]. Furthermore, the isolate SOR9 was resilient to different bile salt concentrations (0.15%, 0.3%, 0.5%, and 1%) in aerobic condition [Figure 1b]. The effect of gastric juices on isolate SOR9 after a total incubation period of 1 h for lysozyme, 3 h for pepsin, and 4 h for pancreatin showed survival level of 8.29 ± 0.046 CFU/mL, 7.68 ± 0.046 CFU/mL, and 7.486 ± 0.027 CFU/mL, respectively, in simulated GI passage [Table 1].
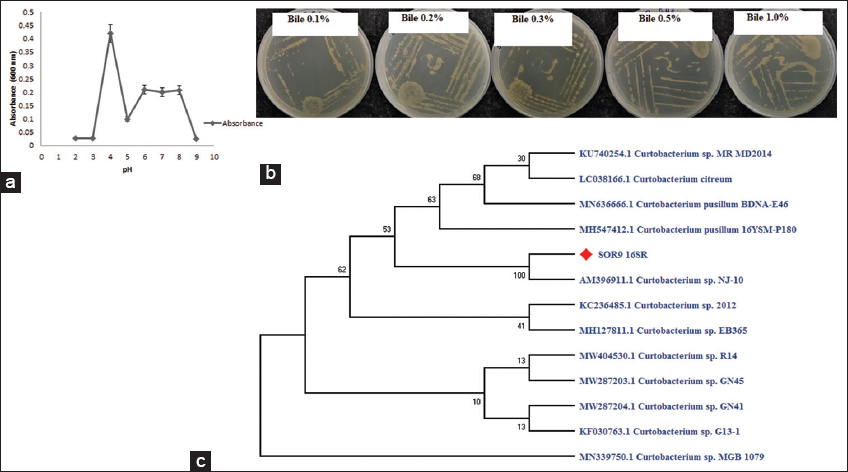 | Figure 1: (a) pH profile of Curtobacterium sp. SOR9. (b) Bile salt resistance exhibited by Curtobacterium sp.SOR9 provides 0.1–1% of bile. (c) The phylogenetic tree represents the position of SOR9 based on 16S rRNA gene sequences. [Click here to view] |
Table 1: Tolerance of Curtobacterium sp. SOR9 to gastric juice.
| Resistance to the simulated human GI tract (CFU/mL) | ||
|---|---|---|
| Lysozyme | Pepsin | Pancreatin |
| 8.29±0.046 | 7.68±0.046 | 7.486±0.027 |
GI: Gastrointestinal
3.3. Biofilm Assay
The test tube method has been conducted to investigate biofilm development qualitatively by isolating SOR9. With an absorbance of 0.392, it produced a weak biofilm (an absorbance between 0 and 1 indicates weak biofilm growth).
3.4. Auto-Aggregation and Hydrophobicity
A key probiotic feature is auto-aggregation, which occurs when two cells coexist, helping in the production of biofilms. Bacterial cells stick tightly to the mucosal cells of the host intestine due to their hydrophobicity and conduct physiological tasks. Most of the time, aggregation potential is linked to the adherence of cell characteristics [21]. Hence, the prospective isolate SOR9 was tested for auto-aggregation and hydrophobicity. It was noted that the isolate SOR9 exhibited both auto-aggregation and hydrophobicity in the current study which is represented in Table 2.
Table 2: Curtobacterium sp. SOR9 adhesion to methanol and n-hexane and auto-aggregation.
| Adhesion (%) | Auto-aggregation (%) in (h) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Methanol | n-Hexane | 1 | 2 | 3 | 4 | 24 | 28 |
| SOR 9 | 17.5±1.03 | 0 | 53.30±0.98 | 33.37±1.03 | 21.64±1.17 | 71.44±1.11 | 0 | 0 |
3.5. Potential Isolate Identification
The 16S rRNA sequencing of the putative isolate SOR 9 (taken from lemon tree leaves) revealed a strong relationship with Curtobacterium sp. The sequence analysis identified SOR9 as Curtobacterium sp. SOR9 (accession no. ON795170). Figure 1c shows a neighbor-joining phylogenetic tree derived from 16S rRNA gene sequence data. Furthermore, no investigations on Curtobacterium sp. as a probiotic have been undertaken yet. This is the first report of Curtobacterium sp. SOR9 being used as a probiotic in the fortification of pomegranate juice.
3.6. TPC of the Juice
Figure 2a shows the TPC of pomegranate juice fortified with Curtobacterium sp. SOR9 and control (without Curtobacterium sp. SOR9). The data confirmed that the TPC values of pomegranate juice fortified with SOR9 were higher than the control. The treated juice showed the highest phenol concentration (650.33 ± 1.47 mg gallic acid equivalent/100 mL) at 48 h. A significant increase in TPC for both juices (control and fortified) has been observed from 24 h to 48 h. The bacterial growth has reached the end of its life cycle after 48 h of fortification, resulting in an insignificant rise in total phenols.
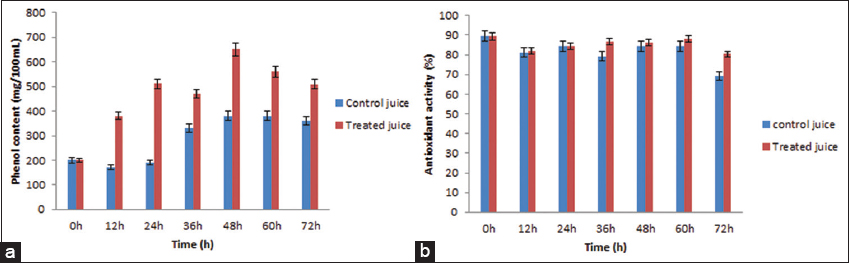 | Figure 2: (a) Total phenolic content of pomegranate juice during fortification (b) antioxidant activity of pomegranate juice during fortification. [Click here to view] |
3.7. DPPH Radical Scavenging Activity of the Juice
The sample’s antioxidant capacity is represented by its DPPH radical scavenging activity. Fortified pomegranate juice with probiotic Curtobacterium sp. SOR9 showed higher antioxidant properties than the control (pomegranate juice without SOR9) [Figure 2b]. The highest antioxidant activity of fortified juice was found to be 88.17 ± 0.89 % at 60 h and for control 84.23 ± 0.94 % at 60 h.
3.8. Impact of Storage Conditions on the Survival of Probiotic Cells in Pomegranate Juice
To confer maximum health benefits to the consumers, the total number of viable probiotic bacterial cells in the probiotic beverage should be >6 log CFU/mL [20,22]. However, beverages are required to be preserved at lower temperature (mostly at 4°C) for longer period to meet consumer demands. This kind of preservation may affect the survival of probiotic bacterial cell in the beverage and the cell count may fall below 6 log CFU/mL. Hence, it is important to inspect the effect of storage condition on the probiotic bacterial cells present in the probiotic beverages. The fortified pomegranate juice was refrigerated at 4°C for four weeks and the viability of probiotic bacterial cells (Curtobacterium sp. SOR9) were investigated at 1-week interval. We observed that, the viability of isolate SOR9 in fortified pomegranate juice was not affected negatively by cold storage. Furthermore, during the storage, viable cell counts increased and reached maximum cell count of more than 7.43 ± 0.035 log CFU/mL [Table 3] at day 3 (72 h) and a slow reduction was observed further. However, the number of viable probiotic bacterial cells of Curtobacterium sp. SOR9 was observed to be >6 log CFU/mL till the end of four weeks of refrigeration.
Table 3: Total microbial count on viability (Log CFU/mL) of Curtobacterium sp. SOR9 during fortification and cold storage of pomegranate juice.
| Time period | Curtobacterium sp. viability |
|---|---|
| Time (h) | |
| 0 | 2.13±0.068 |
| 12 | 2.88±0.19 |
| 24 | 3.35±0.17 |
| 36 | 4.08±0.105 |
| 48 | 5.43±0.14 |
| 60 | 6.54±0.37 |
| 72 | 7.43±0.035 |
| Time (weeks) | |
| 0 | 7.43±0.035 |
| 1 | 7.28±0.05 |
| 2 | 7.06±0.043 |
| 3 | 6.6±0.16 |
| 4 | 6.016±0.016 |
These outcomes showed that the beneficial isolate Curtobacterium sp. SOR9 can survive for four weeks in fermented pomegranate juice at 4 ± 1°C.
This decrease in microbial cell load could be attributed to the sample’s less nutrient obtainability, as well as its low pH and oxygen levels [22].
The above data represent the mean ± standard deviation (p<0.05) by taking triplicate samples with a significant and non-significant value.
4. DISCUSSION
The isolate SOR9 was capable to survive in simulated GI passage. Similar results were observed with strains of Lactobacillus plantarum, Lactobacillus pentosus, Pediococcus acidilactici, and Pediococcus Pentosaceus isolated from fermented sausages [23]. Different types of Bacillus strains also showed similar kind of survival behavior concerning acid tolerance [24]. Further, B. subtilis KKU 213 was able to withstand 1% bile salt [24]. Abid et al. investigated similar acidic environments and the enzymatic effect for B. tequilensis-GM [25].
As a result of solubilization, crystal violet staining can be used to assess biofilm formation quantitatively. The capability of pathogens to form biofilms prevents them from attaching to epithelial cells. Biofilm formation by probiotic bacteria is thought to be a beneficial characteristic as it can encourage colonization and longer persistence in the host’s mucosa, attempting to avoid colonization by pathogenic bacteria [26]. L. plantarum subsp. plantarum JCM1149 can form biofilms [27]. Jones et al. (2009) examined the capacity of some Lactobacillus reuteri strains for the formation of biofilms along with their immunomodulatory action. Likewise, other researchers revealed that probiotic Lactobacillus rhamnosus GG could form biofilms [28].
Adhesion is a complicated function that may be the result of a multistep method involving non-specific mechanisms and a specific ligand-receptor interaction [14]. As a result, we investigated the relationship between a variety of characteristics (hydrophobicity and ability to auto-aggregate) and the adhesiveness of isolate SOR9. This supports the findings of Perez et al. (1998) [29] on Bacillus bifidum and Del Re et al. on Bacillus suis [30].
The isolate SOR9 was identified as Curtobacterium sp. SOR9 by sequence analysis. Similar to our study, previous findings report some Curtobacterium sp., such as C. plantarum and C. jeaccumfaciens, were isolated from plant leaves. Correspondingly, Khan et al. (2019) observed that Curtobacterium sp. produced a variety of phytohormones such as JA, ABA, GAs, IAA, and antioxidants such as peroxidase, reduced glutathione, polyphenolic oxidase, and various organic acids to enhance nutritional benefits for plant health under stress conditions [31]. Another study revealed that Curtobacterium sp. aids in plant growth and enhances growth parameters [16, 32].
During the fortification period, the phenol content of fortified juice containing probiotic Curtobacterium sp. SOR9 was enhanced. As a result, pomegranate juice with Curtobacterium sp. SOR9 has a stronger potential to raise phenol concentration than control pomegranate juice (without Curtobacterium sp. SOR9). The antioxidant activity of phenolic compounds, as well as their involvement in the color and sensory features of food products, is well recognized to have significant human health welfare [33]. Several studies have shown that phenol-containing foods have a major effect on human wellness in preventing diseases like cardiovascular problems, diabetes, and aging [34], as well as have impacts on anticancer, antimicrobial, and antioxidant properties [35]. Kumar et al. (2015) found that during fortification with probiotic L. plantarum, the total polyphenolic content of mango and sapota juice has been enhanced [36].
The results concluded that pomegranate juice with probiotic Curtobacterium sp. SOR9 has higher antioxidant activity in comparison to control pomegranate juice (without Curtobacterium sp. SOR9). A similar finding is reported for other beverages by some researchers such as noni [37], pomegranate [18], jussara juices [38], and cashew apple juice which have validated our findings. The enhancement of antioxidant possessions could be attributed to anthocyanin acylation with phenolic acid or anthocyanin diacylation [39].
The results demonstrated that the beneficial isolate Curtobacterium sp. SOR9 may survive in fortified pomegranate juice at 4 ± 1°C for 4 weeks. Alike outcomes have been obtained with carrots [40,41], bitter gourd, bottle gourd, and beet juice. Saccharomyces cerevisiae boulardii was also evaluated by Fratianni et al. (2014) for its capacity to thrive in berry juice and endure 4 weeks of preservation at 4°C. All of the reports backed up our findings [42].
5. CONCLUSION
This study aimed to assess the functional properties of the probiotic beverage of pomegranate juice, which is rich in vitamins, minerals, and fiber that promotes health benefits. They also contain some protein. It is worth mentioning that the addition of 2% probiotic bacteria Curtobacterium sp. SOR9 considerably enhanced the physicochemical and microbiological properties of the probiotic beverage, demonstrating the highest antioxidant and antibacterial activities. Furthermore, pomegranate juice significantly improved the viability of the probiotic strain, Curtobacterium sp. SOR9, above the recommended counts (> log 6 CFU/g) for health benefits. We vouch that, this is the first study that has focused on developing a probiotic beverage by adding pomegranate juice, and it should be taken as a basis for developing new functional products. Overall, our findings deduced that probiotic bacterial strain Curtobacterium sp. SOR9 isolated from plants exhibits remarkable viability in pomegranate juice. Probiotic beverage produced using Curtobacterium sp. SOR9 can serve health benefits to consumers as its probiotic properties remain unaffected during storage. Further, our findings will significantly give insights to researchers dealing with food and beverages to develop more plant-based probiotic supplementation and provide alternative options to health-conscious consumers who have a version or sensitivity to dairy-based probiotic products.
6. ACKNOWLEDGMENT
The authors express their gratitude towards the Center for Biotechnology, Siksha O Anusandhan (Deemed to be University), Bhubaneswar, for providing lab facilities for conducting the research.
7. AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
8. FUNDING
There is no funding to report.
9. CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
10. ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
11. DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
12. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Nair AS, Dubhashi AV. In-vitro transit tolerance of probiotic Bacillus species in human gastrointestinal tract. Int J Sci Res 2016;5: [CrossRef]
2. Hotel AC, Cordoba A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001;5:1-10.
3. Kanmani P, Kumar RS, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Probiotics and its functionally valuable products-a review. Crit Rev Food Sci Nutr 2013;53:641-58. [CrossRef]
4. Espitia PJ, Batista RA, Azeredo HM, Otoni CG. Probiotics and their potential applications in active edible films and coatings. Food Res Int 2016;90:42-52. [CrossRef]
5. Da Costa GM, de Carvalho Silva JV, Mingotti JD, Barão CE, Klososki SJ, Pimentel TC. Effect of ascorbic acid or oligofructose supplementation on L. paracasei viability, physicochemical characteristics and acceptance of probiotic orange juice. LWT Food Sci Tech 2017;75:195-201. [CrossRef]
6. Speranza B, Campaniello D, Bevilacqua A, Altieri C, Sinigaglia M, Corbo MR. Viability of Lactobacillus plantarum on fresh-cut chitosan and alginate-coated apple and melon pieces. Front Microbiol 2018;9:2538. [CrossRef]
7. Nikolaou A, Tsakiris A, Kanellaki M, Bezirtzoglou E, Akrida-Demertzi K, Kourkoutas Y. Wine production using free and immobilized kefir culture on natural supports. Food Chem 2019;272:39-48. [CrossRef]
8. Sharma A, Bhattacharyya KG. Azadirachta Indica (Neem) leaf powder as a biosorbent for removal of Cd (II) from aqueous medium. J Hazard Mater 2005;125:102-12. [CrossRef]
9. Gómez NC, Ramiro JM, Quecan BX, de Melo Franco BD. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 biofilms formation. Front Microbiol 2016;7:863. [CrossRef]
10. Prasad J, Gill H, Smart J, Gopal PK. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int Dairy J 1998;8:993-1002. [CrossRef]
11. Zago M, Fornasari ME, Carminati D, Burns P, Suàrez V, Vinderola G, et al. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol 2011;28:1033-40. [CrossRef]
12. Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis 2011;15:305-11. [CrossRef]
13. Crow VL, Gopal PK, Wicken AJ. Cell surface differences of lactococcal strains. Int Dairy J 1995;5:45-68. [CrossRef]
14. Del Re B, Sgorbati B, Miglioli M, Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 2000;31:438-42. [CrossRef]
15. Das A, Belgaonkar P, Raman AS, Banu S, Osborne JW. Bioremoval of lead using Pennisetum purpureum augmented with Enterobacter cloacae-VITPASJ1:A pot culture approach. Env Sci Pollut Res 2017;24:15444-53. [CrossRef]
16. Vimal SR, Singh JS, Arora NK, Singh S. Soil-plant-microbe interactions in stressed agriculture management:A review. Pedosphere 2017;27:177-92. [CrossRef]
17. Nwachukwu E, Ezeigbo CG. Changes in the microbial population of pasteurized soursop juice treated with benzoate and lime during storage. Afr J Microbiol Res 2013;7:3992-5.
18. Mousavi ZE, Mousavi SM, Razavi SH, Hadinejad M, Emam-Djomeh Z, Mirzapour M. Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biotechnol 2013;27:1-13. [CrossRef]
19. Cassani L, Gerbino E, Del Rosario Moreira M, Gómez-Zavaglia A. Influence of non-thermal processing and storage conditions on the release of health-related compounds after in vitro gastrointestinal digestion of fiber-enriched strawberry juices. J Funct Foods 2018;40:128-36. [CrossRef]
20. Sharma V, Mishra HN. Fermentation of vegetable juice mixture by probiotic lactic acid bacteria. Nutrafoods 2013;12:17-22. [CrossRef]
21. Boris S, Suarez JE, Barbes C. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J Appl Microbiol 1997;83:413-20. [CrossRef]
22. Yoon KY, Woodams EE, Hang YD. Fermentation of beet juice by beneficial lactic acid bacteria. Lebensm Wiss Technol 2005;38:73-5. [CrossRef]
23. Klingberg TD, Axelsson L, Naterstad K, Elsser D, Budde BB. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int J Food Microbiol 2005;105:419-31. [CrossRef]
24. Khochamit N, Siripornadulsil S, Sukon P, Siripornadulsil W. Antibacterial activity and genotypic-phenotypic characteristics of bacteriocin-producing Bacillus subtilis KKU213:Potential as a probiotic strain. Microbiol Res 2015;170:36-50. [CrossRef]
25. Abid Y, Azabou S, Joulak I, Casillo A, Lanzetta R, Corsaro MM, et al. Potential biotechnological properties of an exopolysaccharide produced by newly isolated Bacillus tequilensis-GM from spontaneously fermented goat milk. LWT 2019;105:135-41. [CrossRef]
26. Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 2003;17:741-54. [CrossRef]
27. Kubota H, Senda S, Nomura N, Tokuda H, Uchiyama H. Biofilm formation by lactic acid bacteria and resistance to environmental stress. J Biosci Bioeng 2008;106:381-6. [CrossRef]
28. Lebeer S, Verhoeven TL, Velez MP, Vanderleyden J, De Keersmaecker SC. Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 2007;73:6768-75. [CrossRef]
29. Pérez PF, Minnaard Y, Disalvo EA, De Antoni GL. Surface properties of bifidobacterial strains of human origin. Appl Environ Microbiol 1998;64:21-6. [CrossRef]
30. Del Re B Busetto A, Vignola G, Sgorbati B, Palenzona DL. Autoaggregation and adhesion ability in a Bifidobacterium suis strain. Lett Appl Microbiol 1998;27:307-10. [CrossRef]
31. Khan N, Bano A, Babar MA. Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PloS One 2019;14:e0213040. [CrossRef]
32. Singh JS, Koushal S, Kumar A, Vimal SR, Gupta VK. Book review:Microbial inoculants in sustainable agricultural productivity-Vol. II:Functional application. Front Microbiol 2016;7:2105. [CrossRef]
33. Porto MR, Okina VS, Pimentel TC, Garcia S, Prudencio SH. Beet and orange mixed juices added with Lactobacillus acidophilus. Nutr Food Sci 2018;48:76-87. [CrossRef]
34. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxi Med Cell Long 2009;2:270-8. [CrossRef]
35. Kumar N, Goel N. Phenolic acids:Natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst) 2019;24:e00370. [CrossRef]
36. Kumar BV, Sreedharamurthy M, Reddy OV. Probiotication of mango and sapota juices using Lactobacillus plantarum NCDC LP 20. Nutrafoods 2015;14:97-106. [CrossRef]
37. Wang CY, Ng CC, Su H, Tzeng WS, Shyu YT. Probiotic potential of noni juice fermented with lactic acid bacteria and bifidobacteria. Int J Food Sci Nutr 2009;60 Suppl 6:98-106. [CrossRef]
38. Braga AR, de Souza Mesquita LM S, Martins PL, Habu S, de Rosso VV. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J Food Compost Anal 2018;69:162-70. [CrossRef]
39. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins:Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 2017;61:1361779. [CrossRef]
40. Kun S, Rezessy-SzabóJM, Nguyen QD, Hoschke Á. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Proc Biochem 2008;43:816-21. [CrossRef]
41. Daneshi M, Ehsani MR, Razavi SH, Labbafi M, Rezaee MS. Effect of cold storage on viability of probiotic bacteria in carrot fortified milk. J Nutr Food Sci 2012;2:1000162. [CrossRef]
42. Fratianni F, Cardinale F, Russo I, Iuliano C, Tremonte P, Coppola R, et al. Ability of synbiotic encapsulated Saccharomyces Cerevisiae boulardii to grow in berry juice and to survive under simulated gastrointestinal conditions. J Microencapsul 2014;31:299-305. [CrossRef]