1. INTRODUCTION
Mesenchymal stem cells (MSCs) are more prevalent in regenerative medicine for more than 10 years and are being used in clinical practice mainly due to their immunomodulatory properties and capability to treat tissue damage [1]. One of the most prominent sources of MSCs is the human umbilical cord (UC) [2], which is considered medical waste. In addition to its apparent benefits, such as a painless collecting method and rapid self-renewal, UC-MSCs have demonstrated their ability to differentiate into three germ layers, accumulate in damaged or inflammatory regions, promote tissue healing, and influence immune response [3]. Furthermore, access to UC-MSCs has not been hampered by ethical issues. Although some differentiation capacities are known to be partial, UC-MSCs, like MSCs produced from other sources, show a specific capacity for self-renewal while preserving their multipotency, that is, the ability to develop into adipocytes, osteocytes, chondrocytes, neurons, and hepatocytes [4,5]. Furthermore, due to their immunomodulatory qualities, UC-MSCs have also garnered a lot of attention as being touted as a modern, adaptable tool for immunotherapy, and regenerative medicine. Furthermore, they are known to exhibit higher growth and stemness potential [6]. Due to all these properties, the MSCs from the umbilical cord are known to be an ideal viable candidate for allogeneic cell therapy [7].
Despite the advantages and benefits of using UC-MSCs for clinical treatments, one of the major problems accounted for in the culturing of MSCs for clinical transplantation involves the usage of culture medium supplemented with animal serum [8], as there is pathogenic contaminant risk in fetal bovine serum (FBS), variability in the serum batches, and the immunological reactions they create [9]. Several studies have shown the usage of human platelet lysate (hPL) as an alternative to fetal bovine serum in the culturing of MSCs [10]. Platelet granules are the richest sources of growth factors and cytokines released by the freeze-thaw mediated lysis of the platelets, thus making the hPL a suitable alternative for FBS in the culturing of MSCs [9].
Several clinical studies worldwide have used MSCs derived from various individual donors for diseases such as myocardial infarction, meniscus injury, stroke, graft-versus-host disease, limb ischemia, and other types of autoimmune disorders (www.clinicaltrials.gov) [11]. However, there is always a need for a large number of MSCs for transplantation. Hence, in this study, we aimed (i) to culture the UC-MSCs from individual donors and also to pool the cells from 3 different donors, expanding, and studying the characterization and chromosomal abnormalities of these cells up to passage 10, (ii) culturing of the cells in stir tank reactor (3D culture) and studying the characterization and chromosomal abnormalities of these cells, and (iii) culturing and maintaining of the UC-MSCs in Xeno free media containing hPL.
2. MATERIALS AND METHODS
2.1. Collection of the hUCT
hUCTs were collected from three healthy donors after full-term gestation (>37 weeks) following normal/cesarean birth. Approximately 30 cm of the cord was collected aseptically and transported from the hospital to the centralized processing facility at Chennai in the transport medium containing DPBS (Himedia), 250 μg/mL EDTA (Sigma), and 100 μg/mL Ofloxacin (Ranbaxy) at 2–8°C in a temperature controlled carrier within 48 h of collection. Informed written consent forms were obtained from the donors and all the procedures were performed by the protocol approved by the Institutional Stem Cell Research Committee. The eligibility criteria for all three participants include blood tests for transfusion transmissible infectious diseases, age of the mother to be 18–30 years, gestational age not <36 weeks, normal and cesarean (Preferable) delivery, and baby weight should not be <2.5 kg.
2.2. Isolation of Mesenchymal Stem Cells from hUCT
Mesenchymal stem cells (MSCs) were isolated from the hUCT samples by an explant culturing method as detailed in our previous paper [12]. Briefly, the cord tissue samples were removed from the transport medium and washed in an antibiotic solution containing 50 μg/mL of vancomycin (Gland pharma), 100 μg/mL of ofloxacin (Ranbaxy), and 250 μg/mL of amphotericin B (Himedia). The cord tissue was minced into small pieces (~5–7 mm) and cultured in 60 mm Petri dishes containing 5 mL of culture medium (Alpha MEM [Caisson, USA], supplemented with 5 % hPL, vancomycin [50 μg/mL], amphotericin B [250 μg/mL] ultra-glutamine [2 mm], and heparin [Sigma, 5000 IU/mL]) and incubated for 3 days at 37°C with 5 % CO2. Medium change was performed on day 3 and day 10 followed by every 3rd day with cell morphology being observed regularly until confluency is reached. When the cells reached 70–80% confluence, they were harvested by trypsinization using trypLE express (Gibco) and the cells were counted using the automated cell counter. Around 20 million cells were harvested from each donor.
2.3. Sequential Expansion and Pooling of the MSCs
Around 3 million cells at passage 0 (P0) from each donor were expanded to P1. After reaching 70–80% confluence approximately 15 million cells were obtained. One dish from each donor will be expanded to P2 for sequential passage till P10, while the cells from the other dish are pooled from all the 3 donors and then sequentially passaged till P10. 1 million cells from the sequential passage of each donor as well as the pooled cells from each passage were used for the testing parameters, namely, cell count and viability, trilineage differentiation potential, karyotyping, flow cytometry analysis, and chromosomal microarray (CMA). The remaining cells were cryopreserved at 1 million cells/mL. Similarly at P2, the cells were pooled from each donor and then expanded till P10.
2.4. Immunophenotypic Analysis by Flow Cytometry
The immunophenotype for all three individuals as well as the pooled donor MSCs at each passage level from P0 to P10 was analyzed by fluorescence-activated cell sorting (FACS) (FACS canto II, BD Biosciences). A human MSC Analysis Kit (BD Stemflow) was used to examine the cell phenotype, which includes the CD panel as advised by ISCT. Fluorochrome-conjugated antibodies against specific antigens, namely, CD105PerCP-Cy5.5, CD90 FITC as positive markers, and CD31 as negative expression markers were incubated with MSCs. To calculate the percentage of positive cells using flow cytometry, cells were washed with 1X PBS, centrifuged at 1200 rpm for 5 min, and then resuspended in 200 mL of 1X PBS. The data were, then, analyzed using FlowJo software.
2.5. Karyotypic Analysis
Karyotyping was carried out by standard procedure. Briefly, colcemid was added when the cells were 80% confluent, and the cells were, then, incubated overnight in a 5% CO2 incubator. Then, the cells were trypsinized, subjected to hypotonic solution treatment, and then fixed in methanol. The cells were also prepared for Giemsa staining after being spread out on the surface of clean glass slides. Using an automated imaging system for cytogenetics, individual metaphase spreads were taken, and the band quality was assessed under a phase-contrast microscope (Olympus BX63) (CytoVision; Applied Imaging Corporation). Using FACS and a BD CycletestTM Plus kit (BD Biosciences), DNA ploidy analysis was carried out. The DNA index (DI) is derived from the ratio of the modal value of the MSCs’ G0/G1 peak to that of the PBMNC (control).
2.6. Trilineage Differentiation of MSCs
MesenCultTM osteogenic/adipogenic/chondrogenic differentiation kit, stemcell technologies, USA, was used for the assays, which were carried out by the manufacturer’s instructions. Briefly, the cells were plated in a 35 mm dish at a density of 5000 cells/cm2 for osteogenic and adipogenic differentiation. The differentiation test medium for osteogenic or adipogenic differentiation was added in place of the growth medium after 4 days. The media was changed every 3rd day for three weeks. Alizarin Red S staining was used to visualize the mineralized matrix created by osteoblasts after the cells were fixed in 4% paraformaldehyde. Oil Red O staining followed by counterstaining with hematoxylin was used to examine the adipogenic differentiation of the fixed cells. For chondrogenic differentiation, 10 μL droplets of micro mass culture from a suspension of 16 million cells/mL were cultured in a 35 mm plate. After 2 hours, media replenishment was performed, and the culture was retained for 4 days. The cells were subjected to a chondrogenic differentiation test medium for 3 weeks, with medium changes performed every 3rd day. Chondrogenic spheroids were recovered, fixed in 4% paraformaldehyde, and dyed in 1% alcian blue.
2.7. Expansion in Spinner Flask with Microcarrier
The expansion of the MSCs in the spinner flask was carried out with 450 cm2 for a 50 ml culture. From the microcarrier stock concentration, 1.25 g of microcarrier of star plus were transferred to the spinner flask. Microcarriers were presoaked with 35 ml of 5% PL culture medium and kept in a CO2 incubator for 3 h. Presoaked media from the spinner flask was removed and added with 20 ml of culture medium. Mesenchymal stem cells (P3) were seeded 3000 cells/cm2 in the spinner flask containing 1250 mg of microcarriers (450 cm2) in 20 mL of 5% platelet lysate culture medium. For the initial attachment of the cells, intermittent shaking was carried out for 10–20 s manually and kept static for an hour. The cycle was repeated for 36 h. After 36 h, RPM was set at 25 and incubated in the CO2 incubator. After 24 h, cell count analysis was carried out by withdrawing 0.5 mL samples from the spinner flask into a 1.5 mL microcentrifuge tube and keeping static for 5 min to allow microcarriers to settle down. After 36 h, the spinner flask was made up to 50 mL. The rpm was set between 25 and 35 until the end of the experiment. 50% of media change was performed on day 4 and day 6. About 80% complete media change was performed on Day 8, 9, and 10. Analysis of cell growth on the spinner flask was carried out daily.
Harvesting cells from the spinner flask involve the following steps. Briefly, the stirring was stopped and the microcarriers were made to settle by leaving it undisturbed. The spent medium was carefully decanted and the microcarriers were rinsed with 30 mL of DPBS. Again the microcarriers were allowed to settle and DPBS was carefully removed. This step was repeated one more time. 12 mL of trypsin was added to the spinner flask and incubated in static condition for 10–12 min in the CO2 incubator. 30 mL of culture medium was added to the flask for trypsin inactivation and stirred at 90–100 rpm using a magnetic stirrer in the CO2 incubator to detach the cells completely from the microcarriers. After the cell detachment, 40 mL media containing cells and microcarriers were separated by passing through a 100-micron cell strainer. Retained microcarriers on the cell strainer were washed with 5 mL of culture medium to maximize the cell yield. The cell suspension was then centrifuged at 1200 rpm for 5 min. The supernatant was discarded and resuspended with 5–10 mL of culture medium. Cell count was performed using an automated cell counter and tested for immunophenotypic analysis by flow cytometer, trilineage differentiation, and karyotypic analysis.
2.8. Microbial Testing
For the detection of bacterial (aerobic and anaerobic) and fungal contaminations, routine sterility tests were carried out during all stages of growth using BACTEC (BD Biosciences).
3. RESULTS AND DISCUSSION
3.1 Explant Culturing of hUCT-MSCs and their Sequential Passaging Till P10
Mesenchymal stem cells were isolated from umbilical cord tissue using the explant culture method as this method is found to be more beneficial than the enzymatic digestion method in terms of least time consumption and cost-effectiveness [13]. Furthermore, it is found that bFGF secreted from the umbilical cord tissue pieces plays an important role in the stimulation of MSC growth in explant culturing [14]. Explant methods, on the other hand, rely on the MSCs’ capacity to move from umbilical cord tissues and adhere to the surface of culture vessels, making them more affordable and straightforward than enzymatic digestion protocols [15]. Explant techniques vary from one another in a variety of ways, including the size of the explants [14], the kind of culture vessels (plates, Petri dishes, or flasks), the supplements supplied to the culture media [15], and the amount of time needed to switch the medium for the first time [16]. Many laboratories create and refine their isolation and expansion protocols in this area [17]. In the present study, we observed the migration of cells from the umbilical cord tissue to happen by day 10 and 80% confluency was accomplished by around 19 days with a total cell yield of 30.04 ± 3.81 × 106 cells from three individual donors. The isolated cells from all the three individual donors were further sequentially passaged till P10, and also, the cells were pooled from these three donors at P1 and P2 and expanded till P10 and the viable cell count obtained at each passage along with the viability percentage is given in Figures 1 and 2. The viability percentage at each passage level was found to be >90%.
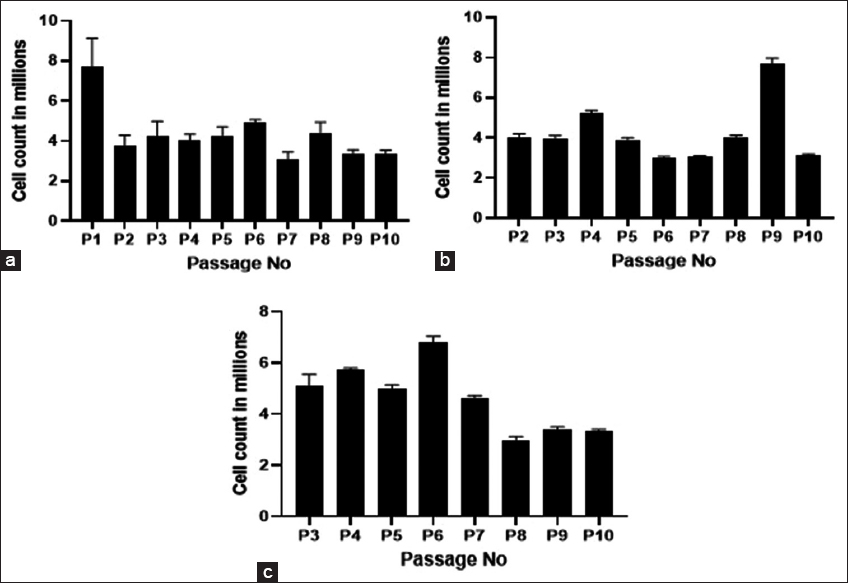 | Figure 1: Viable cell count from individual donors at different passages (a) viable cell count of pooled donors from P1 (b); and viable cell count of pooled donors from P2 (c). [Click here to view] |
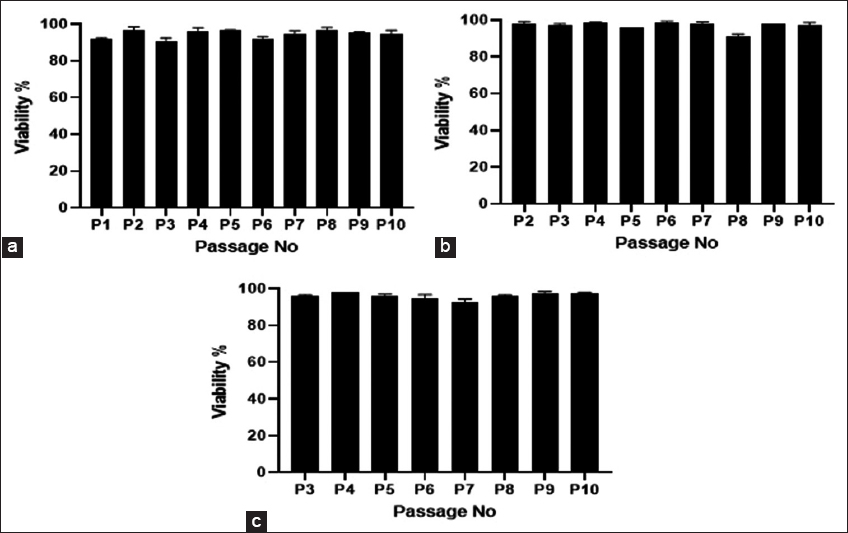 | Figure 2: Viability percentage from individual donors at different passages (a); viability percentage of pooled donors from P1 (b); and viability percentage of pooled donors from P2 (c). [Click here to view] |
The MSCs obtained from the explant cultures were pooled and maintained to generate the master cell bank (MCB). Donor pooling not only reduces donor heterogeneity but also boosts the MCB’s dose capacity and prevents manufacturing fluctuation from lot to lot [18]. Furthermore, maximizing the number of doses from a single master bank is essential for improving batch consistency, as sampling mode and location play a critical role in contributing to cellular heterogeneity.
3.2. Characterization of MSCs
UC-MSCs derived from individual donors and pooled donors were characterized for their cell surface markers, trilineage differentiation potential, and chromosomal alterations using karyotyping. Flow cytometry analysis of the UC-MSCs from individual donors and pooled donors showed higher expressions of CD105 and CD90 (>90%) and low expression of CD31 (<3%) and viability using 7AAD to be >90% for all the passages and the results for the same are shown in Tables 1 and 2. Thus, the isolated MSCs are positive for CD105 and CD90 and negative for CD31 surface markers.
Table 1: Flow cytometry analysis of MSCs from individual donors at different passages for CD105, CD90, CD31, and 7AAD.
| Passage No. | Donor 1 | Donor 2 | Donor 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD105 | CD90 | CD31 | 7AAD | CD105 | CD90 | CD31 | 7AAD | CD105 | CD90 | CD31 | 7AAD | |
| P0 | 92.1 | 99.6 | 2.31 | 95.4 | 88.1 | 99.7 | 1.65 | 93.3 | 96.9 | 99.6 | 1.41 | 94 |
| P1 | 94.3 | 98.4 | 2.85 | 95.5 | 98.2 | 99.9 | 2.64 | 94 | 98.8 | 99.3 | 1.59 | 96.8 |
| P2 | 98.8 | 99.7 | 1.85 | 97.5 | 98.2 | 99.9 | 1.49 | 98.3 | 99.1 | 99.5 | 1.36 | 94.6 |
| P3 | 99.1 | 99.8 | 2.82 | 98.6 | 98.1 | 99.9 | 2.7 | 98.5 | 99.2 | 100 | 2.44 | 98.3 |
| P4 | 98.6 | 100 | 1.26 | 99.3 | 98.1 | 99.6 | 1.1 | 98.8 | 98.6 | 98.8 | 1.07 | 98.6 |
| P5 | 99.7 | 99.9 | 1.22 | 97.8 | 99.7 | 100 | 1.8 | 98.5 | 99.5 | 99.7 | 1.9 | 98.5 |
| P6 | 99.9 | 100 | 1.78 | 97 | 99.8 | 100 | 2.34 | 97.8 | 99.9 | 100 | 1.07 | 98 |
| P7 | 99.6 | 100 | 1.58 | 99.3 | 99.9 | 100 | 1.31 | 99.5 | 99.6 | 100 | 1.25 | 99.2 |
| P8 | 99 | 99.9 | 0.831 | 97.7 | 99.3 | 100 | 1.8 | 98.2 | 99.5 | 100 | 0.905 | 97.3 |
| P9 | 99.8 | 100 | 0.199 | 98.7 | 99.8 | 99.9 | 1.66 | 99.1 | 99 | 100 | 0.492 | 97.7 |
| P10 | 99.9 | 100 | 0.983 | 99 | 99.5 | 100 | 1.65 | 98.1 | 99.9 | 100 | 0.524 | 98.3 |
MSCs: Mesenchymal stem cells
Table 2: Flow cytometry analysis of MSCs from pooled donors at P1 and P2 for CD105, CD90, CD31, and 7AAD.
| Passage No. | P1 Pool | P2 Pool | ||||||
|---|---|---|---|---|---|---|---|---|
| CD105 | CD90 | CD31 | 7AAD | CD105 | CD90 | CD31 | 7AAD | |
| P0 | NA | NA | NA | NA | NA | NA | NA | NA |
| P1 | 95.9 | 99.8 | 1.38 | 98.8 | NA | NA | NA | NA |
| P2 | 97 | 99 | 2.25 | 97.8 | 96.6 | 98.6 | 1.74 | 96.2 |
| P3 | 99.5 | 99.8 | 2.09 | 97.2 | 99.1 | 99.4 | 2.71 | 99 |
| P4 | 99.9 | 100 | 2.79 | 96.6 | 98.5 | 100 | 1.26 | 98.5 |
| P5 | 99.6 | 100 | 1.15 | 99.3 | 99.1 | 100 | 0.849 | 97.8 |
| P6 | 99.1 | 99.8 | 1.06 | 98 | 99.9 | 100 | 1.89 | 96.6 |
| P7 | 99.1 | 99.4 | 0.589 | 98.8 | 99.9 | 100 | 1.62 | 99.5 |
| P8 | 99.8 | 100 | 0.637 | 98.9 | 99.9 | 100 | 1.75 | 98.9 |
| P9 | 99.9 | 100 | 1.64 | 99.7 | 99.8 | 99.8 | 0.886 | 99.3 |
| P10 | 99.3 | 99.9 | 1.83 | 98.6 | 99.9 | 100 | 0.873 | 99.5 |
MSCs: Mesenchymal stem cells
Trilineage differentiation of MSCs was induced using the corresponding inducing media for osteogenic, chondrogenic, and adipogenic differentiation and was stained using Alizarin S Red, Alcian blue, and Oil red O staining. MSCs from individual donors and pooled donors were found to be differentiated into osteogenic lineage which is determined by the positive staining for alizarin red [Figure 3], indicating the presence of calcium deposits, adipogenic lineage being determined by the positive staining for Oil Red O [Figure 4], indicating the accumulation of lipid vacuoles and chondrogenic lineage which is determined by the positive staining for alcian blue [Figure 5] indicating the presence of amino glycans at passages P0, P3, P5, P7, and P10, thus showing their ability to differentiate into the trilineages without any alterations till passage 10 both from the individual and the pooled donor MSCs.
 | Figure 3: Osteogenic differentiation of mesenchymal stem cells from individual donors and P1 and P2 pooled donors at Passages P0, P3, P5, P7, and P10. [Click here to view] |
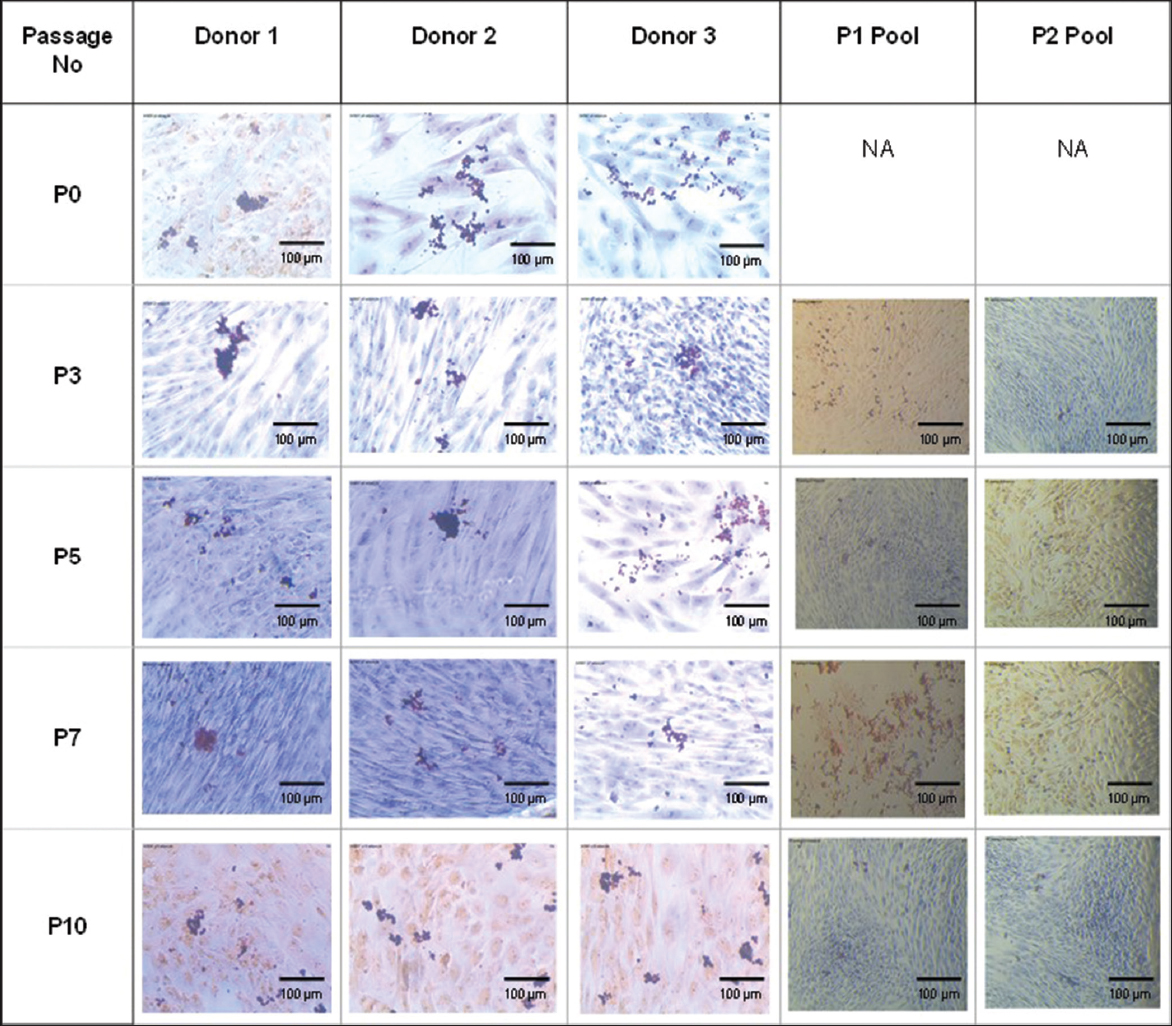 | Figure 4: Adipogenic differentiation of mesenchymal stem cells from individual donors and P1 and P2 pooled donors at Passages P0, P3, P5, P7, and P10. [Click here to view] |
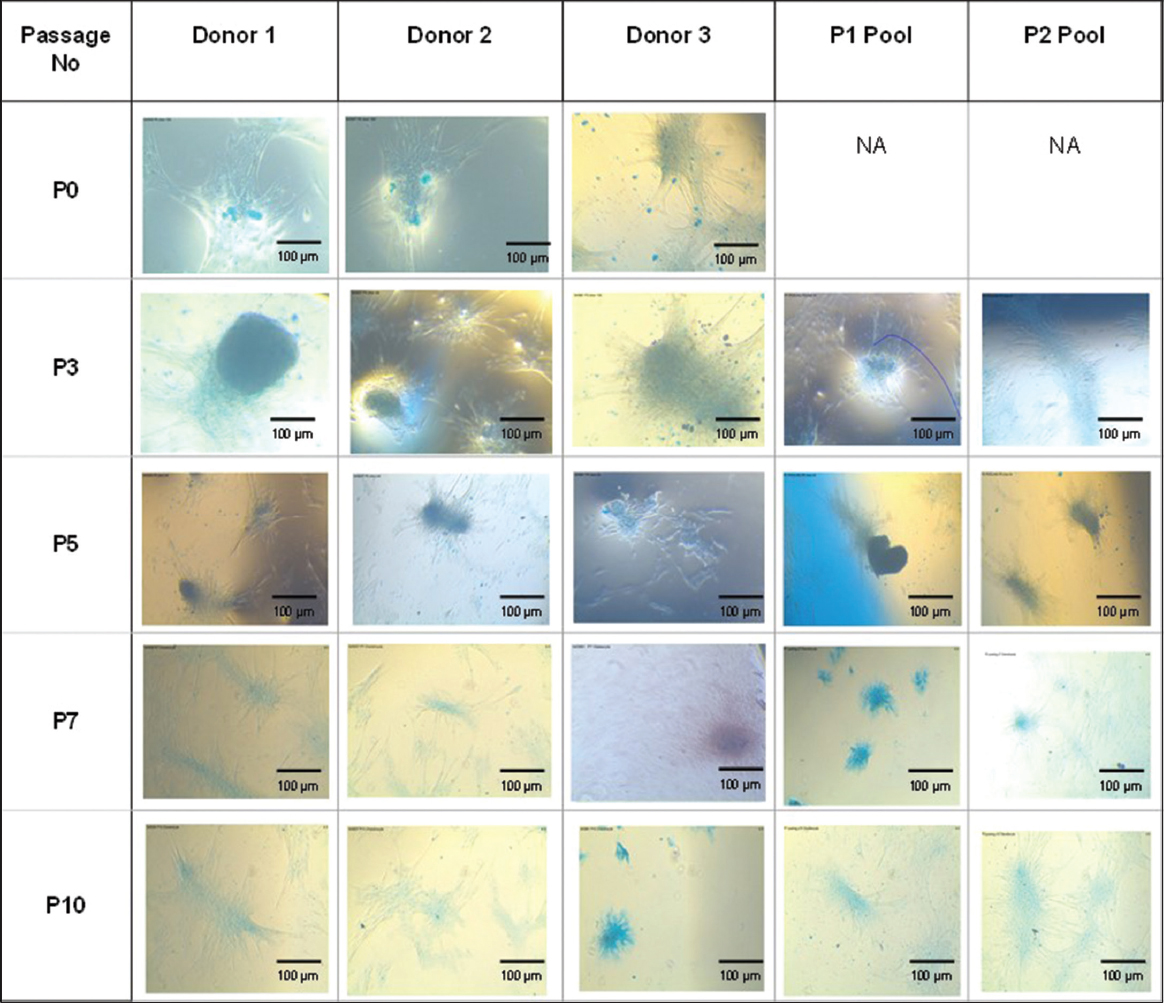 | Figure 5: Chondrogenic differentiation of mesenchymal stem cells from individual donors and P1 and P2 pooled donors at Passages P0, P3, P5, P7, and P10. [Click here to view] |
In accordance with other studies [13,19], the isolated UC-MSCs demonstrated the trilineage differentiation potential and correlated well with the typical positive and negative phenotypic and molecular profile (CD90+, CD105+, and CD31-) [20,21], demonstrating that the protocol used did not change the expression of MSC surface markers during isolation and expansion period till P10 from both individual and pooled donors.
Karyotyping is one of the essential steps in the characterization of UC-MSCs. At all the stages of passages from P0 to P10 from both individual and pooled donors, the chromosomal stability of the isolated UC-MSCs was assessed using traditional cytogenetic analysis detailed in the above methods section. In general, UC-MSCs at all the passages were identified as having normal diploid karyotypes, demonstrating that a typical chromosomal plate consisted of 23 pairs of sister chromatids, and had the conventional appearance of four arms linked at the centromere [Figure 6]. As a result, the separated MSCs retained a normal karyotype and showed no signs of chromosomal aberrations in terms of numbers or structure. The isolated MSCs metaphase is determined to have a typical diploid karyotype. The absence of chromosomal abnormalities in G-banded karyotyping indicates the UC-MSCs continued chromosomal stability on subsequent cell culture passages. Even though karyotyping can identify chromosomal stability in MSCs [22,23], some chromosomal abnormalities call for a more sensitive approach [24].
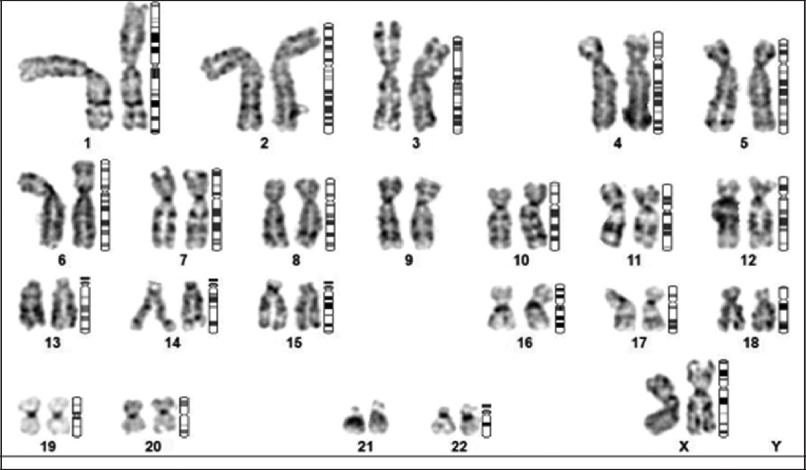 | Figure 6: Representative image showing the absence of abnormal chromosomal structure and number from chromosomal analysis of mesenchymal stem cells from individual and pooled donors. [Click here to view] |
3.3. Expansion of MSCs in Spinner Flask Bioreactor with Microcarriers
As the expansion of MSCs on a large scale is essential in the case of clinical transplantation of large numbers of MSCs, the MSCs at P4 passage were pooled from all the three donors and they were expanded in spinner flask bioreactors. Figure 7 shows the number of viable cell count/mL obtained from day 1 to day 11 and it was observed that there was a steady lag phase in the cell numbers until day 6 and the number of cells started to increase logarithmically from day 7 onwards, thus showing the increased proliferation of the MSCs in the bioreactor. Furthermore, the MSCs were cultured in the xeno-free medium containing hPL, thus making the cells more efficient for transplantation.
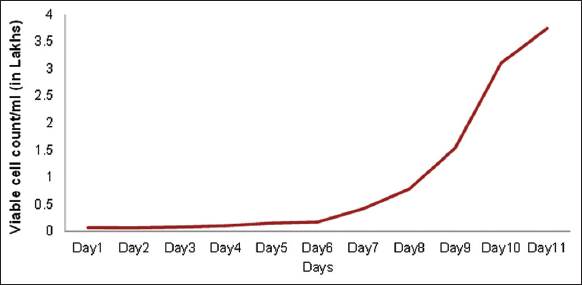 | Figure 7: Viable cell count/mL from pooled P4 cells grown in spinner flask. [Click here to view] |
Studies have shown a comparison of the MSCs grown on a large scale utilizing FBS and hPL and have shown that hPL is found to significantly increase cell proliferation compared to FBS and has also shortened the population doubling time without affecting the cell viability, phenotype, and their differentiation potential [25-28]. At the end of the spinner flask bioreactor, MSCs expanded were characterized for the presence of cell surface markers using flow cytometry, trilineage differentiation potential, and the chromosomal stability which were assessed using karyotyping. MSCs pooled at the P4 passage and expanded in the spinner flask bioreactor showed a population of >98% to be positive for the expression of CD105, CD90, and CD73 whereas <0.5% of the cells expressed the negative marker CD31 with a viability of 93.8% using 7AAD [Figure 8]. It is interesting to note that the expression levels of CD105 are not altered on culturing in the spinner flask bioreactor which contradicts other reports [29-32], while the expressions of other positive and negative markers are in line with other studies. MSCs expanded in the spinner flask have the potential to differentiate into osteogenic, adipogenic, and chondrogenic lineages and this was confirmed by their positive staining for Alizarin red, Oil Red O, and Alcian blue, respectively [Figure 9]. Furthermore, the MSCs expanded in the spinner flask and exhibited normal karyology [Figure 10]. The majority of studies on the expansion of human MSCs, including those utilizing bioreactor systems, have concentrated on the quality assessment of the expanded cells using immunophenotype analysis by flow cytometry and differentiation potentials as the minimum criteria and our study has proven the same for the MSCs expanded in the planar cultures and the spinner flask bioreactors.
 | Figure 8: Characterization of mesenchymal stem cells grown in spinner flask for their cell surface markers using flow cytometry. [Click here to view] |
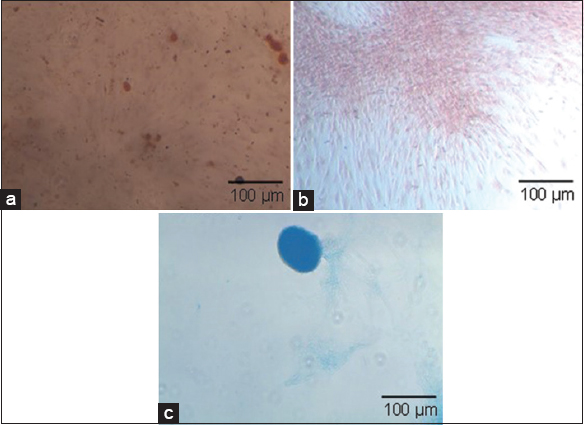 | Figure 9: Trilineage differentiation of pooled P4 mesenchymal stem cells grown in spinner flasks (a) adipogenic differentiation; (b) osteogenic differentiation; and (c) chondrogenic differentiation. [Click here to view] |
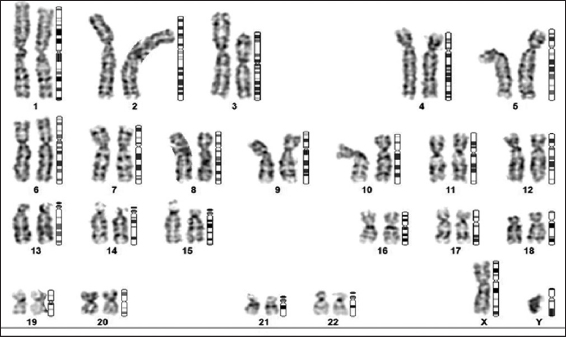 | Figure 10: Chromosomal analysis of the pooled P4 mesenchymal stem cells grown in spinner flask using karyotyping analysis showing the absence of abnormalities. [Click here to view] |
Overall, these findings are consistent with our group’s earlier findings that MSCs primary properties are successfully preserved during their culturing in this xeno-free stirred culture method [12].
4. CONCLUSION
This study illustrates the successful pooling of MSCs from three different donors to reduce the donor-dependent variables without altering the cell viability, characterization in terms of surface markers, trilineage differentiation, and chromosomal abnormalities. Furthermore, culturing the pooled cells in the stir tank reactor enables the large-scale production of the cells for further clinical trials and transplantations.
5. AUTHORS’ CONTRIBUTIONS
Chirayu Padhiar: concept and design, admin, technical or material support, execution, drafting the manuscript; Arvind Kumar Dhanraj and Muthuraman Muthuchamy: Revision of draft, Srividhya Raghavan: Graph plotting; Mayur Abhaya: Data compilation; Wilson Aruni: supervision, and final approval.
6. FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
7. CONFLICTS OF INTEREST
This declaration is made by all the authors: we have no known conflicting interests or personal connections that might have seemed to affect the research documented in this publication.
8. ETHICAL APPROVALS
The study protocol was approved by IAEC (Approval number:Ref:135/IRB-IBSEC /SIST Dated:19th November 2019).
9. DATA AVAILABILITY
Data are available on request to the corresponding author.
10. PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells:A new trend for cell therapy. Acta Pharmacol Sin 2013;34:747-54. [CrossRef]
2. Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells:A new era for stem cell therapy. Cell Transplant 2015;24:339-47. [CrossRef]
3. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells:Their advantages and potential clinical utility. World J Stem Cells 2014;6:195. [CrossRef]
4. Hsieh JY, Fu YS, Chang SJ, Tsuang YH, Wang HW. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of the umbilical cord. Stem Cells Dev 2010;19:1895-910. [CrossRef]
5. Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells 2008;26:591-9. [CrossRef]
6. Beeravolu N, Khan I, McKee C, Dinda S, Thibodeau B, Wilson G, et al. Isolation and comparative analysis of potential stem/progenitor cells from different regions of human umbilical cord. Stem Cell Res 2016;16:696-711. [CrossRef]
7. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation:Mechanisms and therapeutic potential. Trends Pharmacol Sci 2020;41:653-64. [CrossRef]
8. Nguyen LT, Tran NT, Than UT, Nguyen MQ, Tran AM, Do PT, et al. Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum-and xeno-free conditions. Stem Cell Res Ther 2022;13:15. [CrossRef]
9. Karnieli O, Friedner OM, Allickson JG, Zhang N, Jung S, Fiorentini D, et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017;19:155-69. [CrossRef]
10. Hagen A, Holland H, Brandt VP, Doll CU, Häußler TC, Melzer M, et al. Platelet lysate for mesenchymal stromal cell culture in the canine and equine species:Analogous but not the same. Animals 2022;12:189. [CrossRef]
11. Gong W, Han Z, Zhao H, Wang Y, Wang J, Zhong J, et al. Banking human umbilical cord-derived mesenchymal stromal cells for clinical use. Cell Transplant 2012;21:207-16. [CrossRef]
12. Padhiar C, Aruni AW, Abhaya M, Muthuchamy M, Dhanraj AK, Ganesan V, et al. GMP compliant clinical grade and xenofree manufacturing of human Wharton's jelly derived mesenchymal stem cell from pooled donors. Biochem Eng J 2022;184:108470. [CrossRef]
13. Hassan G, Kasem I, Soukkarieh C, Aljamali M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells:Using explant method and umbilical cord blood serum. Int J Stem Cells 2017;10:184-92. [CrossRef]
14. Hendijani F, Sadeghi-Aliabadi H, Javanmard SH. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton's jelly matrix. Cell Tissue Bank 2014;15:555-65. [CrossRef]
15. Yoon JH, Roh EY, Shin S, Jung NH, Song EY, Chang JY, et al. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton's jelly. BioMed Res Int 2013;2013:42?. [CrossRef]
16. Capelli C, Gotti E, Morigi M, Rota C, Weng L, Dazzi F, et al. Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy 2011;13:786-801. [CrossRef]
17. Iftimia-Mander A, Hourd P, Dainty R, Thomas RJ. Mesenchymal stem cell isolation from human umbilical cord tissue:Understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobank 2013;11:291-8. [CrossRef]
18. Widholz B, Tsitlakidis S, Reible B, Moghaddam A, Westhauser F. Pooling of patient-derived mesenchymal stromal cells reduces inter-individual confounder-associated variation without negative impact on cell viability, proliferation and osteogenic differentiation. Cells 2019;8:633. [CrossRef]
19. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007;25:1384-92. [CrossRef]
20. Cooper K, SenMajumdar A, Viswanathan C. Derivation, expansion and characterization of clinical grade mesenchymal stem cells from umbilical cord matrix using cord blood serum. Int J Stem Cells 2010;3:119-28. [CrossRef]
21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [CrossRef]
22. Ben-David U, Mayshar Y, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011;9:97-102. [CrossRef]
23. SensebéL, Tarte K, Galipeau J, Krampera M, Martin I, Phinney DG, et al. Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell 2012;10:9-10. [CrossRef]
24. Chen KG, Hamilton RS, Robey PG, Mallon BS. Alternative cultures for human pluripotent stem cell production, maintenance, and genetic analysis. J Vis Exp 2014;89:e51519. [CrossRef]
25. Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Aziz ZA, Abdullah M, et al. Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy 2011;13:1221-33. [CrossRef]
26. Haack-Sørensen M, Juhl M, Follin B, Søndergaard RH, Kirchhoff M, Kastrup J, et al. Development of large-scale manufacturing of adipose-derived stromal cells for clinical applications using bioreactors and human platelet lysate. Scand J Clin Lab Invest 2018;78:293-300. [CrossRef]
27. Carmelo JG, Fernandes-Platzgummer A, Diogo MM, Da Silva CL, Cabral JM. A xeno-free microcarrier-based stirred culture system for the scalable expansion of human mesenchymal stem/stromal cells isolated from bone marrow and adipose tissue. Biotechnol J 2015;10:1235-47. [CrossRef]
28. Mizukami A, Fernandes-Platzgummer A, Carmelo JG, Swiech K, Covas DT, Cabral JM, et al. Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells. Biotechnol J 2016;11:1048-59. [CrossRef]
29. Mark P, Kleinsorge M, Gaebel R, Lux CA, Toelk A, Pittermann E, et al. Human mesenchymal stem cells display reduced expression of CD105 after culture in serum-free medium. Stem Cells Int 2013;2013:698076. [CrossRef]
30. De Soure AM, Fernandes-Platzgummer A, Moreira F, Lilaia C, Liu SH, Ku CP, et al. Integrated culture platform based on a human platelet lysate supplement for the isolation and scalable manufacturing of umbilical cord matrix-derived mesenchymal stem/stromal cells. J Tissue Eng Regen Med 2017;11:1630-40. [CrossRef]
31. Tozetti PA, Caruso SR, Mizukami A, Fernandes TR, da Silva FB, Traina F, et al. Expansion strategies for human mesenchymal stromal cells culture under xeno-free conditions. Biotechnol Prog 2017;33:1358-67. [CrossRef]
32. Tabatabaei M, Mosaffa N, Nikoo S, Bozorgmehr M, Ghods R, Kazemnejad S, et al. Isolation and partial characterization of human amniotic epithelial cells:The effect of trypsin. Avicenna J Med Biotechnol 2014;6:10-20.