1. INTRODUCTION
The genus Ficus universally known as fig, belongs to the family Moraceae [1] and is one of the largest plant genera, with more than 750 species distributed worldwide [2-4]. In India, 115 species of Ficus are distributed throughout the country, and the maximum diversity of the species lies in the North-East region [5]. Ficus species have a diversity of habitations; few are hemi epiphytes, large woody climbers, and trees and shrubs. Most of the Ficus species are good fodder sources. A large number of vertebrates depend for food on Ficus species [6].
Ficus palmata Forssk. is a multiuse tree that belongs to Moraceae family, found growing wild in the Himalayan region, native of North-Western India and Rajasthan regions, Garhwal and Kumaun region, Uttarakhand, Nepal up to 1550 m above the sea level. F. palmata occasionally occurs in the forest but grows well around the villages, fields, and wastelands. F. palmata is a deciduous tree with minor unisexual flowers and deep violet to black fruit inside which numerous, round, and very small seeds are found. The whole fruit and the seeds can be eaten either in the immature stage by cooking as a vegetable or after maturing as fruit. It is one of the top edible wild fruits with medicinal and nutritional properties [7,8].
Several studies show the importance of F. palmata in treating several diseases [9] but very few on its regeneration status, phenological events, fruit/seed maturity, and germination. Shifting in the timing of fruit seed maturation time can severely impact the regeneration of this species. There appear to be no baseline data to compare the changes in the timing of phenological events of this important species coupled with regeneration information.
Ficus species are wild edible species, and the dependency for food on different species of Ficus is very high. In the Himalayan mountains, regeneration of the fodder/edible fruit species is a significant problem. Regeneration of the species, survival, and growth of seedlings depend upon human disturbance, trampling by cattle, and cattle browsing [10]. The successful regeneration of forest species is characterized by a sufficient population of saplings, seedlings, and adults [11].
We studied the regeneration status of fruit/seed-related traits, the timing of fruit/seed maturation, and germination, which is critically needed to develop baseline data to fully comprehend the impact of climate change on this struggling species. (1) In view of the importance of this species-poor regeneration and scanty information on seed/fruit maturity indices, the present study was planned to document the most suitable time for fruit/seed collection and determines the effect of different collection dates on fruit/seed characters and germination essential for its multiplication. (2) Timing of major phenological events was carried out to determine the impact of climatic change compared to previous studies. (3) Nutrient analysis of leaves and fruits was done to determine the suitability of the leaves/fruits of the species as fodder so that large-scale plantations of the species can be undertaken to meet the needs of green fodder during the lean summer months in the hill areas.
2. MATERIALS AND METHODS
2.1. Study Site
The study areas are located between 29’ 22” N latitude and 79’ 38” E longitude. The research was carried out over 2 years (2017 and 2018). Three sites (S1, S2 and S3) were selected between 1136 m~1704 msl. altitude on northeast and northwest aspect [Table 1]. The associated species were Ficus nemoralis Vern., Quercus leucotrichophora A. Camus, Ficus roxburghii Wall., Grewia optiva Drumm., Pinus roxburghii Sarg., Aesculus indica Wall., Cupressus torulosa D. Don ex Lamb., Prunus cerasoides D. Don., Pyrus pashia Buch. and Cedrus deodara Rox. were found as main associates. The texture of the soil was sandy clay loam across all the three sites. The climate of the study sites is subtropical monsoon with high temperatures towards lower elevation and lower temperatures towards high elevation. Rainfall is governed by the southwest monsoon; annual rainfall was 1337.50~1536.40 mm (approx.) during the study period, of which 90% occurred from mid-June to mid-September. In both years, May was the warmest month of the year and December the coldest month, with temperatures ranging from 2.89°C to 25.9°C (Source: S. M. R. A. G. I. C. Tallital, Nainital).
Table 1: Details of selected sites of Ficus palmata.
| S. No. | Site name | Aspect | Elevation (msl.) | Coordinates | Density (ind/ha) |
|---|---|---|---|---|---|
| 1 | Site1 (bhumiyadhar) (nainital district) | North west | 1704 | N 30°29’70” E 79°13’15” | 66 |
| 2 | Site 2 (gethiya) (nainital district) | North east | 1136 | N 29°22’10” E 79°30’28” | 78 |
| 3 | Site 3 (katyari) (almora district) | North east | 1530 | N 29°35’29” E 79°38’30” | 122 |
2.2. Regeneration Status
For calculating the regeneration of the species 10 quadrats each of 10 × 10 m were placed across all the sites [12]. Density of trees, saplings, and seedlings was estimated following [13].
2.3. Phenology
The phenophases were observed from bud formation to fruit/seed formation. During the field visit, the phenophases were recorded for selected species. To record observation of different phenological events, 05 superior individuals were marked and frequent field visits were made [14]. Phenological records were collected from the last week of March 2017 to the 2nd week of January 2018.
2.4. Maturity Indices
The species produced flowers/fruits twice in the year, Mar-May and Oct-Dec. However, only the data for the period between March and May has been presented. As the fruits from October to December produced non-viable seeds which failed to germinate. Fruit collection was started from the 2nd week of April up to the availability of fruits from marked trees for all three sites at week intervals. Fruits were collected at 1-week intervals directly from the selected trees. Fruits from all trees were combined at one collection date to create a composite sample. Fruits were manually de-pulped to extract seeds. Three replicates of 25 fruits/seeds were taken from the composite sample to determine the different morphological characters of fruits/seeds (size, color, and fresh weight). Fruit and seed weight (100 fruits/seeds) was measured by electronic balance (Model No. PGB 301 accuracy + 0.001 mg Wensar), and fruit and seed size (25 fruits/seeds) were expressed in mm² (length and width) was estimated using (Model No. CD-6” accuracy + 0.02 mm Mitutoyo Co.) digital Vernier caliper. Moisture content% was estimated based on three replicates of fruits and seeds (25) each and assessed on a fresh weight basis by drying at 103 ± 2°C for 16 ± 1 h and then the samples were reweighted according to [15,16]. For germination four replicates of hundred seeds were kept at the top of germination paper in petri-dishes at room temperature. The appearance of the first radicle germination was considered to have started and was studied for 40 days, following [17], the seed germination% was calculated.
 |
The data were analyzed using analysis of variance [18].
2.5. Viability
For viability retention seeds of F. palmata collected at maturity were stored under two conditions in airtight plastic containers placed at room temperature (during day18–26°C and night 3–13°C) and refrigerator (1 and 3°C) [1]. The viability was checked by germinating 3 replicates of 100 seeds every 30 days up to the time seeds germinated (in both year-1 and year-2) [19].
3. RESULTS
3.1. Regeneration Status
The tree density of F. palmata ranged between 22 and 122 ind/ha across all the studied sites. Seedlings were completely absent in all the study sites. Saplings were present in all selected sites except site 1 but the density was low. The total tree density of the site varied between 297 and 554 ind/ha with 1.91 and 26.2 m2/ha−1 TBA across all the sites.
3.2. Phenology
The leaf initiation in F. palmata started in the 3rd week of March and was complete by the 3rd week of April. Flowering and fruiting occurred 2 times a year 1st-time, fruits were visible between the 3rd week of March and the 3rd week of May, and a 2nd time between the 3rd week of October and the 2nd week of December. Seed dispersal also occurred 2 times in a year, and the period of seed dispersal in both seasons was short. The leaf fall started in the 1st week of December and was completed by the 1st week of January [Figure 1].
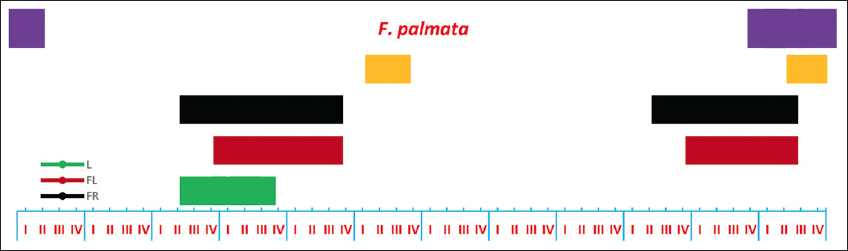 | Figure 1: Phenological events of Ficus palmata (I-IV) weeks of each month I= week 1, II week 2, III = week 3, IV = week 4. L= Leafing; FL = Flowering; FR= Fruiting; SD= Seed dispersal; LF= Leaf fall. [Click here to view] |
 | Figure 2: (a) A tree (10 m), (b) leaf initiation, (c), (e) seed (1.30), (d) leaf fall and seeds of Ficus palmata, (f) fruiting (30.45) (g) flowering. [Click here to view] |
3.3. Fruit/Seed Characteristics
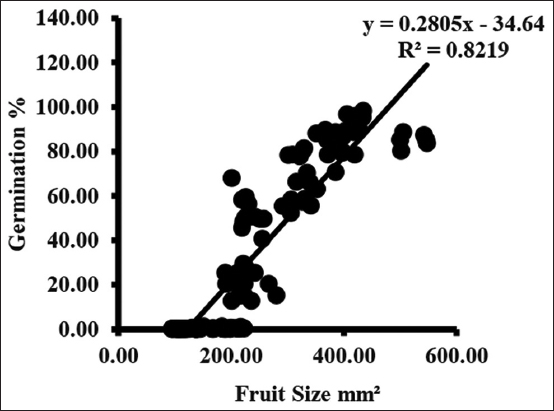
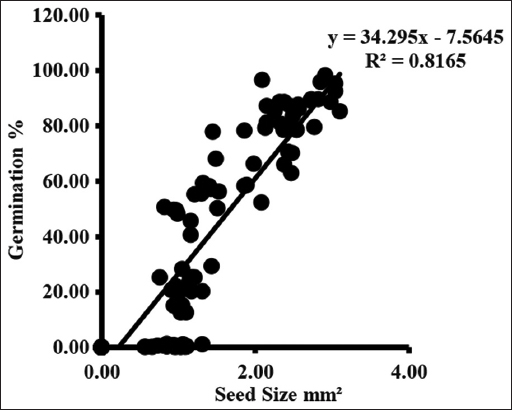
The color of the fruit in the first collection was light green in the 2nd week of April. The fruit turned dark purple at the final collection last May week [Table 2]. From initial to final collection, the fruit size ranged between 103.13 ± 5.37 (S1) and 502.26 ± 1.53 (S3) mm2 across all the sites. The seeds during the first two collections were extremely soft and immature. The mean seed size during the third collection ranged between 0.60 ± 0.03 (S2) and 1.06 ± 0.05 (S2) mm2 across all the sites.
Table 2: Variation in physical parameters of fruits of Ficus palmata over the collection period in Yr-1 (2017) and Yr-2 (2018) for site 1, site 2, and site 3.
| Sites | DOC | CLR | Yr-1 | Yr-2 | ||||
|---|---|---|---|---|---|---|---|---|
| Fruit size mm2 | Weight of 100 fruits (g) | No. of fruits in 100 g | Fruit size mm2 | Weight of 100 fruits (g) | No. of fruits in 100 g | |||
| Site 1 | 11 April | LG | 103.13±5.37 | 43.30±0.85 | 280.0±5.77 | 106.68±0.88 | 43.41±0.83 | 260.00±5.77 |
| 18 April | LG | 140.37±2.46 | 61.50±0.31 | 220.00±5.77 | 141.70±6.04 | 44.46±0.47 | 270.00±8.82 | |
| 25 April | DG | 155.55±6.48 | 115.83±1.53 | 121.67±1.67 | 152.58±1.21 | 61.63±0.34 | 173.33±8.82 | |
| 02 May | P | 221.47±8.96 | 136.97±8.82 | 91.67±6.01 | 201.20±5.70 | 113.90±3.51 | 83.33±3.33 | |
| 09 May | P | 255.10±2.05 | 155.83±3.09 | 77.33±1.20 | 227.99±7.24 | 148.34±2.20 | 57.67±0.88 | |
| 16 May | DP | 386.45±1.23 | 156.40±2.48 | 73.67±2.03 | 373.15±2.22 | 158.07±4.17 | 53.33±3.33 | |
| 23 May | DP | 409.52±5.27 | 157.17±1.97 | 67.33±1.20 | 397.38±1.76 | 160.34±0.74 | 43.33±3.33 | |
| Site 2 | 11 April | LG | 112.56±4.10 | 31.40±2.43 | 323.33±3.33 | 111.78±1.48 | 31.67±2.66 | 313.33±6.67 |
| 18 April | LG | 174.90±6.60 | 96.67±2.40 | 213.33±3.33 | 134.55±4.22 | 43.87±0.41 | 243.33±3.33 | |
| 25 April | DG | 199.76±7.61 | 107.33±4.37 | 121.00±0.58 | 194.37±9.96 | 95.37±4.68 | 113.33±6.67 | |
| 02 May | P | 263.20±1.17 | 137.53±6.87 | 97.00±1.53 | 200.43±9.56 | 109.93±0.29 | 93.33±3.33 | |
| 09 May | P | 301.77±5.01 | 163.63±0.43 | 84.67±2.40 | 220.55±2.62 | 121.25±4.69 | 90.00±5.77 | |
| 16 May | DP | 322.00±6.47 | 167.70±0.15 | 76.33±2.19 | 312.33±3.13 | 158.30±1.67 | 53.33±3.33 | |
| 23 May | DP | 413.47±9.84 | 169.53±7.35 | 69.67±0.33 | 412.34±5.61 | 160.23±3.58 | 53.33±3.33 | |
| Site 3 | 11 April | LG | 111.21±7.25 | 31.73±2.60 | 320.00±5.77 | 106.41±4.07 | 31.37±2.63 | 306.67±6.67 |
| 18 April | LG | 118.97±9.16 | 43.07±0.09 | 256.67±3.33 | 110.20±4.18 | 42.73±0.32 | 246.67±3.33 | |
| 25 April | DG | 211.15±2.36 | 95.47±3.09 | 125.00±2.89 | 204.40±9.71 | 92.23±4.53 | 133.33±3.33 | |
| 02 May | P | 213.22±6.41 | 114.07±3.34 | 123.67±1.86 | 216.34±2.75 | 115.33±2.47 | 113.33±3.33 | |
| 09 May | P | 217.67±8.10 | 141.27±0.07 | 122.00±2.00 | 232.91±3.94 | 143.23±0.41 | 93.33±3.33 | |
| 16 May | DP | 342.16±5.23 | 151.27±0.07 | 70.00±0.00 | 321.85±3.64 | 152.83±1.50 | 53.33±3.33 | |
| 23 May | DP | 502.26±1.53 | 162.30±2.55 | 63.67±1.86 | 414.80±3.61 | 161.17±4.90 | 50.00±0.58 | |
DOC: Denotes date of collection, CLR: Color, DG: Dark green, LG: Light green, P: Purple, DP: Dark purple.
In both the years, the fruit size between initial to the final collection was 103.13 ± 5.37 and 409.52 ± 5.27mm2 throughout collection change in fruit size was 306.39 mm2, and the seed size between initial to the final collection was 0.81 ± 0.04 and 3.00 ± 0.04 throughout collection change in seed size was 2.19 mm2 at (S1). The fruit size between initial to the final collection was 111.78 ± 1.48 and 413.47 ± 9.84 mm2 over the period of collection change in fruit size was 301.69 mm2, and seed size between initial to the final collection was 0.60 ± 0.03 and 2.79 ± 0.12 over the period of collection change in seed size was 2.19 mm2 at (S2). The fruit size between initial to final collection was 106.41 ± 4.07 and 502.26 ± 1.53 mm2 over the period of collection change in fruit size was 395.85 mm2 and seed size between initial to final collection was 1.00 ± 0.05 and 2.44 ± 0.04 over the period of collection change in seed size was 1.44 mm2 at (S3).
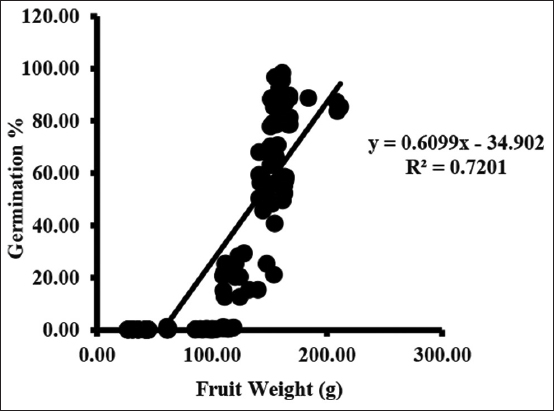
At site 1 the weight of 100 fruits between initial to final collection was 43.30 ± 0.85 and 160.34 ± 0.74 g over the period of collection change in fruit weight was 117.04 g and weight of 100 seeds between initial to final collection was 0.07 ± 0.01 and 0.17 ± 0.01 g over the period of collection change in seed weight was 0.1 g. At site 2 the weight of 100 fruits between initial to final collection was 31.40 ± 2.43 and 169.53 ± 7.35 g over the period of collection change in fruit weight was 138.13 g and weight of 100 seeds between initial to final collection was 0.05 ± 0.01 and 0.25 ± 0.01 g over the period of collection change in seed weight was 0.2 g. At site 3 the weight of 100 fruits between initial to final collection was 31.37 ± 2.63 and 162.30 ± 2.55 g over the period of collection change in fruit weight was 130.93 g and weight of 100 seeds between initial to final collection was 0.06 ± 0.01 and 0.23 ± 0.01g over the period of collection change in seed weight was 0.17 g across both the years. Across all the sites and years the largest fruit size was in (S3) Yr-1. Across all the sites and years the heaviest fruit was recorded at (S2) Yr-1 [Tables 2,3]. Across all the sites and years the largest seed size was in (S1) Yr-2. Across all the sites the heaviest seed was recorded at (S2) Yr-1.
Table 3: Variation in physical parameters of seeds of Ficus palmata over the collection period in Yr-1 (2017) and Yr-2 (2018) for site 1, site 2, and site 3 (Im=Seeds were immature inside the fruit).
| Sites | DOC | CLR | Yr-1 | Yr-2 | ||||
|---|---|---|---|---|---|---|---|---|
| Seed size mm2 | Weight of 100 seeds (g) | No. of seeds in 100 g | Seed size mm2 | Weight of 100 seeds (g) | No. of seeds in 100 g | |||
| Site 1 | 11 April | Im | Im | Im | Im | Im | Im | Im |
| 18 April | Im | Im | Im | Im | Im | Im | Im | |
| 25 April | OW | 0.93±0.07 | 0.07±0.01 | 86733.33±2.03 | 0.81±0.04 | 0.07±0.02 | 86900.00±10.12 | |
| 02 May | OW | 1.00±0.03 | 0.09±0.01 | 94500.00±0.58 | 0.88±0.06 | 0.09±0.01 | 94500.00±7.77 | |
| 09 May | LB | 1.02±0.07 | 0.10±0.01 | 95300.00±1.73 | 0.99±0.10 | 0.11±0.01 | 97100.00±10.97 | |
| 16 May | B | 2.36±0.03 | 0.13±0.01 | 98600.00±4.93 | 2.25±0.07 | 0.12±0.02 | 97700.00±12.17 | |
| 23 May | B | 2.55±0.23 | 0.16±0.01 | 210533.33±6.64 | 3.00±0.04 | 0.17±0.01 | 119600.00±12.17 | |
| Site 2 | 11 April | Im | Im | Im | Im | Im | Im | Im |
| 18 April | OW | 0.60±0.03 | 0.07±0.01 | 75500.00±6.35 | Im | Im | Im | |
| 25 April | OW | 1.00±0.01 | 0.08±0.01 | 76366.67±9.77 | 1.06±0.05 | 0.05±0.01 | 76066.67±9.94 | |
| 02 May | LB | 1.07±0.04 | 0.12±0.01 | 86566.67±9.53 | 1.09±0.12 | 0.07±0.01 | 81966.67±8.41 | |
| 09 May | LB | 1.72±0.26 | 0.16±0.01 | 95933.33±2.03 | 1.23±0.11 | 0.08±0.01 | 85766.67±11.67 | |
| 16 May | B | 2.35±0.11 | 0.18±0.01 | 97600.00±6.93 | 1.54±0.18 | 0.11±0.01 | 94500.00±11.02 | |
| 23 May | B | 2.79±0.12 | 0.25±0.01 | 98100.00±6.08 | 2.63±0.08 | 0.15±0.01 | 96200.00±14.43 | |
| Site 3 | 11 April | Im | Im | Im | Im | Im | Im | Im |
| 18 April | Im | Im | Im | Im | Im | Im | Im | |
| 25 April | OW | 1.00±0.05 | 0.17±0.01 | 85466.67±6.12 | 1.03±0.03 | 0.06±0.01 | 85800.00±10.41 | |
| 02 May | OW | 1.12±0.11 | 0.17±0.01 | 93700.00±5.29 | 1.15±0.02 | 0.09±0.01 | 86133.33±12.72 | |
| 09 May | LB | 1.44±0.06 | 0.19±0.01 | 96400.00±6.35 | 1.42±0.06 | 0.12±0.01 | 94000.00±9.17 | |
| 16 May | B | 2.44±0.03 | 0.22±0.02 | 119600.33±7.54 | 1.76±0.16 | 0.14±0.01 | 96333.33±6.64 | |
| 23 May | B | 2.44±0.04 | 0.23±0.01 | 120133.33±7.86 | 2.42±0.17 | 0.17±0.01 | 210366.67±13.20 | |
DOC: Denotes date of collection, CLR: Color, OW: Off-white, LB: Light brown, B: Brown.
Analysis of variance (ANOVA) showed that fruit size, weight of 100 fruits and number of fruits in 100 g varied significantly across years, sites and dates (P < 0.05). The interactions between year × site, year × date, site × date, and year × site × date were also significant for fruit size, weight of 100 fruits and number of fruits in 100 g. ANOVA showed that size, weight, and number of seeds in 100 g were significant across years and dates (P < 0.05). The interactions between year × site, year × date, site × date and year × site × date were significant for seed size, weight of 100 seeds, and number of seeds (P < 0.05) [Tables 4,5].
Table 4: Analysis of variance (ANOVA) for different fruit parameters across different collection dates (number), sites, and years of Ficus palmata.
| Characters | Source | Type III Sum of Square | df | Mean Square | F-Value |
|---|---|---|---|---|---|
| Fruit size (mm2) | Year | 12354.607 | 1 | 12354.607 | 102.605** |
| Site | 2033.226 | 2 | 1016.613 | 8.443** | |
| Date | 1623433.472 | 7 | 231919.067 | 1.926** | |
| Year×site | 2644.489 | 2 | 1322.245 | 10.981** | |
| Year×date | 6150.654 | 6 | 1025.109 | 8.514** | |
| Site×date | 35470.172 | 12 | 2955.848 | 24.548** | |
| Year×site×date | 30109.453 | 12 | 2509.121 | 20.838** | |
| Weight of 100 fruits (g) | Year | 4475.006 | 1 | 4475.006 | 148.906** |
| Site | 1512.932 | 2 | 756.466 | 25.171** | |
| Date | 301693.971 | 7 | 43099.139 | 1.434** | |
| Year×site | 2563.155 | 2 | 1281.577 | 42.645** | |
| Year×date | 2814.375 | 6 | 469.062 | 15.608** | |
| Site×date | 3114.919 | 12 | 259.577 | 8.637** | |
| Year×site×date | 4427.374 | 12 | 368.948 | 12.277** | |
| Number of fruits in 100 g | Year | 672.071 | 1 | 672.071 | 13.519** |
| Site | 4499.190 | 2 | 2249.595 | 45.251** | |
| Date | 974341.587 | 7 | 139191.655 | 2.800** | |
| Year×site | 1023.857 | 2 | 511.929 | 10.298** | |
| Year×date | 7636.984 | 6 | 1272.831 | 25.603** | |
| Site×date | 22033.365 | 12 | 1836.114 | 36.934** | |
| Year×site×date | 7441.587 | 12 | 620.132 | 12.474** |
NS: Non significant,
Significant at 5% (P<0.05).
Table 5: Analysis of variance (ANOVA) for different seed parameters across different collection dates (number), sites, and years of Ficus palmata.
| Characters | Source | Type III sum of square | df | Mean square | F-value |
|---|---|---|---|---|---|
| Seed size (mm2) | Year | 0.477 | 1 | 0.477 | 18.193** |
| Site | 0.179 | 2 | 0.090 | 3.420NS | |
| Date | 108.211 | 7 | 15.459 | 589.991** | |
| Year×site | 0.455 | 2 | 0.228 | 8.690** | |
| Year×date | 1.179 | 6 | 0.197 | 7.500** | |
| Site×date | 1.984 | 12 | 0.165 | 6.311** | |
| Year×site×date | 0.873 | 12 | 0.073 | 2.778** | |
| Weight of 100 seeds (g) | Year | 0.055 | 1 | 0.055 | 277.648** |
| Site | 0.023 | 2 | 0.012 | 57.752** | |
| Date | 0.515 | 7 | 0.074 | 369.008** | |
| Year×site | 0.028 | 2 | 0.014 | 69.161** | |
| Year×date | 0.014 | 6 | 0.002 | 11.951** | |
| Site×date | 0.020 | 12 | 0.002 | 8.157** | |
| Year×site×date | 0.021 | 12 | 0.002 | 8.886** | |
| Number of seeds in 100 g | Year | 1.172 | 1 | 1.172 | 6.590** |
| Site | 2.066 | 2 | 1.033 | 5.809** | |
| Date | 2.844 | 7 | 4.062 | 2.284** | |
| Year×site | 3.220 | 2 | 1.610 | 9.052** | |
| Year×date | 2.185 | 6 | 3.642 | 2.048** | |
| Site×date | 2.344 | 12 | 1.953 | 1.098** | |
| Year×site×date | 2.771 | 12 | 2.309 | 1.298** |
NS: Non significant,
Significant at 5% (P<0.05).
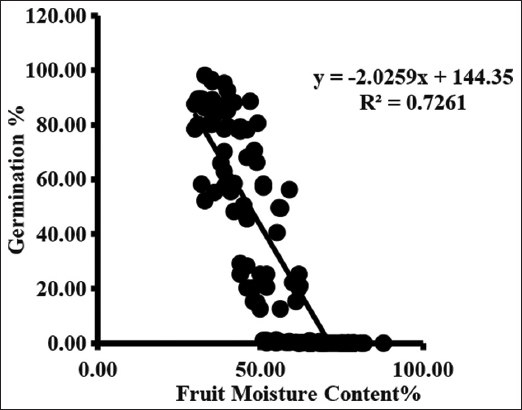
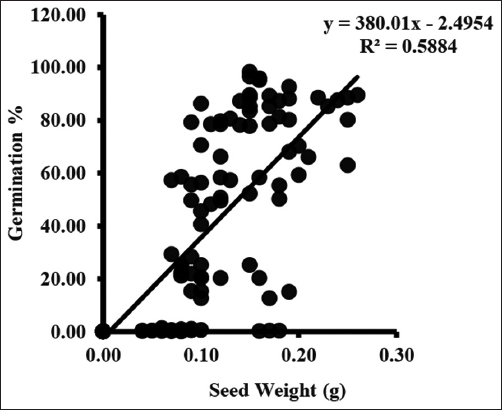
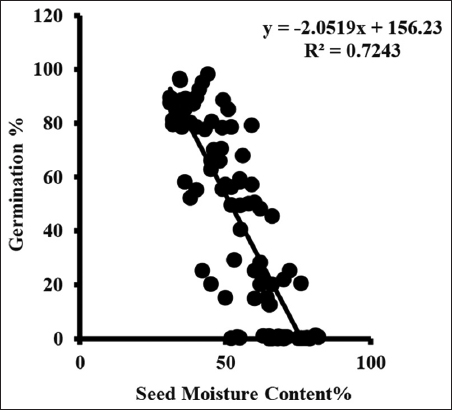
Germination started from the third collection and varied from 0.09 ± 0.02 to 0.69 ± 0.30%. Germination rate increases with each collection and maximum germination occurred in seed of final collection. Maximum germination ranged between 84.68 ± 2.42 and 95.33 ± 1.64% when seed moisture content was between 36.33 ± 0.88 and 42.33 ± 0.88% [Figure 3].
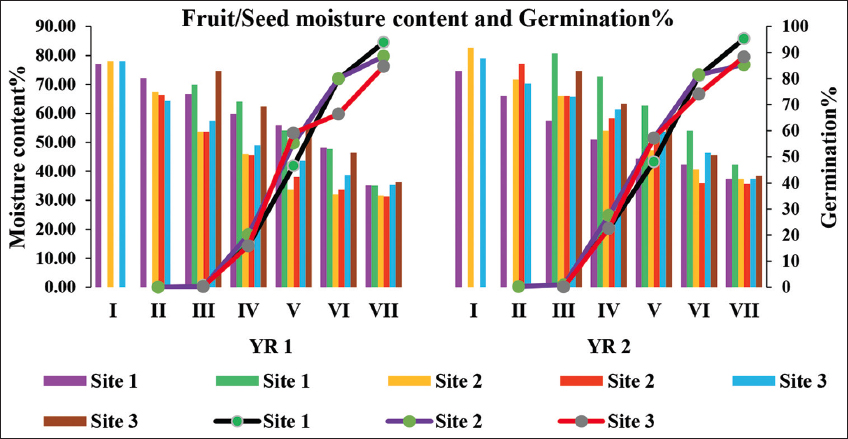 | Figure 3: Fruit/Seed Moisture content% and Germination% of Ficus palmata. (SMC denotes Seed Moisture Content, FMC: Fruit Moisture Content and GER: Germination). [Click here to view] |
In both years germination of stored seed at room temperature declined after 3 months 10.42 ± 1.42% and 10.11 ± 1.09% and germination under room temperature decreased drastically after 60 days in both the years. In both years, there was no germination in seed stored in refrigerator after 6 months. The best temperature for storage was 1–3°C in refrigerator [Figure 4].
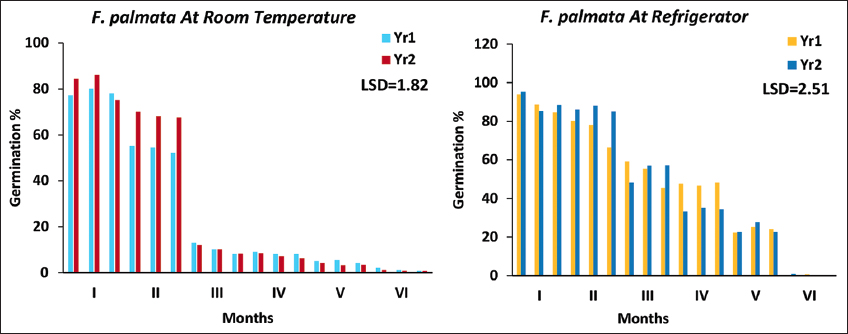 | Figure 4: Germination% at room temperature and refrigerator of Ficus palmata. [Click here to view] |
ANOVA showed that fruit and seed moisture content varied significantly across years, sites and dates (P < 0.05). The interaction between site × date, year × site, and year × site × date was significant for moisture content and germination of fruit and seed (P < 0.05) [Table 6].
Table 6: Analysis of variance (ANOVA) for fruit and seed moisture content and germination across different collection dates (number), sites, and years of Ficus palmata.
| Characters | Source | Type III sum of square | df | Mean square | F-value |
|---|---|---|---|---|---|
| Fruit moisture content % | Year | 283.470 | 1 | 283.470 | 36.927** |
| Site | 256.327 | 2 | 128.163 | 16.695** | |
| Date | 26085.043 | 7 | 3726.435 | 485.431** | |
| Year×site | 1273.090 | 2 | 636.545 | 82.921** | |
| Year×date | 39.114 | 6 | 6.519 | 0.849NS | |
| Site×date | 534.109 | 12 | 44.509 | 5.798** | |
| Year×site×date | 429.705 | 12 | 35.809 | 4.665** | |
| Seed moisture content % | Year | 283.260 | 1 | 283.260 | 51.038** |
| Site | 111.274 | 2 | 55.637 | 10.025** | |
| Date | 75637.651 | 7 | 10805.379 | 1.947** | |
| Year×site | 174.383 | 2 | 87.191 | 15.710** | |
| Year×date | 3707.182 | 6 | 617.864 | 111.327** | |
| Site×date | 5763.137 | 12 | 480.261 | 86.534** | |
| Year×site×date | 5539.661 | 12 | 461.638 | 83.178** | |
| Germination % | Year | 184.598 | 1 | 184.598 | 18.825** |
| Site | 633.800 | 2 | 316.900 | 32.317** | |
| Date | 162532.42 | 7 | 23218.918 | 2.368** | |
| Year×site | 1170.638 | 2 | 585.319 | 59.689** | |
| Year×date | 331.275 | 6 | 55.213 | 5.630** | |
| Site×date | 1363.055 | 12 | 113.588 | 11.583** | |
| Year×site×date | 1136.284 | 12 | 94.690 | 9.656** |
NS: Non significant,
Significant at 5% (P<0.05).